Abstract
Background
Here we ask whether platelet GPIb and GPIIb/IIIa receptors modulate platelet sequestration and activation during GalTKO.hCD46 pig lung xenograft perfusion.
Methods
GalTKO.hCD46 transgenic pig lungs were perfused with heparinized fresh human blood. Results from perfusions in which αGPIb Fab (6B4, 10mg/L blood, n=6), αGPIIb/IIIa Fab (ReoPro, 3.5mg/L blood, n=6), or both drugs (n=4) were administered to the perfusate were compared to 2 additional groups in which the donor pig received DDAVP, 3μg/kg (to pre-deplete pVWF, the main GPIb ligand), with or without αGPIb (n=6 each).
Results
Platelet sequestration was significantly delayed in αGPIb, αGPIb+DDAVP and αGPIb+αGPIIb/IIIa groups. Median lung “survival” was significantly longer (>240 vs. 162min reference, p=0.016), and platelet activation (as CD62P and βTG) were significantly inhibited, when pigs were pre-treated with DDAVP, with or without αGPIb Fab treatment. Pulmonary vascular resistance rise was not significantly attenuated in any group, and was associated with residual thromboxane and histamine elaboration.
Conclusions
The GPIb-VWF and GPIIb/IIIa axes play important roles in platelet sequestration and coagulation cascade activation during GalTKO.hCD46 lung xenograft injury. GPIb blockade significantly reduces platelet activation and delays platelet sequestration in this xenolung rejection model, an effect amplified by adding αGPIIb/IIIa blockade or depletion of VWF from pig lung.
Keywords: lung, xenotransplantation, ex-vivo perfusion, coagulation, platelet activation, GPIb, GPIIb/IIIa, Glycoprotein, Fab, GalTKO.hCD46
Introduction
In both in vivo transplantation and ex vivo perfusion models, introduction of α-galactosyl-transferase knockout (GalTKO) and human complement (e.g. hCD46, hCD55, hCD59) or coagulation (e.g. hCD39, hTBM, hEPCR) pathway regulatory gene additions have contributed to substantially improved performance of xenogeneic organs in recent years (1-4). Particularly for the lung, even with these modifications, activation and sequestration of platelets and leukocytes remain prominent, implicating them in GalTKO.hCD46 lung xenograft injury and failure. Swine lung vasculature is particularly prone to releasing large amounts of von Willebrand Factor (VWF) (5). Under “physiologic” conditions, human platelet glycoprotein Ib (GPIb) only becomes adhesive to human VWF in presence of shear stress (6) or in presence of thrombin and low shear stress (7). In contrast, non-human primate and human GPIb receptors bind pig VWF even in absence of shear stress. As a consequence, primate platelets adhere to pig endothelium, and become activated, without a requirement for shear stress (8-10) or GPIb conformational pre-activation. Targeting this pathway is one logical approach to prevent “non-physiologic” adhesion of primate platelets to porcine endothelium. Biologically appropriate (“physiologic”) platelet adhesion mechanisms also presumably contribute to platelet sequestration when pig lungs encounter human blood, driven by various pro-coagulant and cell activation events attendant to this interaction.
Various approaches have been evaluated to target the GPIb-VWF axis in xenotransplant models. TMVA, a GPIb-binding protein from the venom of Trimeresurus mucrosquamatus, and aurin tricarboxylic acid (ATA) have shown efficacy to prevent thrombosis and prolong survival in several xenograft models (10-13). However, these compounds are not specific and can be associated with significant side-effects when injected to baboons (14). VWF deficient pigs, developed to study one form of human hemophilia, were initially evaluated in context of early xenograft models using wild type pigs (15-17), and demonstrated an important role for VWF in lung xenograft injury (17). However, the bleeding phenotype associated with complete VWF deficiency is a significant barrier to introducing such modification into modern genetically engineered pig colonies developed for xenotransplantation. Partial depletion of porcine endothelial VWF-stores by pretreatment of donor swine with 1-deamino-8-d-arginine vasopressin (DDAVP), an analog of vasopressin, was validated as a way to reduce the content of VWF in pulmonary xenografts using a clinically relevant drug (18-20). Importantly, a humanized αGPIb Fab fragment, 6B4, which selectively blocks the binding site of platelet GPIb by VWF, appears safe and effective to block GPIb-mediated platelet activation in an arterial thrombosis injury baboon model (21-22).
Here we explore how GPIb blockade, DDAVP, and inhibition of GPIIb/IIIa (fibrin-mediated ligation of platelets) influence the platelet sequestration and activation that is otherwise characteristic of pig lung xenograft rejection by human blood.
Methods and Materials
Animals
Genetically modified pigs (6-15kg) lacking the alpha-Gal epitope (GalTKO) and expressing human membrane cofactor protein (hCD46) were provided by Revivicor (Blacksburg, VA). The generation of these GalTKO.hCD46 pigs (23) and their evaluation in the lung perfusion model (3) have been previously reported; results are shown here as reference group. All procedures were approved by the IACUC at the University of Maryland School of Medicine, and were conducted in compliance with NIH guidelines for the care and use of laboratory animals.
Surgical Procedure /Lung Harvest
Donor pig anesthesia and surgical organ dissection were performed as previously described (24-25). Prior to flushing the lungs with pneumoplegia (1L; Perfadex, XVIVO, Gothenburg, Sweden), 1-benzylimidazole (5mg/kg BW; Sigma-Aldrich, St. Louis, MO, a thromboxane synthase inhibitor), and synthetic prostacyclin I2 (Remodulin (Treprostinil); 0.06mg/kg BW; United Therapeutics, Silver Springs, MD or Flolan (Epoprostenol); 0.03mg/kg BW; GlaxoSmithKline, Research Triangle Park, NC) were administered intravenously and allowed to circulate for several minutes.
Lung Perfusion
Left and right lungs were separately perfused via the pulmonary artery using side-by-side circuits fashioned from silicon tubing and polyurethane connectors as previously described (25-26). Pulmonary artery flow was measured and recorded with a flowmeter (Transonic Systems, Model TTFM 73-0146, Ithaca, NY). The Digimed System Integrator (Micro-Med, Louisville, KY) continuously recorded PA- and airway pressures via transducers integrated into the perfusion (Ismatec MCP roller pump, IDEX, Wertheim, Germany) and ventilator (Harvard apparatus respirator, model 613, Cambridge, MA) circuits, respectively, using PowerLab 16/35 and LabChart 7 pro (AD Instruments, Colorado Springs, CO). Lung failure was defined as rise of pulmonary vascular resistance (>600 mmHg*min/L), or loss of vascular barrier function, manifest by development of gross tracheal edema prohibiting lung ventilation, loss of oxygen transport (step-up in blood oxygen content across the lung), or loss of >85% of starting reservoir perfusate volume, reflecting massive intraparenchymal sequestration. All results obtained after a lung met failure criteria were censored from analyses of data at subsequent time points.
Perfusate Preparation
Healthy human volunteers donated approximately 450mL of whole blood into a blood collection bag containing citrate phosphate dextrose adenine (CPDA-1). Two units of blood were mixed with four units (~240mL each) of ABO type-compatible thawed plasma. Total initial volume was approximately two liters. After the perfusate was heparinized (3IU/mL blood, Heparin Sodium Injection, Sagent, Schaumburg, IL), CPDA-1 was neutralized by adding CaCl2 (1.3 to 1.6mg/mL blood, American Regent, Shirley, NY), and sodium bicarbonate (~0.84mg/mL blood, Hospira, Lake Forest, IL) was added to achieve physiologic pH. Blood, plasma and drugs were mixed thoroughly prior to dividing the total volume into two parts that were added into the reservoirs of the perfusion circuits in proportion to average relative lung weight (60% to the right lung circuit).
αGPIb Fab Dose Determination
Blood was obtained from healthy, aspirin free human volunteers in sodium citrate (0.011M). Porcine platelet poor plasma (PPP) was prepared by centrifugation and frozen at −80C until use. Human platelet-rich-plasma (PRP) and PPP were obtained by centrifugation. Human platelet aggregation (in hPRP) by PPP from wild-type (WT) and GalTKO.hCD46 pigs (as a source of pVWF) was assessed in a dose-dependent fashion by humanized 6B4 (experiments done by H Deckmyn for WT and Revivivor for GalTKO plasma).
Experimental Groups
GalTKO.hCD46 lungs were perfused with fresh heparinized human blood to which αGPIb 6B4 Fab (n=6) or αGPIIb/IIIa Fab (abciximab [ReoPro], Janssen, Leiden, Netherlands; 3.5mg/L of perfusate; n=6) was added. A third experimental group (n=4) was given the combination of αGPIb and αGPIIb/IIIa at identical dosages. In two further experimental groups (n=6 each), the donor pigs received DDAVP pre-treatment (Desmopressin Acetate, Diamondback Drugs, Scottsdale, AZ; 3μg/kg BW IV on day −1 and prior to organ procurement) and the pig lungs were either perfused with untreated blood or with blood that was treated with αGPIb Fab at the dose listed above. Previously reported untreated GalTKO.hCD46 lungs (n=37) are shown here as reference group (3).
Sampling Regimen
Blood samples were collected after mixing the blood components to establish baseline (“pre”), as well as after adding drug treatment to the experimental group (“post”). To detect and control for any impact the perfusion system on cell activation and sequestration, after collection of “pre” and “post” samples from the pooled blood in each circuit, an additional reference sample was collected after the blood mixture was circulated and warmed in the perfusion circuit for at least 3 minutes (“R0”). The R0 sample served as the reference (“baseline”) to which later samples are compared. The circuit has no consistent effect on parameters reported (“pre” or “post” vs “R0”; data not shown), while the paired lung perfusion system (comparing a drug-treated circuit to the otherwise identical contralateral untreated circuit) largely controls for artifacts attributable to the model. After centrifugal separation of pulmonary vein effluent collected at 5, 15, 30, 60, 120 and 240min following the start of perfusion, serum and plasma samples were stored at −70°C.
Lung tissue samples were collected pre-perfusion, as well as at 10, 30, 60, 120, and 240min following the start of the perfusion, and processed as described below.
Hematologic Analysis
Blood cell counts were performed on perfusate collected in ethylenediaminetetraacetic acid (EDTA) using standard automated techniques (Antech Diagnostics, Rockville, MD). Platelet and neutrophil counts are presented as the percentage of cells remaining compared to baseline (time 0 sample).
Enzyme-Linked Immunosorbent Assays
Beta-thromboglobulin (βTG) and prothrombin fragments 1+2 (F1+2) were measured by commercial enzyme-linked immunosorbent assay (ELISA) in plasma samples collected in CTAD tubes as previously described (24). EDTA plasma was used to measure histamine (ELISA kit Starfish, Gernusco, Italy) and C3a levels. Because the manufacturer of C3a kits recently modified their ELISA to the C3a Plus version (Microvue Complement C3a Plus, Quidel, San Diego, CA), archived GalTKO.hCD46 samples were reanalyzed for this study. Blood, collected at 3 time points (0, 15 and 60min) in EDTA tubes containing 100μl (at 10μg/ml) of meclofenamate (Sigma-Aldrich, St.Louis, MO), was analyzed to measure plasma levels of thromboxane B2 (TXB2 EIA Kit, Cayman Chemical Company, Ann Arbor, MI; Catalog No. 519031).
Flow Cytometry
After collection from the pulmonary vein, blood samples were rapidly fixed in 1% paraformaldehyde and kept at 4C. Within 1-5 days, fixed platelets were washed and 1×106 platelets were stained with monoclonal antibodies against CD41 (platelet marker) (AbD Serotec, Raleigh, NC) and P-selectin (CD62P, activated platelet marker) (BD Pharmingen, San Diego, CA) following the manufacturer’s protocol. In experiments in which αGPIIb/IIIa therapy was used, an antibody against human CD61 (BD Pharmingen) was used instead of CD41 to avoid competition with ReoPro. Two experiments in both αGPIIb/IIIa groups were not informative due to lack of CD61 staining. Samples were acquired on a FACSCalibur (BD Biosciences, San Jose, CA). Platelets were identified by size and CD41/CD61 staining, and then analyzed for expression of CD62P. Results were expressed as percentage of CD62P-positive cells among CD41-positive cells.
Measurement of Wet/Dry Ratio
West zone 2 (located approximately 3cm above the heart) lung tissue weight was measured immediately at experiment termination (“wet”), and after drying, to measure the wet/dry weight ratio (WDR), and compared to lungs obtained from GalTKO.hCD46 pigs euthanized at Revivicor.
Histology
Lung biopsy specimens obtained during the perfusion and terminal samples were trisected, and processed as previously described (24). Formalin-fixed tissue sections from biopsies collected after 60 or 240 minutes were stained with hematoxylin-eosin to assess the incidence of thrombosis, edema and hemorrhage.
Measurement of αGPIb Plasma Levels
Plasma levels of 6B4 Fab were determined by ELISA. In brief, microtiter plates were coated overnight with Fab specific goat anti-mouse IgG (Sigma) (5μg/mL in PBS) and plates were blocked with 3% milk in PBS (2h at room temperature). Two fold serial dilutions of plasma samples were added (starting dilution 1:10, 1.5h at 37°C). Bound 6B4 Fab was detected with HRP-labeled polyclonal goat anti-mouse IgG/IgM/IgA (Sigma) (1:1000, 1h at room temperature) and HRP was visualized via Optical path difference (OPD). Antibodies were diluted in PBS containing 0.3% milk and plates were washed with PBS containing 0.1% Tween 20. A standard curve of known 6B4 Fab concentrations in plasma was used to calculate 6B4 Fab concentrations in experimental samples.
PCR Analysis
Pre-perfusion tissue from DDAVP-treated (n=6) and untreated lungs (n=7) was analyzed for porcine mRNA expression levels of cyto- and chemokines (IL1b, IL6, IL8, TNFα, MCP1), adhesion molecules (CD54, VCAM-1, E- and P-Selectins) and complement factors (C5AR1). In addition mRNA levels of human CD46 were assessed.
Statistical Analysis
Statistical analyses were performed using the statistical package SigmaPlot for Windows (Version 11.0). Unless otherwise indicated, continuous variables were expressed as the mean plus SEM. Variables that were not normally distributed were analyzed using the Mann-Whitney non-parametric test. Those that were normally distributed were assessed with the student t-test. P-values <0.05 were considered statistically significant.
Results
αGPIb Fab Dosing
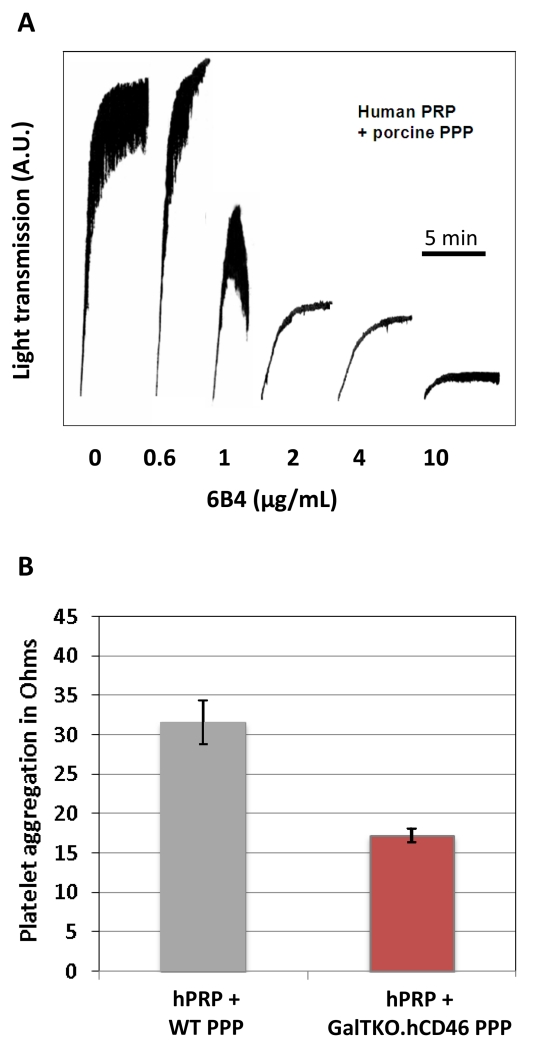
Human platelet aggregation by PPP from WT pigs was inhibited by humanized 6B4, with >90% at 10μg/ml, and >70% inhibition at 2 and 4μg/ml [Fig.1A]. Similar results were obtained using human washed platelets with porcine PPP (data not shown). GalTKO.hCD46 PPP also triggered platelet activation, although platelet aggregation tended to be lower with GalTKO.hCD46 PPP [Fig.1B]. No aggregation was detected with human PPP in the same conditions. Based on these results, a target 6B4 concentration of 10mg/L was chosen to obtain efficient inhibition of GPIb-dependent platelet aggregation.
Figure 1.
Human platelet aggregation by porcine platelet-poor-plasma (PPP). (A) h6B4 inhibits spontaneous aggregation of human platelets by wild-type (WT) porcine PPP in a dose-dependent fashion. Results expressed as light transmission units. (B) Porcine GalTKO.hCD46 PPP also triggers human platelet aggregation, but to a lesser extent compared to WT. Results expressed as impedance units. No aggregation was seen with autologous human PPP. h6B4 also inhibits aggregation of human platelets by GalTKO.hCD46 plasma at 10mg/L (data not shown).
αGPIb Plasma Levels
Initial αGPIb trough levels, measured in samples that were collected after αGPIb administration but before perfusion of the xenogeneic lung, ranged from 4.3 to 11.4μg/ml (average 8.2μg/ml). Final trough levels, measured in blood at the time of lung failure (20, 25, 60, 240, 240, 240min), showed values from 4.8 to 9.9μg/ml (average 7.8μg/ml). Comparing the initial and the final trough levels of free αGPIb within each experiment shows that on average 98.5% (range 82-110%) of the initial Fab is still detectable in the blood at the final time point.
Graft Survival and Function
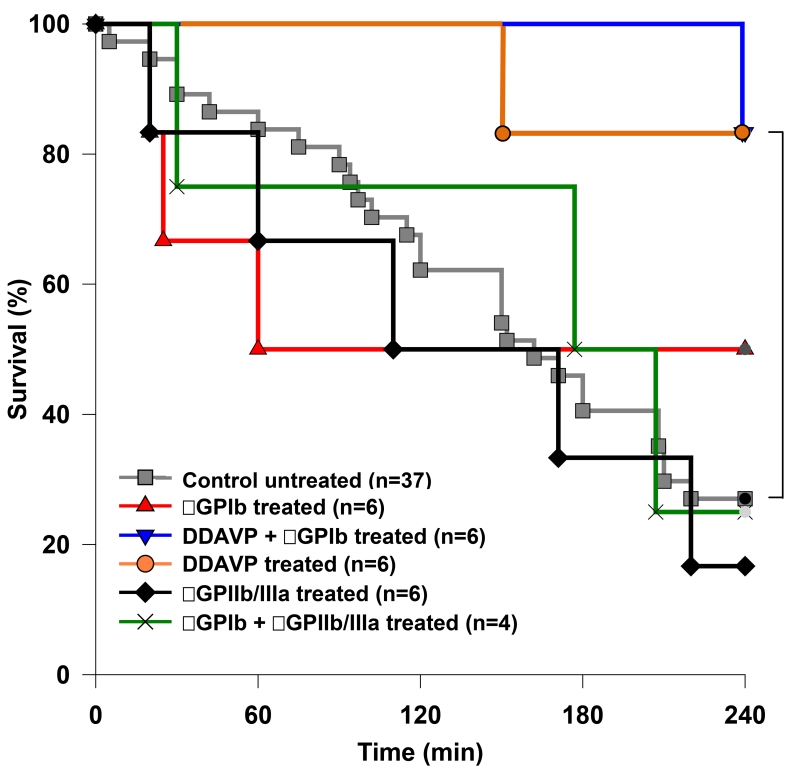
DDAVP-pretreated GalTKO.hCD46 lungs exhibited significantly prolonged survival compared to the reference group (GalTKO.hCD46 with no additional drugs) median survival time (MST) 240 vs. 162min, p=0.016 [Fig.2]). Only one lung in each of the αGPIb+DDAVP and DDAVP groups failed before elective termination at four hours, and in each instance was terminated due to elevated PVR. 50% of the lungs treated with αGPIb Fab alone reached elective termination (MST 150min). The addition of αGPIIb/IIIa Fab did not result in a significant survival prolongation whether in combination with αGPIb or when used alone (MST 191 and 141min, respectively).
Figure 2.
Cumulative survival time of ex vivo perfused lungs. Lung “survival time” represents the time of perfusion at which a lung failure endpoint was met, based on the pre-defined criteria. In groups where the donor pigs were pre-treated with DDAVP, lung survival was significantly prolonged compared to the untreated reference group (MST 240 vs. 162min, * p=0.016).
Table 1 demonstrates that rise in PVR (a surrogate for right heart failure) and loss of vascular barrier function (primarily development of tracheal edema) were the predominant failure modes in lungs treated with various drugs tested here.
Table. 1.
Summary of lung failure mechanisms in the different treatment groups. The table shows that the loss of blood flow through the lungs (elevated PVR) was the predominant lung failure mechanism, followed by the development of trachea edema.
| Control | αGPIb | DDAVP | αGPIb + DDAVP | αGPIIb/IIIa | αGPIb + αGPIIb/IIIa | |
|---|---|---|---|---|---|---|
| Total (n) | 37 | 6 | 6 | 6 | 6 | 4 |
| Failed lungs (n) | 27 | 3 | 1 | 1 | 5 | 3 |
| Rejection reason | n (%) of failed lungs | |||||
|---|---|---|---|---|---|---|
| Trachea edema | 7 (19%) | 2 (33%) | - | - | 1 (17%) | 1 (25%) |
| Loss of perfusate | 2 (5%) | - | - | - | - | - |
| Loss of blood flow | 15 (41%) | 1 (17%) | 1 (17%) | 1 (17%) | 4 (67%) | 2 (50%) |
| Oxygen failure | 3 (8%) | - | - | - | - | - |
The table shows that the loss of blood flow through the lungs (elevated pulmonary vascular resistance) was the predominant lung failure mechanism, followed by the development of trachea edema.
DDAVP, desmopressin (1-desamino-8-d-arginine vasopressin).
Pulmonary Vascular Resistance
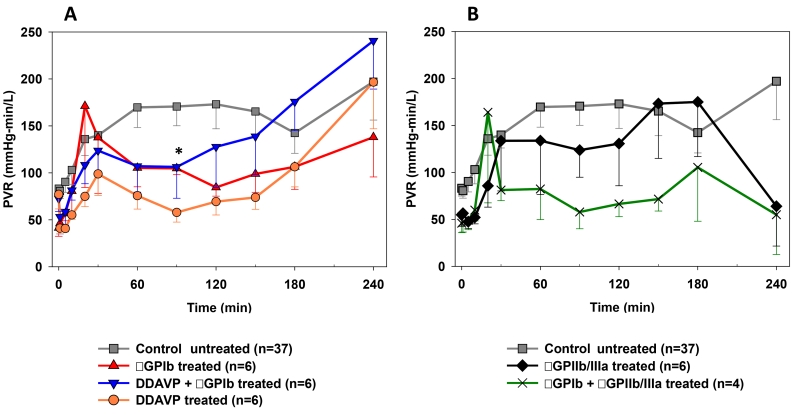
Although each study regimen was associated with a trend toward lower PVR at most time points relative to the reference group, only at the 90 minutes time point the PVR was significantly lower in the DDAVP group when compared to the control experiments (58±10 vs. 171±10mmHg*min/L, p=0.02) [Fig.3A]. Despite the small number of observations, a trend toward reduced pulmonary vascular resistances was also seen in the αGPIb+αGPIIb/IIIa group between 60 and 150 minutes (e.g. at 90min, 58±18 vs. 171±10mmHg*min/L, p=0.09) [Fig.3B].
Figure 3.
Pulmonary vascular resistance (PVR), expressed as a function of time for control and study groups. Time 0 represents measurements obtained during the first minute of lung perfusion. PVR between 60 and 150min of perfusion showed a trend to lower values in the DDAVP (A) and the αGPIb+αGPIIb/IIIa (B) groups, reaching statistical significance only at the 90 min time point in the DDAVP group (* p=0.02).
Complement Activation
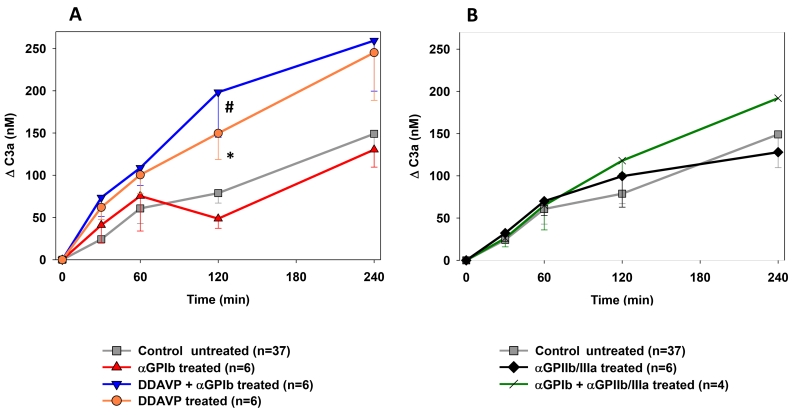
In experiments in which αGPIIb/IIIa or αGPIb were given alone or in combination to the perfusate, complement activation was similar to that found in reference experiments [Fig.4]. The pre-treatment of donor pigs with DDAVP resulted in significantly higher C3a elaboration compared to experiments without pre-treatment (e.g. C3a at 120min αGPIb+DDAVP 198±53 vs. control 79±12, p=0.002; DDAVP 149±31ng/mL vs. control p=0.018) [Fig.4A].
Figure 4.
Plasma levels of the complement activation byproduct C3a, expressed as the concentration of complement fragments produced above the pre-perfusion baseline. Complement activation was significantly higher at 120 min of perfusion in the DDAVP (* p=0.018) and αGPIb+DDAVP groups (# p=0.002) when compared to the reference group (A). αGPIIb/IIIa treatment groups showed similar complement activation to the control (B).
Platelet Sequestration and Activation
Initial absolute platelet numbers ranged from 98 to 124 K/μl; there was no significant difference between groups in initial platelet counts, or activation profile within any group.
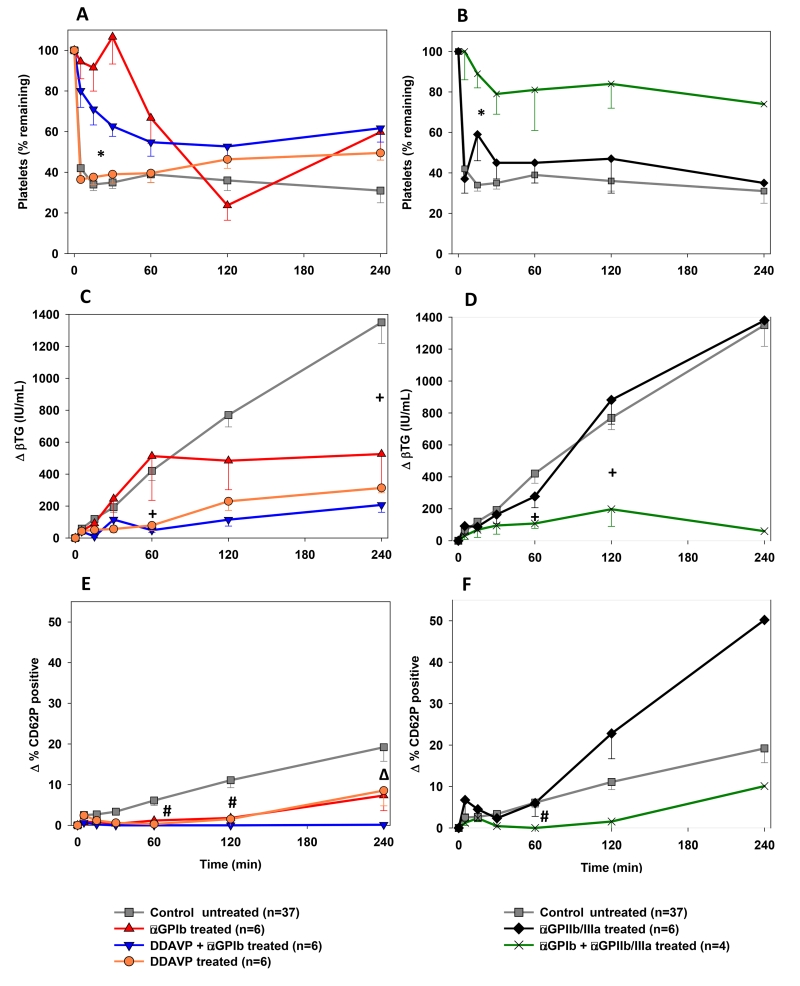
The addition of αGPIb to the perfusate resulted in significantly less platelet sequestration during the first 30 minutes of perfusion in all αGPIb-treated groups. While platelet counts in the αGPIb alone group dropped at subsequent intervals to values similar to those in the reference group [Fig.5A], additional DDAVP pre-treatment of the pigs tended to inhibit this later decline in platelet counts, which remained stable between hours 1 and 4 (% initial platelets remaining at 120min control 36±5 vs. αGPIb 24±10 vs. αGPIb+DDAVP 53±6%, p=0.1 vs. control). Platelet sequestration was largely prevented by adding αGPIb and αGPIIb/IIIa to the perfusate [Fig.5B]: thrombocyte counts remained above 75% of initial counts throughout perfusion (e.g., αGPIb+αGPIIb/IIIa 84±12 vs. control 39±4% at 120min; p=0.015). Neither DDAVP pre-treatment nor αGPIIb/IIIa administration alone significantly attenuated platelet sequestration.
Figure 5.
Platelet and coagulation cascade activation profile. (A, B) Blood platelet counts measured serially by automated counting. Platelet sequestration was expressed as the percentage of platelets remaining in the perfusate at each time point. The addition of αGPIb Fab prevented the initial platelet count drop found at 5 min in non-αGPIb-treated groups and kept platelet sequestration significantly lower during the first half hour of perfusion (*). The combined αGPIb+αGPIIb/IIIa treatment (B) kept platelets at about 80% of the initial count throughout the perfusion time. (C, D) Platelet activation assessed by plasma levels of βTG released from platelet granules. All treatment groups, but the αGPIIb/IIIa alone group, showed significantly reduced βTG values at the 4 h time point or earlier when compared to the reference group (+). (E, F) Platelet activation assessed by measuring the proportion of CD41+ or CD61+ platelets expressing CD62P by flow cytometry. # p<0.05 for DDAVP, αGPIb+DDAVP and αGPIb+αGPIIb/IIIa, Δ p<0.001 for αGPIb+DDAVP vs. reference.
Total (sequestered and circulating) platelet activation, as plasma beta-thromboglobulin (βTG) elaboration, was similar in the reference, αGPIIb/IIIa, and αGPIb monotherapy groups [Fig.5C] (the 4h comparison, 526±218 αGPIb vs. control 1350±133IU/mL, had too few values in either group to allow calculation of statistical significance). βTG increase was significantly inhibited with DDAVP pre-treatment (αGPIb+DDAVP 115±40 vs. control 770±74IU/mL at 120min, p<0.001; DDAVP 230±57IU/mL, p=0.002 vs. control) [Fig.5C] or when αGPIb was combined with αGPIIb/IIIa (197±108 IU/mL at 120min, p=0.006) [Fig.5D] relative to the reference group.
Circulating platelet activation, as measured by CD62P expression, was decreased by DDAVP alone, and in association with αGPIb Fab treatment compared to the untreated reference group [Fig.5E,F]. For example, ΔCD62 at 120min was 1.5±1.5 for DDAVP monotherapy, significantly below the reference (10.2±1.6%, p=0.01); at that interval, αGPIb+DDAVP was 0.1±0.1 (p<0.01), αGPIb+αGPIIb/IIIa measured 1.6±1.5 (too few values for p calculation) and aGPIb monotherapy 2.7±1.4 for αGPIb (p=0.09). With αGPIIb/IIIa monotherapy, platelets showed an increase in CD62P expression during the first 60 minutes not significantly different from that observed in the control group [Fig.5F].
Other parameters associated with lung xenograft failure
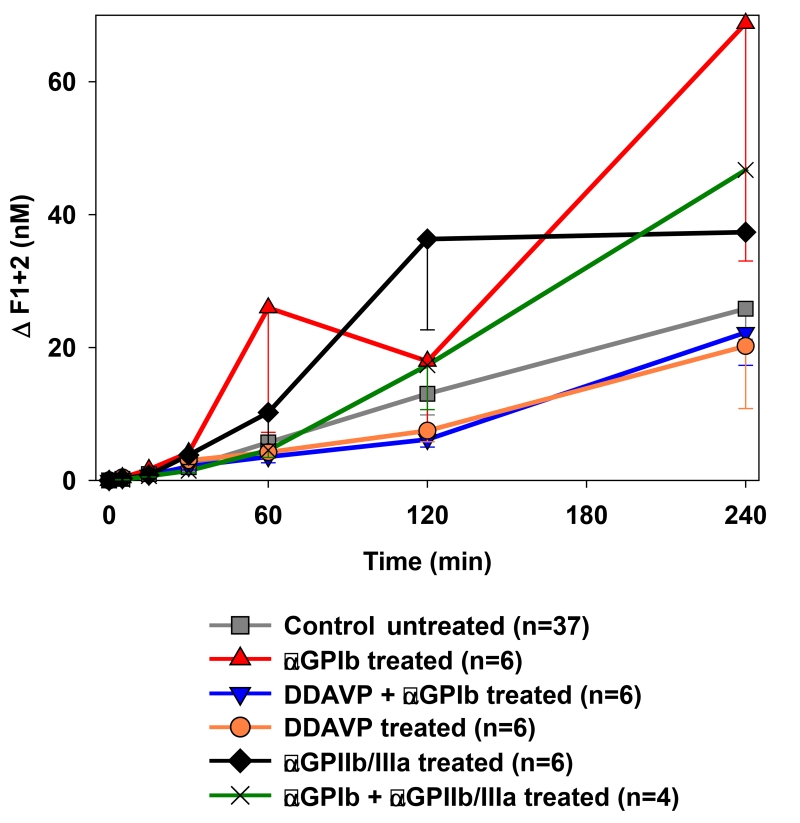
Although lungs from pigs treated with DDAVP tended to be associated with lower thrombin formation (e.g. ΔF1+2 at 2h: αGPIb+DDAVP 6.2±1.2 vs. control 13.0±3.2nM, p=0.31), no significant difference in thrombin formation was detected between groups [Fig.6].
Figure 6.
Activation of the coagulation cascade was detected by the formation of thrombin measured by plasma levels of fragments F1+2. No significant differences were detected between groups.
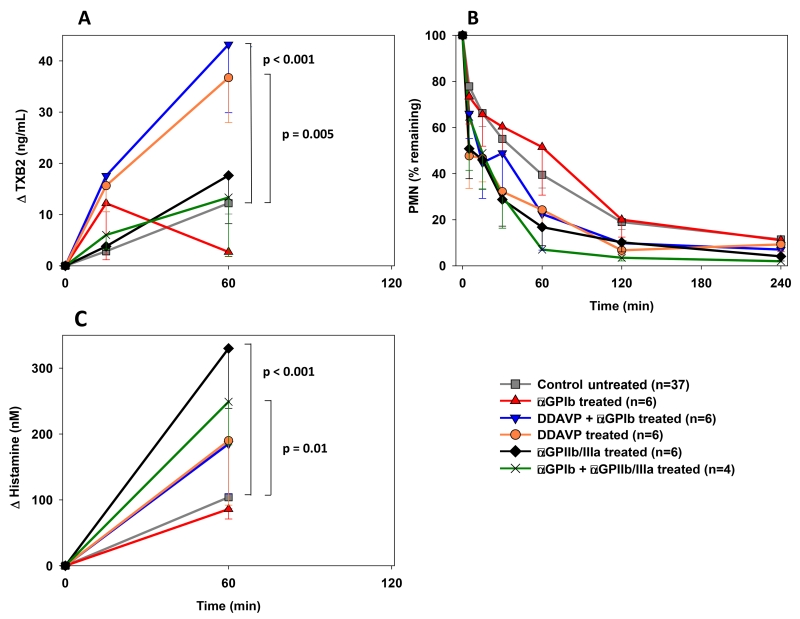
Plasma thromboxane B2 levels rose in both αGPIIb/IIIa-treated groups and in the reference group to a similar degree during the first hour of perfusion [Fig.7A]. TXB2 elaboration was greater at 15 minutes in the DDAVP, αGPIb monotherapy, and αGPIb+DDAVP treatment groups; this effect persisted at 60 minutes (αGPIb+DDAVP 43.2±13.3, p<0.005 vs. DDAVP 36.7±8.8, p<0.001 vs. control 12.2±2.1ng/mL) except for the αGPIb treated group, where half of the lungs, those with very high TXB2 levels, had failed.
Figure 7.
(A) Plasma thromboxane B2 levels during the first hour of perfusion were significantly higher in experiments where the pigs were pretreated with DDAVP when compared to the control experiments (* p<0.005). (B) Neutrophil sequestration from circulation was not significantly different between groups. Data is expressed as change from the baseline and shown as the mean ± SEM of surviving experiments. (C) Histamine levels at 1h of perfusion reached significantly higher values in perfusions where αGPIIb/IIIa was administered (# p<0.0001 for αGPIIb/IIIa and p=0.01 for αGPIb+αGPIIb/IIIa vs. control).
No significant differences in neutrophil sequestration [Fig.7B] or WDR at experimental termination (not shown) were observed when study groups were compared to the reference group.
Histamine was rapidly elaborated in all groups. While αGPIb alone, pre-treatment with DDAVP or the combination did not show a significant difference in histamine elaboration relative to the reference group, αGPIIb/IIIa treatment was associated with a significantly greater rise in histamine elaboration at one hour (ΔHistamine αGPIIb/IIIa 330±66, p<0.001; αGPIb+αGPIIb/IIIa 249±91, p=0.01 vs. control 101±16nM) [Fig.7C].
Histology
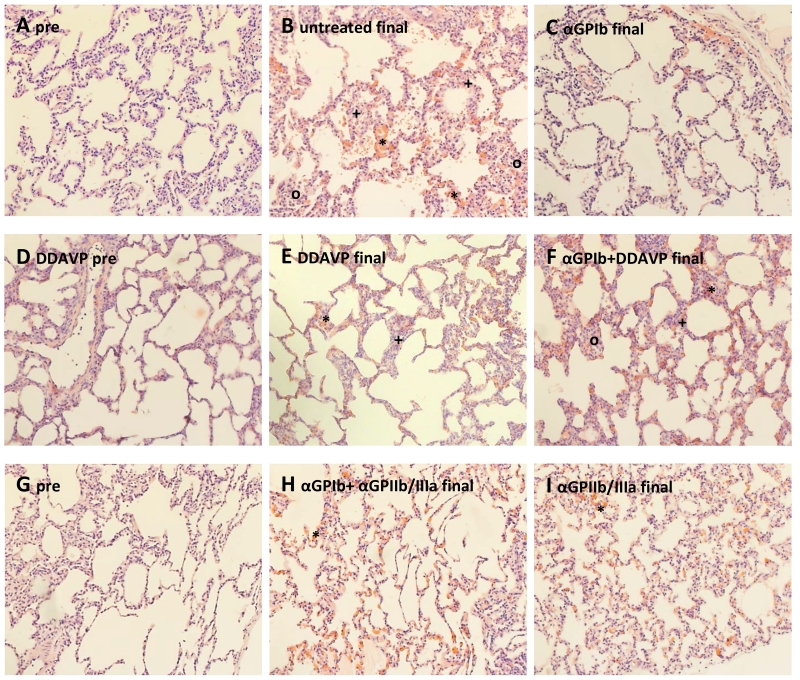
Independent of treatment group, lungs which failed before 120 minutes exhibited alveolar hemorrhage, moderate to severe interalveolar septal thickening and septal congestion (prominent erythrocytes), and interstitial cellular infiltration with 60 minutes, whereas lungs which survived beyond 240 minutes exhibited relatively normal lung architecture at this interval. Histology from an untreated control experiment with preserved function at 4h of perfusion showed relatively normal lung anatomy, with minimal alveolar hemorrhage (*), moderate inter-alveolar septal thickening and septal congestion (+), and little cellular infiltration (o) [Fig.8B] which were attenuated to some degree in association with various treatments (8E,F; 8G,H). Complement, platelet, and IgM staining intensity did not differ significantly between treatment groups (not shown). No intravascular thrombosis was observed in large or small vessels in any group. Sub-pleural and alveolar capillary congestion with erythrocytes is prominent at experimental termination, and is not obviously different between treatment groups.
Figure 8.
Histological analysis of lung biopsies (H&E, 20×) shows (A) a representative pre-perfusion biopsy of the lung pair that was used in the perfusion of which the final tissue biopsies are shown in (B, left lung, untreated) and (C, right lung, αGPIb treated); (D) displays the pre-perfusion lung biopsy of a pig that was pre-treated with DDAVP and (E and F) show final tissue biopsy of the same lung pair after perfusion with blood in the absence (E) or presence of αGPIb (F); (H and I) show final tissue biopsy of the lung shown in (G) after perfusion with αGPIb+αGPIIb/IIIa (H) or αGPIIb/IIIa treated blood (I). All final samples were collected at the 240min time point. (* alveolar hemorrhage, + inter-alveolar septal thickening and septal congestion, o cellular infiltration)
PCR mRNA levels
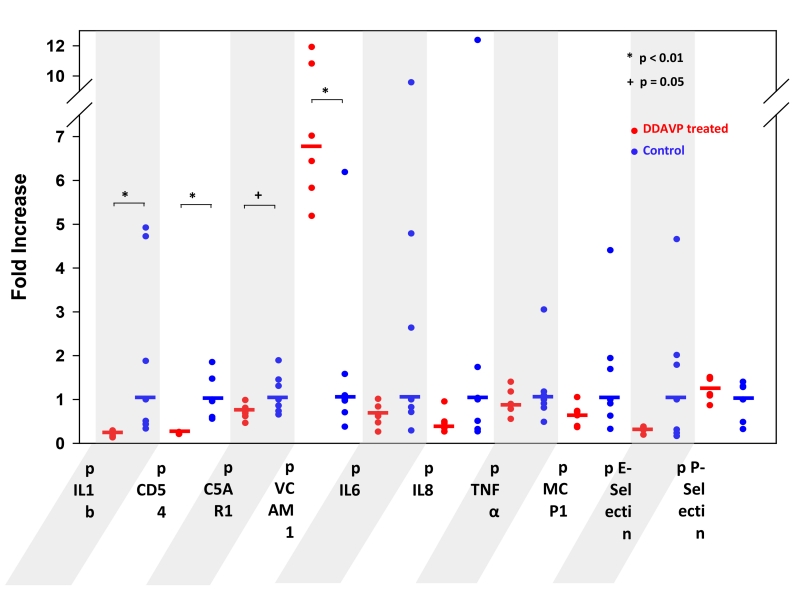
Porcine IL1b, CD54 (ICAM-1), and C5a receptor 1 (C5AR1) mRNA were significantly reduced in association with DDAVP pre-treatment (p≤0.05), while VCAM-1 was significantly upregulated (6.73 fold increase over untreated, p=0.005) [Fig.9]. Levels of hCD46 mRNA were similar in both groups (data not shown).
Figure 9.
PCR analyses of DDAVP-treated (red) lung biopsies, expressed as mRNA level fold-increase over untreated lungs (blue). Significantly reduced mRNA levels in the treated group are observed for porcine IL1b, CD54 and C5AR1. Porcine VCAM-1 shows a significant 6.7-fold increase when DDAVP was given. DDAVP-pretreatment resulted in a slight mRNA level increase for P-Selectin.
Discussion
Multiple lines of evidence demonstrate that activation of platelets and dysregulation of the coagulation cascade play a central role in GalTKO.hCD46 organ xenograft rejection (3,28-30). Pigs that express multiple transgenes that address molecular incompatibilities in the coagulation cascade (e.g. hEPCR, hTBM, hCD39) have been created, and are currently being evaluated in lung perfusion studies to determine the extent to which each intervention successfully controls the targeted pathway (31-35) and modulates organ injury. Our findings to date using these pigs (36-38, LB unpublished observations) show that, despite high expression of these transgenes - and in some instances associated with significant reduction of coagulation cascade activation parameters - platelet activation and sequestration remain prominent features associated with ex vivo lung perfusion (27).
The Fab antibody fragment directed against human GPIb’s VWF binding site, 6B4, inhibits VWF binding to GPIb, and prevents shear-induced VWF binding to platelets, and ristocetin or botrocetin-induced platelet aggregation (19). It addition, it has been shown that 6B4 also inhibits shear-independent binding of human GPIb to porcine VWF (39). Here, we report for the first time our evaluation of this selective GPIb antagonist in xenogeneic lung injury. Specifically, using GalTKO.hCD46 pig lungs, we confirm our hypothesis that VWF and platelet GPIb and GPIIb/IIIa receptors participate significantly in xeno platelet sequestration during pig lung perfusion with human blood.
αGPIb Fab transiently but significantly inhibited platelet sequestration during the first half hour of perfusion, indicating that GPIb interaction with VWF mediates rapid adhesion of the platelets to the endothelial wall, probably caused by non-physiologic binding as previously described (7, 8). Pre-depletion of VWF by DDAVP, alone and in combination with αGPIb, was associated with less thrombocyte sequestration and platelet activation (as βTG) during the last two hours of perfusion, suggesting that this pathway also mediates later pro-thrombotic effects that are a physiologically “appropriate” consequence of endothelial activation or injury. Since anti-non-Gal anti-pig antibody binding, low-level complement activation (as C3a), and coagulation cascade activation (as F1+2) were not targeted in these experiments, endothelial activation and expression of pVWF by one or more of these mechanisms would be expected. Indeed our data show that VWF and GPIb blockade partly mediate “physiologic” platelet sequestration and activation.
Thrombus formation requires aggregation of platelets, which is normally mediated by fibrinogen binding by GPIIb/IIIa (40-41) to cross-link platelets as the platform for a stable clot. Blocking the GPIIb/IIIa receptor alone with αGPIIb/IIIa Fab by itself did not modulate platelet sequestration or activation, measured by plasma βTG and P-selectin expression on platelets, suggesting that coagulation pathway dysregulation and deposition of clotting cascade molecules (tissue factor, thrombin, fibrinogen) is not the principle upstream trigger for platelet adhesion and activation. When combined with inhibition of GPIb, GPIIb/IIIa blockade was associated with sequestration of only about 20% of the initial thrombocytes, and significantly prevented platelet activation even in the absence of VWF pre-depletion, to a degree similar to that seen with αGPIb plus DDAVP. We infer that GPIIb/IIIa activation participates in platelet activation and adhesion downstream from GPIb/VWF, but to a lesser extent and hypothesize that pig genetics or drug treatments which prevent fibrinogen binding will similarly attenuate platelet adhesion and activation.
In prior studies we found that targeting platelet receptors using aurintricarboxylic acid (ATA, an inhibitor of platelet-GPIb interactions with VWF) and SC52012A (a synthetic GPIIb/IIIa inhibiting peptide) reduced complement activation in wild-type pig lung perfusions (11). In contrast, none of the treatment therapies used in this report inhibited complement (C3a). This difference could be related to the multiple “off-target” effects of ATA in the coagulation and complement cascade, compared to the selective blockade of GPIb with 6B4. Alternatively, amplification of complement activation may not depend on platelet activation in the context of a GalKO.hCD46 organ. Although targeting GPIIb/IIIa combined with cobra venom-mediated inhibition of complement was sufficient to prolong cardiac survival in a rodent discordant cardiac xenograft model (42), we confirm that it does not, when used alone, prevent injury to xenogeneic pig lungs.
This study shows for the first time that GalTKO.hCD46 PPP is able to cause dramatic abnormal activation of human platelets in absence of any platelet agonist or sheer stress, therefore justifying the need to target this pathway in genetically modified pigs. Since we found a trend towards lower platelet aggregation with GalTKO.hCD46 PPP compared to WT PPP, further studies will be necessary to determine whether this difference is related to individual differences between animals, differences in VWF concentration or glycosylation between pig genetics, or to the presence of soluble CD46 in PPP.
Thromboxane and histamine release were not prevented by platelet inhibition. Thromboxane is a potent vasoconstrictor and inducer of platelet aggregation. It is produced by platelets, activated through GPIb (amongst others), but not via GPIIb/IIIa ligation (43-44). The fact that thromboxane release was not prevented by GPIb blockade, despite suppression of βTG and P-selectin expression, suggests that platelets may not be the main source of thromboxane in this xenogeneic model, and extends our prior work implicating pulmonary intravascular macrophages (PIMs) as the principal source of thromboxane (45). Together, our findings suggest that thromboxane and histamine synthesis pathways are activated by human blood, independent of platelet adhesion or activation, probably by PIMs, and may require separate therapeutic targeting to control xenogeneic lung injury mechanisms.
Interestingly, although DDAVP pre-treatment was associated with significantly prolonged “survival” relative to the control group, both treatment groups including DDAVP also displayed significantly elevated TXB2 and histamine values during the first hour of perfusion, relatively prolific complement activation (C3a), and high PVR values at 4 hours of perfusion. Although we were unable to measure a difference in VWF content in lung in association with DDAVP pretreatment prior to organ procurement, we hypothesize that the medically-induced Weibel-Palade body release by DDAVP lowered the VWF content of the endothelial cells, and blunted some adhesive (ICAM-1) and proinflammatory (IL-1b, C5AR1) mediators. On the other hand DDAVP may also have primed lung endothelium with respect to VCAM-1-dependent xenogeneic interactions, and appears to have primed pulmonary intravascular macrophages to elaborate enhanced histamine and thromboxane. Thus DDAVP treatment appears to have both pro- and anti-inflammatory effects beyond VWF depletion that contributed to the differences in outcome observed. Meanwhile, our observations using GPIb blockade with DDAVP pre-depletion provide the rationale for genetic generation of pigs with a knock-in of human VWF, ideally with replacement of the porcine VWF loci that ligate hGPIb (46). We hypothesize that this approach will not only be helpful to further improve lung function in xenograft models, but may also attenuate consumptive coagulopathy and thrombotic micrangiopathy that are observed in other organ xenograft models.
Clinically, perioperative treatment with glycoprotein IIb/IIIa antagonists is known to potentially cause significant bleeding complications (47,48) and the use and time of drug administration has to be chosen carefully. In contrast, the 6B4 Fab antibody fragment inhibits shunt-associated platelet aggregation in vivo in baboons without causing thrombocytopenia (10% decrease of platelet counts) or prolongation of bleeding time (28), features suggesting that GPIb blockade will prove safe to use during surgery. Accordingly, we have safely incorporated αGPIb 6B4 therapy routinely in lung (unpublished) and pilot liver (28) xenograft models.
We conclude that, in a xenogeneic perfusion model, the activation and sequestration of platelets is initiated by the binding of porcine VWF to human platelet GPIb. The positive effect of diminishing endothelial VWF content by DDAVP pre-treatment on survival and platelet activation suggests that the non-physiologic activation of human platelets by porcine VWF indeed is one of the main triggers leading to platelet sequestration and consequent xenograft failure. The administration of an αGPIb Fab, but not αGPIIb/IIIa alone, prevented the rapid thrombocytopenia that occurs during lung perfusion. In addition, the significantly reduced platelet activation parameters in the DDAVP treatment groups demonstrate the impact of endothelial VWF content on the outcome. Aggregation of activated platelets, mediated by fibrinogen-GPIIb/IIIa binding, is shown to be secondary to the VWF-GPIb binding, leading to a delayed platelet sequestration as observed in the αGPIb group. When both adhesion and aggregation of platelets were blocked, prevention of platelet sequestration was sustained. Results of our study demonstrate that 1) activation and sequestration of platelets play an important role in xenogeneic rejection mechanisms, that 2) blocking or diminishing the activating triggers can improve the function and “survival” of GalTKO.hCD46 pulmonary xenografts perfused with human blood, and that 3) residual lung injury phenomena, associated with coagulation cascade dysregulation, histamine and thromboxane elaboration, and sequestration of other cell populations, are mediated by mechanisms independent of GPIb and GPIIb/IIIa.
Acknowledgements
Funding for this study was supported by NIH NIAID U19 AI 090959, sponsored research agreement with Revivicor, Inc and unrestricted gift support from United Therapeutics, Inc. to the University of Maryland, Baltimore Foundation.
This work was supported by the University of Maryland Clinical Translational Science Institute and the University of Maryland General Clinical Research Center. We are grateful to Katleen Broos for her help with the production of 6B4.
Glossary
List of non-standardized abbreviations
- αGPIb
anti-Glycoprotein Ib Fab
- αGPIIb/IIIa
anti-Glycoprotein IIb/IIIa Fab
- DDAVP
Desmopressin (1-desamino-8-D-arginine vasopressin)
- EGF
early graft failure
- ELISA
enzyme-linked immunosorbent assay
- F1+2
Prothrombin fragments 1 + 2
- Fab
Fragment antigen-binding (Fab fragment)
- HAR
hyperacute rejection
- HALR
hyperacute lung rejection
- hCRP
human complement regulatory protein
- MST
Median survival time
- PMN
polymorphonuclear leukocyte
- PVR
pulmonary vascular resistance
- WDR
Wet/Dry Ratio
Footnotes
Disclosures:
RNP serves without compensation on Revivicor’s Scientific Advisory Board.
DLA is CEO and a full-time employee of Revivicor, Inc.
CJP is head of xenotransplantation research and development at Revivicor, Inc.
Revivicor, Inc. is a wholly owned subsidiary of United Therapeutics, Inc.
Author Contributions
Concept and design: LB, RNP, AMA.
Data collection: LB, AR, ER, IIS, DH, TZ, SD, DP, FA, ES, EK, XC, ES, YZ, GB, SDM.
Critical scientific contributions: LB, AR, HD, CJP, DLA, RNP, AMA.
Providing of αGPIb Fab: IIS, SM, HD.
Providing of transgenic pigs: DLA, CJP.
Data analysis and interpretation: LB, AR, RNP, AMA.
Statistics: LB, AR.
Drafting article: LB, AR.
Critical revision: LB, IIS, HD, DLA, RNP, AMA.
Approval of article: LB, AR, HD, DLA, RNP, AMA.
References
- 1.MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14(2):488–9. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.YAMADA K, YAZAWA K, SHIMIZU A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 3.BURDORF L, STODDARD T, ZHANG T, et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am J Transplant. 2014;14(5):1084–95. doi: 10.1111/ajt.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HARRIS DG, QUINN KJ, DAHI S, et al. Lung xenotransplantation: recent progress and current status. Xenotransplantation. 2014;21(6):496–506. doi: 10.1111/xen.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HOLZKNECHT ZE, COOMBES S, BLOCHER BA, et al. Immune complex formation after xenotransplantation: evidence of type III as well as type II immune reactions provide clues to pathophysiology. Am J Pathol. 2001;158:627–637. doi: 10.1016/S0002-9440(10)64004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GOTO S, SALOMON DR, IKEDA Y, RUGGERI ZM. Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J Biol Chem. 1995;270:23352–23361. doi: 10.1074/jbc.270.40.23352. [DOI] [PubMed] [Google Scholar]
- 7.COSEMANS JM, SCHOLS SE, STEFANINI L, et al. Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow. Blood. 2011;117(2):651–60. doi: 10.1182/blood-2010-01-262683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GACA JG, LESHER A, AKSOY O, et al. The role of porcine von Willebrand factor-baboon platelet interactions in disseminated intravascular coagulation associated with pulmonary xenotransplantation. Transplantation. 2002;74:1596–1603. doi: 10.1097/00007890-200212150-00018. [DOI] [PubMed] [Google Scholar]
- 9.MAZZUCATO M, DE MARCO L, PRADELLA P, MASOTTI A, PARETI FI. Porcine von Willebrand factor binding to human platelets GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75:655–660. [PubMed] [Google Scholar]
- 10.SCHULTE AM, ESCH J, 2ND, CRUZ MA, SIEGEL JB, ANRATHER J, ROBSON SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90(11):4425–37. [PubMed] [Google Scholar]
- 11.PFEIFFER S, ZORN GL, 3RD, ZHANG JP, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75(7):953–9. doi: 10.1097/01.TP.0000058517.07194.90. [DOI] [PubMed] [Google Scholar]
- 12.CHEN G, WEI Q, WANG XM, et al. TMVA, a novel GPIb-binding protein, significantly prevents platelet microthrombi formation and prolongs discordant cardiac xenograft survival. Xenotransplantation. 2004;11(2):203–9. doi: 10.1111/j.1399-3089.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 13.KIM HK, KIM JE, WI HC, et al. Aurintricarboxylic acid inhibits endothelial activation, complement activation, and von Willebrand factor secretion in vitro and attenuates hyperacute rejection in an ex vivo model of pig-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15(4):246–56. doi: 10.1111/j.1399-3089.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 14.ALWAYN IP, APPEL JZ, III, GOEPFERT C, et al. Inhibition of platelet aggregation in baboons: therapeutic implications for xenotransplantation. Xenotransplantation. 2000;7(4):247–57. doi: 10.1034/j.1399-3089.2000.00965.x. [DOI] [PubMed] [Google Scholar]
- 15.MEYER C, WOLF P, ROMAIN N, et al. Use of von Willebrand diseased kidney as donor in a pig-to-primate model of xenotransplantation. Transplantation. 1999;67(1):38–45. doi: 10.1097/00007890-199901150-00006. [DOI] [PubMed] [Google Scholar]
- 16.LAU CL, CANTU E, 3RD, GONZALEZ-STAWINSKI GV, et al. The role of antibodies and von Willebrand factor in discordant pulmonary xenotransplantation. Am J Transplant. 2003;3(9):1065–75. doi: 10.1034/j.1600-6143.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 17.CANTU E, BALSARA KR, LI B, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7(1):66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 18.KIM YT, LEE HJ, LEE SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15(1):27–35. doi: 10.1111/j.1399-3089.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- 19.KANG HJ, LEE G, KIM JY, et al. Pre-treatment of donor with 1-deamino-8-d-arginine vasopressin could alleviate early failure of porcine xenograft in a cobra venom factor treated canine recipient. Eur J Cardiothorac Surg. 2005;28(1):149–56. doi: 10.1016/j.ejcts.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 20.KAUFMANN JE, OKSCHE A, WOLLHEIM CB, GÜNTHER G, ROSENTHAL W, VISCHER UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106(1):107–16. doi: 10.1172/JCI9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FONTAYNE A, DE MAEYER B, DE MAEYER M, et al. Paratope and epitope mapping of the antithrombotic antibody 6B4 in complex with platelet glycoprotein Ibalpha. J Biol Chem. 2007;282(32):23517–24. doi: 10.1074/jbc.M701826200. [DOI] [PubMed] [Google Scholar]
- 22.FONTAYNE A, MEIRING M, LAMPRECHT S, et al. The humanized anti-glycoprotein Ib monoclonal antibody h6B4-Fab is a potent and safe antithrombotic in a high shear arterial thrombosis model in baboons. Thromb Haemost. 2008;100(4):670–7. [PubMed] [Google Scholar]
- 23.PHELPS CJ, KOIKE C, VAUGHT TD, et al. Production of alpha 1,3-galactosyltransferase deficient pigs. Science. 2004;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NGUYEN BN, AZIMZADEH AM, SCHROEDER C, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18(2):94–107. doi: 10.1111/j.1399-3089.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SCHROEDER C, ALLAN JS, NGUYEN BN, et al. Hyperacute rejection is attenuated in GalT knockout swine lungs perfused ex vivo with human blood. Transplant Proc. 2005;37(1):512–3. doi: 10.1016/j.transproceed.2004.12.133. [DOI] [PubMed] [Google Scholar]
- 26.BURDORF L, AZIMZADEH AM, PIERSON RN., 3RD Xenogeneic lung transplantation models. Methods Mol Biol. 2012;885:169–89. doi: 10.1007/978-1-61779-845-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.HARRIS DG, QUINN KJ, FRENCH BM, et al. Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015;22(2):102–11. doi: 10.1111/xen.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LAMATTINA JC, BURDORF L, ZHANG T, et al. Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein Ib blockade. Xenotransplantation. 2014;21(3):274–86. doi: 10.1111/xen.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EKSER B, BURLAK C, WALDMAN JP, et al. Immunobiology of liver xenotransplantation. Expert Rev Clin Immunol. 2012;8(7):621–34. doi: 10.1586/eci.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.YEH H, MACHAIDZE Z, WAMALA I, et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21(5):454–64. doi: 10.1111/xen.12111. [DOI] [PubMed] [Google Scholar]
- 31.MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148(3):1106–13. doi: 10.1016/j.jtcvs.2014.06.002. discussion 1113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BURDORF L, RYBAK E, ZHANG T, et al. Human EPCR expression in GalTKO.hCD46 lungs extends survival time and lowers PVR in a xenogenic lung perfusion model. J Heart Lung Transplant. 2013;32:S137. [Google Scholar]
- 33.SIEVERT E, CHENG X, GAO Z, et al. Detecting the presence of hTM in lung tissue of GalTKO.hCD46.hTM transgenic pigs. Xenotransplantation. 2013;20:380. [Google Scholar]
- 34.BOTTINO R, WIJKSTROM M, VAN DER WINDT DJ, et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14(10):2275–87. doi: 10.1111/ajt.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IWASE H, EZZELARAB MB, EKSER B, COOPER DK. The role of platelets in coagulation dysfunction in xenotransplantation, and therapeutic options. Xenotransplantation. 2014;21(3):201–20. doi: 10.1111/xen.12085. [DOI] [PubMed] [Google Scholar]
- 36.BURDORF L, RYBAK E, ZHANG T, et al. Human EPCR expression in GalTKO.hCD46 lungs extends survival time and lowers PVR in a xenogenic lung model. J Heart Lung Transplant. 2013;32:S137. [Google Scholar]
- 37.HARRIS D, BENIPAL P, GAO Z, et al. Activated protein C modulates the thrombotic phenotype of hEPRC receptor transgenic porcine endothelium. Xenotransplantation. 2013;20:345. [Google Scholar]
- 38.HARRIS DG, GAO Z, SIEVERT EP, et al. Transgenic Human Thrombomodulin Expression Reduces Xenogeneic Thrombosis: a Promising Means of Reducing Pig Lung Xenograft Thrombotic Injury. J Heart Lung Transpl. 2014;32(4):S108. [Google Scholar]
- 39.DECKMYN H, SALLES II, PIERSON RN, III, AZIMZADEH AM. Humanized monoclonal antibody 6B4 Fab inhibits GPIb-vWF-mediated human platelet aggregation by porcine plasma. Xenotransplantation. 2009;16(5):370. [Google Scholar]
- 40.BRYCKAERT M, ROSA JP, DENIS CV, LENTING PJ. Of von Willebrand factor and platelets. Cell Mol Life Sci. 2015;72(2):307–26. doi: 10.1007/s00018-014-1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.BLEDZKA K, SMYTH SS, PLOW EF. Integrin αIIbβ3: from discovery to efficacious therapeutic target. Circ Res. 2013;112(8):1189–200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CANDINAS D, LESNIKOSKI BA, HANCOCK WW, et al. Inhibition of platelet integrin GPIIbIIIa prolongs survival of discordant cardiac xenografts. Transplantation. 1996;62(1):1–5. doi: 10.1097/00007890-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 43.YEH CH, CHANG MC, PENG HC, HUANG TF. Pharmacological characterization and antithrombotic effect of agkistin, a platelet glycoprotein Ib antagonist. Br J Pharmacol. 2001;132(4):843–50. doi: 10.1038/sj.bjp.0703865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KROLL MH, HARRIS TS, MOAKE JL, HANDIN RI, SCHAFER AI. von Willebrand factor binding to platelet GpIb initiates signals for platelet activation. J Clin Invest. 1991;88(5):1568–73. doi: 10.1172/JCI115468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.COLLINS BJ, BLUM MG, PARKER RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol. 2001;90(6):2257–68. doi: 10.1152/jappl.2001.90.6.2257. 1985. [DOI] [PubMed] [Google Scholar]
- 46.SCHULTE AM, ESCH J, 2ND, ROBSON SC, KNOEFEL WT, et al. Impact of O-linked glycosylation of the VWF-A1-domain flanking regions on platelet interaction. Br J Haematol. 2005;128(1):82–90. doi: 10.1111/j.1365-2141.2004.05253.x. [DOI] [PubMed] [Google Scholar]
- 47.FERRANDIS R, LLAU JV, MUGARRA A. Perioperative Management of Antiplatelet-Drugs in Cardiac Surgery. Curr Cardiol Rev. 2009;5(2):125–132. doi: 10.2174/157340309788166688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.FERRARIS VA, FERRARIS SP, SAHA SP. Antiplatelet Drugs: Mechanisms and Risks of Bleeding Following Cardiac Operations. Int J Angiol. 2011;20(1):1–18. doi: 10.1055/s-0031-1272544. [DOI] [PMC free article] [PubMed] [Google Scholar]