Abstract
Background
Belimumab is a recombinant, human, IgG1λ monoclonal antibody that targets B-lymphocyte stimulator. The intravenous formulation is indicated for the treatment of active, autoantibody-positive systemic lupus erythematosus (SLE). Belimumab has been formulated for subcutaneous (SC) administration to improve patient convenience. This post-hoc modeling and simulation analysis characterizes the population pharmacokinetics (PK) of SC belimumab, and compares the exposure profiles of the approved belimumab IV dose—10 mg/kg every four weeks—to the 200 mg SC weekly dose in SLE patients, highlighting key pharmacological differences relevant for clinicians.
Methods
Data from two Phase 1 studies in US American and Japanese healthy subjects were analyzed with a non-linear mixed effects modeling approach. The resulting SC population PK model and a previously developed IV population PK model were used to conduct simulation trials in a Phase 3 IV belimumab SLE patient population, comparing chronic exposure profiles and exposure ranges stratified by body weight tertiles for IV vs SC dosing.
Results
The PK of belimumab following SC administration was best described by a linear two-comment model. The estimates for clearance, steady-state volume of distribution, and bioavailability were 208 mL/day, 5250 mL, and 76%, respectively. After four weeks of SC dosing, simulated belimumab concentrations exceeded the steady-state trough concentrations of the IV dosing regimen. At steady state simulated serum profiles demonstrated comparable average belimumab concentrations (Cavg,ss) after IV and SC dosing. Simulated belimumab exposures demonstrated largely overlapping concentration ranges following 200 mg SC weekly and 10 mg/kg IV every four weeks dosing.
Discussion
The predicted Cavg,ss of belimumab in SLE patients was comparable following 200 mg SC weekly and 10 mg/kg IV every four weeks dosing. The simulated belimumab accumulation following SC weekly dosing indicated that administration of a loading dose was not required. Similar Cavg,ss ranges were predicted for fixed dose SC and weight-proportional IV regimens in the simulated SLE population, albeit with a reversed body-size-to-exposure relationship for the SC regimen. These findings provide rheumatologists with a better understanding of expected differences in belimumab exposure when comparing IV and SC dosing regimens.
Keywords: Systemic lupus erythematosus, belimumab, pharmacokinetics, subcutaneous, intravenous
Introduction
B-lymphocyte stimulator (BLyS), also known as B-cell activating factor, is a cytokine that promotes B-cell selection, survival, maturation, and differentiation.1 Elevated levels of BLyS have been observed in patients with systemic lupus erythematosus (SLE),2,3 a systemic autoimmune disease that is characterized by autoantibody production.3 Belimumab is a recombinant, fully human, IgG1λ monoclonal antibody (mAb) that targets the BLyS protein, and thereby inhibits B-cell survival and differentiation.1,4
Intravenous (IV) belimumab, in combination with standard therapy, is indicated for the treatment of adult patients with active, autoantibody-positive SLE. The approved IV dose of belimumab is 10 mg/kg every two weeks for the first three doses, and every four weeks thereafter. In a pooled analysis of two Phase 3 studies the SLE Responder Index response rates at week 52 for both studies combined were 38.6% with placebo vs 46.2% (p = 0.005) and 50.6% (p < 0.001) with belimumab 1 mg/kg and 10 mg/kg, respectively.5 Responder rates peaked in both studies at around 24 weeks for all three treatment arms and, in the Phase 3 study with the more responsive patient population, the difference to placebo was significant as early as week 16 of the 10 mg/kg belimumab arm.6
In order to improve patient convenience, patient independence, and to reduce the healthcare burden of IV belimumab, belimumab was formulated for administration via the subcutaneous (SC) route. Subcutaneous administration is less invasive and takes a few minutes in comparison to an hour administration with IV belimumab. Subcutaneous belimumab can be administered outside of the doctor’s clinic by patients or their caregivers. These advantages of SC administration are particularly important for chronic long term treatment of SLE. After initial clinical development with a 100 mg/mL belimumab SC formulation (ClinicalTrials.gov identifier: NCT00732940) a higher concentration formulation (200 mg/mL) was developed to reduce dosing requirements. Based on a preliminary analysis of data from a Phase 1 study in healthy US subjects (NCT01583530),7 200 mg belimumab SC once weekly was selected for the global belimumab SC Phase 3 study (NCT01484496). Recently, data from a Phase 1 study in a healthy Japanese subject population (NCT01516450) became available.8
In this post-hoc analysis, data from the two Phase 1 studies in US subjects (BEL114488/HGS1006-C1105) and Japanese subjects (BEL116119) were pooled and analyzed with a population pharmacokinetic (popPK) modeling approach. The popPK approach provides an integrated characterization of the combined PK results, including a characterization of the effects of subject characteristics, and enables the subsequent application of simulation methods to demonstrate differences in exposure profiles between chronic IV and SC administration, and predict the effect of SLE patient characteristics on exposure. The objectives of this analysis were to (1) characterize the combined popPK of belimumab following SC administration in healthy subjects for both Phase 1 studies, (2) to determine whether the SC 200 mg weekly dosing would achieve similar steady-state exposure as IV 10 mg/kg every four weeks dosing, based on the popPK parameters, (3) to contrast SC and IV exposure profiles for chronic administration of belimumab in SLE patients, and (4) to compare the predicted exposure variability of SC administration with a fixed dose to the variability of IV weight-proportional dosing in SLE patients.
Methods
Pooled studies
In all, 134 subjects from two Phase 1 studies following SC and IV belimumab administration to healthy US7 and Japanese8 subjects were included in this post-hoc analysis. Both studies were randomized, parallel-group, open-label design.7,8 Dosing schedules, subject numbers, and PK sampling times for these studies are shown in Table 1.
Table 1.
Studies included in the SC belimumab population pharmacokinetic analysis
| Study, subject population | Number of patients (n) | Dose and administration | Pharmacokinetic sampling | Ref. |
|---|---|---|---|---|
| Phase 1 US population | 19–20 subjects per treatment cohort, males and females (∼1:1), total of 118 subjects. | Cohort 1: Single-dose on Day 0, SC 240 mg belimumab (2 × 0.6 mL (2 × 120 mg)). Cohort 2: Single-dose on Day 0, SC 240 mg belimumab (1 × 1.2 mL (1 × 240 mg)). Cohort 3: Single-dose on Day 0, SC 200 mg belimumab (1 × 1.0 mL (1 × 200 mg)). Cohort 4: Single-dose on Day 0, IV 240 mg belimumab (1 h infusion). Cohort 5: Multiple-dose, weekly × 4 (qwk × 4) on Days 0, 7, 14 and 21, SC 240 mg belimumab (2 × 0.6 mL (2 × 120 mg)). Cohort 6: Multiple-dose, qwk × 4 on Days 0, 7, 14 and 21, SC 200 mg belimumab (1 × 1.0 mL (1 × 200 mg)). Randomization was stratified by weight ( < 75 kg vs ≥75 kg) and SC administration site (abdomen vs. thigh). | SC single-dose (cohorts 1, 2 and 3): Days 1–7, 10, 14, 21, 28, 42, 56, and 70. IV single-dose (cohort 4): 5 min, 1 and 3 h post-dose administration and on Days 1, 2, 4, 7, 14, 21, 28, 42, 56, and 70. SC multiple-dose (cohorts 5 and 6): Days 1–6, 7 (prior to 2nd dosing), 14 (prior to 3rd dosing), 21 (prior to 4th dosing and 6 h post-dosing), 22–28, 31, 35, 42, 49, 63, 77, 91, and 119. Serum belimumab concentrations were quantified using validated electrochemiluminescence (ECL)-based method. | 7 |
| Phase 1 Japanese population | Eight subjects per treatment cohort, males, total of 16 subjects. | Cohort 1: Single-dose, IV 200 mg (∼1 h infusion). Cohort 2: Single dose, SC 200 mg (1 × 200 mg). Randomization was stratified by weight (<65 kg vs ≥65 kg). The SC administration site was the thigh. | IV single-dose (cohort 1): Pre-dose and 5 min, 1, and 6 h after post-dose administration, and on Days 1, 2, 4, 7, 14, 21, 28, 42, 56, and 70. SC single-dose (cohort 2): Pre-dose and 6 h post-dose administration and on Days 1, 2, 3, 4, 5, 6, 7, 10, 14, 21, 28, 42, 56, and 70. Serum belimumab concentrations were quantified using validated ECL-based method. | 8 |
Pharmacokinetic modeling and simulation
For a detailed description of the development and validation of the SC belimumab popPK model based on pooled data from the two clinical studies using a non-linear mixed-effects modeling approach, refer to the Supplementary Methods section. Following covariate analysis with the full model approach, the reduced SC popPK model, which only retained statistically significant covariates, was utilized to conduct simulations of 200 mg weekly SC dosing. These simulations were compared with simulations from a previously-developed IV population PK model9 based on the IV dosing approved for SLE. Simulations were conducted using (1) the population PK parameters of the SC and IV popPK model to provide a comparison for chronic exposure profiles for a typical patient, and (2) by incorporating the patient characteristics of the IV SLE Phase 3 trial and random between-subject variability to provide a comparison of the exposure ranges relative to patients’ body weights.
Results
Subcutaneous belimumab population pharmacokinetic model
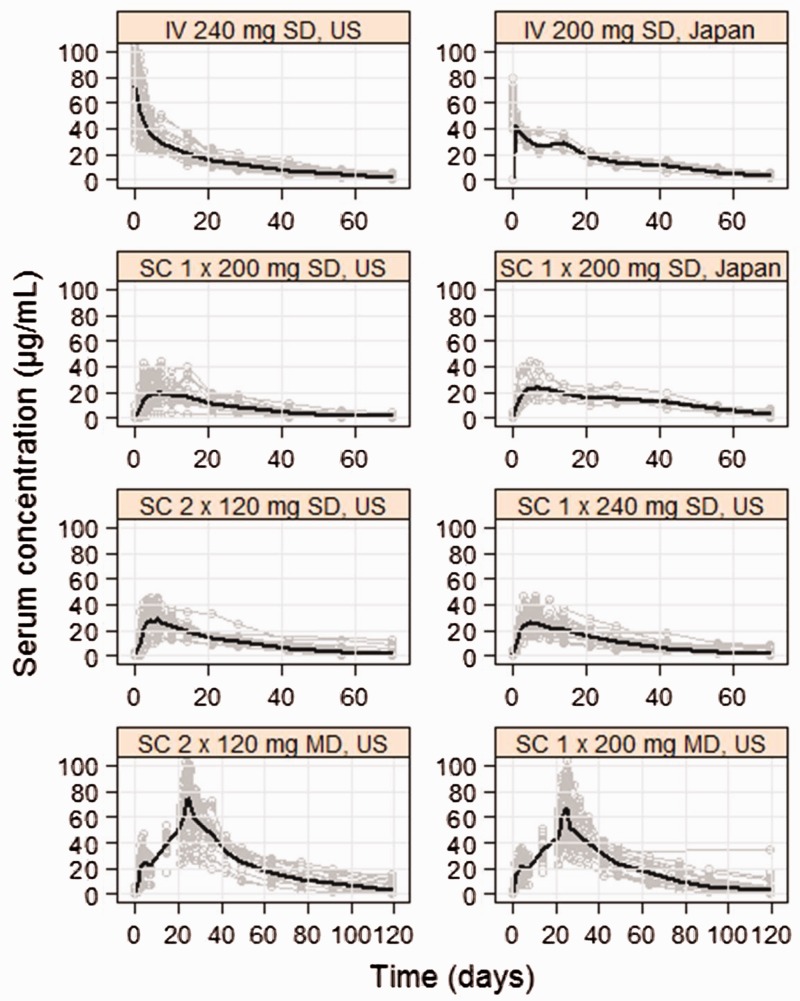
The serum profiles of belimumab following IV and SC administration from the two clinical studies are shown in Figure 1. Similar belimumab peak concentrations were observed between US and Japanese subjects, although increased exposure for Japanese subjects during the elimination phase of the PK profiles was apparent (Figure 1). The PK of SC belimumab was best described by a linear two-compartment model with an absorption lag time in addition to first-order absorption kinetics. Belimumab population PK estimates for the full SC model are shown in Table 2 (see Supplementary Table 1S, for the full set of parameters and Supplementary Methods and Results for model validation). Following SC administration the absorption lag time was 4.25 h (Table 2) and the bioavailability of belimumab was 76% (Table 2). The time after administration to reach maximum belimumab concentration was 5.1 days for the first dose and 2.6 days at steady state. The terminal half-life for belimumab was estimated to be 18.7 days.
Figure 1.
Serum profiles of belimumab following IV single doses (SD) and SC single and multiple doses (MD) in US and Japanese subjects. Gray lines and black lines are individual and geometric mean serum profiles, respectively.
Table 2.
Belimumab pharmacokinetic estimates for the full SC population pharmacokinetic model
| Parameter | Model estimates (%RSE) |
|---|---|
| CL (mL/day) | 208 (3.9) |
| Vss (mL) | 5250 (NA) |
| ka (day−1) | 0.312 (6.6) |
| Lag time (day) | 0.177 (3.4) |
| F | 0.761 (4.6) |
%RSE: percentage relative standard error, calculated as (standard error/mean) × 100%; Vss: volume of distribution at steady-state; NA: not applicable; ka: first-order absorption rate constant; F: bioavailability.
The effects of subject characteristics included in the full SC covariate model were the significant covariates from the IV popPK model,9 which was developed from a large SLE patient population (N = 1603). Among these effects, only baseline body weight on clearance, volume of distribution and intercompartmental clearance, baseline body mass index on volume of distribution, and baseline albumin on clearance (refer to Supplementary Table 1S) were significant in the smaller SC population (N = 134) and retained in the reduced model for simulations.
Predicted belimumab exposure following intravenous (IV) and subcutaneous (SC) chronic dosing
The simulated serum profiles for a typical SLE patient receiving belimumab IV 10 mg/kg every four weeks and SC 200 mg weekly are shown in Figure 2. The accumulation profile indicates that belimumab SC concentrations start exceeding the steady-state trough concentrations of the IV 10 mg/kg dosing regimen (Figure 2) after four weeks of SC dosing. Table 3 shows the minimum steady-state serum concentrations (Cmin,SS), maximum steady-state serum concentrations (Cmax,SS), and average steady-state serum concentrations (Cavg,SS) for belimumab from the IV and SC simulated serum profiles (Figure 2). The average steady-state serum concentration following belimumab SC 200 mg once a week (104 µg/mL) was comparable to the concentrations achieved following belimumab IV 10 mg/kg every four weeks (110 µg/mL) (Table 3). The steady-state trough concentration was 1.73-fold higher and steady-state peak concentration was 2.90-fold lower following SC weekly dosing when compared to IV every four weeks dosing (Figure 2, Table 3). At steady-state, the fluctuation of belimumab serum concentrations was smaller following the SC dosing regimen when compared to the IV dosing regimen (Figure 2 and Table 3).
Figure 2.
Simulated serum profiles for typical SLE patient receiving belimumab IV 10 mg/kg every four weeks (red line) vs SC 200 mg weekly (green line). The dashed line represents the belimumab IV steady-state trough concentration.
Table 3.
Steady-state Cmin, Cmax and Cavg for the simulated belimumab serum profiles following IV and SC dosing
| Dosing regimen | Cmin,SS (µg/mL) | Cmax,SS (µg/mL) | Cavg,SS (µg/mL) |
|---|---|---|---|
| IV 10 mg/kg monthly | 56 | 313 | 110 |
| SC 200 mg once weekly | 97 | 108 | 104 |
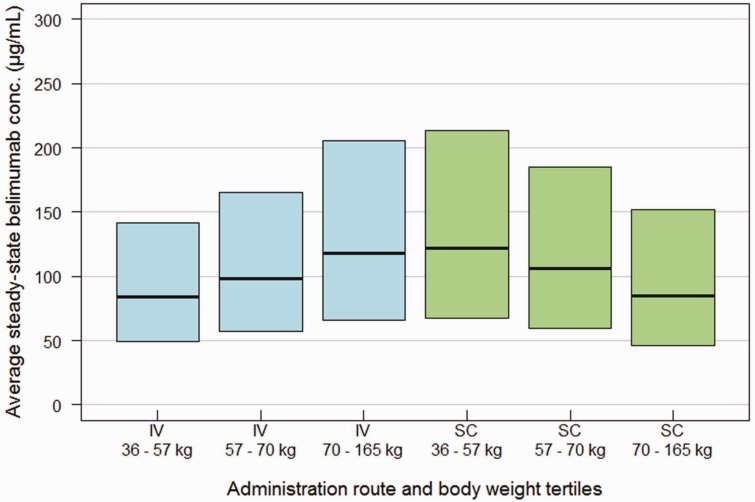
Simulated average steady-state serum concentrations for belimumab stratified by body weight tertile in the SLE patient population following IV 10 mg/kg every four weeks dosing and SC 200 mg weekly dosing are shown in Figure 3. The simulated belimumab exposures demonstrate largely overlapping concentration ranges following SC and IV dosing (Figure 3). An inverse body-weight-to-belimumab exposure relationship was evident following SC weekly dose, where the exposure decreased with an increase in body weight (Figure 3). The opposite was observed following IV every four weeks dosing, where belimumab exposure increased with an increase in body weight (Figure 3).
Figure 3.
Belimumab average steady-state concentrations (median and 90% prediction interval) vs body weight tertile, simulated in the SLE patient population following IV 10 mg/kg every four weeks and SC 200 mg weekly dosing.
Discussion
In this post-hoc analysis, a popPK model integrating results from two Phase 1 studies in healthy subjects was developed, which enabled (1) the characterization of belimumab PK including the effects of subject characteristics following belimumab SC administration, (2) simulation of PK profiles for chronic dosing, and (3) the simulation and assessment of the effect of body size on steady-state exposure for fixed dose SC administration in SLE patients. These characteristics were contrasted with the corresponding characteristics for the approved IV 10 mg/kg dosing regimen to provide a better understanding of the differential pharmacology when switching SLE patients from IV to SC belimumab administration.
The linear two-compartment popPK model identified for belimumab SC included absorption characteristics (76% bioavailability, 0.312 day−1 absorption rate) typical of monoclonal antibodies, which are absorbed predominantly via convective transport through lymphatic vessels.10 Distribution (steady-state volume of distribution, Vss =5250 mL) and elimination (central clearance, CL = 208 mL/day) parameters for SC belimumab in healthy subjects were very similar to parameters of IV belimumab in SLE patients (Vss = 5290 mL, CL = 215 mL/day),9 confirming that, after accounting for the slow absorption from the subcutaneous compartment, belimumab IV and SC pharmacokinetics are comparable. In line with this, the similar elimination half-life (SC popPK = 18.7 days; IV popPK = 19.4 days) indicates similar time periods for IV and SC administration until belimumab concentrations can be considered negligible (e.g. at least five half-lives after the last dose).
The estimated effects of subject characteristics on PK parameters in the full SC covariate model were, except for baseline creatinine clearance, qualitatively consistent with the covariate effects estimated in the IV popPK model9 in terms of effect size, although most covariates were not statistically significant due to the small number of subjects in the SC Phase 1 trials compared to the IV popPK population. Only the effects of baseline body weight, baseline body mass index (BMI), and baseline albumin were statistically significant and were retained in the reduced covariate model used for simulations. The estimated distribution volumes were set to increase proportionally with body weight, and the systemic and intercompartmental clearances were set to increase less than proportionally (with an exponent of 0.75 according to the theory of quarter-power allometric scaling11), leading to lower maximal and average belimumab concentrations, respectively, with higher body weight. For baseline BMI, a negative correlation was evident with volume of distribution, likely accounting for the lesser contribution of adipose tissue to the volume of distribution of monoclonal antibodies compared to lean body mass.
Albumin and IgG bind the neonatal FcRn receptor non-competitively in the acidic endosome after cellular uptake.12 This process comprised of pH dependent binding, subsequent transport back to the cell surface, and release back into the circulation, protects albumin and IgG from lysosomal degradation and thereby extends their half-life. We hypothesize, assuming a constant albumin synthesis rate, that increased albumin levels indicate increased FcRn receptor expression and, consequently, more protection of belimumab and endogenous IgG from lysosomal degradation, explaining the estimated effect of lower belimumab clearance with higher subject albumin level.
Higher belimumab serum exposure was evident across the elimination phase in Japanese subjects following both IV and SC dosing when compared to the US subjects (Figure 1). This is, at least partially, explained by the overall smaller body size of the Japanese subject population (mean = 66.1 kg vs mean = 76.0 kg in the US subject population) and captured by the model as the model-based simulations predict higher median concentrations for this population (see Supplementary Figure 1S). The smaller sample size in the Japanese study variability may also have contributed to the observed higher exposure. Since there was no apparent dose–response relationship for adverse effects in the Phase 3 trials of IV belimumab between 1 mg/kg and 10 mg/kg regimens, it is expected that the higher exposure in Japanese subjects does not pose a safety risk and is, therefore, not clinically relevant. A Phase 3 SC global study with SLE patients from 31 countries including Japan will provide further data.
Simulations with the population parameters of the SC and IV popPK model demonstrated that following SC 200 mg weekly dosing the average steady-state belimumab serum concentrations were comparable to those following IV 10 mg/kg every four weeks dosing, which is currently approved for use in active, autoantibody-positive SLE patients (Figure 2 and Table 3). This integrated analysis of SC Phase 1 data therefore confirms the dose selection for the ongoing SC Phase 3 trial, which aimed to approximately match the average steady-state serum concentrations for the two administration routes. Figure 2 and Table 3 demonstrate that the fluctuations around the average steady-state serum concentration are reduced, and higher trough concentrations are maintained with SC administration due to weekly dosing and slow SC absorption.
Given that IV belimumab demonstrated efficacy for 1 mg/kg dosing,6 and at the end of the first month of SC 200 mg weekly dosing belimumab concentrations are predicted to be higher than minimum steady-state serum concentration for the IV 10 mg/kg regimen (Figure 2), no loading dose regimen was specified for the SC Phase 3 trial.
In order to simulate exposure ranges in SLE patients the reduced SC popPK model was applied to the SLE population of the IV Phase 3 studies.9 The simulated average belimumab concentrations stratified by body weight tertiles (36–57 kg, 57–70 kg, and 70–165 kg) demonstrated largely overlapping concentration ranges following IV 10 mg/kg monthly and SC 200 mg weekly dosing regimens (Figure 3). The inverse body-weight-to-exposure relationship observed following SC dosing is due to the average steady-state serum concentration being proportional to 1/clearance, and clearance increasing with body weight. In the IV case, since dose increases proportionally with body weight, but clearance less than proportionally, the average steady-state serum concentration increases with body size, leading to overall similar exposure ranges for IV and SC administration. This simulation analysis and the qualitative agreement of the estimated effects of subject characteristics on PK parameters indicate that, with weekly 200 mg SC administration, as for 10 mg/kg IV administration, no dose adjustments are required in the targeted SLE population. Results from the global SC Phase 3 study will provide conclusive data on the question of necessity of dose adjustments. In summary, this post-hoc popPK and simulation analysis predicts the chronic exposure in SLE patients from Phase 1 data in healthy volunteers, and enables the SLE healthcare provider to better understand pharmacokinetic differences between belimumab IV and SC dosing.
Supplementary Material
Acknowledgements
The authors wish to thank the study teams and Drs Xiaohua Gong and Xiaobin Li for preparing the NONMEM data files.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr S W.S. Yapa was funded by GSK. Drs D Roth and D Gordon are employees of GSK and hold stock in GSK. Dr Struemper is an employee of PAREXEL on behalf of GSK, and holds stock in GSK.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by GlaxoSmithKline (GSK).
References
- 1.Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 2009; 119: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petri M, Stohl W, Chatham W, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008; 58: 2453–2459. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001; 166: 6–10. [DOI] [PubMed] [Google Scholar]
- 4.Halpern WG, Lappin P, Zanardi T, et al. Chronic administration of belimumab, a BLyS antagonist decreases tissue and peripheral blood B-lymphocyte populations in Cynomolgus Monkeys: pharmacokinetic, pharmacodynamic, and toxicologic effects. Toxicol Sci 2006; 91: 586–599. [DOI] [PubMed] [Google Scholar]
- 5.Manzi S, Sánchez-Guerrero J, Merrill JT, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis 2012; 71: 1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomized, placebo-controlled, Phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 7.Cai WW, Fiscella M, Chen C, John Zhong Z, Friemuth WW, Subich DC. Bioavailability, pharmacokinetics, and safety of belimumab administered subcutaneously in healthy subjects. Clin Pharmacol Drug Dev 2013; 2: 349–357. [DOI] [PubMed] [Google Scholar]
- 8.Shida Y, Takahashi N, Sakamoto T, Endo A, Hirama T. The pharmacokinetics and safety profiles of belimumab after single subcutaneous and intravenous doses in healthy Japanese volunteers. J Clin Pharm Ther 2014; 39: 97–101. [DOI] [PubMed] [Google Scholar]
- 9.Struemper H, Chen C, Cai W. Population pharmacokinetics of belimumab following intravenous adminsitration in patients with systemic lupus erythematosus. J Clin Pharmacol 2013; 53: 711–720. [DOI] [PubMed] [Google Scholar]
- 10.Nathanael DL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010; 49: 633–659. [DOI] [PubMed] [Google Scholar]
- 11.Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 2009; 24: 25–36. [DOI] [PubMed] [Google Scholar]
- 12.Roopenian DC, Sun VZ. Clinical ramifications of the MHC family Fc receptor FcRn. J Clin Immunol 2010; 30: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84: 548–558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.