Abstract
Background
Despite evidence from animal and clinical studies showing the detrimental effects of macrophage migration inhibitory factor (MIF) on bone metabolism, there are no clinical studies relating circulating MIF levels to osteoporosis-related phenotypes. This cross-sectional study investigated the association of plasma MIF with bone mineral density (BMD), bone turnover markers (BTMs), and prevalence of osteoporosis in postmenopausal Korean women.
Methods
A total of 246 women not taking any medications or diagnosed with any diseases that could affect bone metabolism were enrolled. BMD values at the lumbar spine, femoral neck, and total femur, and blood levels of MIF and BTMs were measured in all subjects. Osteoporosis was defined by World Health Organization criteria.
Results
Before and after adjustment for confounding variables, higher MIF levels were significantly associated with lower BMD values at all measured sites and higher levels of all BTMs. All BMD values and BTMs significantly changed in a dose-dependent fashion across increasing MIF quartile. When participants were divided into two groups according to osteoporosis status, postmenopausal women with osteoporosis demonstrated 24.2% higher plasma MIF levels than those without osteoporosis (P=0.041). The odds ratio per each standard deviation increment of MIF levels for prevalent osteoporosis was 1.32 (95% confidence interval, 1.01 to 1.73).
Conclusion
This study provides the first epidemiological evidence that higher plasma MIF may be associated with higher risk of osteoporosis resulting from lower bone mass and higher bone turnover rate, and thus it could be a potential biomarker of poor bone health outcomes in postmenopausal women.
Keywords: Macrophage migration-inhibitory factors, Bone density, Bone remodeling, Bone strength, Osteoporosis
INTRODUCTION
The skeleton is a metabolically active organ that undergoes continuous remodeling throughout life to help it adapt to changing biomechanical forces, as well as remodeling to remove old, microdamaged bone and replace it with new, mechanically stronger bone to help preserve bone strength. In sex hormone-rich conditions, the balance between osteoclastic bone resorption and osteoblastic bone formation during bone remodeling is tightly regulated in a local, coordinated, and sequential manner [1]. However, in sex hormone-deficiency conditions, such as menopause or hormone deprivation therapy, excessive bone resorption that is not matched by an equivalent increase in bone formation leads to bone loss. An imbalance in the regulation of bone remodeling results in many metabolic bone diseases, especially osteoporosis and osteoporotic fractures (OFs). Because osteoporosis and OFs significantly increase mortality and morbidity in elderly individuals, this disorder is a serious, worldwide, public health problem [2,3].
Macrophage migration inhibitory factor (MIF) is a 37.5 kDa homotrimeric protein that acts via its receptor CD74 [4] to exert diverse biological effects and has been implicated in several inflammatory and immune conditions [5,6]. Currently, several pieces of evidence indicate that MIF also plays a critical role in bone metabolism. In fact, MIF knockout (KO) mice showed lower osteoclast numbers within fracture calluses [7], whereas elevated bone resorption is seen in MIF transgenic mice [8]. Furthermore, Swanberg et al. [9] showed that MIF promoter polymorphisms are associated with increased rate of bone loss and higher levels of bone resorption markers in elderly women. Based on these previous studies, it was strongly suggested that MIF may have harmful effects on bone metabolism, by mediating bone remodeling. However, despite the clear implications of the effects of MIF on bone metabolism, no study has examined the relationship between MIF levels and osteoporosis-related phenotypes. Thus, in the current study, we validated previous in vitro and in vivo data and investigated the association between MIF levels and bone mineral density (BMD) at various sites, bone turnover markers (BTMs), and the risk of prevalent osteoporosis in postmenopausal Korean women.
METHODS
Study subjects and protocol
Study subjects were postmenopausal Korean women who visited the Asan Medical Center (AMC; Seoul, Korea) between January 2010 and June 2012. All women either visited the osteoporosis clinic for concerns regarding the possibility of osteoporosis or were referred for osteoporosis that had been diagnosed during routine examinations. Menopause was defined as the absence of menstruation for at least 1 year and was confirmed by measuring serum follicle-stimulating hormone levels. Women who exhibited premature menopause (<40 years of age) and those who had taken drugs that could affect bone metabolism for more than 6 months or within the previous 12 months, such as bisphosphonates, systemic glucocorticoids, or hormone replacement therapy, were excluded from the study. Subjects with diseases that might affect bone metabolism such as diabetes, neoplastic diseases, hyperparathyroidism, rheumatoid arthritis, asthma/chronic obstructive pulmonary disease, or major cardiovascular disease, were also excluded. Osteophyte formation above the fourth grade of the Nathan classification and/or severe facet joint osteoarthritis in the lumbar spine as determined by conventional spine radiographs were also criteria for exclusion from the study. Subjects were excluded if they had a fever (oral temperature ≥38℃) or abnormal findings on complete blood counts of leukocytes (<4.0 or >10.0×109/L) or platelets (<150 or >350×109/L). Finally, subjects with increased serum liver enzyme activities (aspartate aminotransferase or alanine aminotransferase >120 IU/L) or decreased renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2) were excluded. These criteria were used to rule out any systemic illness. This study was approved by the local Institutional Review Board of the AMC. All subjects enrolled in the study provided written informed consent.
We obtained patient information using a self-administered questionnaire to assess smoking habits (current smoker), alcohol intake (≥3 units/day), regular outdoor exercise (≥30 minutes/day), history of medication use, previous medical or surgical procedures, and reproductive status (including menstruation) and using an interviewer-assisted questionnaire to assess parental history of OFs. Height (cm) and weight (kg) were measured using standardized protocols while the subjects were dressed in light clothing and had their shoes off. Body mass index (BMI, kg/m2) was calculated from the height and weight.
Measurement of BMD and definition of osteoporosis
Areal BMD (g/cm2) was measured at the lumbar spine (L1 to L4) and proximal femur (femoral neck, total femur) by dual-energy X-ray absorptiometry using Lunar equipment (running software version 9.30.044, Prodigy, Madison, WI, USA). The precision values of the equipment, in terms of the coefficients of variation (CVs), were 0.67% and 1.25% for the lumbar spine and femoral neck, respectively, which were determined in 17 volunteers who were not enrolled in this study. Each volunteer underwent five scans on the same day, and volunteers were required to get on and off the table between examinations.
BMD measurements provided absolute values for each anatomic site, which were then compared with those of healthy young Korean adults (T-score). The reference population consisted of 590 women between the ages of 20 and 39 years, and the reference BMD values at the lumbar spine, femoral neck, total femur, trochanter, and Ward's triangle were 1.148±0.119, 0.942±0.121, 0.974±0.120, 0.737±0.112, and 0.841±0.138 g/cm2, respectively. According to the World Health Organization definition, osteoporosis was diagnosed as T-score ≤–2.5 standard deviation (SD) at the lumbar spine, femoral neck, or total femur.
Biochemical measurement
Serum calcium concentrations were measured using the cresolphthalein complexone method on a Toshiba 200FR Autoanalyzer (Toshiba Medical Systems Co. Ltd., Tokyo, Japan). The intra- and interassay CVs were 1.24% and 2.06%, respectively, and the reference interval was 2.07 to 2.50 mmol/L. Serum phosphorus concentrations were measured using the phosphomolybdate ultraviolet method (Toshiba 200FR instrument). The intra- and interassay CVs were 1.28% and 2.54%, respectively, and the reference interval was 0.81 to 1.45 mmol/L. Serum concentration of 25-hydroxyvitamin D3 (25-OH-D3) was measured by radioimmunoassay (Cobra II Auto-γ Counting system, Packard Instruments, Downers Grove, IL, USA), with a lower limit of detection of 0.6 ng/mL (1.5 nmol/L). The intra- and interassay CVs for this analysis were less than 3.5%. The glomerular filtration rate (mL/min/1.73 m2), an indicator of renal failure, was calculated using the Cockcroft-Gault formula [10].
To measure the BTMs, fasting blood samples were obtained between 8:00 AM and 10:00 AM in the morning. The serum osteocalcin concentration was measured using an electrochemical-luminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany), with intra- and interassay CVs of 1.2% to 4.0% and 1.7% to 6.5%, respectively. The serum C-terminal telopeptide of type I collagen (CTX) level was also measured using an electrochemical-luminescence immunoassay (Roche Diagnostics GmbH), with intra- and interassay CVs of 1.0% to 4.6% and 1.6% to 4.7%, respectively.
Measurement of plasma MIF levels
Fasting blood samples were obtained. After centrifugation, the supernatants were carefully collected to exclude cellular components. All samples demonstrating hemolysis or clotting were discarded. The plasma samples were stored at –80℃ before determination of the MIF levels. MIF levels were measured by using the MIF enzyme-linked immunosorbent assay kit (R&D Systems Inc., Minneapolis, MN, USA) in accordance with the manufacturer's instructions. The intra- and interassay MIF CVs were 5.2% and 9.17%, respectively. The lower limit of detection of the kit was 0.016 ng/mL. Duplicate samples were assayed and all results were reported as means.
Statistical analysis
Continuous variables are reported as mean±SD and categorical variables as numbers and percentages, unless otherwise specified. To examine the relationship between plasma MIF levels and various BMD values and BTMs, we performed Pearson and partial correlation analysis before and after adjustment for well-known demographic and behavioral factors that might affect bone metabolism, respectively. These factors included age, BMI, current smoking, alcohol intake, regular outdoor exercise, parental history of OF, years since menopause, and 25-OH-D3. The MIF and BTM levels were logarithmically transformed because the distribution was positively skewed. Multivariate-adjusted least squares mean (95% confidence interval [CI]) BMD values and BTMs, according to plasma MIF quartiles, were estimated using analysis of covariance (ANCOVA) after adjusting for confounders. The trends of these variables across the increasing plasma MIF quartiles were estimated using multiple linear regression analysis. Multivariate-adjusted least squares mean MIF levels (95% CI) in terms of osteoporosis status were estimated using ANCOVA after adjusting confounding variables. To generate odds ratios (ORs; 95% CI) per SD increment in plasma MIF concentration for prevalent osteoporosis, we performed multiple logistic regression analyses before and after adjustment for confounding variables. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant.
RESULTS
Correlation between plasma MIF levels and BMD at various sites, and plasma MIF levels and BTM in the study subjects
The clinical characteristics of the 246 postmenopausal Korean women in the study are shown in Table 1. The mean for study subject age was 62.7±6.2 years (range, 50 to 79), and mean plasma MIF level was 25.0±20.4 ng/mL (range, 0.8 to 121.5).
Table 1. Baseline Characteristics of the Study Population.
| Variable | Postmenopausal women (n=246) |
|---|---|
| Age, yr | 62.7±6.2 |
| Height, cm | 155.4±5.2 |
| Weight, kg | 57.0±7.0 |
| Body mass index, kg/m2 | 23.6±2.9 |
| Current smoker | 7 (2.8) |
| Alcohol intake (≥3 U/day) | 37 (15.0) |
| Exercise (≥30 min/day) | 94 (38.2) |
| Parental history of OF | 37 (15.0) |
| Years since menopause, yr | 12.1±6.8 |
| MIF, ng/mL | 25.0±20.4 |
| Bone mineral density, g/cm2 | |
| Lumbar spine | 0.894±0.123 |
| Femoral neck | 0.756±0.094 |
| Total femur | 0.813±0.097 |
| Corrected calcium level, mg/dLa | 8.9±0.4 |
| Phosphorus, mg/dL | 3.8±0.5 |
| Bone turnover marker | |
| Osteocalcin, ng/mL | 30.5±14.3 |
| CTX, ng/mL | 0.560±0.223 |
| 25-OH-D3, ng/mL | 30.5±15.5 |
Values are expressed as mean±SD or number (%).
OF, osteoporotic fracture; MIF, macrophage migration inhibitory factor; CTX, C-terminal telopeptide of type I collagen; 25-OH-D3, 25-hydroxyvitamin D3.
aCorrected calcium level (mg/dL)=total calcium level (mg/dL)+0.8×[4.0 g/dL–serum albumin level (g/dL)] (mg/dL).
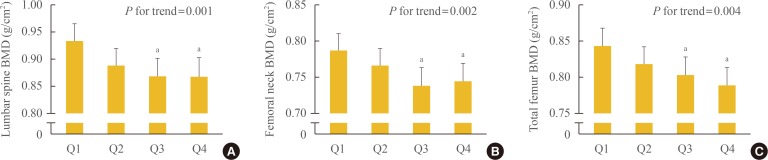
To determine which BMD and BTM values could influence the relationship between MIF levels and bone metabolism, we analyzed the correlations of MIF levels with BMD and BTM values (Table 2). Before and after adjustment for age, BMI, current smoking, alcohol intake, exercise status, parental history of OF, years since menopause, and 25-OH-D3, plasma MIF levels were determined to be inversely correlated with the BMD values at the lumbar spine, femoral neck, and total femur (γ=–0.335 to γ=–0.223, P<0.001 to P=0.001). To better understand the clinical implications of these results, we categorized the subjects into four groups according to the plasma MIF levels. The BMD values at the lumbar spine (Fig. 1A), femoral neck (Fig. 1B), and total femur (Fig. 1C) dose-dependently decreased along with increasing plasma MIF quartiles. A post hoc analysis showed that subjects in the third and fourth quartiles had significantly lower BMD values at all measured sites compared with those in the lowest quartile. When the multivariate-adjusted least squares mean BMD values according to the plasma MIF quartiles were estimated after considering other potential confounders, the statistical significance still persisted at all sites.
Table 2. Correlation of Plasma Macrophage Migration Inhibitory Factor Levels with Bone Mineral Density and Bone Turnover Markers.
| Variable | Unadjusted | Age and BMI adjusted | Multivariable adjusted | |||
|---|---|---|---|---|---|---|
| γ | P valuea | γ | P valueb | γ | P valuec | |
| Bone mineral density, g/cm2 | ||||||
| Lumbar spine | –0.327 | <0.001 | –0.335 | <0.001 | –0.317 | <0.001 |
| Femoral neck | –0.224 | <0.001 | –0.236 | <0.001 | –0.223 | 0.001 |
| Total femur | –0.229 | <0.001 | –0.245 | <0.001 | –0.232 | <0.001 |
| Bone turnover markersd | ||||||
| Osteocalcin | 0.186 | 0.015 | 0.189 | 0.014 | 0.187 | 0.017 |
| CTX | 0.217 | 0.001 | 0.218 | 0.001 | 0.196 | 0.004 |
Values are statistically significant.
BMI, body mass index; CTX, C-terminal telopeptide of type I collagen.
aP values were determined by Pearson's correlation analysis; bP values were determined by partial correlation analysis adjusted for age and BMI; cP values were determined by partial correlation analysis adjusted for age, BMI, current smoking, alcohol intake (≥3 units/day), exercise status (≥30 min/day), parental history of osteoporotic fracture, years since menopause, and 25-hydroxyvitamin D3; dMacrophage migration inhibitory factor and bone turnover markers were log-transformed because their distributions were positively skewed.
Fig. 1. Bone mineral density (BMD) values according to plasma macrophage migration inhibitory factor quartiles after adjusting for confounding variables in the (A) lumbar spine, (B) femoral neck, and (C) total femur. Confounding variables included age, body mass index, current smoking, alcohol intake (≥3 units/day), exercise status (≥30 min/day), parental history of osteoporotic fracture, years since menopause, and 25-hydroxyvitamin D3. The mean estimated BMDs (95% confidence interval) were calculated by analysis of covariance (ANCOVA). The P values for the trends were generated using multiple linear regression analysis. aStatistically significantly different from the lowest quartile (Q1) by ANCOVA.
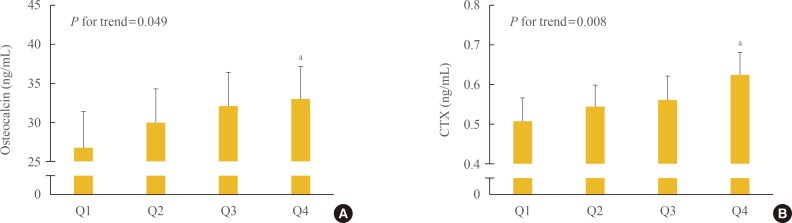
All BTM values were also significantly associated with plasma MIF levels (γ=0.186 to γ=0.218, P=0.001 to P=0.017). Partial correlation analyses showed that these higher BTM values correlated with higher plasma MIF levels independently of confounding variables. The BTMs, according to the plasma MIF quartiles, are presented in Fig. 2. Serum osteocalcin (Fig. 2A) and CTX (Fig. 2B) levels were significantly higher in the highest MIF quartile than in the lowest MIF quartile (P=0.008 to P=0.049). More specifically, the plasma MIF levels positively correlated not only with a bone resorption marker (serum CTX), but also with a bone formation marker (serum osteocalcin). These results suggested that MIF may be associated with the bone turnover rate.
Fig. 2. Bone-specific biochemical turnover markers (BTMs), such as (A) osteocalcin and (B) C-terminal telopeptide of type I collagen (CTX) according to the plasma macrophage migration inhibitory factor quartiles after adjusting for confounding variables. Confounding variables included age, body mass index, current smoking, alcohol intake (≥3 units/day), exercise status (≥30 min/day), parental history of osteoporotic fracture, years since menopause, and 25-hydroxyvitamin D3. The mean estimated BTMs (95% confidence interval) were calculated using analysis of covariance (ANCOVA). The P values for the trends were generated using multiple linear regression analysis. aStatistically significantly different from the lowest quartile (Q1) by ANCOVA.
Differences in plasma MIF levels according to osteoporosis status
We divided our participants into two groups according to osteoporosis status. Before and after adjustment for age and BMI, subjects with osteoporosis demonstrated 29.7% and 28.6% higher plasma MIF levels, respectively (P=0.012 and P=0.017, respectively) (Table 3). After additional adjustment for current smoking habits, alcohol intake, regular outdoor exercise, parental history of OF, years since menopause, and 25-OH-D3, subjects with osteoporosis demonstrated 24.2% higher plasma MIF levels, in comparison to those without osteoporosis, and statistical significance persisted (P=0.041).
Table 3. Differences in Plasma MIF Levels according to Osteoporosis Status.
| Adjusted model | Estimated MIF levels, ng/mL (95% CI)a | ||
|---|---|---|---|
| Subjects with osteoporosis (n=94) | Subjects without osteoporosis (n=152) | P valueb | |
| Unadjusted | 29.08 (24.98–33.19) | 22.42 (19.19–25.64) | 0.012 |
| Age and BMI adjusted | 28.94 (24.82–33.05) | 22.51 (19.27–25.74) | 0.017 |
| Multivariable adjustedc | 28.22 (24.09–32.35) | 22.72 (19.50–25.94) | 0.041 |
MIF, macrophage migration inhibitory factor; CI, confidence interval; BMI, body mass index.
aValues were compared and generated by analysis of covariance; bValues are statistically significant; cMultivariable factors included age, BMI, current smoking, alcohol intake (≥3 units/day), exercise status (≥30 min/day), parental history of osteoporotic fracture, years since menopause, and 25-hydroxyvitamin D3.
Risk of prevalent osteoporosis according to plasma MIF levels
Prior to adjustment of confounding variables, the OR per SD increment in plasma MIF levels for prevalent osteoporosis was 1.387 (95% CI, 1.065 to 1.806), and the statistical significance of this persisted after adjustment for age and BMI (OR, 1.371; 95% CI, 1.051 to 1.788) (Table 4). Even after adjustment for all confounding variables, each SD increment of plasma MIF levels was still associated with a multivariable-adjusted OR of 1.319 (95% CI, 1.007 to 1.729) for prevalent osteoporosis. The OR values of all other covariates in the final multivariable adjusted model, which are important for bone health, were presented as Supplemental Table S1.
Table 4. Multiple Logistic Regression Analyses to Determine the Ability of Plasma MIF Levels to Predict Risk of Osteoporosis.
| OR (95% CI)a | P value | |
|---|---|---|
| Unadjusted | 1.387 (1.065–1.806) | 0.015 |
| Age and BMI adjusted | 1.371 (1.051–1.788) | 0.020 |
| Multivariable adjusted | 1.319 (1.007–1.729) | 0.044 |
Values are statistically significant. Multivariable factors included age, BMI, current smoking, alcohol intake (≥3 units/day), exercise status (≥30 min/day), parental history of osteoporotic fracture, years since menopause, and 25-hydroxyvitamin D3.
MIF, macrophage migration inhibitory factor; OR, odds ratio; CI, confidence interval; BMI, body mass index; SD, standard deviation.
aPer SD increment in plasma MIF levels.
DISCUSSION
We have shown here that circulating MIF levels were inversely correlated with the BMD values at all measured sites and positively correlated with both bone resorption and formation markers, after adjusting for confounding variables. Furthermore, BMD at all measured sites increased and BTMs decreased in a dose-dependent manner across increasing MIF quartiles. Importantly, MIF levels were markedly higher in postmenopausal women with osteoporosis in comparison to those without osteoporosis. Each SD increment of plasma MIF levels was associated with a multivariable-adjusted OR of 1.32 for prevalent osteoporosis. To the best of our knowledge, our current investigation is the first clinical study to report the association between plasma MIF levels and BMD and BTMs in postmenopausal women.
Inflammation has been shown to be involved in the mechanism of osteoporosis [11]. Systemic inflammation contributes to an imbalance between the bone resorption and formation of the osteoclast and osteoblast in bone remodeling, and then the presence and progression of bone damage. Recently, inflammatory molecules such as interleukin (IL)-1β, tumor necrosis factor-α, IL-6, and IL-8 have been considered to be potential biomarkers for osteoporosis [11]. In the present study, we showed that MIF has several harmful effects on bone, and this result is consistent with other experimental study which demonstrated that MIF transgenic mice exhibited low bone mass and abnormal bone microarchitecture [8]. Based on these background results, we supposed that MIF, a proinflammatory cytokine produced by macrophages, plays an important role in the pathogenesis of osteoporosis and that plasma MIF levels could serve as a potential new biomarker for predicting the presence and progression of osteoporosis.
There are other possible mechanisms that may explain the association between plasma MIF levels and bone health. Since establishing the critical role of estrogen deficiency in the pathogenesis of postmenopausal osteoporosis, various studies have focused on elucidating the mechanisms of estrogen in osteoporosis [12]. It is now widely accepted that after menopause the depletion of estrogen causes a high bone-turnover state that leads the bone metabolic balance to resorption, which results in osteoporosis [13]. Similar to the mechanism of estrogen deficiency-induced osteoporosis, our study showed that higher plasma MIF levels were associated with high bone-turnover rates. Furthermore, according to the study performed by Oshima et al. [14], histomorphometry of the bone revealed that ovariectomy-induced bone loss was associated with an increase in osteoclastic bone resorption and, in MIF KO mice, resistance to bone loss after ovariectomy was observed. These results suggested that MIF could be a mediator between estrogen and bone metabolism. In fact, recent studies revealed that estrogen also downregulated the production of MIF in murine macrophages [15]. Since circulating estrogen levels were unavailable in the current study, we were unable to determine these relationships. Further investigation regarding this issue could be highly interesting.
Since bone enables us to move or sustain our bodies, maintaining bone strength is very important, especially in the elderly. Bone strength is made up of two components: bone mass (approximately 70%) and bone quality (approximately 30%) [16,17]. Although BMD is valuable for measuring bone mass, the ability of this measurement to estimate bone quality is limited [18]. There are currently many potential parameters that are tentatively used for estimating bone strength such as sphingosine-1-phosphate [19], periostin [20], sclerostin [21], and homocysteine [22]; however, it is still impossible to assess bone mass and quality at the same time. In our study, MIF significantly correlated with BMDs at all measurement sites as well as with BTMs. Furthermore, all BMD values and BTMs significantly changed in a dose-dependent fashion across increasing MIF quartiles. Regretfully, due to the differences in study population and design, exclusion criteria, and adjustment strategy, etc., it was difficult to directly compare MIF with other biomarkers. However, because BMD and BTM reflect bone mass and bone quality [23], respectively, we anticipate that MIF may be employed as a useful differentiated parameter for assessing bone mass and quality at once.
There are several potential limitations to our present study. Most importantly, because this was a cross-sectional study, we could not determine whether a causal relationship exists between plasma MIF levels and osteoporosis-related phenotypes. Second, due to the high morbidity and mortality, the ultimate goal of bone biology research is to prevent OFs. However, regretfully, we could not investigate the relationship between circulating MIF levels and OFs. Nevertheless, despite this limitation, we believe that this study could be an important beginning of research with MIF on osteoporosis-related phenotypes including fractures and could provide background information for future study. We expect further long-term prospective studies that designate incident OF as a primary endpoint in order to completely understand the clinical meaning of blood MIF in human. Third, the study population consisted of women who visited a referral hospital and, therefore, may not be representative of the general population and could have resulted in selection bias. Fourth, although we considered many confounding factors, we cannot exclude every possibility that the observed association could have resulted from uncontrolled factors that affect MIF and/or bone parameters, such as circulating estrogen levels. Lastly, because MIF can play important roles in inflammatory diseases, our results could be affected by conditions that elevate the levels of circulating MIF. To minimize this possibility, we strictly excluded subjects with certain inflammatory-associated disorders and those with any types of cancers according to the results of our laboratory tests and medical history review. We assume that this approach at least partly compensated for this limitation.
In conclusion, the present study provides evidence that higher blood MIF is associated with the risk of osteoporosis through mediating bone mass and turnover, and also suggests that the circulating MIF levels could be a potential biomarker for predicting poor bone health in postmenopausal women. Further investigational studies with large numbers of subjects will be needed to confirm this possibility.
ACKNOWLEDGMENTS
This study was supported by research funding from the Korean Endocrine Society and by a grant from the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (project no. HI13C1432).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Supplementary materials
Logistic Regression Analyses to Generate ORs of Covariates Included in the Final Multivariable Adjusted Model for the Risk of Osteoporosis
References
- 1.Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965;206:489–490. doi: 10.1038/206489a0. [DOI] [PubMed] [Google Scholar]
- 2.Seeman E. Growth and age-related abnormalities in cortical structure and fracture risk. Endocrinol Metab (Seoul) 2015;30:419–428. doi: 10.3803/EnM.2015.30.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YK, Yoon BH, Koo KH. Epidemiology of osteoporosis and osteoporotic fractures in South Korea. Endocrinol Metab (Seoul) 2013;28:90–93. doi: 10.3803/EnM.2013.28.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bucala R, Shachar I. The integral role of CD74 in antigen presentation, MIF signal transduction, and B cell survival and homeostasis. Mini Rev Med Chem. 2014;14:1132–1138. doi: 10.2174/1389557515666150203144111. [DOI] [PubMed] [Google Scholar]
- 5.Kim BS, Pallua N, Bernhagen J, Bucala R. The macrophage migration inhibitory factor protein superfamily in obesity and wound repair. Exp Mol Med. 2015;47:e161. doi: 10.1038/emm.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin TR. MIF mediation of sepsis. Nat Med. 2000;6:140–141. doi: 10.1038/72230. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Onodera S, Kondo E, Tohyama H, Fujiki H, Yokoyama A, et al. Impaired fracture healing in macrophage migration inhibitory factor-deficient mice. Osteoporos Int. 2011;22:1955–1965. doi: 10.1007/s00198-010-1385-0. [DOI] [PubMed] [Google Scholar]
- 8.Onodera S, Sasaki S, Ohshima S, Amizuka N, Li M, Udagawa N, et al. Transgenic mice overexpressing macrophage migration inhibitory factor (MIF) exhibit high-turnover osteoporosis. J Bone Miner Res. 2006;21:876–885. doi: 10.1359/jbmr.060310. [DOI] [PubMed] [Google Scholar]
- 9.Swanberg M, McGuigan F, Ivaska KK, Gerdhem P, Lerner UH, Bucala R, et al. Polymorphisms in the macrophage migration inhibitory factor gene and bone loss in postmenopausal women. Bone. 2010;47:424–429. doi: 10.1016/j.bone.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 13.Jilka RL. Cytokines, bone remodeling, and estrogen deficiency: a 1998 update. Bone. 1998;23:75–81. doi: 10.1016/s8756-3282(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 14.Oshima S, Onodera S, Amizuka N, Li M, Irie K, Watanabe S, et al. Macrophage migration inhibitory factor-deficient mice are resistant to ovariectomy-induced bone loss. FEBS Lett. 2006;580:1251–1256. doi: 10.1016/j.febslet.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 15.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–S18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 17.Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med. 2008;75:739–750. doi: 10.3949/ccjm.75.10.739. [DOI] [PubMed] [Google Scholar]
- 18.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 19.Lee SH, Lee SY, Lee YS, Kim BJ, Lim KH, Cho EH, et al. Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J Clin Endocrinol Metab. 2012;97:E1421–E1428. doi: 10.1210/jc.2012-1044. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau JC, Sornay-Rendu E, Bertholon C, Chapurlat R, Garnero P. Serum periostin is associated with fracture risk in postmenopausal women: a 7-year prospective analysis of the OFELY study. J Clin Endocrinol Metab. 2014;99:2533–2539. doi: 10.1210/jc.2013-3893. [DOI] [PubMed] [Google Scholar]
- 21.Ardawi MS, Rouzi AA, Al-Sibiani SA, Al-Senani NS, Qari MH, Mousa SA. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: the Center of Excellence for Osteoporosis Research Study. J Bone Miner Res. 2012;27:2592–2602. doi: 10.1002/jbmr.1718. [DOI] [PubMed] [Google Scholar]
- 22.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–2041. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E, Delmas PD. Bone quality: the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Logistic Regression Analyses to Generate ORs of Covariates Included in the Final Multivariable Adjusted Model for the Risk of Osteoporosis