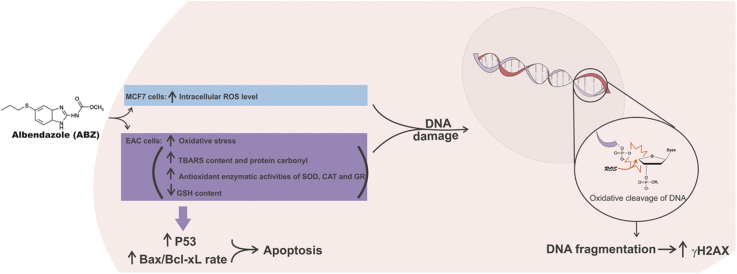
Abstract
This work evaluated the antitumor effects of albendazole (ABZ) and its relationship with modulation of oxidative stress and induction of DNA damage. The present results showed that ABZ causes oxidative cleavage on calf-thymus DNA suggesting that this compound can break DNA. ABZ treatment decreased MCF-7 cell viability (EC50=44.9 for 24 h) and inhibited MCF-7 colony formation (~67.5% at 5 μM). Intracellular ROS levels increased with ABZ treatment (~123%). The antioxidant NAC is able to revert the cytotoxic effects, ROS generation and loss of mitochondrial membrane potential of MCF-7 cells treated with ABZ. Ehrlich carcinoma growth was inhibited (~32%) and survival time was elongated (~50%) in animals treated with ABZ. Oxidative biomarkers (TBARS and protein carbonyl levels) and activity of antioxidant enzymes (CAT, SOD and GR) increased, and reduced glutathione (GSH) was depleted in animals treated with ABZ, indicating an oxidative stress condition, leading to a DNA damage causing phosphorylation of histone H2A variant, H2AX, and triggering apoptosis signaling, which was confirmed by increasing Bax/Bcl-xL rate, p53 and Bax expression. We propose that ABZ induces oxidative stress promoting DNA fragmentation and triggering apoptosis and inducing cell death, making this drug a promising leader molecule for development of new antitumor drugs.
Keywords: Albendazole, Antitumor, Apoptosis, Cell cycle arrest, DNA fragmentation, Oxidative stress
Graphical abstract
Highlights
-
•
The ABZ redox signaling pathway was examined in cancer inhibition.
-
•
The oxidative stress can explain the ABZ antitumor mechanisms of action.
-
•
The ABZ oxidative stress modulation can be used for cancer therapeutics development.
-
•
ABZ can be used as a molecule prototype in possible drug repositioning.
1. Introduction
Cancer is a major public health problem in many parts of the world. In Brazil, about 57,000 of new cases of breast cancer were expected to be diagnosed in 2015 [1]. Standard cancer treatments are surgery, radiation, immunotherapy and/or chemotherapy. To the moment cancer management by chemotherapy is one of the most effective and potent strategies to treat malignant tumors. However, the multidrug resistance, which is the mechanism that many cancer cells develop drug resistance to chemotherapy, has been a significant impediment to successful treatment [2]. On this context, in which increased cancer diagnosis and lack of an optimal treatment is observed, new therapeutic alternatives are required. On the other hand, drug repositioning is an important pharmaceutical strategy for drug development because it is faster and reduces drug risks once the safety and pharmacokinetic profiles of drugs are already well- known. Repositioning can offer a better risk versus-reward trade-off compared to other strategies for drug development. Furthermore, this strategy is economically attractive when compared to the cost of drug development based on de novo drug discovery and development [3].
Albendazole (methyl N-(6-propylsulfanyl-1H-benzimidazol-2-yl) carbamate; ABZ) is a benzimidazole derivative that was introduced in 1982 as an antihelmintic [4]. The mechanism of action of ABZ is related to microtubule inhibition and blocking glucose uptake that causes depletion of glycogen stores and lowering formation of ATP in the larval and adult stages of susceptible parasites. Altogether, it leads to immobilization and death of the parasite [5], [6]. There are some few evidences in the literature supporting ABZ repositioning for cancer therapy. ABZ possesses anti- proliferative effects against several tumor cell lines [7], [8], [9], [10]. Additional reasons to assess ABZ repositioning can be deduced from the fact that targeting the altered redox status of cancer cells is an interesting approach for new cancer therapy.
Cancer cells are under high oxidative stress because they possess altered antioxidant defenses and some researchers claim that reactive oxygen species (ROS) induced by anticancer drugs produce a shift in cellular antioxidant machinery as well as in the mitochondrial membrane potential, which are related to induction of programmed cell death [11]. It is important to stress out that ABZ showed increased ROS production, thereby promoting oxidative stress [12]. And this could be used as approach in cancer treatment considering cancer cells evading the apoptotic signaling [13]. In addition, due to the role of ROS as cellular switches for signaling cascades this property could be related with prooncogenic promotion or antitumorigenic signaling pathways, making the use of redox-modulating as an interesting strategy for anticancer therapy [14].
The aim of this work was to evaluate the antitumor effects of ABZ and relationship with induction of oxidative stress and DNA damage accessed by oxidative cleavage of CT-DNA, intracellular ROS content in MCF-7 cells and the related oxidative stress modulation, as well as the cycle cell arrest in Ehrlich ascites in mice treated with this drug, trying to characterize ABZ as a molecule prototype for possible drug repositioning in cancer therapy.
2. Material and methods
2.1. Chemicals and antibodies
Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS) and antibiotics were purchased from Gibco (USA). Albendazole (PubChem CID: 2082), methotrexate, calf thymus DNA (CT-DNA), agarose, DMSO, 2′,7′-dichlorofluorescein diacetate (DCFH-DA), N-acetyl-l-cysteine (NAC), 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), bovine serum albumin (BSA), ethidium bromide (EtdBr), protease inhibitor cocktail, hydrogen peroxide, trichloroacetic acid, thiobarbituric acid, glutathione oxidized form (GSSG), NADPH and epinephrine purchased from Sigma Aldrich (Brazil). The phosphatase inhibitor cocktail was from Calbiochem (Merck Biosciences). Antibodies against γH2AX, p53, Bax and Bcl-xL were obtained from Santa Cruz Biotechnology, Inc. (USA). Mouse antibody against β-actin was from Millipore (USA). The secondary antibodies and the kit for chemiluminescence detection of HRP-coupled antibodies were from Millipore (USA). PI/RNAse solution kit from Immunostep (Salamanca, Spain).
2.2. Oxidative cleavage of CT-DNA
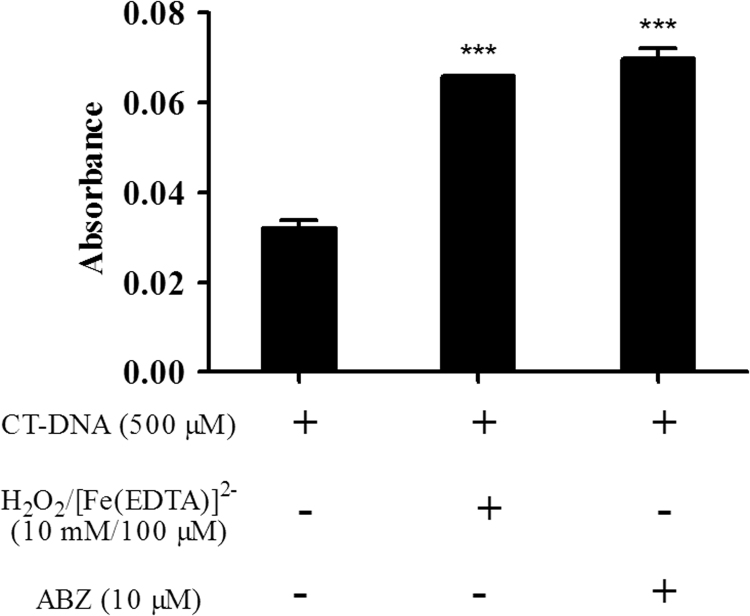
CT-DNA oxidative damage was evaluated using CT-DNA (0.5 mM) in 50 mM phosphate buffer (pH 7.2) incubated with 10 μM albendazole at 37 °C for 24 h. The samples were treated with 2-thiobarbituric acid 1% in 50 mM NaOH and glacial acetic acid, and incubated at 100 °C for 30 min. After cooling, absorbance was measured at 532 nm. The control had all components except albendazole. Fe(EDTA)-2: H2O2 was used for the positive control (100 μM/10 mM) [15].
2.3. Cytotoxicity, anti-proliferative assay, intracellular levels of ROS and mitochondrial membrane potential (ΔΨm) determination
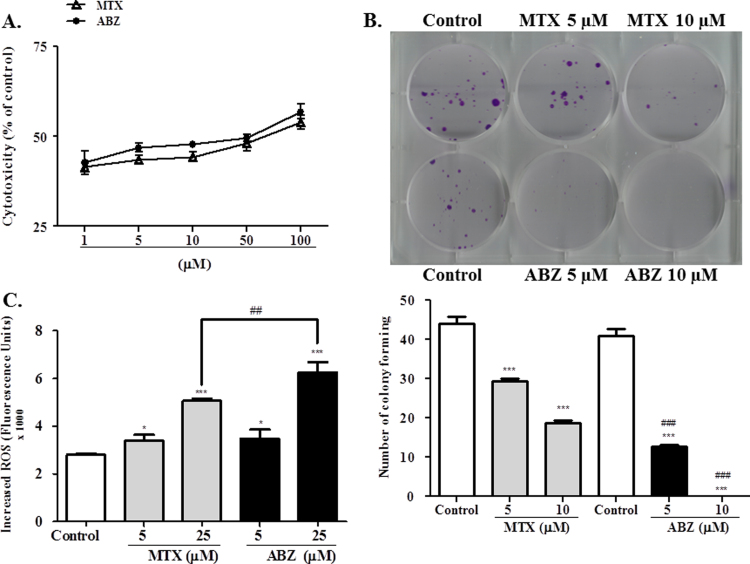
Human breast cancer cell line MCF-7 was purchased from the Rio de Janeiro cell bank, Brazil. Cells were cultured in DMEM high glucose with FBS 10%, penicillin (100 U/mL) and streptomycin (100 μg/mL). MTT assay was used to study the effect of ABZ on cell viability [16]. Cells (104 cells/well) were placed on 96-well plates and after confluence they were exposed to ABZ (0–100 μM) for 24 h. Cells were washed and after 2 h of incubation with MTT (0.5 mg/mL), formazan crystals were solubilized in DMSO. The colored solutions were read at 550 nm. The results were presented as values of concentration EC50.
Proliferation was evaluated in vitro through the colony forming unit assay. MCF-7 cells at density of single cells (500 cells) were allowed to set in six-well plates for 24 h. After, the medium was replaced by other containing of ABZ and MTX at non- cytotoxic concentrations (5 and 10 μM) and incubated for a further 24 h. In control wells, the cells were incubated in medium containing only DMSO 0.1%. After treatment, the cells were washed with warm PBS and fresh medium was provided. The cells were incubated for 16 days when the proliferation was counted in terms of colony forming units (CFUs) [17].
Intracellular ROS content were evaluated as reported by [18]. MCF-7 cells (15,000) were loaded with DCFH-DA (10 μM) in HBSS at 37 °C and incubated for 30 min. Excess DCFH-DA was removed by washing with fresh HBSS. After, the cells were incubated for 1 h with ABZ (5–25 μM) and methotrexate (MTX; at same concentrations), washed twice with HBSS, and then 100 μl of HBSS/well was added. The intensity of fluorescence was measured at 485 nm for excitation and 530 nm for emission using a Multiscan microplate reader.
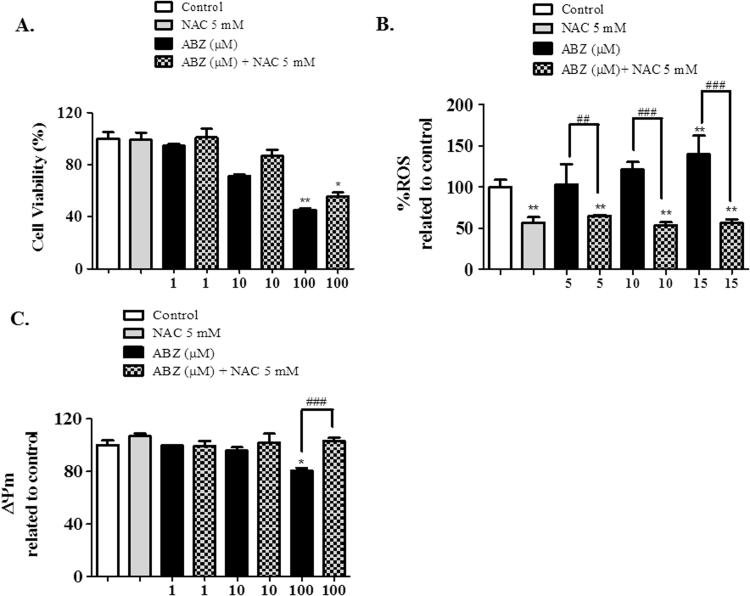
Mitochondrial membrane potential was performed using a fluorescent probe TMRE. MCF-7 cells (104/well) were plated in fluorescence 96-well plate, after confluence the cells were treated with different concentrations of ABZ (1, 10 and 100 µM), NAC (5 mM) or ABZ associated with NAC. After 6 h of treatment the cells were washed once with HBSS and incubated with TMRE (1 µM) during 20 min at 37 °C. After the cells were washed once with HBSS, followed by fluorescence intensity measurement, using excitation peak of 549 nm and emission of 575 nm.
2.4. Ehrlich carcinoma growth inhibition in mice
The antitumor effects of ABZ were evaluated against the Ehrlich ascitic carcinoma inoculated into the abdomen of isogenic Balb-c mice (20±2 g). Animal procedures were conducted in accordance with legal requirements and the approval of the local ethics committee (CEUA/UFSC PP00784) and legal requirements (NIH publication #80-23, revised in 1978). Animals were housed under controlled conditions and had free access to laboratory food and water.
Tumor induction was carried out by inoculating 5×106 cells of Ehrlich carcinoma. Twenty-four hours later mice were divided into 3 groups (n=12): The control was treated with saline (50 μl). The test-group was treated with ABZ 20 mg/kg in the same volume of vehicle (50 μl). MTX (2.5 mg/kg) was used for the positive control [19]. The dose of ABZ was chosen previously considering the maximum saturation point of this drug. The treatment started 24 h after tumor inoculation and the abdominal circumference of all animals was measured (time zero). It was repeated every 24 h during nine days. On the tenth day, the abdominal circumference of all animals was measured. Then, half of each group was euthanized for the evaluation of the ascitic fluid. Tumor growth was determined using the following equation [20]: Inhibition of tumor growth (%)=100- [(variation in waist circumference of the treated group×100)/variation in waist circumference of the control group]. Mice (n=6) from each group were kept alive to determine survival time [21], [22].
2.5. Antioxidant defense and oxidative damage biomarkers in Ehrlich carcinoma
The ascitic fluid of treated Ehrlich ascites-bearing mice was collected and some markers of oxidative stress were analyzed. Lipid peroxidation was assessed by the measurement of substances that react with TBA using the thiobarbituric acid method, as described by [23]. Oxidative damage to proteins was quantified as carbonyl proteins as described by [24]. The content of reduced glutathione (GSH) was determined as described by [25]. Catalase (CAT) activity was determined kinetically through the method described by [26] based on the decomposition of H2O2 and the decrease in absorbance at 240 nm. Superoxide dismutase (SOD) activity was measured by monitoring the oxidation of adrenaline to adrenochrome as described by [27]. Glutathione reductase (GR) activity was determined by measuring the rate of NADPH oxidation at 340 nm [28]. The reaction was monitored spectrophotometrically at 340 nm [29]. In general, the results were normalized by the content of proteins determined by the Lowry method [30].
2.6. DNA damage in Ehrlich ascitic carcinoma cells
The comet assay was performed to evaluate DNA fragmentation in tumor cells from treated mice [31]. Cells were resuspended in low-melting point agarose 0.75% and deposited on the surface of a slide covered by a thin layer of agarose 1.5%. This was allowed to set for 10 min at room temperature. The slides were submerged (2 h) in a lysis solution (2.5 M NaCl; 10 mM Tris; 100 mM EDTA; 1% Triton X-100% and 10% DMSO; pH 10.0) and after submitted to horizontal electrophoresis at 300 mA, 8 °C for 20 min in a tank with buffer (300 mM NaOH; 1 mM EDTA; pH 13). A neutralizing solution (0.4 M Tris HCl; pH 7.5) was added (3 times) followed by washing in water and dryness. After 10 min in a fixing solution (15% TCA; 5% ZnSO4; 5% glycerol) the slides were washed and dried again. Slides were stained with ethidium bromide (0.5 mg/mL) and analyzed under a fluorescence microscope. Each nucleus received an arbitrary value ranging from 0 (undamaged) to 4 (maximal damage) [32].
2.7. Cell death assessmentand cell cycle arrest in Ehrlich carcinoma
Tumor cells in the ascitic fluid (25 μl) were stained using a solution (5 μl) of acridine orange (200 μM) and ethidium bromide (250 μM). Samples were mixed with the staining just prior to microscopy. Cells (300) were visualized and categorized on microscope slides. Live cells appear uniformly green. Early apoptotic cells stain green and contain bright green dots in the nuclei. Late apoptotic cells also incorporate ethidium bromide and therefore stain orange, but, in contrast to necrotic cells, the late apoptotic cells show condensed and often fragmented nuclei [33].
Cell cycle arrest was evaluated using a PI/RNAse solution kit from Immunostep (Salamanca, Spain) using the procedure suggested by the manufacturer. Ehrlich tumor cells (5×105) were carefully washed with PBS, pelleted and fixed by rapid submersion in ice-cold ethanol (70%) with vigorous vortexing. After overnight fixation at −20 °C the cells were washed with PBS, pelleted, resuspended and incubated (15 min, under room temperature) with PI/RNAse solution. Finally, cells were evaluated by the FACSCanto II (BD Biosciences) cytometer. Data were processed using the Flowing Software 2.5.1.
2.8. Western blots analysis
After treatments, Ehrlich tumor cells were carefully washed with PBS and lysed in RIPA buffer (50 mM Tris-Cl; pH 7.4; 150 mM NaCl; 1% NP40; 0,25% Na-deoxycholate and 1 mM phenylmethylsulfonyl fluoride) supplemented with 1% protease inhibitor and 3% phosphatase inhibitor cocktails. After denaturation in Laemmli buffer (60 mM Tris-Cl; pH 6.8; 2% SDS; 10% glycerol; 5% β- mercaptoethanol; 0.01% bromophenol blue), equal amounts of protein (25 μg) from whole cellular homogenates were subjected to SDS-PAGE electrophoresis followed by electroblot to nitrocellulose membranes. After blocking and washing, the membranes were incubated overnight with the primary antibodies, washed again and further incubated with the secondary antibodies. Primary antibodies were: polyclonal rabbit anti-p-Histone H2AX from Santa Cruz Biotechnology (sc-101696), anti- p53 from Santa Cruz Biotechnology (sc-6243), mouse monoclonal antibody against Bax (sc- 7480) and Bcl-xL (sc-8392) were from Santa Cruz Biotechnology Inc. Mouse antibody against β-actin, the secondary antibodies and the kit for chemiluminescence detection of HRP-coupled antibodies were from Millipore (USA).
2.9. Statistical analysis
In vitro assays were performed in triplicate and repeated three times independently. In vivo assays were performed with n=12. Data were recorded as means±S.E.M. or percentages. Data were analyzed by the ANOVA test followed by the Bonferroni test. Comparisons were done using GraphPad Prism software (version 5.01). Values of p<0.05 were considered statistically significant.
3. Results and discussion
In cancer cells the interactions between antitumor drugs and DNA result in cell damage and target cell death [34]. CT-DNA treated with ABZ underwent oxidative damage (Fig. 1) and this was considered positive because DNA damage could provide therapeutic opportunities to treat cancer [35], [36]. Several factors can alter the structure of DNA, such as UV radiation, reactive oxygen and nitrogen species, as well as extrinsic chemical compounds [37]. Drugs able to oxidize DNA at C4 of deoxyribose cause DNA breakage thereby generating products of DNA degradation such as the base propenal, which is a TBA-reactive product [38], [39]. ABZ treatment overgenerates ROS therefore being able to oxidize DNA at C4 of deoxyribose causing DNA breakage which is detected by TBARS, showing that ABZ have a direct action on purified DNA.
Fig. 1.
Evaluation of CT-DNA cleavage in presence of albendazole (ABZ). The analysis was assessed by absorbance of thiobarbituric reactive species (TBARS) in CT-DNA (500 μM) treated with ABZ (10 μM). Negative control (NC): phosphate buffer. Positive control (PC): [Fe(EDTA)]2-/H2O2. Data represent the mean of three independent experiments. Significant at ***p<0.001 compared to the control group.
Treatment with ABZ and MTX inhibited cell viability in a concentration dependent manner (Fig. 2A). The EC50 value was calculated considering experiments performed after 24 h of incubation and the EC50 value obtained were 44.9 and 45.7 μM respectively, suggesting a very high ABZ and MTX cytotoxicity to MCF-7 cells.
Fig. 2.
Evaluation of MCF-7 cell lines treated with albendazole (ABZ) and methotrexate (MTX). (A) In vitro cytotoxicity of ABZ and MTX in MCF-7 cells. EC50 values obtained by the MTT reduction assay after 24 h treatment (1–100 μM). (B) Inhibition of cell proliferation assessed by colony formation assay after treatment with ABZ and MTX at 5 and 10 μM. (C) Content of Intracellular ROS in MCF-7 cells after treatment with ABZ and MTX at 5 and 25 μM, for 1 h. Data represent the mean of three independent experiments. Values are expressed as mean±S.E.M, n=3. * and *** denote statistical difference compared to non-treated cells of the control when p<0.1 and p<0.001, respectively. ## and ### denote statistical difference between ABZ and MTX treatment when p<0.01 and p<0.001, respectively.
In accordance, the anti-proliferative evaluation showed that ABZ and MTX treatments inhibited the MCF-7 colony formation (Fig. 2B), however in comparison with MTX the effect of ABZ at same concentration was more pronounced. The colony formation inhibition after ABZ treatment was approximately 67.5% for 5 μM and at 10 μM no colony formation was observed. MTX at same concentrations inhibited approximately 36% and 50%, respectively.
The content of intracellular ROS increased in MCF-7 cells treated with ABZ (Fig. 2C), at a concentration-dependent manner. These results suggest that ROS overgeneration could be involved in the cytotoxicity effects of MCF-7 cells treated with ABZ, because ROS can activate Bax thereby triggering apoptosis and reducing the cell viability [40], [41].
In attempt to verify if the ABZ ROS generation is affected in presence of antioxidant compounds we analyzed the ABZ effects in presence of N-acetyl-l-cysteine (NAC). The cytotoxic effects observed in MCF-7 cells treated with ABZ were reversed in presence of NAC (Fig. 3A), however NAC is not able to complete abolish ABZ effects upon MCF-7 cells in the higher concentration. The intracellular ROS increased due ABZ treatment (Fig. 3B) is completely reversed in presence of NAC in all treatment. The analysis of mitochondrial membrane potential (ΔΨm) showed that the presence of ABZ (100 µM) decreased the ΔΨm (Fig. 3C) but NAC is able to restored this effect. Loss of ΔΨm renders cells depleted of energy with subsequent death, showing a direct effect of ABZ ROS generation and apoptosis triggering.
Fig. 3.
Evaluation of MCF-7 cell line treated with albendazole (ABZ) and N-acetyl-l-cysteine (NAC). (A) In vitro cytotoxicity of ABZ (1–100 μM) and NAC (5 mM) in MCF-7 cells. MTT reduction assay after 24 h treatment. (B) Content of Intracellular ROS in MCF-7 cells after treatment with ABZ (5, 10 and 15 μM) and NAC (5 mM), for 4 h. (C) Mitochondrial membrane potential (ΔΨm) determination in MCF-7 cells after treatment with ABZ (1–100 μM) and NAC (5 mM), for 6 h. Data represent the mean of three independent experiments. Values are expressed as mean±S.E.M, n=3. * and ** denote statistical difference compared to non-treated cells of the control when p<0.1 and p<0.01, respectively. ## and ### denote statistical difference between ABZ and NAC treatment when p<0.01 and p<0.001, respectively.
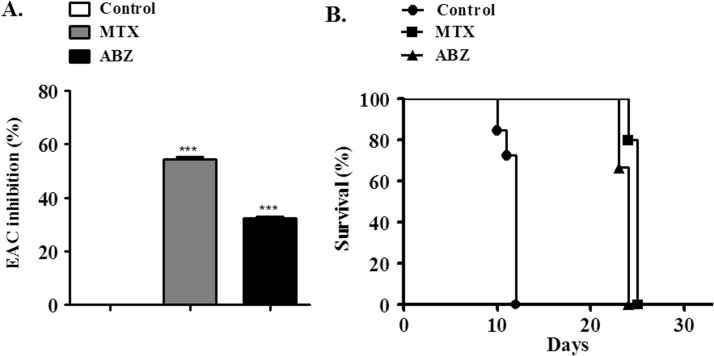
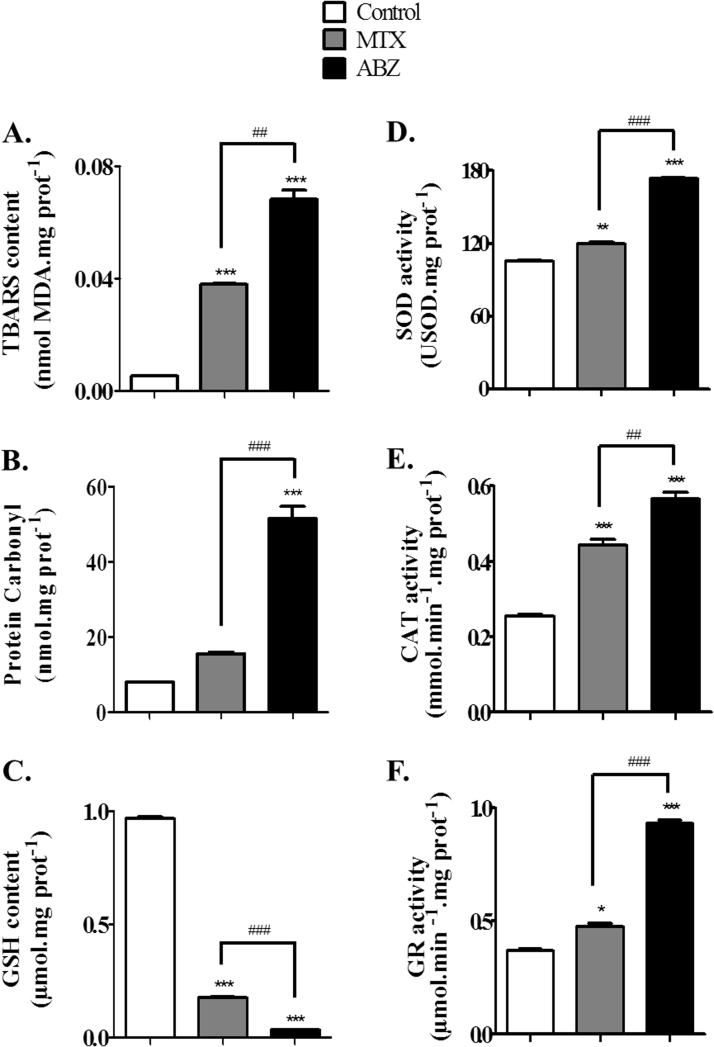
The in vivo assay to verify the antitumor activity using Ehrlich ascites carcinoma-bearing mice showed that ABZ (20 mg/kg) caused 32% of tumor growth inhibition (Fig. 4A), while MTX (2.5 mg/kg) caused nearly 55%. In addition, MTX and ABZ treatments elongated the survival time of the animals (Fig. 4B). The difference between MTX and ABZ effects may be due to the related ABZ low bioavailability, for this reason pharmacokinetic and structure activity studies are needed aiming to improve the solubility of this drug. In attempt to verify if the mechanism beyond ABZ antitumor activity involves ROS biosignaling, oxidative stress markers were analyzed using the ascitic fluid. In this regard, increased oxidative markers were detected, MDA content was 12.9-fold higher compared to controls for ABZ treatment and 7.2-fold higher compared to controls for MTX (Fig. 5A). Similarly, carbonyl protein (Fig. 5B) also increased for ABZ (6.4-fold) and MTX (2.0-fold) compared to controls. However, ABZ showed a more potent ROS generation ability compared to MTX. Accordingly, biomarkers of oxidative stress are directly connected with changes of ROS concentrations [42], [43]. When antioxidant defenses are impaired or overcome, oxidative stress enhances lipid peroxidation and protein carbonyl formation [44]. In some cases, the formation of carbonyl derivatives could be nonreversible, causing conformational changes and decreasing catalytic activity in antioxidant enzymes [45]. Also, it is well known that ROS or oxidants are involved in the induction of apoptosis as well as in necrosis [46].
Fig. 4.
Antitumor activity of albendazole (ABZ, 20 mg/kg) and methotrexate (MTX, 2.5 mg/kg, positive control) against Ehrlich Ascites Carcinoma in balb-c mice. (A) ABZ and MTX growth tumor inhibition analysis. (B) Increased survival time of Ehrlich Ascites Carcinoma-bearing mice treated with ABZ or MTX. Values are expressed as mean±S.E.M, n=12.*** denote statistical difference compared to non-treated cells of the control (saline group) when p<0.001.
Fig. 5.
Markers of oxidative stress and antioxidant defenses in Ascitic fluid from Ehrlich tumor cells from mice treated with Albendazole (ABZ, 20 mg/kg) or methotrexate (MTX, 2.5 mg/kg, positive control). The treatment caused molecular damage on (A) lipids (lipoperoxidation) and (B) proteins (carbonylation) and consuming (C) GSH in tumor cells. The activity of antioxidant enzymes increased: (D) superoxide dismutase (SOD), (E) catalase (CAT) and (F) glutathione reductase (GR). Values are expressed as mean±S.E.M, n=12. Significant at *p<0.05, **p<0.01 and ***p<0.001 compared to control group (saline group). ##p<0.01 and ###p<0.001 indicated significance between the ABZ and MTX groups.
Whereas the levels of the tri-peptide GSH were drastically decreased, all activity of antioxidant enzymatic such as SOD, CAT and GR were increased after treatment with both drugs. Taken together, these findings are indicative of an oxidative stress increased caused by ABZ and MTX treatments, stressing out that ABZ presented a greater effect compared to MTX.
A drastic depletion of GSH content (Fig. 5C) for ABZ (96.77%) and MTX (81.87%) was observed compared to controls, despite the increased of GR activity (2.5 and 1.3-fold compared to controls for ABZ and MTX, respectively), suggesting that ABZ is a potent ROS generator (Fig. 5F). The GSH redox system is important for attenuating oxidative stress and in this process, GSH as a generalist radical scavenger, is converted to oxidized glutathione (GSSG), while GR continuously converts GSSG back to GSH [47]. Accordingly, the increased GR activity observed in both treatments could be justified by ROS overproduction, which is known to increase GR activity [48]. In this context, our results showed that both ABZ and MTX increased ROS generation concomitantly to GSH depletion and GR upregulation.
In cancer cell GSH metabolism is usually upregulated, suggesting that cancer cells depend on GSH metabolism [49]. Therefore, cancer cells appear to rely heavily on GSH reducing equivalents to maintain redox homeostasis to face oxidative challenge, while GSH manipulation by limiting its synthesis and/or inhibiting its redox recycling have been shown to be an effective way of sensitizing this kind of cell clonogens to cell death. The role of GSH redox in cell apoptosis in a variety of cell types [50] indicates the importance in use such strategy in tumor cells growth inhibition.
According to [51] SOD and CAT inhibition is result of tumor growth, while the increased activity of both enzymes is important for tumor inhibition. Accordingly, ABZ and MTX revealed to be potent tumor inhibitors, probably as a consequence of their ability to upregulate SOD and CAT activity (Fig. 5 D and E). ABZ increased 1.64-fold SOD activity and 2.22-fold CAT activity, whereas MTX increased 1.13 and 1.73, respectively. In addition, according to [12], in a comparative study of oxidative stress induced by ABZ, increased SOD activity would render higher H2O2 concentrations inside the cells, which is in agreement with the high CAT activity found under ABZ treatment (Fig. 5E). Moreover, relatively slight increases in intracellular H2O2 can elicit apoptosis and, in accordance to ours results, ABZ promoted increased ROS generation as well as SOD and CAT activity, leading to apoptotic cell death. MXT induced SOD and CAT activity whereas, ABZ revealing a greater enzymatic increase.
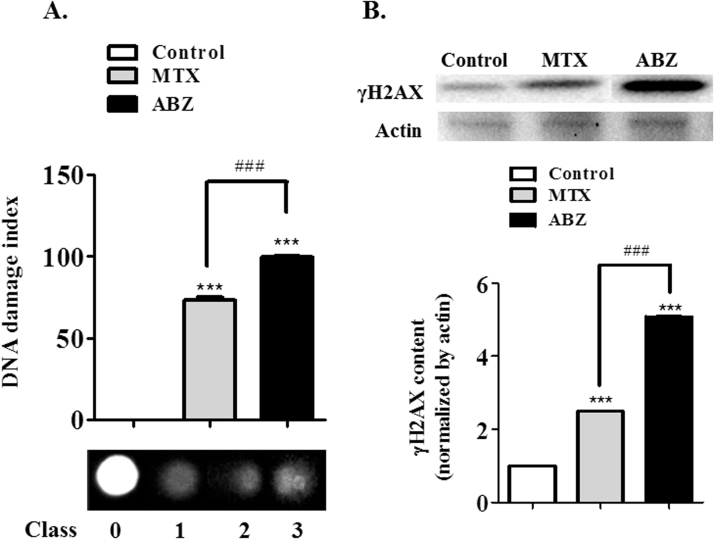
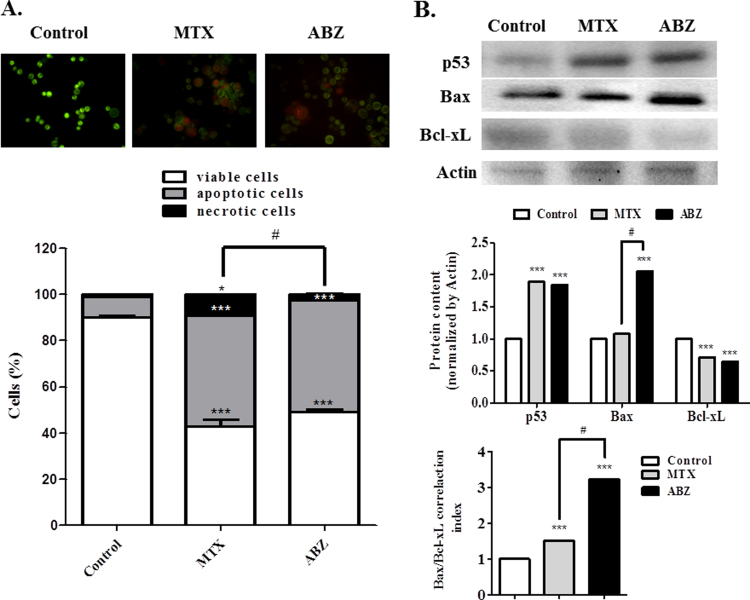
In the context of modulation of oxidative stress by ABZ, DNA damage and increased apoptotic cell death in Ehrlich cells, were both verified after ABZ treatment. DNA fragmentation was measured (Fig. 6A) and the result showed a strong DNA damage in Erhlich cells treated with ABZ (damage index of 99.5), again more pronounced compared to MTX (73.0). These damages could be associated with increased ROS generation promoted by ABZ, affecting DNA cells. This result was confirmed by the immunoblotting assay (Fig. 6B), showing that ABZ treatment increased the content of γH2AX (5.1-fold when compared with controls), an effect again more than MTX treatment (2.5). In agreement, cell death results showed that the DNA fragmentation damage caused by ABZ-induced ROS generation, was probably responsible for the increased cell death by apoptosis. A strong ABZ increase of apoptosis cell (5.4-fold when compared with controls), whereas the viable cell was drastically reduced (Fig. 7A). Besides, MTX increased apoptosis (5.3-fold compared with controls), raised necrosis rate (9.2-fold compared with controls), responses that were not observed after the ABZ treatment. This result was confirmed by the immunoblotting assay (Fig. 7B), ABZ and MTX treatments when compared with controls showed an increase of p53 and pro-apoptotic Bax expression, whereas the anti-apoptotic Bcl-xL expression was diminished.
Fig. 6.
Albendazole (ABZ, 20 mg/kg) and Methotrexate (MTX, 2.5 mg/kg, positive control) effects on DNA of Ascitic fluid from Ehrlich ascitic carcinoma cells. (A) DNA damage indexdetermined by the comet assay and (B) γH2AX was assessed by immunoblotting. Values are expressed as mean±S.E.M. Significant at ***p<0.001 compared to control group (saline group).###p<0.001 indicated significance between the ABZ and MTX groups.
Fig. 7.
Albendazole (ABZ, 20 mg/kg) and Methotrexate (MTX, 2.5 mg/kg, positive control) induce apoptosis and necrosis. (A) Detection of apoptosis by the acridine orange/ethidium bromide staining. (B) Pro and anti-apoptotic protein p53, Bax and Bcl-xL were assessed by immunoblotting. Significant at ***p<0.001, and *p<0.05 compared to control group (saline group). #p<0.05 indicated significance between the ABZ and MTX groups.
According to [52], it is well established that oxidative stress exposure induces DNA damage in cells. γH2AX is a variant of histone H2A, which is phosphorylated at ser139 in response to DNA damage [53]. In this way, ROS can activate p53 [54], which, in accordance to [49], after p53 activation the expression of various proapoptotic factors occurs, including Bax. In turn, Bax activation leads to an upstream of apoptotic pathways converging into a final stage of cell self-destruction. Also, increasing the ratio of mitochondrial Bax/Bcl-xL induces Bax activation and cytochrome c release from mitochondria in the presence of a BH3-only protein, tBid, in a cell-free system [55]. In addition, p53 control apoptosis in response to abnormal proliferative signals and stress including DNA damage [56]. Our results showed an increase of this correlation rate of Bax/Bcl-xL, thereby confirming that ABZ induces mitochondrial-associated pathway of apoptosis (Fig. 7B) in a greater rate compared to MTX. This correlation rate of Bax/Bcl-xL increase is in accordance with p53 increase, because p53-dependent apoptosis is involved with mitochondrial cytochrome c release [56].
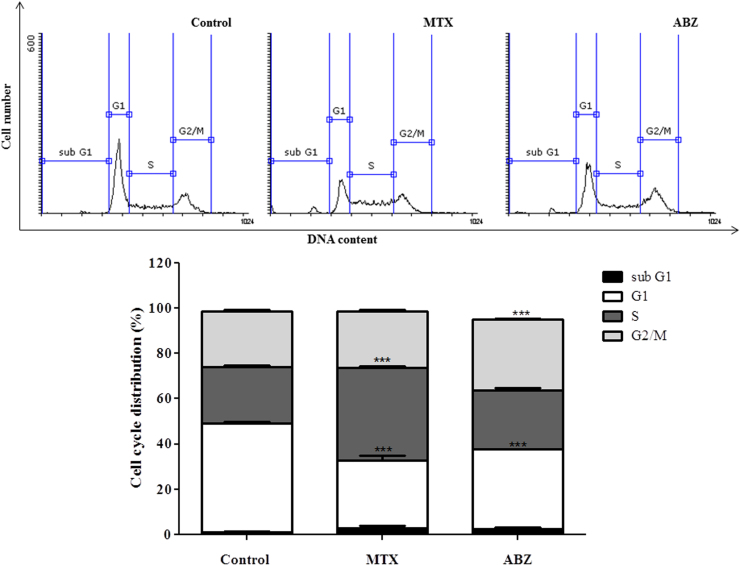
Finally, the cell cycle analysis (Fig. 8A) demonstrating that Ehrlich ascitic carcinoma (EAC) cells treated with albendazole and methotrexate showed a different cell cycle profile compared with non-treated EAC cells (control). Both treatments diminished the number of cells G1 phase compared with control. Ours results (Fig. 8A and B) showed that ABZ besides inhibits G1 phase arrest G2/M phase in EAC carcinomas as expected, suggesting that ABZ mechanism of action in this cellular type appears to be related with tubulin polymerization inhibition [57]. In this context, microtubules (heterodimer composed of α tubulin and β tubulin subunits) are indispensable for the formation and disappearance of the mitotic spindle, in other words, they are responsible for separation of duplicated chromosomes during cell division, while disruption of microtubule dynamics by inhibiting polymerization or preventing depolymerization of tubulin, thereby promoting cell cycle arrest on G2/M phase [57], [58]. MTX analysis showed that G1 phase was inhibited and S phase was arrested. In this context, the EAC growth inhibition treated with MTX could be related with the modulation of pathways including inhibition of G0/G1-S phase cell cycle progression and induction of apoptosis [59], as well as S phase arrest, which is associated with growth-inhibitory activity. Such effects of growth inhibition, induction of apoptosis and S-G2-phase cell cycle arrest have been already described [60] in HL60 promyelocytic leukemia cells treated with resveratrol. Accordingly, [61] showed that Bladder Carcinoma Cell Line Growth Inhibition is mediated by S/G2-M cell cycle arrest and apoptosis using ciprofloxacin.
Fig. 8.
Albendazole (ABZ, 20 mg/kg) and Methotrexate (MTX, 2.5 mg/kg, positive control) influence on the cell cycle regulation. Percentage of EAC cells in sub G1, G1, S and G2/M phases after treatment with ABZ. Significant at **p<0.01 and ***p<0.001 compared to control group (saline group).
4. Conclusion
The ABZ-modulation oxidative stress revealed an interesting approach regarding cancer treatment. ROS generation promoted by ABZ in vitro seems to be responsible for sensitizing the cell death in human breast cancer cells, probably due to the strong inhibition in proliferation and viability of these cells. ABZ treatment in vivo also indicated to be related with oxidative stress pathway, leading to depletion of reduced glutathione levels, to augmentation of important oxidative biomarkers and activity of antioxidant enzymes, as well as promoting EAC cells to an impaired antioxidant defense system in relation to normal cells, thereby inducing activation of apoptosis pathway. Moreover, ABZ is able to break the CT-DNA in vitro, while in EAC cells the drug seems to be involved in epigenetic modification associated with DNA damage by inducing DNA fragmentation, as well as increasing the expression of pro-apoptotic proteins. All these effects are underlined by a significant anticancer in vivo action, indicating ABZ as a molecule prototype in possible drug repositioning for human breast cancer treatment.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
L.S.E.P.W Castro, F. Ourique, V.M.A.S Grienevicius, E.B. Parisotto are recipients of research grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil). RC Pedrosa (Proc. 302404/2011-2) and DWF (Proc. 303234/2015-6) are recipient of research grants from the Conselho Nacional de Pesquisa (CNPq, Brazil). Prof. Dr. Marcelo Farina the group leader of Metals Neurotoxicity Laboratory and Ms Diones C. Bueno for technical support in mitochondrial membrane potential studies.
References
- 1.José Alencar Gomes da Silva National Institute of Cancer (INCA). Estimate/2014: Cancer Incidence in Brazil, in: General Coordination of Prevention and Surveillance (Ed.). Summary of results and comments, Female Breast Cancer, Rio de Janeiro, INCA, 2014, pp. 35.
- 2.Liang X.J., Chen C., Zhao Y., Wang P.C. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol. Biol. 2010;596:467–488. doi: 10.1007/978-1-60761-416-6_21. 〈http://dx.doi.org/10.1007/978-1-60761-416-6_21〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Disco. 2004;3:673–683. doi: 10.1038/nrd1468. 〈http://dx.doi.org/10.1038/nrd1468〉 [DOI] [PubMed] [Google Scholar]
- 4.Upcroft P., Upcroft J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol Rev. 2001;14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. 〈http://dx.doi.org/10.1128/CMR.14.1.150-164.2001〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertram G.K. 5th edition. chapter 55. London; Appleton and Lange: 1992. Clinical Pharmacology of the Anthelmintic Drugs. In: A Publishing Division of Prentice Hall. Basic and Clinical Pharmacology; p. 748. [Google Scholar]
- 6.Martin R.J. Modes of action of anthelmintic drugs. Vet. J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. 〈http://dx.doi.org/10.1016/S1090-0233(05)80005-X〉 [DOI] [PubMed] [Google Scholar]
- 7.Pourgholami M.H., Woon L., Almajd R., Akhter J., Bowery P., Morris D.L. In vitro and in vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001;165:43–49. doi: 10.1016/s0304-3835(01)00382-2. 〈http://dx.doi.org/10.1016/S0304-3835(01)00382-2〉 [DOI] [PubMed] [Google Scholar]
- 8.Khalilzadeh A., Wangoo K.T., Morris D.L., Pourgholami M.H. Epothilone- paclitaxel resistant leukemic cells CEM/dEpoB300 are sensitive to albendazole: Involvement of apoptotic pathways. Biochem Pharm. 2007;74:407–414. doi: 10.1016/j.bcp.2007.05.006. 〈http://dx.doi.org/10.1016/j.bcp.2007.05.006〉 [DOI] [PubMed] [Google Scholar]
- 9.Chu S.W., Badar S., Morris D.L., Pourgholami M.H. Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole. Anticancer Res. 2009;29:3791–3796. [PubMed] [Google Scholar]
- 10.Patel K., Doudican N.A., Schiff P.B., Orlow S.J. Albendazole sensitizes cancer cells to ionizing radiation. Radiat. Oncol. 2011;6:1–7. doi: 10.1186/1748-717X-6-160. 〈http://dx.doi.org/10.1186/1748-717X-6-160〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik N.K., Kaushik N., Park D., Choi E.H. Altered antioxidant system stimulates dielectric barrier discharge plasma-induced cell death for solid tumor cell treatment. PLoS One. 2014;9:e103349. doi: 10.1371/journal.pone.0103349. 〈http://dx.doi.org/10.1371/journal.pone.0103349〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locatelli C., Pedrosa R.C., de Bem A.F., Creczynski-Pasa T.B., Cordova C.A., Wilhelm Filho D. A comparative study of albendazole and mebendazole-induced, time-dependent oxidative stress. Redox Rep. 2004;9:89–95. doi: 10.1179/135100004225004751. 〈http://dx.doi.org/10.1179/135100004225004751〉 [DOI] [PubMed] [Google Scholar]
- 13.Salimi A., Roudkenar M.H., Sadeghi L., Mohseni A., Seydi E., Pirahmadi N., Pourahmad J. Ellagic acid, a polyphenolic compound, selectively induces ROS- mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox. Biol. 2015;6:461–471. doi: 10.1016/j.redox.2015.08.021. 〈http://dx.doi.org/10.1016/j.redox.2015.08.021〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukawera E., Chartier S., Williams V., Pagano P.J., Lapointe R., Grandvaux N. Redox-modulating agents target NOX2-dependent IKKε oncogenic kinase expression and proliferation in human breast cancer cell lines. Redox. Biol. 2015;6:9–18. doi: 10.1016/j.redox.2015.06.010. 〈http://dx.doi.org/10.1016/j.redox.2015.06.010〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun T., Bochu W., Liancai Z. Hydrolytic cleavage of DNA by quercetin manganese(II) complexes. Colloids Surf. B Biointerfaces. 2007;55:149–152. doi: 10.1016/j.colsurfb.2006.11.044. 〈http://dx.doi.org/10.1016/j.colsurfb.2006.11.044〉 [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. 〈http://dx.doi.org/10.1016/0022-1759(83)90303-4〉 [DOI] [PubMed] [Google Scholar]
- 17.Franken N.A.P., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. 〈http://dx.doi.org/10.1038/nprot.2006.339〉 [DOI] [PubMed] [Google Scholar]
- 18.Glorieux C., Dejeans N., Sid B., Beck R., Calderon P.B., Verrax J. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochem Pharm. 2011;82:1384–1390. doi: 10.1016/j.bcp.2011.06.007. 〈http://dx.doi.org/10.1016/j.bcp.2011.06.007〉 [DOI] [PubMed] [Google Scholar]
- 19.Kabel A.M., Abdel-Rahman M.N., El-Sisi Ael D., Haleem M.S., Ezzat N.M., El Rashidy M.A. Effect of atorvastatin and methotrexate on solid Ehrlich tumor. Eur. J. Pharmacol. 2013;713:47–53. doi: 10.1016/j.ejphar.2013.04.049. 〈http://dx.doi.org/10.1016/j.ejphar.2013.04.049〉 [DOI] [PubMed] [Google Scholar]
- 20.Farias M.S., Pich C.T., Kviecinski M.R., Bucker N.C., Felipe K.B., Da Silva F.O., Gunther T.M., Correia J.F., Rios D., Benites J., Valderrama J.A., Calderon P.B., Pedrosa R.C. Substituted 3acyl2phenylamino1,4naphthoquinones intercalate into DNA and cause genotoxicity through the increased generation of reactive oxygen species culminating in cell death. Mol. Med Rep. 2014;10:405–410. doi: 10.3892/mmr.2014.2160. 〈http://dx.doi.org/10.3892/mmr.2014.2160〉 [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457. 〈http://dx.doi.org/10.1080/01621459.1958.10501452〉 [Google Scholar]
- 22.Gupta M., Mazumder U.K., Rath N., Mukhopadhyay D.K. Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich Ascites Carcinoma. J. Ethnopharmacol. 2000;72:151–156. doi: 10.1016/s0378-8741(00)00227-0. 〈http://dx.doi.org/10.1016/S0378-8741(00)00227-0〉 [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. 〈http://dx.doi.org/10.1016/0003-2697(79)90738-3〉 [DOI] [PubMed] [Google Scholar]
- 24.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.-G., Ahn B.-W., Shaltiel S., Stadtman E.R. [49] Determination of carbonyl content in oxidatively modified proteins. Methods Enzym. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. 〈http://dx.doi.org/10.1016/0076-6879(90)86141-H〉 [DOI] [PubMed] [Google Scholar]
- 25.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 26.Aebi H. [13] Catalase in vitro. Methods Enzym. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. 〈http://dx.doi.org/10.1016/S0076-6879(84)05016-3〉 [DOI] [PubMed] [Google Scholar]
- 27.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 28.Carlberg I., Mannervik B. [59] Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. 〈http://dx.doi.org/10.1016/S0076-6879(85)13062-4〉 [DOI] [PubMed] [Google Scholar]
- 29.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 30.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. 〈http://dx.doi.org/10.1016/0014-4827(88)90265-0〉 [DOI] [PubMed] [Google Scholar]
- 32.Ross G.M., McMillan T.J., Wilcox P., Collins A.R. The single cell microgel electrophoresis assay (comet assay): technical aspects and applications: Report on the 5th LH Gray Trust Workshop, Institute of Cancer Research, 1994. Mutat. Res. 1995;337:57–60. doi: 10.1016/0921-8777(95)00007-7. 〈http://dx.doi.org/10.1016/0921-8777(95)00007-7〉 [DOI] [PubMed] [Google Scholar]
- 33.Kasibhatla S., Amarante-Mendes G.P., Finucane D., Brunner T., Bossy-Wetzel E., Green D.R. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006;3:4493. doi: 10.1101/pdb.prot4493. 〈http://dx.doi.org/10.1101/pdb.prot4493〉 [DOI] [PubMed] [Google Scholar]
- 34.Bischoff G., Hoffmann S. DNA-binding of drugs used in medicinal therapies. Curr. Med Chem. 2002;9:321–348. doi: 10.2174/0929867023371085. 〈http://dx.doi.org/10.2174/0929867023371085〉 [DOI] [PubMed] [Google Scholar]
- 35.Lord C.J., Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287–294. doi: 10.1038/nature10760. 〈http://dx.doi.org/10.1038/nature10760〉 [DOI] [PubMed] [Google Scholar]
- 36.Hosoya N., Miyagawa K., Targeting D.N.A. damage response in cancer therapy. Cancer Sci. 2014;105:370–388. doi: 10.1111/cas.12366. 〈http://dx.doi.org/10.1111/cas.12366〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikhed Y., Görlach A., Knaus U.G., Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. 2015;5:275–289. doi: 10.1016/j.redox.2015.05.008. 〈http://dx.doi.org/10.1016/j.redox.2015.05.008〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciriolo M.R., Peisach J., Magliozzo R.S. A Comparative Study of the Interactions of Bleomycin with Nuclei and Purified DNA. J. Biol. Chem. 1989;264:1443–1449. [PubMed] [Google Scholar]
- 39.Jiang Q., Xiao N., Shi P., Zhu Y., Guo Z. Design of artificial metallonucleases with oxidative mechanism. Coord. Chem Rev. 2007;251:1951–1972. 〈http://dx.doi.org/10.1016/j.ccr.2007.02.013〉 [Google Scholar]
- 40.Portt L., Norman G., Clapp C., Greenwood M., Greenwood M.T. Anti- apoptosis and cell survival: a review. Biochim Biophys. Acta. 1813;238–259:2011. doi: 10.1016/j.bbamcr.2010.10.010. 〈http://dx.doi.org/10.1016/j.bbamcr.2010.10.010〉 [DOI] [PubMed] [Google Scholar]
- 41.Ryter S.W., Kim H.P., Hoetzel A., Park J.W., Nakahira K., Wang X., Choi A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. 〈http://dx.doi.org/10.1089/ars.2007.9.49〉 [DOI] [PubMed] [Google Scholar]
- 42.Barker C.W., Fagan J.B., Pasco D.S. Downregulation of P4501A1 and P4501A2 mRNA expression in isolated hepatocytes by oxidative stress. J. Biol. Chem. 1994;269:3985–3990. [PubMed] [Google Scholar]
- 43.Ahmad I., Pacheco M., Santos M.A. Enzymatic and nonenzymatic antioxidants as an adaptation to phagocyte-induced damage in Anguilla anguilla L. following in situ harbor water exposure. Ecotoxicol. Environ. Saf. 2004;57:290–302. doi: 10.1016/S0147-6513(03)00080-0. 〈http://dx.doi.org/10.1016/S0147-6513〉(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 44.Stepić S., Hackenberger B.K., Hackenberger D.K., Velki M., Lončarić Ž. Impact of oxidative stress indicated by thiobarbituric acid reactive substances (TBARS) and protein carbonyl levels (PC) on ethoxyresorufin-O-deethylase (EROD) induction in common carp (Cyprinus carpio) Water Air Soil Poll. 2012;223:4785–4793. 〈http://dx.doi.org/10.1007/s11270-012-1234-1〉 [Google Scholar]
- 45.Parvez S., Raisuddin S. Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch) Environ. Toxicol. Pharm. 2005;20:112–117. doi: 10.1016/j.etap.2004.11.002. 〈http://dx.doi.org/10.1016/j.etap.2004.11.002〉 [DOI] [PubMed] [Google Scholar]
- 46.Vairetti M., Ferrigno A., Bertone R., Richelmi P., Bertè F., Freitas I. Apoptosis vs. necrosis: glutathione-mediated cell death during rewarming of rat hepatocytes. Biochim Biophys. Acta. 1740;367–374:2005. doi: 10.1016/j.bbadis.2004.11.022. 〈http://dx.doi.org/10.1016/j.bbadis.2004.11.022〉 [DOI] [PubMed] [Google Scholar]
- 47.Yao J.K., Leonard S., Reddy R. Altered glutathione redox state in schizophrenia. Dis. Markers. 2006;22:83–93. doi: 10.1155/2006/248387. 〈http://dx.doi.org/10.1155%2F2006%2F248387〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frasier C.R., Moukdar F., Patel H.D., Sloan R.C., Stewart L.M., Alleman R.J., La Favor J.D., Brown D.A. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res. 2013;98:47–55. doi: 10.1093/cvr/cvt009. 〈http://dx.doi.org/10.1093/cvr/cvt009〉 [DOI] [PubMed] [Google Scholar]
- 49.Zhou D., Shao L., Spitz D.R. Chapter One – Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. 〈http://dx.doi.org/10.1016/B978-0-12-420117-0.00001-3〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. 〈http://dx.doi.org/10.1016/j.freeradbiomed.2009.12.022〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta M., Mazumder U.K., Kumar R.S., Sivakumar T., Vamsi M.L.M. Antitumor activity and antioxidant status of Caesalpinia bonducella against ehrlich ascites carcinoma in Swiss Albino Mice. J. Pharm. Sci. 2004;94:177–184. doi: 10.1254/jphs.94.177. 〈http://doi.org/10.1254/jphs.94.177〉 [DOI] [PubMed] [Google Scholar]
- 52.Shiloh Y., Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013;14:197–210. 〈http://doi.org/10.1038/nrm3546〉 [PubMed] [Google Scholar]
- 53.Vijayakurup V., Carmela S., Carmelo D., Corrado T., Srinivas P., Gopala S. Phenethyl caffeate benzo[kl]xanthene lignan with DNA interacting properties induces DNA damage and apoptosis in colon cancer cells. Life Sci. 2012;91:1336–1344. doi: 10.1016/j.lfs.2012.10.013. 〈http://doi.org/10.1016/j.lfs.2012.10.013〉 [DOI] [PubMed] [Google Scholar]
- 54.Ditch S., Paull T.T. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. 〈http://doi.org/10.1016/j.tibs.2011.10.002〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu F.T., Goff L.K., Hao J.H., Newland A.C., Jia L. Increase in the ratio of mitochondrial Bax/Bcl-XL induces Bax activation in human leukemic K562 cell line. Apoptosis. 2004;9:377–384. doi: 10.1023/b:appt.0000025815.78761.5c. 〈http://doi.org/10.1023/B:APPT.0000025815.78761.5c〉 [DOI] [PubMed] [Google Scholar]
- 56.Benchimol S. p53-dependent pathways of apoptosis. Nat. Cell Death Differentiation. 2001;8:1049–1051. doi: 10.1038/sj.cdd.4400918. [DOI] [PubMed] [Google Scholar]
- 57.Arora S., Wang X.I., Keenan S.M., Andaya C., Zhang Q., Peng Y., Welsh W. J. novel microtubule polymerization inhibitor with potent antiproliferative and antitumor activity. Cancer Res. 2009;69:1910–1915. doi: 10.1158/0008-5472.CAN-08-0877. 〈http://doi.org/10.1158/0008-5472.CAN-08-0877〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin J.W., Son J.Y., Kang J.K., Han S.H., Cho C.K., Son C.G. Trichosanthes kirilowii tuber extract induces G2/M phase arrest via inhibition of tubulin polymerization in HepG2 cells. J. Ethnopharmacol. 2008;115:209–216. doi: 10.1016/j.jep.2007.09.030. 〈http://doi.org/10.1016/j.jep.2007.09.030〉 [DOI] [PubMed] [Google Scholar]
- 59.Qin C., Morrow D., Stewart J., Spencer K., Porter W., Smith R., Phillips T., Abdelrahim M., Samudio I., Safe S. A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1- Bis(3′-indolyl)−1-(p-substituted phenyl)methanes. Mol. Cancer Ther. 2004;3 247-60. [PubMed] [Google Scholar]
- 60.Joe A.K., Liu H., Suzui M., Vural M.E., Xiao D., Weinstein I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 61.Aranha O., Wood D.P., Jr., Sarkar F.H. Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in a human transitional cell carcinoma of the bladder cell line. Clin. Cancer Res. 2000;6:891–900. [PubMed] [Google Scholar]