Abstract
Importance
The Million Hearts initiative emphasizes the “ABCS” - aspirin, blood pressure control, cholesterol management, and smoking cessation. Evidence for the effects of drugs used to achieve the ABCS has not been comprehensively synthesized in primary atherosclerotic cardiovascular disease (ASCVD) prevention.
Objective
To compare the efficacy and safety of aspirin, blood pressure-lowering therapy, statin, and tobacco cessation drugs on fatal and non-fatal ASCVD outcomes in primary ASCVD prevention.
Evidence Review
Structured search of the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA), MEDLINE, EMBASE, and PROSPERO International Prospective Systematic Review Trial Register to identify systematic reviews published from January 1, 2005, to June 17, 2015, that reported the effect of aspirin, BP-lowering therapy, statin, or tobacco cessation drugs on ASCVD events in individuals without prevalent ASCVD. Additional studies were identified by searching the reference lists of included systematic reviews, meta-analyses, and health technology assessment reports. Reviews were selected according to predefined criteria and appraised formethodologic quality using the Assessment of Multiple Systematic Reviews (AMSTAR) tool (range, 0–11). Studies were independently reviewed for key participant and intervention characteristics. Outcomes that were meta-analyzed in each included review were extracted. Qualitative synthesis was performed, and data were analyzed from July 2 to August 13, 2015.
Findings
From a total of 1967 reports, 35 systematic reviews of randomized clinical trials were identified, including 15 reviews of aspirin, 4 reviews of BP-lowering therapy, 12 reviews of statins, and 4 reviews of tobacco cessation drugs. Methodologic quality varied, but 30 reviews had AMSTAR ratings of 5 or higher. Compared with placebo, aspirin (relative risk [RR], 0.90; 95%CI, 0.85–0.96) and statins (RR, 0.75; 95%CI, 0.70–0.81) reduced the risk for ASCVD. Compared with placebo, BP-lowering therapy reduced the risk for coronary heart disease (RR, 0.84; 95%CI, 0.79–0.90) and stroke (RR, 0.64; 95%CI, 0.56–0.73). Tobacco cessation drugs increased the odds of continued abstinence at 6 months (odds ratio range, 1.82 [95%CI, 1.60–2.06] to 2.88 [95%CI, 2.40–3.47]), but the direct effects on ASCVD were poorly reported. Aspirin increased the risk for major bleeding (RR, 1.54; 95%CI, 1.30–1.82), and statins did not increase overall risk for adverse effects (RR, 1.00; 95%CI, 0.97–1.03). Adverse effects of BP-lowering therapy and tobacco cessation drugs were poorly reported.
Conclusions and Relevance
This overview demonstrates high quality evidence to support aspirin, blood pressure-lowering therapy, and statins for primary ASCVD prevention and tobacco cessation drugs for smoking cessation. Treatment effects of each drug can be used to enrich clinician-patient discussions in primary ASCVD prevention.
INTRODUCTION
In 2011, the United States Department of Health and Human Services launched the Million Hearts initiative to prevent 1 million heart attacks and strokes over 5 years.1 Million Hearts includes a clinical emphasis on the ABCS, that is, aspirin for high-risk patients, blood-pressure control, cholesterol management, and smoking cessation. Projections estimate that optimizing ABCS management could reduce the burden of atherosclerotic cardiovascular disease (ASCVD) substantially.2 To support the Million Hearts goal and identify new models of care delivery and payment, the Center for Medicare and Medicaid Innovation launched the Million Hearts Cardiovascular Risk Reduction Model in 2016, a cluster randomized payment model test to evaluate the effect of value-based payment to incentivize ASCVD risk assessment and reduction. This effort requires rigorous and transparent quantification of treatment effects for aspirin, blood pressure-lowering therapy, statins, and tobacco cessation drugs in primary ASCVD prevention.3 Despite the wealth of evidence synthesis activities within individual drug classes, we are not aware of any reports that have summarized the efficacy and safety of all 4 drug classes highlighted in the Million Hearts initiative for primary ASCVD prevention.
An overview of systematic reviews is a novel approach to appraise and synthesize results from multiple systematic reviews into a single, useful document that can be used to guide health care providers and policy makers.4–7 To address this evidence gap, we performed an overview of systematic reviews to compare the efficacy and safety of aspirin, blood pressure-lowering therapy, statin, and tobacco cessation drugs on fatal and non-fatal outcomes for primary ASCVD prevention.
METHODS
This overview followed guidelines outlined by the Cochrane Collaboration to synthesize effects of multiple interventions for an overarching clinical question using data from published systematic reviews.4 We established a protocol and published it in PROSPERO.8 The clinical question guiding this overview is presented in PICOTSS (patient, intervention, comparators, outcomes, timing, setting, and study design) format (Box). We also performed a supplemental systematic review to evaluate for potential interactions when combination drug therapy is used but found none (detailed search methods and results outlined in eAppendix 1 and 2 in the Supplement).
Box 1. Patients, Interventions, Comparators, Outcomes, Timing, Setting, and Study design (PICOTSS).
Patients
Adults ≥ 18 years of age
People without prevalent atherosclerotic cardiovascular disease (ASCVD)
Systematic reviews or trials that included people with Alzheimer’s disease, end-stage renal disease, macular degeneration, and aortic stenosis were excluded
Interventions
Aspirin
BP-lowering therapy
Statin
Tobacco cessation drug (i.e. nicotine replacement therapy, varenicline, buproprion)
Comparators
Placebo
Usual care
Outcomes
Primary outcomes
All-cause mortality
Fatal and nonfatal cardiovascular events, including myocardial infarction and stroke (ASCVD)
Adverse events as reported by study authors
Secondary outcomes
Fatal and non-fatal ischemic heart disease events, including myocardial infarction, angina, and coronary revascularization)
Fatal and non-fatal cerebrovascular events, including stroke and transient ischemic attack
Total and non-fatal ASCVD events
Total and low-density lipoprotein (LDL) cholesterol
Systolic and diastolic BP
Health-related quality of life
Direct Costs
Timing
Studies of any duration
Setting
Any setting
Study design
Systematic reviews of randomized and quasi-randomized clinical trials
Review Eligibility Criteria
We included systematic reviews of randomized and quasi-randomized clinical trials comparing the pooled treatment effects of aspirin, blood pressure-lowering therapy, statin, or tobacco cessation drugs against placebo or usual care in adults (≥ 18 years of age) without ASCVD. For systematic reviews that included a combination of individuals with and without prevalent ASCVD, we included reviews that reported effect estimates for participants defined as “primary prevention.” When reports included both primary and secondary prevention populations, we included only those reports with <10% of the population with prevalent ASCVD.9 Systematic reviews or trials in which drugs were used to treat or control chronic conditions (e.g., Alzheimer’s disease, rheumatoid arthritis, renal disease, macular degeneration, and aortic stenosis) were excluded.
Outcomes
The primary outcomes for our review were (1) all-cause mortality; (2) fatal and nonfatal cardiovascular events, including myocardial infarction and stroke (ASCVD); and (3) adverse events as reported by the authors of included reviews. Secondary outcomes were (1) fatal and nonfatal ischemic heart disease events, including myocardial infarction, angina, and coronary revascularization; (2) fatal and nonfatal cerebrovascular events, including stroke and transient ischemic attack; (3) total nonfatal ASCVD events; (4) total and low-density lipoprotein cholesterol levels; (5) systolic and diastolic BP; (6) health-related quality of life using validated instruments; and (7) direct costs. Secondary outcomes were: (1) fatal and non-fatal ischemic heart disease events, including myocardial infarction, angina, and coronary revascularization; (2) fatal and non-fatal cerebrovascular events, including stroke and transient ischemic attack; (3) total non-fatal ASCVD events; (4) total and low-density lipoprotein (LDL) cholesterol; (5) systolic and diastolic blood pressure; (6) health-related quality of life using validated instruments; and (7) direct costs.
Search Strategy
We searched the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA), MEDLINE, EMBASE, and PROSPERO International Prospective Systematic Review Trial Register from January 1, 2005, to June 17, 2015. We limited retrieval to English language systematic reviews. One of us (M.A.B.) who was an experienced information specialist performed all searches. Detailed search strategies with explanations of databases and search filters are included in eAppendix 1 in the Supplement.
We identified additional eligible studies by searching the reference lists of included systematic reviews, meta-analyses, and health technology assessment reports. We contacted study authors when necessary to identify further information that we may have missed.
Study Selection and Data Extraction
Two of us (K.N.K. and M.D.H.) independently performed all tasks for study selection, data extraction, evidence synthesis, and quality assessment. Any discrepancies here and throughout were resolved through consensus or recourse to a third investigator (D.M.L.-J.).
We screened titles and abstracts and then full texts to identify relevant systematic reviews for inclusion. For studies that fulfilled the inclusion criteria, we independently abstracted key participant and intervention characteristics and reported data on prespecified outcomes using standardized data extraction templates. We also extracted pooled effect estimates for outcomes that were meta-analyzed in each included review. We reported dichotomous data as risk ratios (RRs) or odds ratios (ORs) with 95% confidence intervals (CI). We reported continuous data as mean differences (MDs) with 95% CI.
Evidence Synthesis
Data were analyzed from July 2 to August 13, 2015. We independently assessed the methodological quality of each systematic review using the Assessment of Multiple SysTemAtic Reviews (AMSTAR) tool.10 Because many systematic reviews included information from overlapping trials, we did not perform a separate meta-analysis of pooled effect estimates. Instead, we performed a qualitative synthesis for each drug intervention and reported the treatment effect from the most comprehensive and highest-quality systematic reviews as recommended by the Cochrane Collaboration and as has been performed in other overviews.4,6,11,12
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to rate the overall quality of the evidence.13 We created an adapted “Summary of Findings” table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions to convey key information about the best treatment effect for each drug intervention and overall confidence in this estimate.4
Secondary Analyses
We reported results that evaluated potential differences in drug effects between men and women, and people with and without diabetes mellitus when these results were presented by review authors. We were unable to quantify differences by race/ethnicity due to limitations of data availability and reporting.
Differences between protocol and overview
We revised our search strategy by using a “precision maximizing filter” after our initial search, which used a “sensitivity- and specificity-balancing filter,” produced unfeasibly large results. We also restricted our retrieval to English language because of time limitations for project completion. Lastly, we elected to include systematic reviews of tobacco cessation drugs that included some trials involving participants with known vascular disease. We made this decision because reviews addressed smoking cessation as the primary efficacy outcome and provided limited data on cardiovascular outcomes or mortality.
RESULTS
Search results
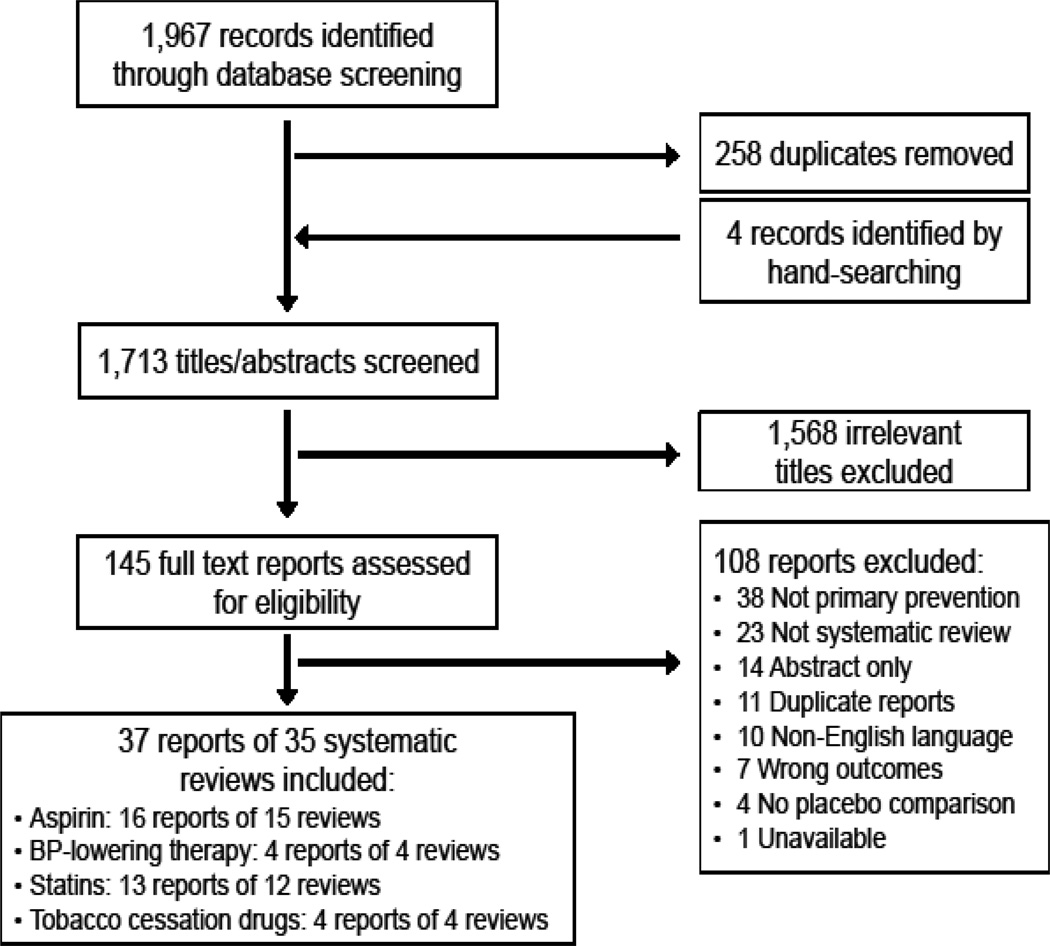
We performed 4 independent searches for systematic reviews evaluating the effects of: (1) aspirin, (2) blood pressure-lowering therapy, (3) statin, and (4) tobacco cessation drugs in the primary prevention of ASCVD. We present a PRISMA study flow in aggregate (Figure) and by drug class (eAppendix 3 in the Supplement).
Figure 1.
PRISMA flowchart for overview of systematic reviews of primary prevention drugs
Our search identified 1,967 records, of which 145 records were evaluated as full-text articles after title and abstract screening. In total, we selected 35 systematic reviews, available as 37 reports for inclusion. There were: 15 systematic reviews evaluating the effect of aspirin,14–29 4 systematic reviews of BP-lowering therapy,30–33 12 systematic reviews of statins,34–46 and 4 systematic reviews of tobacco cessation drugs in a primary prevention setting.47–50 Reasons for exclusion of each full text report and references to the excluded study are presented by drug class in eAppendix 4 in the Supplement.
Aspirin
Characteristics of included reviews
A summary of the 15 aspirin systematic reviews is provided in eAppendix 3 in the Supplement. Type 2 diabetes-specific treatment effects were provided in 6 reviews.18–20,24,28,29 The latest search ran through 2013.17 The reviews included data from 6 to 9 primary prevention trials with 95,456 to 102,621 participants. Trials generally included adults age 40 to 70 years, and weighted mean (SD) age of participants in the most comprehensive report was 57 (4) years.25
Trial quality assessment and review quality
Authors of included studies used a variety of criteria to judge trial quality, including risk of bias assessments, Jadad scores, Delphi process, and the United States Preventive Services Task Force quality criteria. Trial quality was generally assessed as “high”, and risk of bias was categorized as “low.” Systematic review quality, as measured by AMSTAR rating, ranged from a score of 5/11 to 11/11. The most comprehensive and highest rated systematic review was a Health Technology Assessment by Sutcliffe et al. 2013.25
Efficacy/cardiovascular benefits
Treatment effects of aspirin on all-cause mortality, composite cardiovascular events, and individual component cardiovascular events were broadly comparable across all systematic reviews. The systematic review by Sutcliffe et al.25 reported a 6% reduction in all-cause mortality (RR 0.94, 95% CI 0.88–1.00) and 10% reduction in major cardiovascular events (RR 0.90, 95% CI 0.85–0.96).25 Treatment effects on other outcomes are provided in eAppendix 3 in the Supplement.
Systematic reviews that reported type 2 diabetes-specific treatment effects demonstrated similar relative benefits compared with the general primary prevention group; however, all the upper 95% CIs included the possibility of no improvement. Sex-stratified analyses were reported in 4 systematic reviews,14,16,27,29 of which one was an individual participant data (IPD) meta-analysis of 6 primary prevention aspirin.14 These analyses demonstrated similar reductions in the composite cardiovascular disease outcome in men (RR 0.88, 95% CI 0.78–0.98) and women (RR 0.88, 95% CI 0.76–1.01). However, the effects were driven by a reduction in major coronary events in men (RR 0.77, 95% CI 0.67–0.89) and ischemic stroke in women (RR 0.77, 95% CI 0.59–0.99).
Safety, Adverse Effects, and Other Secondary Outcomes
Reviews varied in their definition of clinically important bleeding, but all 15 reported increased risk of bleeding and other hemorrhagic complications with aspirin therapy. The analysis from the Antithrombotic Treatment Trialists’ Collaboration, which accounted for differences in person-years of follow-up and used a standardized definition for “major bleeding” in pooled trials (major gastrointestinal and extracranial bleeding that were fatal or required blood transfusion), reported a 54% increased risk of major bleeding with aspirin therapy (RR 1.54, 95% CI 1.30–1.82) and a 32% increased risk of hemorrhagic stroke (RR 1.32, 95% CI 1.00–1.75).14 Data on other bleeding risks are shown in eAppendix 3 in the Supplement. Data on health-related quality of life and direct costs were not reported in the identified systematic reviews.
GRADE assessment
We rated the quality of evidence for the effect of aspirin on all-cause mortality as moderate, downgrading because of imprecision of treatment effect. We rated the quality of evidence for the effect of aspirin on reducing major cardiovascular events as high. We also rated the quality of evidence for the effect of aspirin on increasing major bleeding as high.
Blood pressure-lowering therapy
Characteristics of included reviews
We identified 4 systematic reviews of blood pressure-lowering therapy in primary ASCVD prevention,30–33 of which 2 of these reviews30,33 reported the effects of blood pressure reduction in individuals with “mild” or grade 1 hypertension (systolic blood pressure 140–149 mmHg and diastolic blood pressure 90–99 mmHg). Diabetes-specific treatment effects were not reported. The latest search ran through 2014 and included individual participant data from the Blood Pressure Lowering Treatment Trialists’ Collaboration (BPLTTC).33 The most comprehensive systematic reviews included 25 trials with 163,131 participants31 and 27 trials with 108,297 participants.32 Only 8 trials overlapped between these 2 reviews owing to variations in search strategies, search time frames, inclusion criteria, and classification of primary prevention by authors of the included reviews. The mean age of participants ranged from 30 to 80 years, and weighted mean age in the most comprehensive systematic review was 62 years.32 A summary of systematic review characteristics and detailed characteristics from the full data abstraction are provided in eAppendix 3 in the Supplement.
Trial quality assessment and review quality
Authors of the included reviews used a variety of criteria to judge trial quality, including risk of bias and GRADE assessments. The authors report a range of quality assessments from “very low” to “high” quality for trials evaluating the effects of calcium channel blockers and diuretics. AMSTAR ratings ranged from a score of 4/11 to 9/11.
Efficacy and Cardiovascular benefits
Treatment effects of BP-lowering therapy on all-cause mortality, composite cardiovascular outcomes, and individual component cardiovascular outcomes were broadly comparable across systematic reviews. The systematic review by Law et al.,32 the most comprehensive review, reported an 11% reduction in all-cause mortality (RR 0.89, 95% CI 0.85–0.95), 16% reduction in coronary heart disease events (RR 0.84, 95% CI 0.79–0.90), and 36% reduction in stroke (RR 0.64, 95% CI 0.56–0.73).32 Treatment effects standardized to a 10 mmHg reduction in systolic BP and 5 mmHg reduction in diastolic BP were also reported (eTable 2).
The effects of BP-lowering treatments in persons with “mild” or grade 1 hypertension were reported in 2 systematic reviews.31,33 The review by Sundström et al.33 was an update to the Diao et al. review30 and included individual participant data from 8 additional trials (10 comparisons) from the Blood Pressure Lowering Treatment Trialists’ Collaboration for a total of 13 trials and 15,266 participants with grade 1 hypertension. Sundström et al.33 reported a 22% reduction in all-cause mortality (OR 0.78, 95% CI 0.67–0.92), a 14% reduction in total cardiovascular events (OR 0.86, 95% CI, 0.74 to 1.01), a 25% reduction in cardiovascular death (OR 0.75, 95% CI 0.57–0.98), and a 28% reduction in stroke (OR 0.72, 95% CI 0.55–0.94). Overall, cardiovascular event rates were low in the trials, so effect estimates for total cardiovascular events and coronary events were imprecise.33
Type 2 diabetes mellitus-specific treatment effects were not reported for BP-lowering treatment. Sundström et al.33 reported no interaction by sex for all-cause mortality, total CVD events, coronary heart disease events, stroke events, or heart failure events, but a borderline interaction was present for CVD mortality (OR 0.58 95%CI 0.41–0.81 for men; OR 1.19 95%CI 0.74–1.91 for women; p=0.02).
Safety, Adverse Effects, and Other Secondary Outcomes
Only 1 systematic review30 reported an increased risk of treatment withdrawals due to adverse events and this was derived from one trial (RR 4.80, 95% CI 4.14–5.57). However, risk of bias for this outcome was high. The updated search by Sundström et al.33 noted that data on treatment withdrawal were “limited” but equally common in the active and control groups when available. Data on health-related quality of life and direct costs were not reported in any review.
GRADE assessment
We rated the quality of evidence for the effect of BP-lowering therapy on reducing all-cause mortality, coronary heart disease, and stroke as high. We rated the quality of evidence for the effect of BP-lowering therapy on treatment withdrawals as low, downgrading because of study limitations and inconsistency.
Statins
Characteristics of included reviews
We identified 13 reports of 12 systematic reviews of trials that investigated the effects of statins in primary prevention.34–46 Type 2 diabetes mellitus-specific treatment effects were reported in 3 reviews36,39,41 and sex-specific treatment effects were reported in 3 systematic reviews.34,37,43 The latest search ran through 2012.45
The 2 most comprehensive reviews included 18 to 20 primary prevention trials involving 56,934 to 63,899 participants.42,45 Mean age of participants generally ranged from 50 to 75 years, and mean age in the most comprehensive review was 57 years.45 Two reports from the Cholesterol Treatment Trialists’ (CTT) Collaboration37,38 were included in our overview. One of these reviews reported treatment effects 70,025 participants without prevalent vascular disease37 and the other reported treatment effects for 53,152 participants with a 5-year predicted risk of a major vascular event 10% or less (>90% without prevalent vascular disease). A summary of systematic review characteristics is provided in eAppendix 3 in the Supplement.
Trial quality assessment and review quality
The authors reported that trials generally had low risk of bias, but many were funded by pharmaceutical companies.45 AMSTAR ratings ranged from a score of 5/11 to 11/11. The most comprehensive and highest rated systematic review was by Taylor et al.45
Efficacy and cardiovascular benefits
Treatment effects of statins on all-cause mortality, total cardiovascular disease events, myocardial infarction, and stroke were broadly comparable across all systematic reviews. The systematic review by Taylor et al.45 reported a 14% reduction in all-cause mortality (OR 0.86, 95% CI 0.79–0.94), 25% reduction in major cardiovascular events (RR 0.75, 95% CI 0.70–0.81), and reductions in fatal and nonfatal coronary heart disease and stroke events.45 Mean baseline (SD) low-density lipoprotein cholesterol (LDL) in CTT trials was 143.1 (27.1) mg/dL and mean (SD) difference in LDL between statin regimen and control was 41.8 mg/dL. Treatment effects standardized per 40.6 mg/dl (1 mmol/L) reduction in LDL cholesterol were also reported by the CTT for all-cause mortality (RR 0.91, 95% CI 0.85–0.97) and major vascular events (RR 0.75, 95% CI 0.70–0.80).38
Effects of statin treatment in participants with diabetes mellitus were reported in 3 systematic reviews and demonstrated similar proportional benefits compared with the general primary prevention group.36,39,41 Sex-specific treatment effects were also reported in 3 systematic reviews,34,37,43 of which one was an individual participant data meta-analysis.37 After adjusting for baseline differences in prognostic characteristics and 5-year vascular risk, no sex-specific heterogeneity was seen.37 Additional statin treatment effects are provided in eAppendix 3 in the Supplement.
Safety, Adverse effects, and other Secondary Outcomes
Data on adverse effects of statin treatment were included in 7 systematic reviews and included risks of cancer, creatine kinase elevation, rhabdomyolysis, liver enzyme elevation, hemorrhagic stroke, type 2 diabetes, and serious adverse effects.34,35,37,38,42,45,46 Taylor et al.45 reported no evidence of increased risk of overall adverse effects (defined as cancers, myalgia and rhabdomyolysis, type 2 diabetes, hemorrhagic stroke, and other adverse effects leading to treatment discontinuation) among individuals treated with statin compared to control/placebo (RR 1.00, 95% CI 0.97–1.03). However, there was an 18% increased risk for the individual outcome of type 2 diabetes for those treated with statin (RR 1.18, 95% CI 1.01–1.39).
Data on health-related quality of life were not reported. Data on cost-effectiveness were reported in Taylor et al45 from 3 statin trials, all demonstrating cost-effectiveness of statin therapy in primary prevention. In the West of Scotland Coronary Prevention Study (WOSCOPS), statin treatment led to 2,460 years of life at £8,121 (or $12,788) per life-year gained. In the Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, statin therapy had a cost-effectiveness of £25,796 (or $40,315) per quality adjusted life year. Last, in an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS), Taylor et al.45 reported an incremental cost effectiveness ratio of £2,320 (or $3,626) per quality adjusted life year at 10 years.
GRADE assessment
We rated the quality of evidence for the effect of statins on reducing the risk of all-cause mortality, major cardiovascular events, coronary heart disease events, and stroke as high. We rated the quality of evidence for the safety of statins as moderate, downgrading because of indirectness of evidence.
Tobacco cessation drugs
Characteristics of included reviews
We included 4 systematic reviews of tobacco cessation drugs.47–50 None of the reviews differentiated between trials that included participants with and without prevalent vascular disease. Moreover, reviews reported limited information about the effects of tobacco cessation drugs on cardiovascular outcomes or risk factors. The primary efficacy outcome for most reviews was continuous smoking cessation at 6 months. Diabetes-specific or sex-specific treatment effects were not reported. The latest search ran through 2012.48 Mean age among participants in the most comprehensive systematic review was 57 years.48
The most comprehensive systematic review was an overview of several Cochrane systematic reviews by Cahill et al. addressing drug therapy for smoking cessation.48 This overview synthesized information from 267 trials of 101,804 participants. Among the trials, there were 150 trials of nicotine replacement therapy, 72 trials of antidepressants (primarily buproprion hydrochloride), and 24 trials of nicotine receptor partial agonists (primarily varenicline tartarate). A summary of systematic review characteristics is provided in eAppedix 3 in the Supplement.
Trial quality assessment and review quality
Authors reported that trials of nicotine replacement therapy and bupropion did not clearly describe methods of randomization or allocation concealment and were therefore at “high risk of bias.” Trials of varenicline were more contemporary and generally lower risk of bias, although they were industry funded.48 AMSTAR ratings ranged from 7/11 to 11/11, and the highest rated systematic review was Cahill et al48 (eAppendix 3 in the Supplement).
Efficacay and smoking cessation
The overview by Cahill et al.48 reported treatment effects of tobacco cessation drugs on 6 months of continuous smoking abstinence, which was primarily biochemically verified. The overview reported that nicotine replacement therapy, buproprion, and varenicline all increased the odds of smoking cessation at 6 months compared to placebo (nicotine replacement therapy OR 1.84, 95% CI 1.71–1.99; bupropion OR 1.82, 95% CI 1.60–2.06; and varenicline 2.88, 95% CI 2.40–3.47). Cahill et al.48 also reported the effects of a limited number of tobacco cessation drugs on cardiovascular disease events. However, individual studies were underpowered for this endpoint, events were poorly reported, and many trials included participants with prevalent CVD. Treatment estimates for buproprion and varenicline for smoking cessation outcomes are listed in eAppendix 3 in the Supplement.
Safety, Adverse effects, and Other Secondary Outcomes
Cahill et al.48 also reported the effects of tobacco cessation drugs on serious adverse events for a limited number of therapies. For nicotine replacement therapy, limited information on serious adverse effects was reported in trials.48 Data for buproprion and varenicline were reported (RR 1.29, 95% CI 0.99–1.69 and RR 1.06, 95% CI 0.72–1.55, respectively).48 Data on health-related quality of life and direct costs were not reported.
GRADE Assessment
We rated the quality of evidence for the effect of varenicline on smoking cessation as high and the quality of evidence for the effect of buproprion and nicotine replacement therapy on smoking cessation as moderate, downgrading because of study limitations. We rated the quality of evidence for the safety of tobacco cessation drugs as moderate, downgrading because of indirectness of evidence. We rated the quality of evidence for the effect of tobacco cessation drugs on all-cause mortality and cardiovascular outcomes as low, downgrading because of study limitations, inconsistency, and imprecision.
DISCUSSION
Principal findings
We performed an overview of systematic reviews that synthesized evidence of the efficacy and safety of drugs that can be used in primary ASCVD prevention to achieve targets set by the Million Hearts initiative and that will be used in the Million Hearts Cardiovascular Risk Reduction model. There is high quality evidence that aspirin, BP-lowering therapy, and statins reduce the risk of ASCVD events from 10% to 25% among individuals without prevalent ASCVD. There is also high quality evidence that BP-lowering therapy and statins reduce the risk of all-cause mortality by 11% and 14%, respectively. There was no heterogeneity in treatment effect among subgroups of individuals with diabetes mellitus or by sex. There is moderate to high quality evidence that tobacco cessation drugs increase the odds of continued abstinence by 88% to 188%, but the direct effects on cardiovascular events are uncertain. A summary of findings table is presented in the Table.
Table 1.
Summary of key findings for the effect of drugs for primary prevention of atherosclerotic cardiovascular diseases.
| Outcomes | Relative risk (95% CI) |
Quality of evidence (GRADE) |
Comment |
|---|---|---|---|
| Aspirin | |||
| All-cause mortality | RR 0.94 (95% CI 0.88–1.00) | Moderate | Downgraded due to imprecision |
| Major cardiovascular events | RR 0.90 (95% CI 0.85–0.96) | High | NA |
| Adverse effects (Major bleeding) |
RR 1.54 (95% CI 1.30–1.82) | High | NA |
| BP-lowering therapies | |||
| All-cause mortality | RR 0.89 (95% CI 0.84–0.95) | High | NA |
| Coronary heart disease (CHD) events | RR 0.84 (95% CI 0.79–0.90) | High | NA |
| CHD events, standardized to a BP reduction of 10 mmHg/5mmHg |
RR 0.79 (95% CI 0.72–0.86) | High | NA |
| Stroke events | RR 0.64 (95% CI 0.56–0.73) | High | NA |
| Stroke events, standardized to a BP reduction of 10 mmHg/5mmHg |
RR 0.54 (95% CI 0.45–0.65) | High | NA |
| Adverse effects (Treatment withdrawal) |
RR 4.80 (95%CI 4.14–5.57) | Low | Downgraded due to study limitations, inconsistency |
| Statins | |||
| All-cause mortality | OR 0.86 (95% CI 0.79–0.94) | High | NA |
| Major cardiovascular events | RR 0.75 (95% CI 0.70–0.81) | High | NA |
| Major vascular events per 1 mmol/L of LDL reduction |
RR 0.75 (95% CI 0.70–0.80) | High | NA |
| CHD events | RR 0.73 (95% CI 0.67–0.80) | High | NA |
| Stroke events | RR 0.78 (95% CI 0.68–0.89) | High | NA |
| Adverse effects (all) |
RR 1.00 (95% CI 0.97–1.03) | Moderate | Downgraded due to indirectness of evidence |
| Adverse effects diabetes) |
RR 1.18 (95% CI 1.01–1.39) | Moderate | Downgraded due to indirectness of evidence |
| Tobacco cessation drugs | |||
| Nicotine replacement therapy, continuous smoking abstinence at ≥6 months |
OR 1.84 (95% CI 1.71–1.99) | Moderate | Downgraded due to study limitations |
| Buproprion hydrochloride, continuous smoking abstinence at ≥6 months |
OR 1.82 (95% CI 1.60–2.06) | Moderate | Downgraded due to study limitations |
| Varenicline tartarate, continuous smoking abstinence at ≥6 months |
OR 2.88 (95% CI 2.40–3.47) | High | NA |
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Adverse effects of drug therapy were either not reported or poorly reported in many systematic reviews, and many review authors noted “under-reporting” of adverse events in the individual trials. Our search demonstrated high quality evidence that aspirin increases the risk of major bleeding by 54% and moderate quality evidence that statins do not increase the overall risk of adverse events, but risk for type 2 diabetes mellitus was increased among individuals taking statins. Adverse effects of BP-lowering therapy and tobacco cessation drugs were poorly reported.
Strengths
Our overview has several strengths. First, we focused this evidence synthesis on systematic reviews and meta-analyses of randomized clinical trials, because randomized clinical trials represent the highest-quality evidence to determine the effects of health care interventions. Second, we used a comprehensive, transparent search strategy to identify studies and followed a prespecified protocol to guide our evidence synthesis, noting any deviations from protocol. Third, we performed all title screening, data extraction, and quality assessments in duplicate to minimize potential bias in generation of this overview. Fourth, we used a validated instrument (the AMSTAR tool) to assess the methodologic quality of included systematic reviews and factored this quality assessment to guide our conclusions regarding the effects of pharmacologic interventions. This systematic process, with study quality assessment using standardized tools, could be used as a potential model for more rapid development of trustworthy guidelines.
Limitations
Our overview also has important limitations to acknowledge. First, we did not retrieve data from primary trials and therefore were limited to the information and judgments of the authors who wrote the systematic reviews. Selection criteria, search strategies, and definitions of primary prevention often varied between reviews, and authors of the included reviews often used different criteria to define primary prevention, which led to different numbers of trials for systematic reviews of the same drug. Second, our conclusions were limited by the available data. Although data were generally well reported for drug efficacy, limited information was reported on safety, particularly for BP-lowering therapy and tobacco cessation drugs. Third, we had limited ability to comment on the potential of differential treatment effects by race/ethnicity. Nevertheless, several reviews noted consistent proportional treatment effects regardless of baseline characteristics, suggesting that treatment effect does not demonstrate heterogeneity. Fourth, our treatment effect estimates were limited by the short-term horizon of the clinical trials. Thus, we may have underestimated the potential added benefits and risks of sustained treatment. Fifth, our overview does not include evidence from recent trials like the Japanese Primary Prevention Project (JPPP)51 or cost-effectiveness analyses of long-term statin use from WOSCOPS.52 However, inclusion of these studies would not have changed our overall conclusions. For example, the point estimate for treatment effect from low-dose aspirin in the JPPP trial was similar to our reported effect, though with wider confidence intervals (HR 0.94, 95% CI 0.77–1.15) and primary prevention statin therapy was also shown to be cost-effective at 15 years of follow-up. Finally, our overview does not provide information on the added effects of lifestyle interventions such as diet, exercise, and weight loss in combination with drug therapy. However, essentially all included clinical trials of ASCVD prevention included background recommendations of therapeutic lifestyle change in combination with study drugs.
Conclusions
This overview of systematic reviews demonstrates high quality evidence to support aspirin, BP-lowering therapy, and statins for primary ASCVD prevention and tobacco cessation drugs for smoking cessation. It provides reliable, evidence-based pooled estimates for these interventions on the lowered risk of primary ASCVD events and best-available evidence for the effect of tobacco cessation drugs on continuous abstinence at 6 months. These treatment effects can be used to enrich discussions between health care professionals and patients.
Supplementary Material
Acknowledgments
Dr. Huffman reports grants from Center for Medicare and Medicaid Innovation via subcontract from the MITRE Corporation, during the conduct of the study; grants from World Heart Federation, outside the submitted work; and serves as an associate editor for JAMA Cardiology and the coordinating editor of the Cochrane Heart Group US Satellite. Dr. Lloyd-Jones reports grants from Center for Medicare and Medicaid Innovation via subcontract from the MITRE Corporation, during the conduct of the study.
Funding/Support: This study was funded by the Center for Medicare and Medicaid Innovation and MITRE Corporation.
Role of Funder/Sponsor: Design and conduct of the study: The work was performed to provide a synthesis of available evidence to support development of a longitudinal cardiovascular risk calculator that will be used in the Million Hearts Cardiovascular Risk Reduction Model. The Center for Medicare and Medicaid Innovation (CMMI) provided an initial description of key questions. However, the final protocol was developed independently by the authors. Collection, management, analysis, and interpretation of the data: The authors were solely responsible for data collection, management, analysis, and interpretation. Members of CMMI and MITRE Corporation reviewed a draft version of a full, more detailed report, and the final version used by the group explicitly addressed comments/suggestions provided. Final data interpretation, including judgments of evidence quality, were solely the responsibility of the authors. Preparation, review, or approval of the manuscript: This manuscript was prepared solely by the authors, with no review or approval by the sponsor. Decision to submit the manuscript for publication: The sponsor was involved in the decision to submit the manuscript for publication in JAMA to provide transparency regarding the evidence base for the new cardiovascular risk calculator.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Additional Contributions: Janet S. Wright, MD, Executive Director, Million Hearts initiative, Centers for Disease Control provided input in preparing this manuscript.
References
- 1.Frieden TR, Berwick DM. The "Million Hearts" initiative--preventing heart attacks and strokes. N. Engl. J. Med. 2011;365(13):e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 2.Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am. J. Prev. Med. 2010;38(6):600–609. doi: 10.1016/j.amepre.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Sanghavi DM, Conway PH. Paying for prevention: A novel test of medicare value-based payment for cardiovascular risk reduction. JAMA. 2015;314(2):123–124. doi: 10.1001/jama.2015.6681. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JPT, Green Se. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. [Accessed 2 February 2016]. Available from http://www.cochrane-handbook.org. [Google Scholar]
- 5.Lavis JN. How can we support the use of systematic reviews in policymaking? PLoS Med. 2009;6(11):e1000141. doi: 10.1371/journal.pmed.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson D, Russell K, Becker L, Klassen T, Hartling L. The evolution of a new publication type: Steps and challenges of producing overviews of reviews. Research synthesis methods. 2010;1(3–4):198–211. doi: 10.1002/jrsm.30. [DOI] [PubMed] [Google Scholar]
- 7.Baker PR, Costello JT, Dobbins M, Waters EB. The benefits and challenges of conducting an overview of systematic reviews in public health: a focus on physical activity. J Public Health (Oxf) 2014;36(3):517–521. doi: 10.1093/pubmed/fdu050. [DOI] [PubMed] [Google Scholar]
- 8.PROSPERO International prospective register of systematic reviews. [Accessed 2 February 2016]; Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015023444. [Google Scholar]
- 9.Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2011;(1):CD001561. doi: 10.1002/14651858.CD001561.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung A, Weir M, Mayhew A, Kozloff N, Brown K, Grimshaw J. Overview of systematic reviews of the effectiveness of reminders in improving healthcare professional behavior. Systematic reviews. 2012;1:36. doi: 10.1186/2046-4053-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger JS, Lala A, Krantz MJ, Baker GS, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: a meta-analysis of randomized trials. Am. Heart J. 2011;162(1):115–124.e112. doi: 10.1016/j.ahj.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295(3):306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 17.Brotons C, Benamouzig R, Filipiak KJ, Limmroth V, Borghi C. A systematic review of aspirin in primary prevention: is it time for a new approach? Am J Cardiovasc Drugs. 2015;15(2):113–133. doi: 10.1007/s40256-014-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butalia S, Leung AA, Ghali WA, Rabi DM. Aspirin effect on the incidence of major adverse cardiovascular events in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2011;10:25. doi: 10.1186/1475-2840-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvin AD, Aggarwal NR, Murad MH, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care. 2009;32(12):2300–2306. doi: 10.2337/dc09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ. 2009;339:b4531. doi: 10.1136/bmj.b4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju N, Sobieraj-Teague M, Hirsh J, O'Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am. J. Med. 2011;124(7):621–629. doi: 10.1016/j.amjmed.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Raju NC, Eikelboom JW. The aspirin controversy in primary prevention. Curr. Opin. Cardiol. 2012;27(5):499–507. doi: 10.1097/HCO.0b013e328356ae95. [DOI] [PubMed] [Google Scholar]
- 23.Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012;172(3):209–216. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 24.Stavrakis S, Stoner JA, Azar M, Wayangankar S, Thadani U. Low-dose aspirin for primary prevention of cardiovascular events in patients with diabetes: a meta-analysis. Am. J. Med. Sci. 2011;341(1):1–9. doi: 10.1097/MAJ.0b013e3181f1fba8. [DOI] [PubMed] [Google Scholar]
- 25.Sutcliffe P, Connock M, Gurung T, et al. Aspirin for prophylactic use in the primary prevention of cardiovascular disease and cancer: a systematic review and overview of reviews. Health Technol. Assess. 2013;17(43):1–253. doi: 10.3310/hta17430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8(12):e81970. doi: 10.1371/journal.pone.0081970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009;150(6):405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- 28.Younis N, Williams S, Ammori B, Soran H. Role of aspirin in the primary prevention of cardiovascular disease in diabetes mellitus: a meta-analysis. Expert Opin Pharmacother. 2010;11(9):1459–1466. doi: 10.1517/14656561003792538. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2010;87(2):211–218. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews. 2012;8:CD006742. doi: 10.1002/14651858.CD006742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fretheim A, Odgaard-Jensen J, Brors O, et al. Comparative effectiveness of antihypertensive medication for primary prevention of cardiovascular disease: systematic review and multiple treatments meta-analysis. BMC Medicine. 2012;10 doi: 10.1186/1741-7015-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundstrom J, Arima H, Jackson R, et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann. Intern. Med. 2015;162(3):184–191. doi: 10.7326/M14-0773. [DOI] [PubMed] [Google Scholar]
- 34.Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statin therapy in patients without cardiovascular disease: Meta-analysis of randomized controlled trials. Eur. Heart J. 2009;30:510–511. [Google Scholar]
- 35.Bukkapatnam RN, Gabler NB, Lewis WR. Statins for primary prevention of cardiovascular mortality in women: a systematic review and meta-analysis. Prev Cardiol. 2010;13(2):84–90. doi: 10.1111/j.1751-7141.2009.00059.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Feng B, Chen ZW. Statins for primary prevention of cardiovascular and cerebrovascular events in diabetic patients without established cardiovascular diseases: a meta-analysis. Experimental and clinical endocrinology & diabetes. 2012;120(2):116–120. doi: 10.1055/s-0031-1297968. [DOI] [PubMed] [Google Scholar]
- 37.Cholesterol Treatment Trialists C. Fulcher J, O'Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 38.Cholesterol Treatment Trialists C. Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332(7550):1115–1124. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costanzo P, Perrone-Filardi P, Petretta M, et al. Impact of gender in primary prevention of coronary heart disease with statin therapy: A meta-analysis. J. Am. Coll. Cardiol. 2009;53(10):A210. doi: 10.1016/j.ijcard.2008.08.001. [abstract] [DOI] [PubMed] [Google Scholar]
- 41.de Vries FM, Denig P, Pouwels KB, Postma MJ, Hak E. Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients a meta-analysis. Drugs. 2012;72(18):2365–2373. doi: 10.2165/11638240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J. Am. Coll. Cardiol. 2008;52(22):1769–1781. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 43.Petretta M, Costanzo P, Perrone-Filardi P, Chiariello M. Impact of gender in primary prevention of coronary heart disease with statin therapy: a meta-analysis. Int. J. Cardiol. 2010;138(1):25–31. doi: 10.1016/j.ijcard.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 2010;170(12):1024–1031. doi: 10.1001/archinternmed.2010.182. [DOI] [PubMed] [Google Scholar]
- 45.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006;166(21):2307–2313. doi: 10.1001/archinte.166.21.2307. [DOI] [PubMed] [Google Scholar]
- 47.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(4):CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebbert J, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews. 2011;(2):CD004306. doi: 10.1002/14651858.CD004306.pub4. [DOI] [PubMed] [Google Scholar]
- 50.Secretariat MA. Population-based smoking cessation strategies: A summary of a select group of evidence-based reviews. Ontario Health Technology Assessment Series. 2010;10(1) [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312(23):2510–2520. doi: 10.1001/jama.2014.15690. [DOI] [PubMed] [Google Scholar]
- 52.McConnachie A, Walker A, Robertson M, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur. Heart J. 2014;35(5):290–298. doi: 10.1093/eurheartj/eht232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.