Abstract
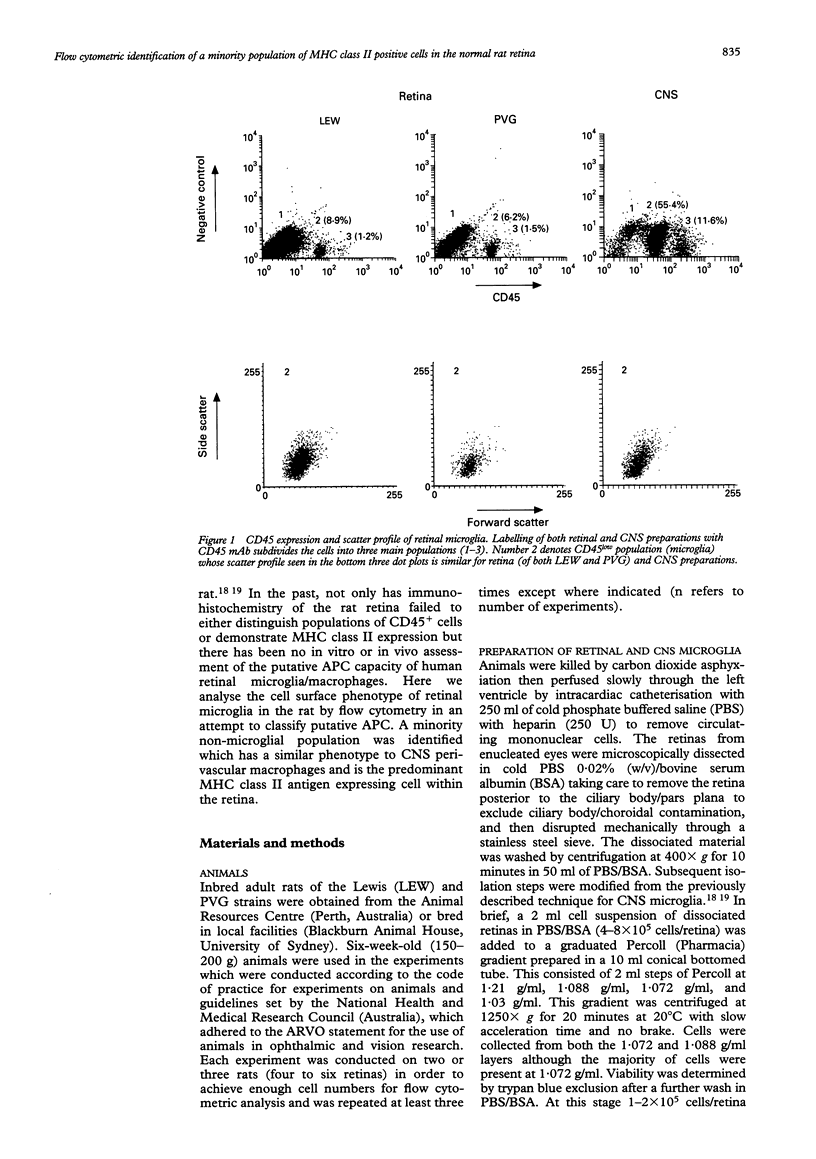
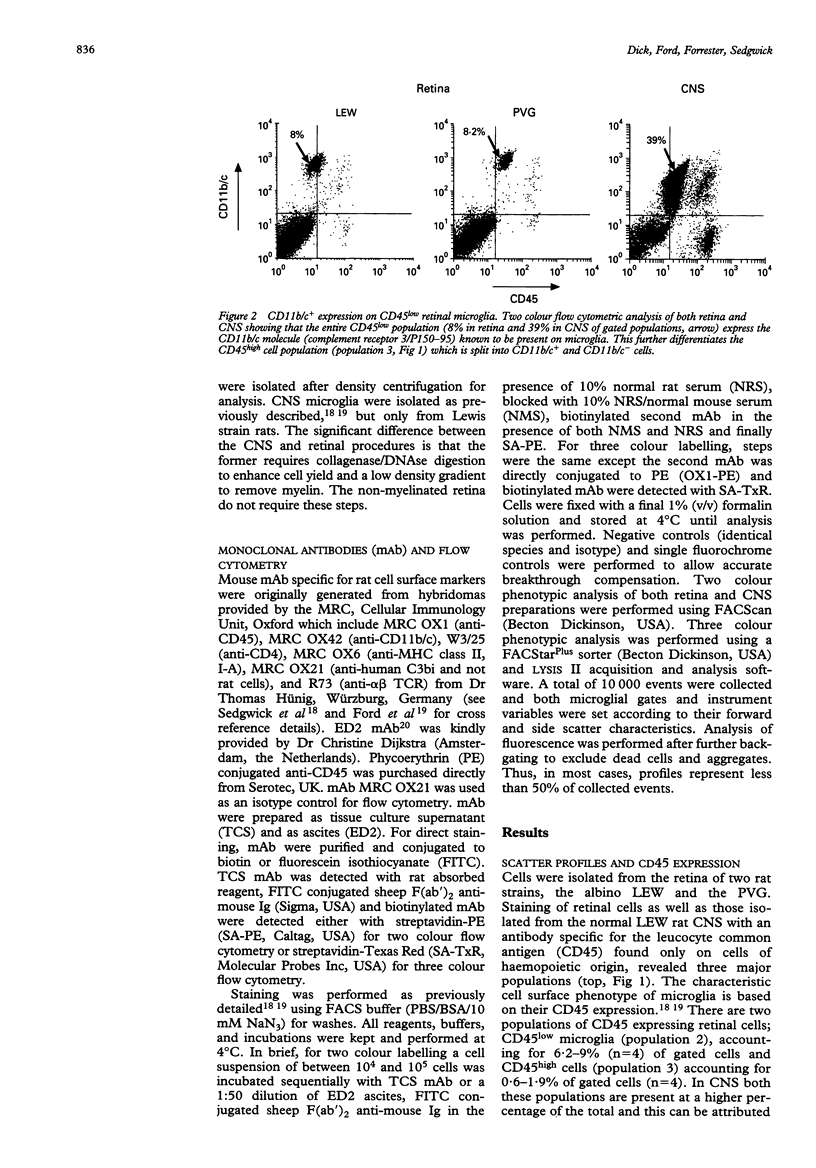
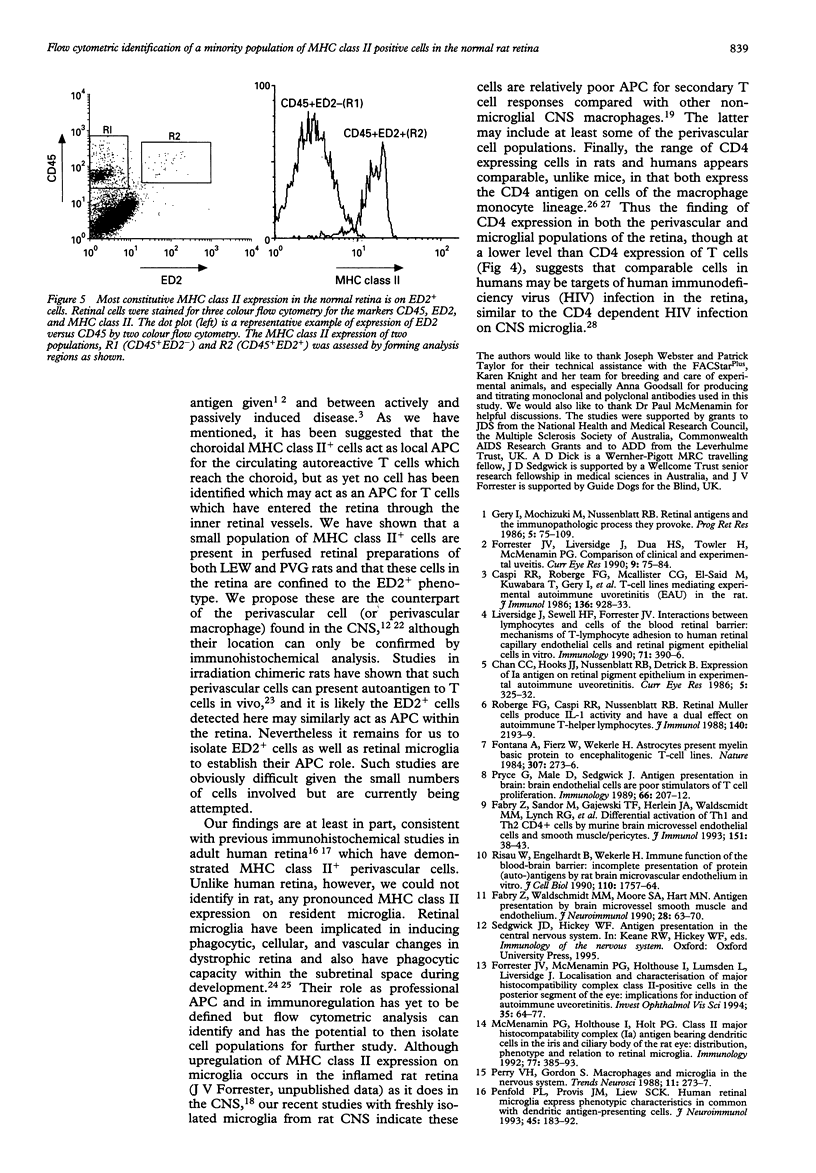
AIMS--This study aimed to isolate and classify by flow cytometry, the cell surface phenotype of microglia in the normal rat retina with a view to identifying putative antigen presenting cells (APC) within the retina, which has to date not been possible by immunohistochemistry. METHODS--Normal rat retinal microglia were isolated and classified using a modification of an isolation technique employing graduated Percoll density gradient cell separation and flow cytometric phenotypic criteria used for CNS microglia. RESULTS--Retinal microglia can be defined by flow cytometry on the basis of their CD45lowCD11b/c+CD4low cell surface expression. Constitutive MHC class II expression in the normal rat retina was confined almost exclusively to a very minor population of cells expressing neither low (microglia) nor high levels of CD45. Three colour flow cytometric analysis confirmed that these MHC class II positive cells were ED2+. CONCLUSIONS--Using this sensitive isolation technique we have identified the cell surface characteristics of ramified, resident microglia, and found that they do not constitutively express MHC class II. There is, however, constitutive MHC class II expression on a phenotypically distinct population of cells (CD45low/highED2+). We propose these cells are the counterpart of the perivascular macrophages found in the CNS which present antigen to extravasating T cells, although their exact retinal location can only be confirmed by immunohistochemical analysis. The role of parenchymal microglia as APC remains undefined. Future isolation of microglia and putative perivascular cells using this technique will help identify the role these cells play in the initiation and perpetuation of immune responses within the retina.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chan C. C., Hooks J. J., Nussenblatt R. B., Detrick B. Expression of Ia antigen on retinal pigment epithelium in experimental autoimmune uveoretinitis. Curr Eye Res. 1986 Apr;5(4):325–330. doi: 10.3109/02713688609020059. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Liversidge J., Dua H. S., Towler H., McMenamin P. G. Comparison of clinical and experimental uveitis. Curr Eye Res. 1990;9 (Suppl):75–84. doi: 10.3109/02713689008999424. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., McMenamin P. G., Holthouse I., Lumsden L., Liversidge J. Localization and characterization of major histocompatibility complex class II-positive cells in the posterior segment of the eye: implications for induction of autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):64–77. [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. A., Watkins B. A., Kufta C., Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991 Feb;65(2):736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liversidge J., Sewell H. F., Forrester J. V. Interactions between lymphocytes and cells of the blood-retina barrier: mechanisms of T lymphocyte adhesion to human retinal capillary endothelial cells and retinal pigment epithelial cells in vitro. Immunology. 1990 Nov;71(3):390–396. [PMC free article] [PubMed] [Google Scholar]
- Loeffler K. U., McMenamin P. G. Evaluation of subretinal macrophage-like cells in the human fetal eye. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1628–1636. [PubMed] [Google Scholar]
- McMenamin P. G., Crewe J., Morrison S., Holt P. G. Immunomorphologic studies of macrophages and MHC class II-positive dendritic cells in the iris and ciliary body of the rat, mouse, and human eye. Invest Ophthalmol Vis Sci. 1994 Jul;35(8):3234–3250. [PubMed] [Google Scholar]
- McMenamin P. G., Holthouse I., Holt P. G. Class II major histocompatibility complex (Ia) antigen-bearing dendritic cells within the iris and ciliary body of the rat eye: distribution, phenotype and relation to retinal microglia. Immunology. 1992 Nov;77(3):385–393. [PMC free article] [PubMed] [Google Scholar]
- Penfold P. L., Madigan M. C., Provis J. M. Antibodies to human leucocyte antigens indicate subpopulations of microglia in human retina. Vis Neurosci. 1991 Oct;7(4):383–388. doi: 10.1017/s0952523800004879. [DOI] [PubMed] [Google Scholar]
- Penfold P. L., Provis J. M., Liew S. C. Human retinal microglia express phenotypic characteristics in common with dendritic antigen-presenting cells. J Neuroimmunol. 1993 Jun;45(1-2):183–191. doi: 10.1016/0165-5728(93)90179-3. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Pryce G., Male D., Sedgwick J. Antigen presentation in brain: brain endothelial cells are poor stimulators of T-cell proliferation. Immunology. 1989 Feb;66(2):207–212. [PMC free article] [PubMed] [Google Scholar]
- Risau W., Engelhardt B., Wekerle H. Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990 May;110(5):1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge F. G., Caspi R. R., Nussenblatt R. B. Glial retinal Müller cells produce IL-1 activity and have a dual effect on autoimmune T helper lymphocytes. Antigen presentation manifested after removal of suppressive activity. J Immunol. 1988 Apr 1;140(7):2193–2196. [PubMed] [Google Scholar]
- Sedgwick J. D., Schwender S., Imrich H., Dörries R., Butcher G. W., ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B. Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia. 1993 Jan;7(1):68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]
- Thanos S. Sick photoreceptors attract activated microglia from the ganglion cell layer: a model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res. 1992 Aug 14;588(1):21–28. doi: 10.1016/0006-8993(92)91340-k. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]


