ABSTRACT
The activated estrogen (E2) receptor α (ERα) is a potent transcription factor that is involved in the activation of various genes by 2 different pathways; a classical and non-classical. In classical pathway, ERα binds directly to E2-responsive elements (EREs) located in the appropriate genes promoters and stimulates their transcription. However, in non-classical pathway, the ERα can indirectly bind with promoters and enhance their activity. For instance, ERα activates BRCA1 expression by interacting with jun/fos complex bound to the AP-1 site in BRCA1 promoter. Interference with the expression and/or functions of BRCA1, leads to high risk of breast or/and ovarian cancer. HTLV-1Tax was found to strongly inhibit BRCA1 expression by preventing the binding of E2–ERα complex to BRCA1 promoter. Here we examined Tax effect on ERα induced activation of genes by the classical pathway by testing its influence on E2-induced expression of ERE promoter-driven luciferase reporter (ERE-Luc). Our findings showed that E2 profoundly stimulated this reporter expression and that HTLV-1Tax significantly induced this stimulation. This result is highly interesting because in our previous study Tax was found to strongly block the E2-ERα-mediated activation of BRCA1 expression. ERα was found to produce a big complex by recruiting various cofactors in the nucleus before binding to the ERE region. We also found that only part of the reqruited cofactors are required for the transcriptional activity of ERα complex. Chip assay revealed that the binding of Tax to the ERα complex, did not interfere with its link to ERE region.
KEYWORDS: BRCA-1, ERα, ERE, HTLV-1, Tax
Introduction
Various gene expressions in mammary tissues are controlled by the ovarian estrogen estradiol (E2) through its interaction and activation of either the estrogen receptor ER-α or ER-β.1,2 Activated ER-α is a transcriptional factor which stimulates the expression of different genes by 2 different pathways: (a) classical pathway, the E2 liganded ER-α homodimerizes and binds directly to the appropriate gene promoters at estrogen response elements (EREs) and activates the expression of the gene after recruitment of appropriate co-activators and co-factors which cooperatively stimulate the transcription of the respective gene.3,4 (b) Non-classical pathway, the E2 liganded ER-α homodimerizes and binds indirectly to the promoters of the target genes by interacting with DNA-bound protein complexes and stimulate the recruitment of various co-activators and co-factors, which enhance the transcription of these genes.5,6 For instance, although the promoter of the breast cancer susceptibility gene BRCA1 lacks the consensus EREs,7 it is activated by the E2-liganded ERα through its interaction with the Jun/Fos transcription factor which is bound to the DNA-bound activator protein (AP)-1 transcription complexes located in the BRCA1 promoter.6,8 In fact, the E2- ERα binds first to p300/CBP co-factor and then this complex interacts with the bound Jun/Fos transcription factor to AP-1 in BRCA1 promoter through direct interaction of p300/CBP with Jun/Fos and thereby activating the transcription of BRCA1 gene.1 It seems to be that E2 is not only inducing the recruitment of ERα to its appropriate promoters, but it also has an effect on gene expression by activating mitogen-activated protein kinases (MAPKs). These kinases phosphorylate the ER-α and other transcription factors and thereby increasing their affinity to bind with other required cofactors,1,4,9 such as specific protein 1 (Sp1), cyclic AMP responsive element binding (CREB) protein,1 non-liganded aromatic hydrocarbon receptor (AhR),10 E2F transcription factor family,11,12 members of the steroid receptor co-activators 1 and 3 [SRC11 and SRC313] and certain other non-classical co-factors.3
Most (90–95%) cases of breast cancer are sporadic, and their incidence is affected by many factors associated with lifestyle and exposure to environmental and occupational pollutants.14-20 However, 5–10% of breast cancer cases are of hereditary origin.21 Several genes have been identified, which their dysfunction enhances the risk for the development of breast cancer.22 However, dysfunction of BRCA1 gene is the most prevalent cause for breast cancer development. This gene encodes a phosphoprotein which acts as a multifunctional tumor suppressor.23 This tumor suppressor participates in many cellular processes like ubiquitination,24 gene expression,25 genome stabilization (by securing proper centrosome amplification26 and mitotic spindle checkpoint27 and enhancing DNA damage repair,28-30 chromatin remodeling,31 induction of cell cycle arrest32 and induction apoptosis.33 Although these functions are essential for normal cells of most organs, BRCA1 dysfunction is linked mainly with breast and ovarian cancers,34 likely because of the response of these organs to E2 and the mutual regulatory effects of BRCA1 and ERα on each other.35 About 45% of the cases of hereditary breast cancer are linked with germline mutations in the BRCA1 gene.36 About 30% to 40% of sporadic breast cancers, exhibit lower or absent levels of BRCA1 in the absence of mutations in the BRCA1 gene.37,38 Because changes in BRCA1 regulation may increase the possibility of breast cancer development, it is important to examine the molecular events that regulate normal BRCA1 expression. The increase in BRCA1 expression in response to E2 treatment39,40 may represent a feedback mechanism that represses ER-signaling during the early stages of breast tumorigenesis.41 This speculation is supported by previous studies showed that BRCA1 protein represses the transcriptional activity of E2- ER-α.42
Human T-cell leukemia virus type-1 (HTLV-1), originally identified as the causative agent of the aggressive malignancy adult T-cell leukemia (ATL)43 and the neurological progressive inflammatory syndrome, called tropical spastic paraparesis or HTLV-1 associated myelopathy (TSP/HAM),44 was later etiologically implicated with a wide range of additional severe clinical disorders.45 Although the pathogenic mechanism of HTLV-1 is not completely clear,33 it is believed that most of HTLV-1 pathogenic activities are due mainly to the multi-functionality of its Tax protein,46,47 which is an oncoprotein, most of whose activities are diametrically opposing to those of BRCA1. For example, through its ability to functionally inactivate p53,48 Tax may potentially repress the p53-dependent BRCA1 transcriptional activities.49-51 Tax may also block CBP/p300-dependent BRCA1 transcriptional activity52 by using its high affinity to the KIX domain of these co-activators to sequester them,53,54 as has been shown for several other CBP/p300-dependent transcription factors.55,56 Likewise, Tax may inhibit the induction of BRCA1 expression by the E2-activated ERα/p300 complex.5 BRCA1-mediated cell cycle arrest32,57 and apoptosis33 can be reversed by the ability of Tax to prevent cell cycle arrest and apoptosis47 through constitutive NF-ҝB activation57 and the induction of other anti-apoptotic factors such as Bcl-2,47 while suppressing pro-apoptotic elements like Bax, Caspases.47,58 Tax also interferes with centrosome amplification,59 the mitotic spindle checkpoint,60 and DNA repair,61 which are all facilitated by BRCA1.26-29 Therefore, we speculate that if expressed in breast cells, Tax would be able to block the expression and most of the activities of BRCA1. This speculation is supported by our previous study,62 which show, for the first time, that Tax inhibits basal and E2-stimulated BRCA1 expression in breast cells by preventing the binding of ER-α transcriptional complex to BRCA1 promoter. It thus appears likely that in breast cells Tax can potentially create a BRCA1-deficient microenvironment that usually leads to breast cancer development.
In the present study we focused Tax effect on the transcriptional activity of E2-ER-α through the classical pathway and compared it with its activity on BRCA1 through the non-classical pathway.
Materials and methods
Cells and culture conditions
In this study we used the weakly invasive ER-α positive MCF-7 epithelial-like breast cancer cells,63 obtained from Etta Livne, and the non-tumorigenic immortalized breast epithelial MCF-10A cells,64 given by Yacob Weinstein, both from our department.
The MCF-7 cells were maintained in Dulbeco's Modified Eagle's Medium (DMEM) supplemented with 2mM L-glutamine and 10% fetal bovine serum (FBS). The MCF-10A cells were cultured in DMEM (Biological industries, Israel) containing 5% horse serum (Biological industries, Israel), 0.5 mg/ml hydrocortisone (Sigma-Aldrich), 20 ng/ml epidermal growth factor (Sigma-Aldrich), 100 ng/ml cholera toxin (Sigma-Aldrich), 10 mg/ml insulin (Sigma-Aldrich). Jurkat T-cells were maintained in RPM11640 medium with 10% FBS. All above media were supplemented with 1% penicillin/streptomycin.
Plasmids and transfection
The reporter firefly luciferase (Luc) driven by the BRCA1 promoter (BRCA1-Luc) and Luc reporter driven by estrogen response elements (EREs-Luc) were provided by Haim Werner (Clinical Biochemistry, Tel-Aviv University, Israel). The CBP and p300 plasmids were provided by addgene company. The ERα-expressing pCDNA3 vector65 was from Michael Danilenko (Clinical Biochemistry, Ben-Gurion University, Beer-Sheva, Israel). The plasmid expressing the Renilla luciferase, was purchased from Promega (Madison WI, USA).66 Different CMV promoter-driven Tax mutants were kindely supplied by Francoise Bex (Universite Libre de Bruxelles, Belgium)54 as follow: a) TaxM22 carrying the T130A, L131S dual nucleotide substitutions, capable of binding CREB, CBP/p300 and the p300/CBP-associated factor (p/CAF), but unable to dissociate the NF-κB factors from their IκB inhibitors, b) TaxM47 carrying the substitution L319R, L320S, capable of dissociating the NF-κB factors from their IκBs and binding CREB and CBP/p300, but incapable of binding p/CAF, which was essential for activating CREB pathway, thus it could not activate CREB pathway.67 TaxV89A mutant can bind p/CAF and activate the NF-κB factors, but can not bind the CBP/p300, was kindely provided by Chou-Zen Giam (Uniformed Service University, Bethesda, USA).54
The plasmids were transfected by jetPRIMTM kit (Polyplus, www.polyplus-transfection.com) according to the manufacturer's instructions. The transfection efficiency, determined with GFP-expressing plasmid, was found by FACS analysis to range in our cells between 70 to 80% (not shown). Each transfection mixture included the pRL-renilla plasmid (0.2 μg) as control for transfection efficiency. The cells were harvested at 24 hr post-transfection for measuring the enzymatic activities. 20 nM Estrogen (E2, Sigma-Aldrich Chemical Co.) was added to the cultures 5 hr before cell harvest. The Luc activity was normalized to that of renilla and presented as fold of the relevant control.
Antibodies, cell fractionation, co-immunoprecipitation and western blot analyses
Monoclonal antibodies against BRCA1, Tax, ER-α, CBP, CREB and Ps2 were all purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Whole cell extracts and sub-cellular fractions were prepared by NucBuster Kit (Calbiochem, Catalog No. 71183–3) according to the Manufacturer's protocol.
For co-immunoprecipitation assays, aliquots of the nuclear extracts (200 μg protein) were immunoprecipitated with the specified mouse antibodies and analyzed by Western blot for co-precipitated proteins with the respective rabbit antibodies as previously described.68
For direct Western analyses, aliquots of the tested extracts (80 μg protein) were analyzed with the respective antibodies as described elsewhere.69
Measurement of pS2 mRNA levels
Total cellular RNA was extracted from serum starved MCF-7 cells using RNA extraction kit (Invitrogen). Total RNA was then reverse transcribed for cDNA generation using random primers and Moloney murine leukemia virus reverse transcriptase (both from Invitrogen) as described by the manufacturer. A quantitative real time PCR was conducted to measure pS2 mRNA expression as previously described70 using the AB 7300 real-time PCR system. Primer sequences for pS2 were: forward 5′- CATGGAGAACAAGGTGATCTG-3′ and reverse 5′- CAGAAGCGTGTCTGAGGTGTC-3′, as previously described.70 The primers sequences for the endogenous reference gene, β-actin, were: forward 5′-TGA GCG CGG CTA CAG CTT-3′, reverse 5′-TCC TTA ATG TCA CGC ACG ATT T-3′. Relative pS2 mRNA expression was quantified using the standard curve method. The presented results are the mean ± SD of 3 independent experiments.
Chromatin immunoprecipitation (ChIP)
MCF-7 (2 × 107) cells were transfected with Tax expressing plasmid by jetPRIMTM kit and at 24 hr post-transfection the cells were treated with E2 for 5 hr. The chromatin mmunoprecipitation was performed by EZ Chip kit (Millipore) according to the manufacturer's instruction. The BRCA1 promoter region flanking the Sp/AP-1/CRE binding sites (171 base pairs) in the obtained DNA was amplified by real time PCR, using the following primers: forward: 5′-GACAGATGGGTATTCTTTGACG-3′ and reverse: 5′-GCATATTCCAGTTCC TATCACGAG-3′.71
The ERE region of the pS2 promoter was amplified using the following primers:
forward: PS2 5′-TATGAATCACTTCTGCAGTGAG-3′ and reverse PS2 5′- GAGCGTTAGATAACATTTGCC-3′
Results
3.1 Effect of HTLV-1 Tax varients on the E2-induced BRCA1-Luc and ERE-Luc expression. In extention to our previous findings62 which proved the potential of HTLV-1 Tax protein to significantly prevent the E2 induced BRCA1 expression, we examined in this study the effect of HTLV-1 Tax on the E2 induced expression of genes controlled by promoters containing the ERE sequences in comparison with its effect on BRCA1 expression. This was done by examining the effect of Tax-expressing vector on the expression of ERE-Luc or BRCA1-Luc in MCF-7 cells. It is known that major part of Tax activity on the expression and functionality of different genes is done through CREB or NF-κB-associated pathways47,72 and by recruitment of transcriptional co-activator/co-factors.
Therefore, we also examined whether Tax exerts its effects on ERE-Luc expression through any of these pathways by using the wild type (w.t.)Tax and its mutants TaxM22, TaxM47 and TaxV89A described in “Materials and Methods” section. For this, MCF-7 were co-transfected with either a plasmid expessing ERE-Luc or BRCA1-Luc together with each of the Tax varients and examined at 24h post transfection for Luc activity. The results presented in Figure 1 showed that while none of the Tax variants affected the basal level of BRCA1 expression (Fig. 1A), the w.t.Tax increased the basal level of ERE expression without E2 treatment (Fig. 1B), however the 3 tested Tax varients (M22, M47 and TaxV89A) had no significant effect on this basal level (Fig. 1B). In contrast to the strong inhibitory effect of w.t.Tax and its varients (M22, M47) on E2 induced BRCA1 expression (left panel of Fig. 1A), the w.t.Tax highly stimulated the E2 induced ERE expression (left panel of Fig. 1B). However, 2 of the tested Tax varients (M22, M47) caused only moderate increase in this E2 induced ERE expression, while TaxV89A had no effect (left panel of Fig. 1B). These results may indicate for a synergestic stimulation of ERE-Luc expression by E2 and w.t.Tax, indicating that their stimulatory effects on ERE expression were affected by each other. Furthermore, this experiment may indicate for the involvement of both CREB and NF-κB-associated pathways in Tax stimulation of ERE expression. This is in contrast to Tax effect on E2 induced expression of BRCA1 where we excluded the involvement of both CREB and NF-κB pathways in Tax inhibition of BRCA1 expression (Fig. 1A) and.62 However, TaxV89A, which is unable to bind the CBP/p300 co-factors,54 has no effect on E2 induced ERE-Luc expression (Fig. 1B). Therefore, it seems that Tax interaction with CBP/p300 is required for either of Tax activities: inhibition of E2 induced BRCA1 expression and stimulation of E2 induced ERE expression.
Figure 1.
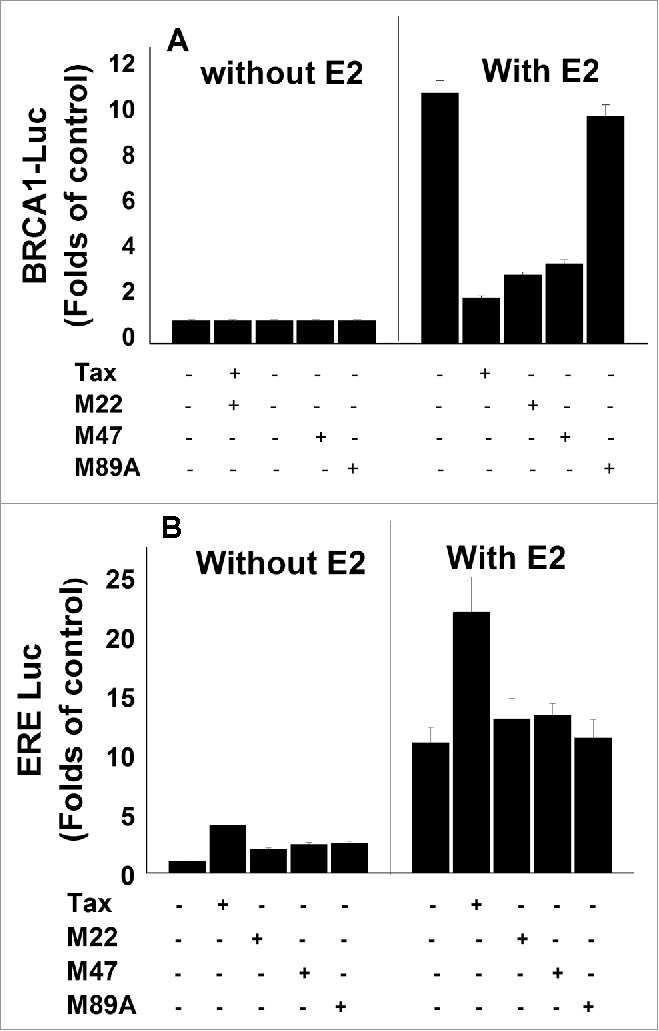
Effect of HTLV-1 Tax varients on the E2-induced BRCA1-Luc and ERE-Luc expression. MCF-7 were co-transfected with either a plasmid expessing BRCA1-Luc (1.5 μg) (A) or ERE-Luc (1.5 μg) (B) alone or together with the indicated combinations of Tax varients (M22, M47 and M89A) expressing plasmids without (left lane) or with (right lane) E2 treatment. The E2 was added to the cultures 5 hr before harvesting the cells for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
3.2 Effect of CBP/p300 and cyclin T1 on E2- ERα transcriptional activities with or without Tax. For elucidating the mechanism of Tax effect on E2- ERα induced ERE expression compared to its effect on E2- ERα induced BRCA1 expression, we examined the involvement of different co-activators such as CBP/p300 and the positive transcription elongation factor (p-TEFb) in this processes. p-TEFb is a protein kinase involved in RNA polymerase II elongation of cellular and viral genes. It composed of catalytic subunit CDK9 and regulatory subunit cyclin T1.73 It was previously found that p-TEFb is recruited to the HTLV-1 LTR promoter by direct interaction of cyclin T1 with Tax.73 The association of p-TEFb with HEXIMI protein through cyclin T1 blocked the CDK9 kinase activity of p-TEFb.74-76 We examined the effect of elevating the cellular level of these co-activators (CBP/p300 and p-TEFb- cyclin T1), by their ectopic expression in MCF-7 cells, on ERE and BRCA1 expression.
Our results (Fig. 2A) showed that both Tax and ectopic CBP/p300 had no significant effect on the basal expression of the BRCA1 reporter in the E2-non-treated MCF-7 cells. However, the results presented in lanes 4–6 of this figure show that introducing ectopic cyclin T1 caused minor but significant increase in BRCA1 reporter basal expression and that Tax has no effect on this cyclin T1 induced expression while HEXIMI completely reduced it to the basal level. The right panel of Figure 2A shows that ectopic CBP/p300 co-activators further enhanced the E2-stimulated expression of the BRCA1 reporter and strongly alleviated the Tax-induced strong inhibition of the E2-stimulated reporter expression. However, ectopic cyclinT1 significantly enhanced the E2-stimulated expression of the BRCA1 reporter but was not able to alleviate the Tax-induced inhibition of this reporter.
Figure 2.
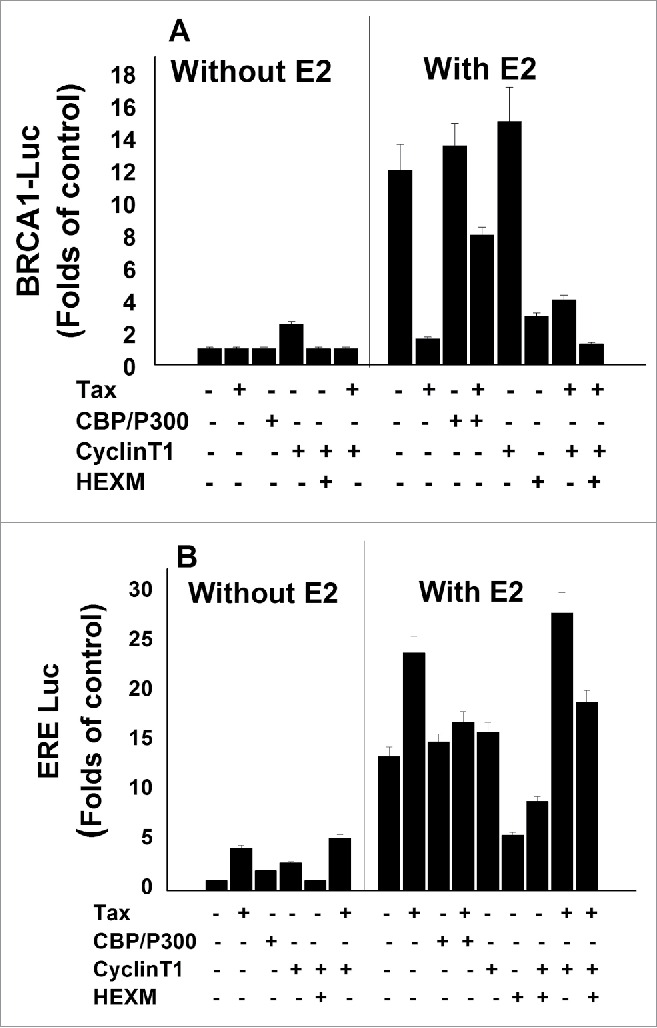
Effect of CBP/p300 and cyclin T1 on E2- ERα transcriptional activities with or without Tax. MCF-7 were co-transfected with either a plasmid expessing BRCA1-Luc (A) or ERE-Luc (B) alone or with the indicated combinations of w.t.Tax, CBP/ p300, cyclineT1, HEXIMI expressing plasmids without (left lane) or with (right lane) E2 treatment. The E2 was added to the cultures 5 hr before harvesting the cells for analyzing the reporter expression. The presented results are an average of 3 repeated experiments ± SE.
In contrast, the results presented in the left panel of Figure 2B show that Tax significantly elevated the ERE reporter basal expression and that ectopic cyclinT1 further increased this activity while ectopic CBP/p300 have no significant on this Tax effect. In the right panel of Figure 2B it can be seen that the strong Tax enhancement of E2-stimulated expression of the ERE reporter was significantly abolished by ectopic CBP/p300 co-activators while ectopic cyclin T1 caused further stimulation of this Tax enhancement. Furthermore, introducing HEXIMI to the E2 treated cells significantly reduced the E2-stimulated expression of the ERE reporter, a reduction which was mostly alleviated by ectopic cyclin T1.
Collectively, the results depicted in Figure 2 suggest that Tax promotes the E2-stimulated expression of the ERE dependent genes (unlike its inhibitory effect on the E2-induced BRCA1 expression) by a mechanism involving the CBP/p300 co-activators. It seems also that cyclin T1 is required for these Tax activities.
3.3 Tax effect on the physical association of E2-ERα with other transcriptional cofactors. Our previous data62 showed that Tax did not prevent the binding of CBP/p300 to ERα but rather physically associated with the ERα-CBP/p300 complex to form an ERα-CBP/p300-Tax complex through direct interaction with the CBP/p300 co-activators. It is known that the CBP/p300 co-activators have several domains on their proteins for physical association with other transcription-modulating factors.77,78 It is, therefore, not surprising that Tax associates with the ERα-CBP/p300 complex through binding to the co-activators rather than through binding to the ERα protein. Various studies have shown that the E2-ERα requires for its transcriptional activity the recruitment of different co-factors, such as the specific protein 1 (Sp1), the cyclic AMP responsive element binding (CREB) protein [15], the non-liganded aromatic hydrocarbon receptor (AhR) [16], the member of the steroid receptor co-activators 1 and 3 [SRC1 [15] and SRC3 [18]] and certain other non-classical co-factors [12]. In this study we tried to find out whether these different cofactors are recruited to ERα before or after its interaction with its promoter and to examine the effect of Tax on the recruitment of these cofactors to E2-ERα complex. Our coimmunoprecipitation analyses presented in Figure 3 show that the immunoprecipitates pulled by mouse ERα specific antibody included practically about the same amounts of various tested cofactors (AhR, CBP, SRC1 and 53BP1) regardless of whether or not the cell were treated with E2 and that Tax did not prevent the binding of either of these cofactors to ERα but rather physically associated with the ERα complex. These results also indicate that the recruitment of the different co-factors to ERα forming the transcriptional complex seems to occurs in the nucleus before the binding of ERα with its promoters regardless if it is activated or not with E2.
Figure 3.
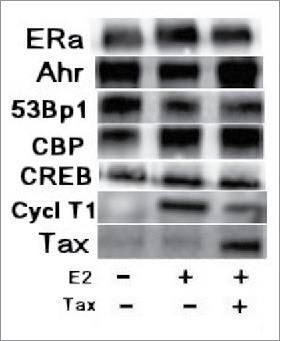
Tax effect on the physical association of E2-ERα with other transcriptional cofactors. MCF-7 cells were or not transfected with 1.5 μg of w.t.Tax expressing plasmid. The indicated cells were treated with E2 at 5 hr before extracting the cells for coimmunoprecipitation (co-IP) analyses. The whole cell extracts were immunoprecipitated with ERα mouse specific monoclonal antibody as indicated in the figure. The various immunoprecipites were analyzed by Western blot analysis with ERα, Ahr, 53Bp1, CBP, Src and Tax rabbit specific monoclonal antibodies.
3.4 Effect of Tax on the ERα complex binding to ERE and BRCA1 promoter. Our previous published results showed that Tax prevented the final step of the E2-ERα-CBP/p300 complex binding to the AP-1 DNA site at the BRCA1 promoter.62 In the present study we examined whether Tax prevents also the binding of other tested cofactors to the AP-1 DNA site at the BRCA1 promoter or still they can directly bind to this promotor not through binding to ERα. This was done by the ChIP analysis, which confirmed that w.t.Tax and its variants (TaxM22 and TaxM47) blocked the various tested cofactors to the AP-1 site of the BRCA 1 promoter, whereas Tax(V89A) had no effect on the binding of these cofactors to BRCA 1 promoter (Fig. 4A). In fact, these results are not surprising since it seems that all these tested cofactors are attached to AP-1 site of the BRCA 1 promoter by binding to ERα. These results are also in agreement with our above results (Fig. 3) which showed association between the tested cofactors and ERα.
Figure 4.
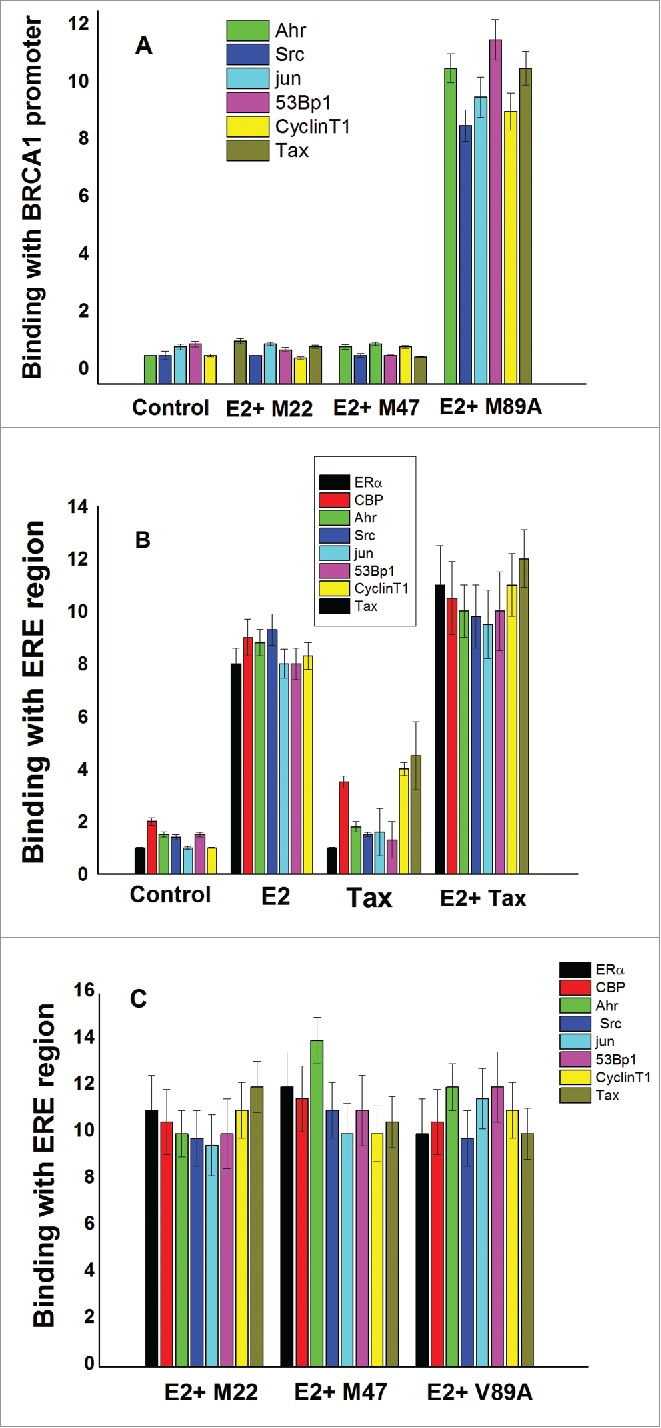
Effect of Tax on the ERα complex binding to ERE and BRCA1 promoter. MCF-7 cells which were or not transfected with 1.5 μg of Tax variants [w.t.Tax, TaxM22, TaxM47 or Tax(V89A)] as indicated in the figure. The cells were treated with E2 at 5 hr before their extraction for examining the binding of the appropriate proteins to BRCA1 promoter (A) or ERE region (B and C) by CHIP assay as described in Materials and Methods section. Control cells were not transfected with Tax and not treated with E2. The presented results are an average of 3 repeated experiments ± SE.
Next we examined the binding of Tax and its varients to ERE region of pS2 gene promotor.79 in control untreated and E2 treated cells. In addition, we tested the effect of Tax and its varients on the binding of E2-ERα complex and the other related cofactors to this ERE region. Our results showed that Tax, CBP and cyclinT1 significantly bind to ERE region in control E2 untreated cells which were transfected with wtTax while the other tested factors (ERα, Ahr, Src, Jun and 53pp1) did not show any significant binding in these cells (Fig. 4B). However, E2 treated cells which were transfected with wtTax (Fig. 4B) or Tax variants (TaxM22, TaxM47 and Tax(V89A) (Fig. 4C) showed high levels of binding of all tested factors (including Tax and ERα proteins) to ERE region.
3.5 Effect of Tax on pS2 gene expression. To demonstrate the relevance of Tax effect on ERE expression to a more physiological situation, it was important to examine the effect of Tax on the expression of endogenous genes rather than transfected reporter genes. Therefore, Tax effect on pS2 gene expression, which is an endogenous ERE responsive gene, was tsted. MCF7 cells were transfected with a plasmid expressing Tax, with or without E2 treatment. pS2 mRNA and protein expression levels at 24h post transfection were examined in the total cellular fractions by RT-PCR and Western blot analysis respectively. Our results presented in Figure 5 show that both E2 and Tax markedly elevated pS2 gene mRNA and protein levels.
Figure 5.
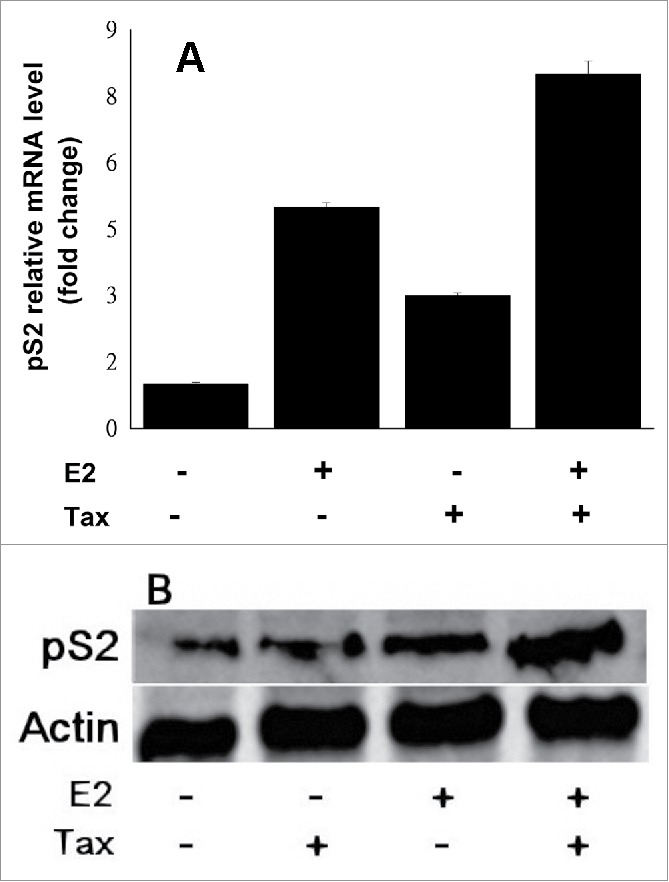
Effect of Tax and E2 on pS2 gene expression. MCF7 cells were transfected with a plasmid expressing Tax, with or without E2 treatment. pS2 mRNA (A) and protein (B) expression levels at 24h post transfection were examined in the total cellular fractions by RT-PCR (as detailed in Material and Methods section) and Western blot analysis (with anti pS2 monoclonal antibody) respectively.
3.6 Are all the various cofactors, which are part of the ERα-E2 complex, required for the transcriptional activity of the ERα-E2 complex?. As it known various cofactors are recruited to the ERα-E2 complex and part of them were tested in this study as shown in Figure 3. We examined whether all the requited cofactors are realy required for the transcriptional activity of this ERα-E2 complex or not by silencing these co-factors synthesis with their specific shRNAs and examining the expression of BRCA-Luc (Fig. 6A), ERE-Luc (Fig. 6B), BRCA1 and pS2 proteins (Fig. 6C). The results presented in Figure 6 show that silencing the expression of Ahr, 53Bp1or CREB had minor effect on the E2 induced expression of BRCA1 or ERE, while slicing of CBP or cyclinT1 strongly reduced this expression.
Figure 6.
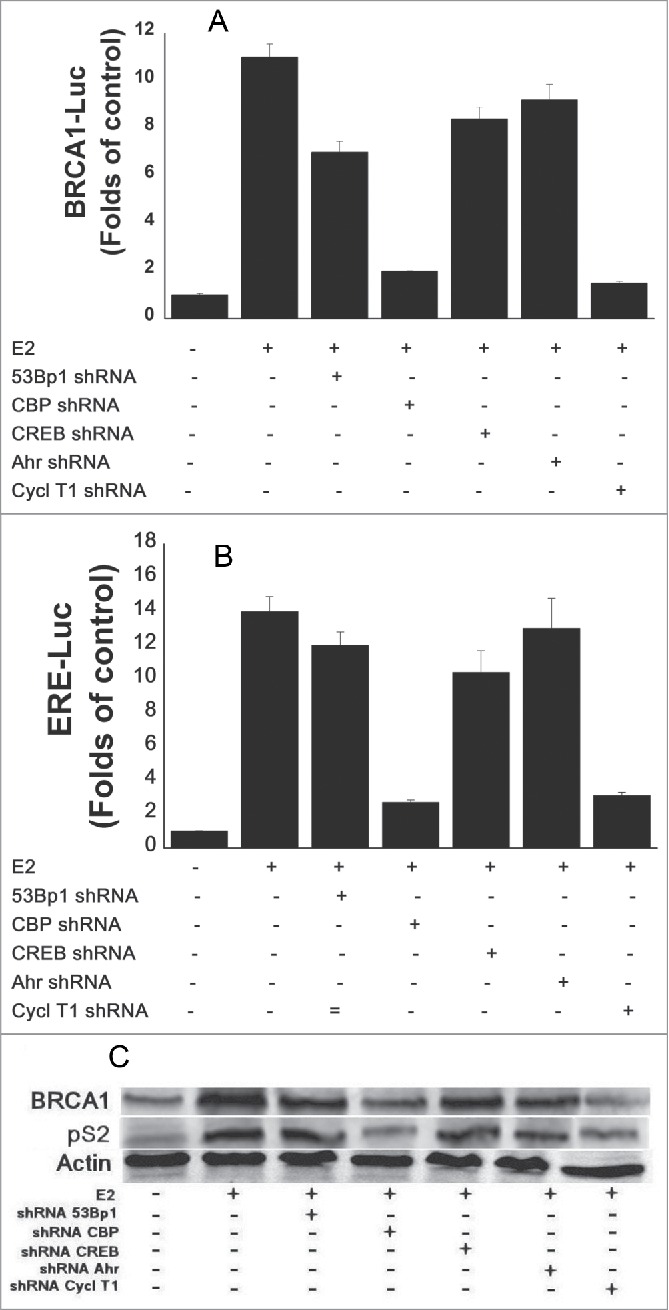
Effect of various co-factors silencing on BRCA1 and pS2 expression. MCF-7 cells were transfected with the shRNAs of the indicated cofactors and treated with E2 w 5 hr before extracting the cells for western blot analysis. The whole cell extracts of the cells were examined for BRACA1 and pS2 expression by western blot analysis with anti BRCA1 and pS2 monoclonal antibodies.
Discussion
The effect of HTLV-1 Tax oncoprotein on the expression of estrogen-responsive genes and its molecular mechanism were investigated in this study. In agreement with previous studies (), we showed that E2 strongly induced ERE-Luc expression as an indication for stimulation of estrogen-responsive genes expression. We also confirmed that this activation was excerted via the classical ERα pathway by showing that the E2-ERα complex directly attached to the ERE region of the pS2 gene promotor (Fig. 4). HTLV-1 Tax is known to induce or prevent the expression of various cellular genes through its effects on different transcriptional factors.80-83 Here we proved for the first time that HTLV-1 Tax strongly stimulate the E2-ERα mediated activation of ERE expression (Fig. 1B). This stimulatory effect of Tax on ERE expression may indicate for a synergestic effect between HTLV-1 Tax and E2-ERα complex probably by increasing the recruitement of required cofactors, such as cyclinT1 which have high affinity to Tax,84,85 to E2-ERα complex. These results are in contrast with our previous data showed that the HTLV-1 Tax oncoprotein drastically antagonizes the E2-ERα mediated activation of BRCA1.62 Also, it should be noted that while Tax has no effect on the basal expression of BRCA1, it significantly stimulate the basal expression of ERE (Fig. 1B).
It is known that Tax is able to block or enhance the expression of different genes by competing for their recruited transcriptional co-activators (such as CBP/p300 and cyclin T1, which are essential also for gene expression) or by mediating the recruitement of these co-activators respectively.72,77,78,86-88 In this study, we observed that increasing the intracellular level of CBP/p300 or cyclin T1 co-factors by ectopic overexpression significantly reduced the Tax-inhibitory effect of the E2-stimulated BRCA1 expression (Fig. 2A). However, only ectopic overexpression of CBP/p300 caused significant reduction of the Tax-stimulatory effect of the E2-stimulated ERE expression (Fig. 2B) while overexpression of cyclin T1 caused further stimulation of this Tax enhancement. Also, HEXIMI, which competing for the binding of cyclin T1, significantly reduced the E2-stimulated expression of the ERE reporter, a reduction which was significantly prevented by ectopic cyclin T1expression. All these results together may indicate for the involvement of CBP/p300 and cyclin T1 co-activators in the mechanism of Tax effect on the E2-stimulated expression of the ERE dependent genes. In our previous study62 we proved that Tax was able to bind to the CBP/p300 co-factors without separating them from ERα and thereby forming an ERα-CBP/p300-Tax tertiary complex. We also proved that this tertiary complex was not formed by direct contact of Tax with ERα molecule, but through binding with the CBP/p300 factors which have been shown to contain several domains for binding of Tax and certain other transcription factors.72,77,78,86 This finding can explain our results presented in Figure 2B which showed that ectopic expression of CBP/p300 significantly reduced the Tax enhancement of E2-stimulated expression of the ERE reporter. According to that, high level of CBP/p300 enables ERα and Tax to separatedly form ERα-CBP/p300 and Tax-CBP/p300 complexes without competing with each other for the CBP/p300 co-activators and avoiding also Tax from physically interacting with the ERα-CBP/p300 complex.62 However, ectopic cyclin T1 expression caused further stimulation of this Tax enhancement (Fig. 2B) which might indicate that cyclin T1 is not interfering with the binding of Tax to ERα complex.
Our resuls also indicated that the recruitment of the different tested co-factors to ERα forming the transcriptional complex occured in the nucleus before the binding of ERα with its promoters regardless if it is activated or not with E2 (Fig. 3). These results are in contrast with previous publications which suggested that the recruitment of different co-factors to the transcriptional factors comlexes happens after the binding of the transcriptional factors to their appropriate promotors.89-91 But still our results did not exclude the possibility that the reqruitment of cofactors can also occure after the binding of the transcriptional factors to their promotors. Our results showed also that not all the reqruited cofactors are highly essential for the transcriptional activity of E2- ERα complex (Fig. 6).
Our previous results62 provided strong indications that Tax inhibits the E2- ERα-CBP/p300-mediated BRCA1-activation by blocking the access of the whole transcriptional complex to the AP1 site at the BRCA1 promoter. However, in this study our CHIP results proved that Tax did not interfer with the binding of the E2- ERα transcriptional complex to the ERE region (Fig. 4).
Thus on one hand, HTLV-1 Tax was found to profoundly inhibit the breast tumor suppressor BRCA1 expression in breast cells (through the non-classical pathway),62 however on the other hand, it strongly stimulates the E2-ERα transcriptional activity through the classical pathway (Fig. 1B). As it known, some of the genes, which are activated by E2-ERα transcriptional complex through the classical pathway, stimulate cells replication.3,92 So, it seems that HTLV-1 Tax is able to enhance breast epithelial cell replication, through ERα classical pathway and probably other pathways such as NFkB,93 and at the same time to block the E2-ERα induced expression, and possibly functions, of BRCA1. These various HTLV-1 Tax activities in breast epithelial cells may enhance breast cancer development.
So, based on these above data it is possible to speculate that penetration of HTLV-1 into breast epithelial cells would likely sensitize them to high risk for malignant transformation by environmental and other source of carcinogens.
It is known that milk of HTLV-1 infected women is rich with HTLV-1 producing T-lymphocytes and infected breast epithelial cells.94,95 It had been previously shown that breast epithelial cells can be infected with HTLV-1 by co-cultivating them with HTLV-1 producing T-cells and these infected epithelial cells can also infect other epithelial.94,96-99 Unfortunately, still there is no serious epidemiological studies were done to examine possible involvement of HTLV-1 in breast cancer. There are only 2 poorly designed studies have been reported so far in the literature that has come up with unconvincing negative data.100,101 Therefore, intensive and well-designed epidemiological studies for examining the involvement of HTLV-1 in breast cancer are still required, focusing on the incidence of breast cancer among HTLV-1 infected population of old women with habits of prolonged breast-feeding.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Hockings JK, Degner SC, Morgan SS, Kemp MQ, Romagnolo DF. Involvement of a specificity proteins-binding element in regulation of basal and estrogen-induced transcription activity of the BRCA1 gene. Breast Cancer Res 2008; 10(2):R29; PMID:18377656; http://dx.doi.org/ 10.1186/bcr1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 2005; 19:1951-9; PMID:15705661; http://dx.doi.org/ 10.1210/me.2004-0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Girault I, Bieche I, Lidereau R. Role of estrogen receptor α transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas 2006; 54:342-51; PMID:16822624; http://dx.doi.org/ 10.1016/j.maturitas.2006.06.003 [DOI] [PubMed] [Google Scholar]
- [4].Kushner PJ, Agard D, Feng WJ, Lopez G, Schiau A, Uht R, Webb P, Greene G. Oestrogen receptor function at classical and alternative response elements. Novartis Found Symp 2000; 230:20-6; PMID:10965500; http://dx.doi.org/ 10.1002/0470870818.ch3 [DOI] [PubMed] [Google Scholar]
- [5].Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, Hager JA, Beliakoff J, Whitesell LJ, Bowden GT, Romagnolo DF. An estrogen receptor-a/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: repressive effects of p53 on BRCA-1 transcription. Neoplasia 2005; 7:873-82; PMID:16229810; http://dx.doi.org/ 10.1593/neo.05256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, Hager JA, Beliakoff J, Whitesell LJ, Bowden GT, Romagnolo DF. An estrogen receptor-a/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: repressive effects of p53 on BRCA-1 transcription. Neoplasia 2005; 7:873-82; PMID:16229810; http://dx.doi.org/ 10.1593/neo.05256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marks JR, Huper G, Vaughn JP, Davis PL, Norris J, McDonnell DP, Wiseman RW, Futreal PA, Iglehart JD. BRCA1 expression is not directly responsive to estrogen. Oncogene 1997; 14:115-21; PMID:9010238; http://dx.doi.org/ 10.1038/sj.onc.1200808 [DOI] [PubMed] [Google Scholar]
- [8].Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997. September 5; 277(5331):1508-10; PMID:9278514; http://dx.doi.org/ 10.1126/science.277.5331.1508 [DOI] [PubMed] [Google Scholar]
- [9].Merchant JS, Oh K, Klerman LV. Sp1 phosphorylation by Erk 2 stimulates DNA binding. Biochem Biophys Res Commun 1999. January 19; 254(2):454-61; PMID:9918860; http://dx.doi.org/ 10.1006/bbrc.1998.9964 [DOI] [PubMed] [Google Scholar]
- [10].Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res 2006; 66:2224-32; PMID:16489025; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1619 [DOI] [PubMed] [Google Scholar]
- [11].Wang W, Schneider-Broussard R, Kumar A, MacLeod M, Johnson D. Regulation of BRCA1 Expression by the Rb-E2F Pathway. J Biol Chem 2000; 275:4532-6; http://dx.doi.org/ 10.1074/jbc.275.6.4532 [DOI] [PubMed] [Google Scholar]
- [12].Wang A, Schneider-Broussard R, Kumar AP, MacLeod MC, Johnson DG. Regulation of BRCA1 expression by the Rb-E2F pathway. J Biol Chem 2000; 275:4532-6; PMID:10660629; http://dx.doi.org/ 10.1074/jbc.275.6.4532 [DOI] [PubMed] [Google Scholar]
- [13].Corkery D, Thillainadesan G, Coughlan N, Mohan RD, Isovic M, Tini M, Torchia J. Regulation of the BRCA1 gene by an SRC3/53BP1 complex. BMC Biochem 2011; 12:50-62; PMID:21914189; http://dx.doi.org/ 10.1186/1471-2091-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nagata C, Mizoue T, Tanaka K, Tsuji I, Wakai K, Inoue M, Tsugane S. Tobacco smoking and breast cancer risk: An evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 2006; 36:387-94; PMID:16766567; http://dx.doi.org/ 10.1093/jjco/hyl031 [DOI] [PubMed] [Google Scholar]
- [15].Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol 2005; 35:213-25; PMID:16054983; http://dx.doi.org/ 10.1016/j.alcohol.2005.04.005 [DOI] [PubMed] [Google Scholar]
- [16].Shrubsole MJ, Gao YT, Dai Q, Shu XO, Ruan ZX, Jin F, Zheng W. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer 2004; 110:605-9; PMID:15122595; http://dx.doi.org/ 10.1002/ijc.20168 [DOI] [PubMed] [Google Scholar]
- [17].Coyle YM. The effect of environment on breast cancer risk. Breast Cancer Res Treat 2004; 84:273-88; PMID:15026625; http://dx.doi.org/ 10.1023/B:BREA.0000019964.33963.09 [DOI] [PubMed] [Google Scholar]
- [18].Snedeker SM. Chemical exposures in the workplace: effect on breast cancer risk among women. AAOHN J 2006; 54:270-9; PMID:16800404; http://dx.doi.org/ 10.1177/216507990605400604 [DOI] [PubMed] [Google Scholar]
- [19].Bernstein L, Patel AV, Ursin G, Sullivan-Halley J, Press MF, Deapen D, Berlin JA, Daling JR, McDonald JA, Norman SA, et al.. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst 2005; 97:1671-9; PMID:16288120; http://dx.doi.org/ 10.1093/jnci/dji374 [DOI] [PubMed] [Google Scholar]
- [20].Ganmaa D, Willett WC, Li TY, Feskanich D, van Dam RM, Lopez-Garcia E, Hunter DJ, Holmes MD. Coffee, tea, caffeine and risk of breast cancer: A 22-year follow-up. Int J Cancer 2008; 122:2071-6; PMID:18183588; http://dx.doi.org/ 10.1002/ijc.23336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Emery J, Lucassen A, Murphy M. Common hereditary cancers and implications for primary care. Lancet 2001; 358:56-63; http://dx.doi.org/ 10.1016/S0140-6736(00)05257-0 [DOI] [PubMed] [Google Scholar]
- [22].Sunpaweravong S, Sunpaweravong P. Recent developments in critical genes in the molecular biology of breast cancer. Asian J Surg 2005; 28:71-5; PMID:15691805; http://dx.doi.org/ 10.1016/S1015-9584(09)60265-7 [DOI] [PubMed] [Google Scholar]
- [23].Yarden RI, Papa MZ. BRCA1 at the crossroad of multiple cellular pathways: approaches for therapeutic interventions. Mol Cancer Ther 2006; 5:1396-404; PMID:16818497; http://dx.doi.org/ 10.1158/1535-7163.MCT-05-0471 [DOI] [PubMed] [Google Scholar]
- [24].Wu W, Koike A, Takeshita T, Ohta T. The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div 2008; 3:1-10; PMID:18179693; http://dx.doi.org/ 10.1186/1747-1028-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rosen EM, Fan S, Ma Y. BRCA1 regulation of transcription. Cancer Lett 2006; 236:175-85; PMID:15975711; http://dx.doi.org/ 10.1016/j.canlet.2005.04.037 [DOI] [PubMed] [Google Scholar]
- [26].Deng CX. Roles of BRCA1 in centrosome duplication. Oncogene 2002; 21:6222-7; PMID:12214252; http://dx.doi.org/ 10.1038/sj.onc.1205713 [DOI] [PubMed] [Google Scholar]
- [27].Wang RH, Yu H, Deng CX. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc Natl Acad Sci USA 2004; 101:17108-13; PMID:15563594; http://dx.doi.org/ 10.1073/pnas.0407585101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yun MH, Hiom K. Understanding the functions of BRCA1 in the DNA-damage response. Biochem Soc Trans 2009; 37:597-604; PMID:19442256; http://dx.doi.org/ 10.1042/BST0370597 [DOI] [PubMed] [Google Scholar]
- [29].Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res 2005; 3:531-9; PMID:16254187; http://dx.doi.org/ 10.1158/1541-7786.MCR-05-0192 [DOI] [PubMed] [Google Scholar]
- [30].Hartman AR, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet 2002; 32:180-4; PMID:12195423; http://dx.doi.org/ 10.1038/ng953 [DOI] [PubMed] [Google Scholar]
- [31].Ye Q, Hu YF, Zhong H, Nye AC, Belmont AS, Li R. BRCA1-induced large-scale chromatin unfolding and allele-specific effects of cancer-predisposing mutations. J Cell Biol 2001; 155:911-22; PMID:11739404; http://dx.doi.org/ 10.1083/jcb.200108049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu B, Kim ST, Kastan MB. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol Cell Biol 2001; 21:3445-50; PMID:11313470; http://dx.doi.org/ 10.1128/MCB.21.10.3445-3450.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thangaraju M, Kaufmann SH, Couch FJ. BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J Biol Chem 2000; 275:33487-96; PMID:10938285; http://dx.doi.org/ 10.1074/jbc.M005824200 [DOI] [PubMed] [Google Scholar]
- [34].Parvin JD. Overview of history and progress in BRCA1 research: the first BRCA1 decade. Cancer Biol Ther 2004; 3:505-8; PMID:15197353; http://dx.doi.org/ 10.4161/cbt.3.6.839 [DOI] [PubMed] [Google Scholar]
- [35].Rosen EM, Fan S, Isaacs C. BRCA1 in hormonal carcinogenesis: basic and clinical research. Endocr Relat Cancer 2005; 12:533-48; PMID:16172191; http://dx.doi.org/ 10.1677/erc.1.00972 [DOI] [PubMed] [Google Scholar]
- [36].Rosen EM, Fan S, Pestell RG, Goldberg ID. BRCA1 gene in breast cancer. J Cell Physiol 2003; 196:19-41; PMID:12767038; http://dx.doi.org/ 10.1002/jcp.10257 [DOI] [PubMed] [Google Scholar]
- [37].Taylor J, Lymboura M, Pace PE, A'hern RP, Desai AJ, Shousha S, Coombes RC, Ali S. An important role for BRCA1 in breast cancer progression is indicated by its loss in a large proportion of non-familial breast cancers. Int J Cancer 1998; 21:334-42; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [38].Wilson CA, Ramos L, Villaseñor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen JJ, Scully R, Livingston D, et al.. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet 1999; 21:236-40; PMID:9988281; http://dx.doi.org/ 10.1038/6029 [DOI] [PubMed] [Google Scholar]
- [39].Gudas JM, Nguyen H, Li T, Cowan KH. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res 1995; 55:4561-5; PMID:7553629. [PubMed] [Google Scholar]
- [40].Romagnolo D, Annab LA, Thompson TE, Risinger JI, Terry LA, Barrett JC, Afshari CA. Estrogen upregulation of BRCA1 expression with no effect on localization. Mol Carcinog 1998. June; 22(2):102-9; PMID:9655254; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [41].Fan S, Ma YX, Wang C, Yuan RQ, Meng Q, Wang JA, Erdos M, Goldberg ID, Webb P, Kushner PJ, et al.. p300 Modulates the BRCA1 inhibition of estrogen receptor activity. Cancer Res 2002; 62:141-51; PMID:11782371 [PubMed] [Google Scholar]
- [42].Welcsh PL, Lee MK, Gonzalez-Hernandez RM, Black DJ, Mahadevappa M, Swisher EM, Warrington JA, King MC. BRCA1 transcriptionally regulates genes involved in breast tumorigenesis. Proc Natl Acad Sci U S A 2002. May 28; 99(11):7560-5; PMID:12032322; http://dx.doi.org/ 10.1073/pnas.062181799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yoshida M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 2005; 24:5931-7; PMID:16155600; http://dx.doi.org/ 10.1038/sj.onc.1208981 [DOI] [PubMed] [Google Scholar]
- [44].Grassi MF, Olavarria VN, Kruschewsky RA, Mascarenhas RE, Dourado I, Correia LC, de Castro-Costa CM, Galvao-Castro B. Human T cell lymphotropic virus type 1 (HTLV-1) proviral load of HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients according to new diagnostic criteria of HAM/TSP. J Med Virol 2011; 83:1269-74; PMID:21567429; http://dx.doi.org/ 10.1002/jmv.22087 [DOI] [PubMed] [Google Scholar]
- [45].Ohshima K. Pathological features of diseases associated with human T-cell leukemia virus type I. Cancer Sci 2007; 98:772-8; PMID:17388788; http://dx.doi.org/ 10.1111/j.1349-7006.2007.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Azran I, Schavinsky-Khrapunsky Y, Aboud M. Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity. Retrovirology 2004; 1:20-43; PMID:15310405; http://dx.doi.org/ 10.1186/1742-4690-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Azran I, Schavinsky-Khrapunsky Y, Priel E, Huleihel M, Aboud M. Implications of the evolution pattern of human T-cell leukemia retroviruses on their pathogenic virulence. Int J Molec Med 2004; 14:909-15; PMID:15492865. [PubMed] [Google Scholar]
- [48].Tabakin-Fix Y, Azran-Shaish I, Levy O, Aboud M. Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: Mechanisms and clinical implications. Carcinogenesis 2006; 27:673-81; PMID:16308315; http://dx.doi.org/ 10.1093/carcin/bgi274 [DOI] [PubMed] [Google Scholar]
- [49].Zhang H, Somasundaram K, Peng Y, Tian H, Zhang H, Bi D, Weber BL, El-Deiry WS. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene 1998; 16:1713-21; PMID:9582019; http://dx.doi.org/ 10.1038/sj.onc.1201932 [DOI] [PubMed] [Google Scholar]
- [50].Somasundaram K, MacLachlan TK, Burns TF, Sgagias M, Cowan KH, Weber BL, El-Deiry WS. BRCA1 signals ARF-dependent stabilization and coactivation of p53. Oncogene 1999; 18:6605-14; PMID:10597265; http://dx.doi.org/ 10.1038/sj.onc.1203284 [DOI] [PubMed] [Google Scholar]
- [51].MacLachlan TK, Takimoto R, El-Deiry WS. BRCA1 directs a selective p53-dependent transcriptional response towards growth arrest and DNA repair targets. Mol Cell Biol 2002; 22:4280-92; PMID:12024039; http://dx.doi.org/ 10.1128/MCB.22.12.4280-4292.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pao GM, Janknecht R, Ruffner H, Hunter T, Verma IM. CBP/p300 interact with and function as transcriptional coactivators of BRCA1. Proc Natl Acad Sci USA 2000; 97:1020-5; PMID:10655477; http://dx.doi.org/ 10.1073/pnas.97.3.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yan JP, Garrus JE, Giebler HA, Stargell LA, Nyborg JK. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J Mol Biol 1998; 281:395-400; PMID:9698555; http://dx.doi.org/ 10.1006/jmbi.1998.1951 [DOI] [PubMed] [Google Scholar]
- [54].Harrod R, Kuo Y, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam C. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem 2000; 275:11852-7; PMID:10766811; http://dx.doi.org/ 10.1074/jbc.275.16.11852 [DOI] [PubMed] [Google Scholar]
- [55].Van Orden K, Yan JP, Ulloa A, Nyborg JK. Binding of the human T-cell leukemia virus Tax protein to the coactivator CBP interferes with CBP-mediated transcriptional control. Oncogene 1999; 18:3766-72; PMID:10391685; http://dx.doi.org/ 10.1038/sj.onc.1202703 [DOI] [PubMed] [Google Scholar]
- [56].Riou P, Bex F, Gazzolo L. The human T cell leukemia/lymphotropic virus Type 1 Tax protein represses MyoD-dependent transcription by inhibiting MyoD-binding to the KIX domain of p300. A potential mechanism for Tax-mediated repression of the transcriptional activity of basic helix-loop-helix factors. J Biol Chem 2000; 275:10551-60; PMID:10744749; http://dx.doi.org/ 10.1074/bc.275.14.10551 [DOI] [PubMed] [Google Scholar]
- [57].Peloponese JM, Yeung ML, Jeang KT. Modulation of nuclear factor-kB by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol Res 2006; 34:1-12; PMID:16720895; http://dx.doi.org/ 10.1385/IR:34:1:1 [DOI] [PubMed] [Google Scholar]
- [58].Kawakami A, Nakashima T, Sakai H, Urayama S, Yamasaki S, Hida A, Tsuboi M, Nakamura H, Ida H, Migita K, Kawabe Y, et al.. Inhibition of caspase cascade by HTLV-I Tax through inhibition of NF-kB nuclear translocation. Blood 1999; 94:3847-54; PMID:10572100 [PubMed] [Google Scholar]
- [59].Pumfery A, de La Fuente C, Kashanchi F. HTLV-1 Tax: Centrosome amplification and cancer. Retrovirology 2006; 3:50; PMID:16899128; http://dx.doi.org/ 10.1186/1742-4690-3-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sasaki M, Sugimoto K, Tamayose K, Ando M, Tanaka Y, Oshimi K. Spindle checkpoint protein Bub1 corrects mitotic aberrancy induced by human T-cell leukemia virus type I Tax. Oncogene 2006; 25:3621-7; PMID:16449967; http://dx.doi.org/ 10.1038/sj.onc.1209404 [DOI] [PubMed] [Google Scholar]
- [61].Marriott SJ, Semmes OJ. Impact of HTLV-1 Tax on cell cycle progression and cellular DNA damage repair response. Oncogene 2005; 24:5986-95; PMID:16155605; http://dx.doi.org/ 10.1038/sj.onc.1208976 [DOI] [PubMed] [Google Scholar]
- [62].Shukrun M, Jabareen A, Abou-Kandil A, Chamias R, Aboud M, Huleihel M. HTLV-1 Tax oncoprotein inhibits the estrogen-induced-ER α-Mediated BRCA1 expression by interaction with CBP/p300 cofactors. PLoS One 2014. February 21; 9(2):e89390; PMID:24586743; http://dx.doi.org/ 10.1371/journal.pone.0089390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat Feb 2004; 83:249-89; http://dx.doi.org/ 10.1023/B:BREA.0000014042.54925.cc [DOI] [PubMed] [Google Scholar]
- [64].Bartek J, Bartkova J, Kyprianou N, Lalani EN, Staskova Z, Shearer M, Chang S, Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc Natl Acad Sci U S A 1991; 88:3520-4; PMID:1708884; http://dx.doi.org/ 10.1073/pnas.88.9.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Veprik A, Khanin M, Hermoni KL, Danilenko M, Levy Y, Sharoni Y. Polyphenols, isothiocyanates and carotenoid derivatives enhance estrogenic activity in bone cells but inhibit it in breast cancer cells. Am J Physiol Endocrinol Metab 2011. August Aug [Epub ahead of print]; PMID:21878663. [DOI] [PubMed] [Google Scholar]
- [66].Bartholin L, Guindon S, Martel S, Corbo L, Rimokh R. Identification of NF-kB responsive elements in follistatin related gene (FLRG) promoter. Gene Dev 2007; 393:153-62 [DOI] [PubMed] [Google Scholar]
- [67].Smith M, Green W. Identification of HTLV-1 tax transactivator mutants exhibiting novel transcriptional phenotypes. Gene Dev 1990; 4:1875-85; PMID:2276622; http://dx.doi.org/ 10.1101/gad.4.11.1875 [DOI] [PubMed] [Google Scholar]
- [68].Torgeman A, Mor-Vaknin N, Zelin E, Ben-Aroya Z, Lochelt M, Flugel RM, Aboud M. Sp1-p53 heterocomplex mediates activation of HTLV-I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate that is antagonized by protein kinase C. Virology 2001; 281:10-20; PMID:11222091; http://dx.doi.org/ 10.1006/viro.2000.0779 [DOI] [PubMed] [Google Scholar]
- [69].xMor-Vaknin N, Torgeman A, Galron D, Lochelt M, Flugel RM, Aboud M. The long terminal repeats of human immunodeficiency virus type-1 and human T-cell leukemia virus type-I are activated by 12-O-tetradecanoylphorbol-13-acetate through different pathways. Virology 1997; 232:337-44; PMID:9191847; http://dx.doi.org/ 10.1006/viro.1997.8566 [DOI] [PubMed] [Google Scholar]
- [70].Demizu Y, Misawa T, Nagakubo T, Kanda Y, Okuhira K, Sekino Y, Naito M, Kurihara M. Structural development of stabilized helical peptides as inhibitors of estrogen receptor (ER)-mediated transcription. Bioorganic Medicinal Chem 2015; 23:4132-8; http://dx.doi.org/ 10.1016/j.bmc.2015.06.067 [DOI] [PubMed] [Google Scholar]
- [71].Hockings JK, Degner SC, Morgan SS, Kemp MQ, Romagnolo DF. Involvement of a specificity proteins-binding element in regulation of basal and estrogen-induced transcription activity of the BRCA1 gene. Breast Cancer Resi 2008; 10:R29. Epub 2008 Mar 31; http://dx.doi.org/ 10.1186/bcr1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Azran-Shaish I, Tabakin-Fix Y, Huleihel M, Bakhanashvili M, Aboud M. HTLV-1 tax-induced NF-kB activation is synergistically enhanced by 12-O-tetradecanoylphorbol-13-acetate: mechanism and implications for Tax oncogenicity. J Mol Med 2008; 86:799-814; PMID:18425496; http://dx.doi.org/ 10.1007/s00109-008-0335-1 [DOI] [PubMed] [Google Scholar]
- [73].Cho WK, Zhou M, Jang MK, Huang K, Jeong SJ, Ozato K, Brady JN. Modulation of the Brd4/P-TEFb Interaction by the Human T-Lymphotropic Virus Type 1 Tax Protein. J Virol 2007; 81:11179-86; PMID:17686863; http://dx.doi.org/ 10.1128/JVI.00408-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yik JH, Chen R, Pezda AC, Zhou Q. Compensatory contributions of HEXIM1 and HEXIM2 in maintaining the balance of active and inactive positive transcription elongation factor b complexes for control of transcription. J Biol Chem 2005. April Apr; 280(16):16368-76; PMID:15713661; http://dx.doi.org/ 10.1074/jbc.M500912200 [DOI] [PubMed] [Google Scholar]
- [75].Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 2001; 414:317-22; PMID:11713532; http://dx.doi.org/ 10.1038/35104575 [DOI] [PubMed] [Google Scholar]
- [76].Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell 2005; 19:535-45; PMID:16109377; http://dx.doi.org/ 10.1016/j.molcel.2005.06.029 [DOI] [PubMed] [Google Scholar]
- [77].Ramirez JA, Nyborg JK. Molecular characterization of HTLV-1 Tax interaction with the KIX domain of CBP/p300. J Mol Biol 2007; 372:958-69; PMID:17707401; http://dx.doi.org/ 10.1016/j.jmb.2007.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Scoggin KE, Ulloa A, Nyborg JK. The oncoprotein Tax binds the SRC-1-interacting domain of CBP/p300 to mediate transcriptional activation. Mol Cell Biol 2001; 21:5520-30; PMID:11463834; http://dx.doi.org/ 10.1128/MCB.21.16.5520-5530.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res 1982; 10:7895-903; PMID:6897676; http://dx.doi.org/ 10.1093/nar/10.24.7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Trevisan R, Daprai L, Paloschi L, Vajente N, Chieco-Bianchi L, Saggioro D. Antiapoptotic effect of human T-cell leukemia virus type 1 tax protein correlates with its creb transcriptional activity. Exp Cell Res 2006; 1390-400; PMID:16483570; http://dx.doi.org/ 10.1016/j.yexcr.2006.01.009 [DOI] [PubMed] [Google Scholar]
- [81].Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell 2003; 11:695-707; PMID:12667452; http://dx.doi.org/ 10.1016/S1097-2765(03)00090-X [DOI] [PubMed] [Google Scholar]
- [82].Mocquet V, Neusiedler J, Rende F, Cluet D, Robin JP, Terme JM, Duc Dodon M, Wittmann J, Morris C, Le Hir H, et al.. The human T-lymphotropic virus type 1 tax protein inhibits nonsense-mediated mRNA decay by interacting with INT6/EIF3E and UPF1. J Virol 2012; 86(14):7530-43; PMID:22553336; http://dx.doi.org/ 10.1128/JVI.07021-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Terme JM, Wencker M, Favre-Bonvin A, Bex F, Gazzolo L, Duc Dodon M, Jalinot P. Cross talk between expression of the human T-cell leukemia virus type 1 Tax transactivator and the oncogenic bHLH transcription factor TAL1. J Virol 2008; 82(16):7913-22; PMID:18495761; http://dx.doi.org/ 10.1128/JVI.02414-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cho WK, Jang MK, Huang K, Pise-Masison CA, Brady JN. Human T-lymphotropic virus type 1 Tax protein complexes with P-TEFb and competes for Brd4 and 7SK snRNP/HEXIM1 binding. J Virol Dec 2010; 84(24):12801-9; doi: 101128/JVI00943-10 Epub Oct 6 2010; http://dx.doi.org/ 10.1128/JVI.00943-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhou M, Lu H, Park H, Wilson-Chiru J, Linton R, Brady JN. Tax interacts with P-TEFb in a novel manner to stimulate human T-lymphotropic virus type 1 transcription. J Virol 2006; 80(10):4781-91; PMID:16641271; http://dx.doi.org/ 10.1128/JVI.80.10.4781-4791.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhang J, Yamada O, Kawagishi K, Araki H, Yamaoka S, Hattori T, Shimotohno K. Human T-cell leukemia virus type 1 Tax modulates interferon-α signal transduction through competitive usage of the coactivator CBP/p300. Virology 2008; 379(2):306-13; PMID:18678383; http://dx.doi.org/ 10.1016/j.virol.2008.06.035 [DOI] [PubMed] [Google Scholar]
- [87].Iwanaga R, Ozono E, Fujisawa J, Ikeda MA, Okamura N, Huang Y, Ohtani K. Activation of the cyclin D2 and cdk6 genes through NF-kappaB is critical for cell-cycle progression induced by HTLV-I Tax. Oncogene 2008; 27(42):5635-42; PMID:18504428; http://dx.doi.org/ 10.1038/onc.2008.174 [DOI] [PubMed] [Google Scholar]
- [88].Hayashibara T, Yamada Y, Mori N, Harasawa H, Sugahara K, Miyanishi T, Kamihira S, Tomonaga M. Possible involvement of aryl hydrocarbon receptor (AhR) in adult T-cell leukemia (ATL) leukemogenesis: constitutive activation of AhR in ATL. Biochem Biophys Res Commun 2003; 300(1):128-34; PMID:12480531; http://dx.doi.org/ 10.1016/S0006-291X(02)02793-6 [DOI] [PubMed] [Google Scholar]
- [89].Goi C, Little P, Xie C. Cell-type and transcription factor specific enrichment of transcriptional cofactor motifs in ENCODE ChIP-seq data. BMC Genomics 2013; 14(Suppl 5):S2; PMID:24564528; http://dx.doi.org/ 10.1186/1471-2164-14-S5-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liu Z, Merkurjev D, Yang F, Li W, Oh S, Friedman MJ, Song X, Zhang F, Ma Q, Ohgi KA, et al.. Enhancer activation requires trans-recruitment of a mega transcription factor complex. Cell 2014; 159(2):358-73; PMID:25303530; http://dx.doi.org/ 10.1016/j.cell.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Colleran A, Collins PE, Carmody RJ. Assessing sites of NF-κB DNA binding using chromatin immunoprecipitation. Methods Mol Biol 2015; 1280:47-59; PMID:25736743; http://dx.doi.org/ 10.1007/978-1-4939-2422-6_4 [DOI] [PubMed] [Google Scholar]
- [92].Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE, Price JS. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J Biol Chem 2013; 288(13):9035-48; PMID:23362266; http://dx.doi.org/ 10.1074/jbc.M112.405456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Martín M. Molecular biology of breast cancer. Clin Transl Oncol 2006; 8(1):7-14; http://dx.doi.org/ 10.1007/s12094-006-0089-6 [DOI] [PubMed] [Google Scholar]
- [94].Southern S, Southern P. Cellular mechanism for milk-borne transmission of HIV and HTLV. Adv Exp Med Biol 2002; 503:183-90; PMID:12026019; http://dx.doi.org/ 10.1007/978-1-4615-0559-4_21 [DOI] [PubMed] [Google Scholar]
- [95].LeVasseur RJ, Southern SO, Southern P. Mammary epithelial cells support and transfer productive human T-cell lymphotropic virus infections. J Hum Virol 1998; 1(3):214-23; PMID:10195245 [PubMed] [Google Scholar]
- [96].Moreira-Ramos K, Castro FM, Linhares-Lacerda L, Savino W. Can thymic epithelial cells be infected by human T-lymphotropic virus type 1? Mem Inst Oswaldo Cruz 2011; 106(6):759-62; PMID:22012233; http://dx.doi.org/ 10.1590/S0074-02762011000600018 [DOI] [PubMed] [Google Scholar]
- [97].Zhou M, Lu H, Park H, Wilson-Chiru J, Linton R, Brady JN. Tax interacts with P-TEFb in a novel manner to stimulate human T-lymphotropic virus type 1 transcription. J Virol 2006; 80(10):4781-91; PMID:16641271; http://dx.doi.org/ 10.1128/JVI.80.10.4781-4791.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liu B, Li Z, Mahesh SP, Kurup SK, Giam CZ, Nussenblatt RB. HTLV-1 infection of human retinal pigment epithelial cells and inhibition of viral infection by an antibody to ICAM-1. Invest Ophthalmol Vis Sci 2006; 47(4):1510-5; PMID:16565386; http://dx.doi.org/ 10.1167/iovs.05-1277 [DOI] [PubMed] [Google Scholar]
- [99].Jack N, Edwards J, Dhanessar W, Benjamin H, Bartholomew C. A study of HTLV-I infection and breast cancers in Trinidad and Tobago. Int J Cancer 2000; 85(2):298-9; PMID:10629093; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [100].Jack N, Edwards J, Dhanessar W, Benjamin H, Bartholomew C. A study of HTLV-I infection and breast cancers in Trinidad and Tobago. Int J Cancer 2000; 85:298-9; PMID:10629093; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [101].Arisawa K, Soda M, Akahoshi M, Fujiwara S, Uemura H, Hiyoshi M, Takeda H, Kashino W, Suyama A. Human T-cell lymphotropic virus type-1 infection and risk of cancer: 15.4 year longitudinal study among atomic bomb survivors in Nagasaki, Japan. Cancer Sci 2006; 97(6):535-9; PMID:16734733; http://dx.doi.org/ 10.1111/j.1349-7006.2006.00212.x [DOI] [PMC free article] [PubMed] [Google Scholar]


