Abstract
The mechanism of calcium uptake, translocation and accumulation in Poaceae has not yet been fully understood. To address this issue, we conducted genome-wide comparative in silico analysis of the calcium (Ca2+) transporter gene family of two crop species, rice and sorghum. Gene annotation, identification of upstream cis-acting elements, phylogenetic tree construction and syntenic mapping of the gene family were performed using several bioinformatics tools. A total of 31 Ca2+ transporters, distributed on 9 out of 12 chromosomes, were predicted from rice genome, while 28 Ca2+ transporters predicted from sorghum are distributed on all the chromosomes except chromosome 10 (Chr 10). Interestingly, most of the genes on Chr 1 and Chr 3 show an inverse syntenic relationship between rice and sorghum. Multiple sequence alignment and motif analysis of these transporter proteins revealed high conservation between the two species. Phylogenetic tree could very well identify the subclasses of channels, ATPases and exchangers among the gene family. The in silico cis-regulatory element analysis suggested diverse functions associated with light, stress and hormone responsiveness as well as endosperm- and meristem-specific gene expression. Further experiments are warranted to validate the in silico analysis of the predicted transporter gene family and elucidate the functions of Ca2+ transporters in various biological processes.
Key words: in silico comparison, calcium transporter, rice, sorghum, genomic annotation, synteny
Introduction
Calcium plays a central role in plants in the regulation of growth and development by acting as a second messenger in the signal transduction pathways. The membrane-associated calcium (Ca2+) transporter proteins are essential to maintain calcium homeostasis under normal physiological conditions and provide tolerance against various stresses. There are three major classes of Ca2+ transporter proteins: channels, ATPases (pumps) and exchangers 1, 2, 3, 4, 5, 6. Members of these transporter proteins may differ in their cellular and tissue distribution and the regulation by other signaling pathways 7, 8. The spatial and temporal regulation of calcium concentration in plant cells depends on the coordinated activities of channels and active transporters located on different organelles and membranes (9). Calcium channels, pumps and exchangers, which differ in their cellular distribution and mechanism of transport, operate the complex and tight regulation of calcium homeostasis. Specific isoforms of these proteins are responsible for increasing or reducing free calcium in the cytosol (10).
Whereas diffusion of molecules across membranes (either intracellular or across the plasma membrane) is mediated by calcium channels, the calcium pump (ATPase) is a membrane-bound Ca2+ transporter that uses energy derived from ATP hydrolysis to transport Ca2+ across membranes against their concentration gradient (11). There are two major Ca2+ ATPase families 12, 13: P-type IIA and P-type IIB. The P-type IIA family lacks N-terminal auto-regulatory domain while the IIB family of plant is characterized by the presence of an auto-inhibitory N-terminal domain containing a Ca2+/CaM-binding site and a serine phosphorylation site (14). Calcium exchanger is a secondary active transporter using energy from the flow of one ion (for example, Na+) down its concentration gradient to transport Ca2+ against its concentration gradient (10). Ca2+ transporters on various membranes play an important role in orchestrating diverse biological processes. The results of electrophysiological studies and molecular analyses indicate the existence of many species of Ca2+ transporter proteins 15, 16. Although a burgeoning number of Ca2+ transporters have been identified, it is often difficult to associate functions with particular transporters. Thus, there is a dire need of in-depth understanding of structural and functional roles of such transporter family, which can be elucidated by comparing the Ca2+ transporter gene family in the grass species of rice and sorghum.
Grasses, covering 20% of the earth’s land surface, are ecologically well adapted (17). This group of plants is especially important to agriculture, contributing a large portion of the calories consumed in the human diet (18). Both rice and sorghum belong to the Poaceae family and represent two types of carbon metabolism: C3 and C4, respectively. The evolution of C4 photosynthesis in the Sorghum lineage involved redirection of C3 progenitor genes as well as recruitment and functional divergence of both ancient and recent gene duplicates (19). Analysis of the sorghum sequence provides new insights into the recruitment of C3 genes to the C4 pathway, allowing us to identify more orthologs in cereals.
Though there exists great diversity among cereals in terms of genome size, ploidy level and chromosome numbers, attempts have been made to reveal the existing synteny and colinearity on the basis of comparative genomics (19). A total of 19,929 (57.8%) sorghum gene models were collinear with rice. About 60% of sorghum genes are located in syntenic regions to rice and orthologous relationships are well established by genetic markers as well as whole-genome comparisons 19, 20. The identification of specific genes and their function would help to understand the development, evolution and differences of crop plants. Additionally, the comparative analysis of sorghum and rice will also offer new insights into the role of different Ca2+ transporters like calcium channel, pump and exchanger. Furthermore, examination of comparative synteny mapping between two monocot model species will allow understanding how genes evolve within monocot species and thus allow better identification of target genes for Ca2+ signaling. In view of the un-availability of sufficient information on the molecular mechanism(s) associated with calcium nutrition, transport and accumulation in cereal grains, we focused on comparing the Ca2+ transporter gene family in the grass species rice and sorghum.
In the present study, we performed genome-wide in silico identification of Ca2+ transporter gene family of sorghum from its recently sequenced genome (19), with annotation for chromosomal location(s), gene structure and phylogenetic tree construction. The putative functions of the predicted Ca2+ transporter genes in sorghum were investigated by analyzing the cis-regulatory elements present in the promoter region of these genes. Moreover, the comparative phylogeny and syntenic mapping with rice transporter gene family were also analyzed.
Results and Discussion
Genome-wide annotation of Ca2+ transporter gene family
The availability of sequenced genome of sorghum (19) provided ample opportunity to annotate and predict the complete set of information of Ca2+ transporter genes using various bioinformatics tools. For the identification of transporter genes, BLAST 21, 22 search was conducted to analyze the complete genome sequence of rice and sorghum, respectively. The genome-wide search revealed presence of 28 transporter genes from sorghum genome, including 1 channel, 15 ATPases and 12 exchangers (Table 1). In comparison, 31 transporter genes were found as annotated from the whole genome of rice, consisting of 1 channel, 14 ATPases and 16 exchangers (Table 2). It shows that fewer Ca2+ transporters were identified in sorghum than in rice. This could be due to the fewer chromosomes in sorghum (10 chromosomes) as compared to rice (12 chromosomes).
Table 1.
Calcium transporters in sorghum
| Serial No. | Locus | Co-ordinate* | No. of conserved domains | Chromosome No. | Function |
|---|---|---|---|---|---|
| 1 | Sb03g031110 | 59449940 - 59431785 | 2 | 3 | Two Pore Ca channel |
| 2 | Sb03g045370 | 72613513 - 72619278 | 6 | 3 | Ca ATPase 3 |
| 3 | Sb08g001260 | 1231390 - 1237532 | 6 | 8 | Ca ATPase, IIB type |
| 4 | Sb05g002380 | 2551588 - 2556879 | 6 | 5 | Ca ATPase, IIB type |
| 5 | Sb01g014620 | 13926528 - 13931352 | 5 | 1 | Ca ATPase 2 |
| 6 | Sb09g024300 | 53852476 - 53858301 | 6 | 9 | Ca ATPase 6 |
| 7 | Sb01g038990 | 62459145 - 62464238 | 5 | 1 | Ca ATPase, IIB type |
| 8 | Sb07g026810 | 61947637 - 61958992 | 6 | 7 | Ca ATPase, IIB type |
| 9 | Sb06g027770 | 56594227 - 56606829 | 6 | 6 | Ca ATPase, IIB type |
| 10 | Sb09g001850 | 1851129 - 1855578 | 5 | 9 | Ca ATPase, IIB type |
| 11 | Sb07g028160 | 63171206 - 63175489 | 6 | 7 | Ca ATPase, IIB type |
| 12 | Sb01g043620 | 66764553 - 66770308 | 6 | 1 | Ca ATPase, IIB type |
| 13 | Sb02g028935 | 64048135 - 64069090 | 6 | 2 | Ca ATPase, IIB type |
| 14 | Sb01g021870 | 26371223 - 26374261 | 4 | 1 | Ca ATPase, IIB type |
| 15 | Sb04g005130 | 4956445 - 4976039 | 3 | 4 | Ca ATPase, IIB type |
| 16 | Sb06g029175 | 57842933 - 57844648 | 1 | 6 | Ca ATPase1, IIA type |
| 17 | Sb04g010130 | 13357947 - 13369114 | 2 | 4 | CAX 1c |
| 18 | Sb04g003135 | 2940231 - 2942365 | 2 | 4 | Ca exchanger 2 |
| 19 | Sb01g033220 | 56450631 - 56454742 | 2 | 1 | Ca exchanger |
| 20 | Sb01g021270 | 24787055 - 24788792 | 1 | 1 | Ca exchanger |
| 21 | Sb08g022240 | 54041794 - 54043632 | 1 | 8 | Na/Ca exchanger |
| 22 | Sb06g031080 | 59454144 - 59458446 | 2 | 6 | Ca exchanger |
| 23 | Sb03g008600 | 9201460 - 9203592 | 1 | 3 | K-dependent Na/Ca exchanger |
| 24 | Sb03g013753 | 17913353 - 17913868 | 1 | 3 | Na/Ca exchanger |
| 25 | Sb09g030750 | 59382798 - 59385885 | 1 | 9 | CAX 1 |
| 26 | Sb04g011451 | 17193273 - 17194308 | 1 | 4 | Ca exchanger |
| 27 | Sb01g012910 | 11968709 - 11970895 | 1 | 1 | Ca exchanger |
| 28 | Sb04g008850 | 10445502 - 10462382 | 1 | 4 | Ca exchanger |
Note: “Co-ordinate” indicates the co-ordinates (5′-3′) of coding sequences in a chromosome.
Table 2.
Calcium transporters in rice
| Serial No. | Locus | Co-ordinate | No. of conserved domains | Chromosome No. | Function |
|---|---|---|---|---|---|
| 1 | Os01g48680 | 27919763 - 27906603 | 2 | 1 | Two Pore Ca Channel |
| 2 | Os01g71240 | 41219326 - 41225966 | 5 | 1 | Ca ATPase 11, IIB type |
| 3 | Os02g08018 | 4208367 - 4203438 | 2 | 2 | Ca ATPase 10, IIB type |
| 4 | Os03g17310 | 9640836 - 9635147 | 4 | 3 | Ca ATPase 2, IIA type |
| 5 | Os03g42020 | 23340016 - 23345128 | 4 | 3 | Ca ATPase 2, IIB type |
| 6 | Os03g52090 | 29903956 - 29882993 | 5 | 3 | Ca ATPase 3, IIA type |
| 7 | Os03g10640 | 5433293 - 5438896 | 5 | 3 | Ca ATPase 2, IIB type |
| 8 | Os04g51610 | 30399000 - 30387339 | 5 | 4 | Ca ATPase 9, IIB type |
| 9 | Os05g41580 | 24284626 - 24291437 | 6 | 5 | Ca ATPase 4, IIB type |
| 10 | Os05g02940 | 1083959 - 1085686 | 2 | 5 | Ca ATPase 2, IIA type |
| 11 | Os08g40530 | 25656887 - 25650557 | 2 | 8 | Ca ATPase 9, IIB type |
| 12 | Os10g28240 | 14613997 - 14610890 | 4 | 10 | Ca ATPase 13, IIB type |
| 13 | Os11g04460 | 1858363 - 1864021 | 4 | 11 | Ca ATPase 4, IIB type |
| 14 | Os12g04220 | 1782572 - 1788562 | 5 | 12 | Ca ATPase 4, IIB type |
| 15 | Os12g39660 | 24452031 - 24457112 | 5 | 12 | Ca ATPase 2, IIB type |
| 16 | Os01g11414 | 6133239 - 6137905 | 5 | 1 | Ca exchanger |
| 17 | Os01g37690 | 21075484 - 21071420 | 1 | 1 | vacuolar cation/proton exchanger 1a |
| 18 | Os02g04630 | 2070492 - 2075369 | 2 | 2 | Na/Ca exchanger 4 |
| 19 | Os02g21009 | 12432344 - 12448156 | 2 | 2 | vacuolar cation/proton exchanger 1c |
| 20 | Os02g43110 | 25952315 - 25951872 | 2 | 2 | Na/Ca exchanger 1 |
| 21 | Os03g01330 | 235750 - 234794 | 1 | 3 | Na/K/Ca exchanger 1 |
| 22 | Os03g08230 | 4195311 - 4193392 | 0 | 3 | Ca exchanger |
| 23 | Os03g27960 | 16060007 - 16063229 | 2 | 3 | vacuolar cation/proton exchanger 2 |
| 24 | Os03g45370 | 25612199 - 25613926 | 2 | 3 | Ca exchanger |
| 25 | Os04g55940 | 33130222 - 33133575 | 2 | 4 | vacuolar cation/proton exchanger 3 |
| 26 | Os05g51610 | 29530679 - 29526503 | 2 | 5 | vacuolar cation/proton exchanger 1b |
| 27 | Os10g30070 | 15542987 - 15544780 | 2 | 10 | Na/K/Ca exchanger 6 |
| 28 | Os11g01580 | 334289 - 333096 | 2 | 11 | Na/K/Ca exchanger 6 |
| 29 | Os11g05070 | 2216489 - 2213647 | 1 | 11 | Na/K/Ca exchanger 6 |
| 30 | Os11g43860 | 26019832 - 26017041 | 1 | 11 | Ca exchanger |
| 31 | Os12g42910 | 26635589 - 26637352 | 2 | 12 | Ca exchanger |
Multiple sequence alignment
To investigate the sequence features of Ca2+ transporter proteins in rice and sorghum, we performed multiple sequence alignment of amino acid sequences for the 59 identified Ca2+ transporter proteins (Figure S1). Sequence alignment of calcium channel, ATPase and exchanger by ClustalW indicated an overall homology amongst their own respective groups. High conservedness was observed in calcium channel between sorghum and rice. In all Ca2+ ATPases, alignment revealed one conserved motif, DKTGTLT (highlighted in Figure S1B). This conserved region locates on motif 2 in Ca2+ ATPase, which contains E1-E2 ATPase phosphorylation site as shown in Table 3. However, other motifs in Ca2+ transporters show degeneracy in various regions of protein sequences. In calcium exchangers, only a few conserved amino acids were observed between sorghum and rice.
Table 3.
Multilevel consensus sequences of the motifs defined by MEME software
| Motif | Width | E value | Multilevel consensus sequences |
|---|---|---|---|
| 1 | 50 | 9.3e-980 | MGIQGTEVAKESSDMIIMDDNFSTIVNVARWGRSVYNNIQKFIQFQLTVN |
| 2 | 50 | 4.5e-920 | PLAVTLCLAFAMKKMMNDKALVRHLSACETMGSATCICSDKTGTLTTNHM |
| 3 | 44 | 5.0e-753 | TAVQLLWVNMIMDTLGALALATEPPNDNMMKRPPVGRREPFITN |
| 4 | 50 | 4.1e-822 | YTCIGIVGIKDPCRPGVKDAVETCMSAGIKVRMVTGDNINTAKAICRECG |
| 5 | 50 | 7.5e-635 | YKQSLQFKHLDKEKKKIQVQVTRDGYRQKVSIYDLVPGDIVHLKIGDQVP |
| 6 | 28 | 8.5e-476 | RKMFGHVVAVTGDGTNDAPALHEADIGL |
| 7 | 36 | 4.9e-454 | GTKVQDGYCKMLVTAVGMRTEWGKLMATISEDGDDE |
| 8 | 39 | 1.4e-507 | CATVSLYFCIATEGWPKGWYDPPGIIGSILLPVMNTAPS |
| 9 | 50 | 2.4e-576 | VFNEFNSREMEKINVFRGIFKNWIFMGIIAITVVFQFIIIEFLGKFANTV |
| 10 | 39 | 6.7e-451 | AIEGPEFREKSPEEMRELIPKIQVMARSSPNDKHTLVKH |
Phylogenetic tree
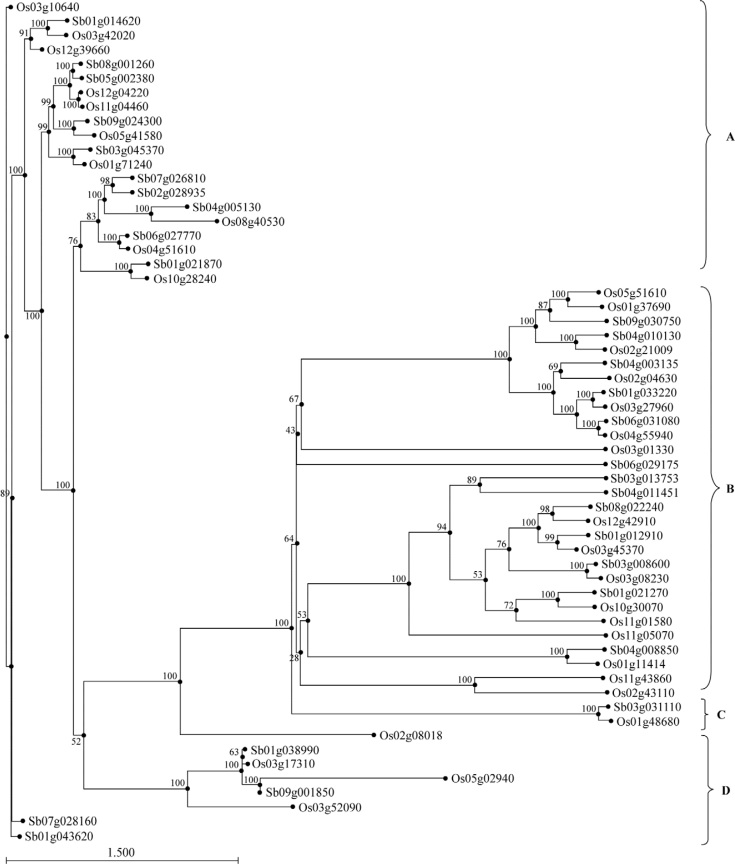
To examine the phylogenetic relationship among transporters in rice and sorghum, a rooted tree was constructed by aligning their amino acid sequences (Figure 1). As is attested by the bootstrap values quoted on the nodes, the phylogenetic analysis of transporters across both species clearly reveals four groups: (A) P-type IIB Ca2+ ATPases, (B) calcium exchangers, (C) calcium channels and (D) P-type IIA Ca2+ ATPases. The difference (sequence-wise and hence in domains) between Ca2+ exchangers and Ca2+ ATPases is evident from the segregation between their respective clusters. Ca2+ ATPases were further separated as P-type IIA and IIB on the basis of their amino acid composition. In spite of being very few in number, as compared to IIB Ca2+ ATPases (Cluster A) and calcium exchangers (Cluster B), IIA Ca2+ ATPases do form a separate cluster with significant bootstrap values (Cluster D). It is also noteworthy that the two calcium channels (Cluster C) are a subset of the calcium exchangers (Cluster B). This is expected since calcium channels and exchangers share much structural and functional similarity. In addition, most of the members belonging to the same cluster also share one or more conserved domains. Therefore, a majority of the Ca2+ transporters in rice are expected to be functional orthologs of the Ca2+ transporters in sorghum. Furthermore, all clusters contain members from the two different species, although rice is a C3 plant whereas sorghum has C4 metabolic pathway. The Ca2+ transporters of these two plants cluster together with respect to their orthology, which suggests that the structure and function of most of these genes might have remain conserved during evolutionary timescale across the plants employing C3 or C4 metabolic pathway.
Figure 1.
Phylogenetic tree of calcium transporter proteins from rice and sorghum. The phylogenetic tree was constructed by unweighted pair group method with arithmetic mean (UPGMA) using MEGA version 4.0.02. The calcium transporters were classified into four groups: A. IIB type calcium ATPases, B. calcium exchanger, C. calcium channel and D. IIA type calcium ATPases.
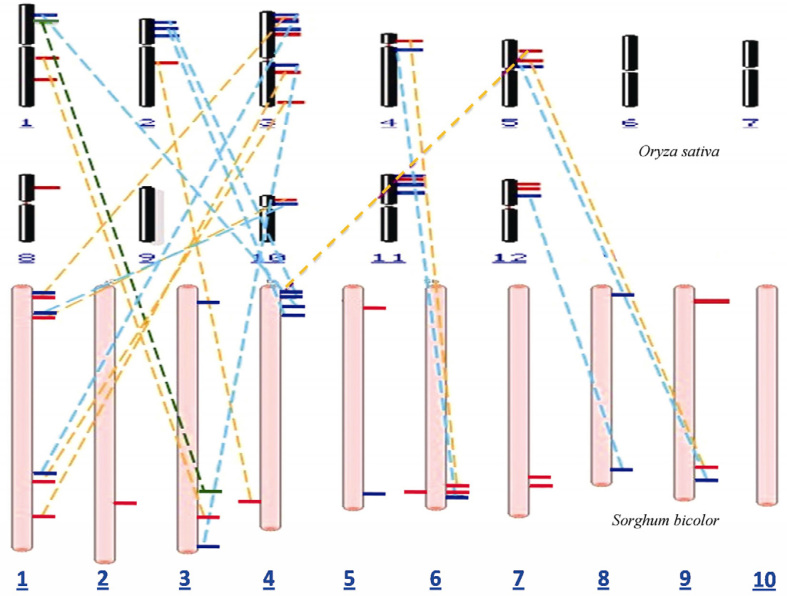
Chromosomal distribution of Ca2+ transporters and the synteny between rice and sorghum
At the whole-genome level, there is some colinearity between rice and sorghum. The orthologs from Ca2+ transporter gene families in both rice and sorghum were mapped and their corresponding chromosome locations are summarized in Table 1, Table 2 and Figure 2. The comparative synteny map also revealed the expansion and inversion of some chromosome regions in sorghum. The genome sizes of the grass family vary dramatically from 400 Mb in rice to 730 Mb in sorghum. In rice, the 31 identified Ca2+ transporter genes were found to be distributed on 9 out of 12 chromosomes, while in sorghum the 28 Ca2+ transporters were distributed on all the chromosomes except chromosome 10 (Chr 10). In the case of rice, maximum 8 (4 ATPases and 4 exchangers) transporters were found to be located on Chr 3, followed by 4 on Chr 1, 2 and 11, respectively, and 3 on each of Chr 5 and 12. Chr 10 contains 2 Ca2+ transporters (1 ATPase and 1 exchanger) while Chr 8 contains only 1 ATPase. No Ca2+ transporters were found on Chr 6, 7 and 9. In the case of sorghum, maximum 7 transporters (4 ATPases and 3 exchangers) were found to be located on Chr 1, followed by 4 on each of Chr 3 and 4, and 3 on each of Chr 6 and 9, while there is only 1 ATPase on Chr 2. Most of the Ca2+ ATPases and exchangers are distributed on Chr 1, 3 and 4 in sorghum.
Figure 2.
Genomic distribution and syntenic mapping of calcium transporter genes. Chromosomes from rice and sorghum were drawn in black and pink with length to approximate scale. The distribution of calcium channel (green), ATPase (red) and exchanger (blue) on different chromosomes in rice and sorghum was depicted. In addition, the synteny of calcium channel, ATPase and exchanger genes between rice and sorghum was indicated by the dashed lines in dark green, brown and cyan, respectively.
Alignment of the sorghum genome map with those of other cereals has revealed extensive macro colinearity, especially between sorghum and rice 23, 24, 25, 26. However, the distribution of the Ca2+ transporter genes on the 9 chromosomes in both sorghum and rice is not uniform, and some transporter genes are found in clusters in various regions on certain chromosomes. Most of the genes located on Chr 1 in sorghum show syntenic relationship with those located on Chr 3 in rice, while genes on Chr 3 in sorghum are syntenic with those on Chr 1 of rice. Similar trend was observed for Ca2+ transporter genes in this study. Calcium channel and ATPase genes located on Chr 3 in sorghum show synteny with those on Chr 1 in rice. Conversely, all the Ca2+ transporters on Chr 3 in rice show the syntenic relationship with those on Chr 1 in sorghum except one Ca2+ ATPase gene, which shows synteny with Ca2+ ATPase on Chr 3 in sorghum. In addition, syntenic relationship was also noticed between one calcium exchanger present on Chr 1 in rice and that on Chr 4 of sorghum, while one ATPase and one exchanger found on Chr 10 in rice show syntenic relationship with those on Chr 1 in sorghum. Furthermore, Ca2+ transporter genes on Chr 2 in rice show syntenic relationship with those on Chr 4 in sorghum, and one ATPase and one exchanger present on Chr 4 in rice show synteny with those on Chr 6 in sorghum, respectively. These Ca2+ transporters with colinearity also share some common motifs and much structural and functional similarity.
Therefore, the rice–sorghum syntenic map provides a reliable and well documented source of data that could be exploited in the future to locate the most conserved syntenic segments, which will also be well positioned for in-depth genome sequence analysis and comparative analysis of the role of Ca2+ transporters in other cereals.
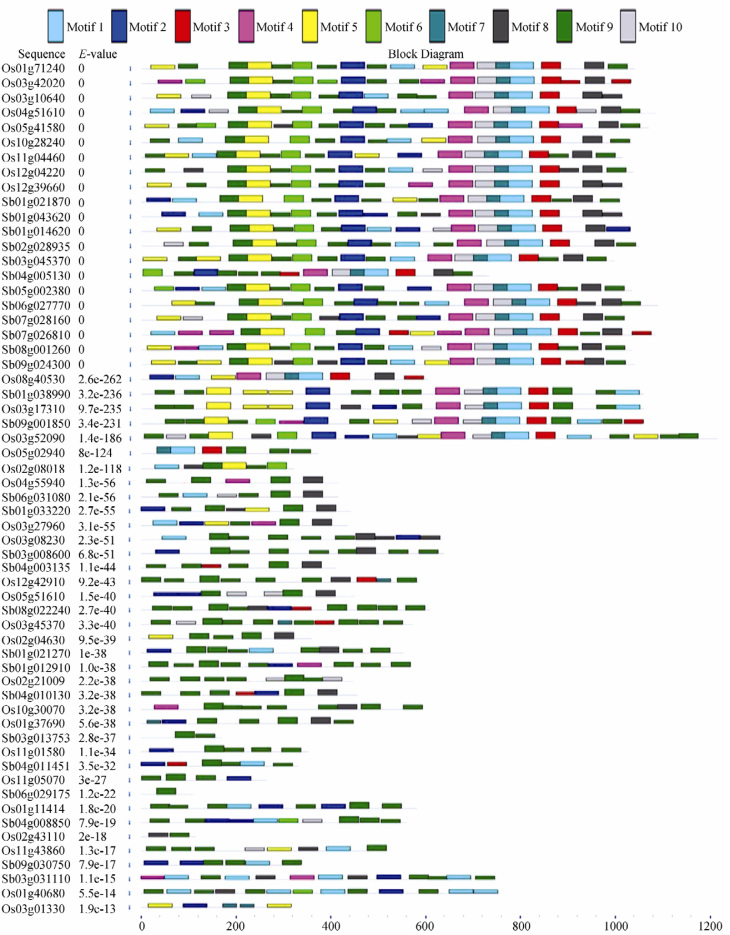
Analysis of conserved motifs
Every calcium ATPase contains all 10 motifs while calcium exchangers contain only a few motifs (Figure 3), out of which two exchangers (1 in rice and 1 in sorghum) contain only one single motif that appears twice. Motif 8 is found in all the calcium transporters, which is functionally related to the N-myristoylation site. Motifs 1 and 2 are also most commonly observed in all transporters, containing N-glycosylation site and E1-E2 ATPase phosphorylation site, respectively. Motif 3 contains cation transporting ATPase motif, which is present in only a few exchangers but completely absent in calcium channels. Motif 4 containing both N-myristoylation site and protein kinase C phosphorylation site is present in a few exchangers from both species and in channels from sorghum, but is absent in channels from rice. On the other hand, Motif 5 containing casein kinase II phosphorylation site is present in channels from rice but absent in those from sorghum. In addition, Motif 6 containing CHAP domain (cysteine, histidine-dependent amidohydrolase/peptidase) is missing in all channels and exchangers except two (Os03g45370 and Os03g01330) from rice. Furthermore, Motifs 7 and 10 are commonly observed in all transporters, both have casein kinase II phosphorylation site. Motif 9, which contains 2 functional sites, N-myristoylation site and C-terminus cation transporting ATPase, was observed in all calcium transporters. Multilevel consensus sequences for the motifs defined by MEME software is shown in Table 3.
Figure 3.
Block diagram of multilevel consensus sequences for the MEME defined motifs of transporter proteins. Ten motifs were obtained using MEME software. Different motifs are indicated by filled boxes with different colors and numbered 1 to 10 in order. Names of all different proteins and E values are shown to the right of the image. Scale bar below the image indicates the relative sizes of the motifs.
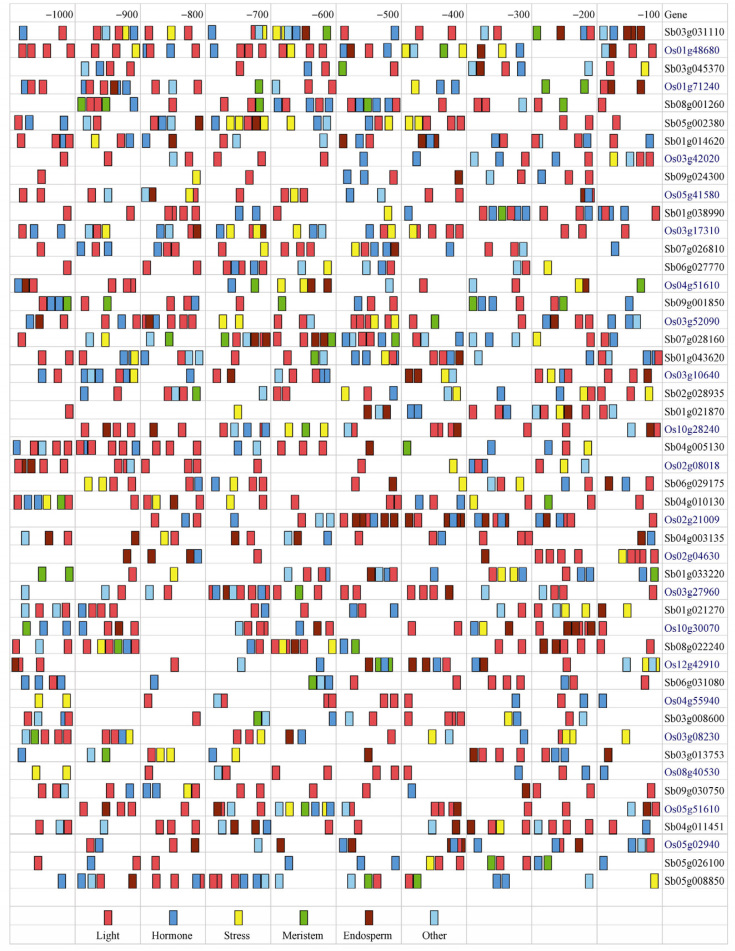
Cis-regulatory elements analysis
The cis-regulatory element analysis was carried out by retrieving nucleotide sequences 1,000 bp upstream of the initiation codon of 31 and 28 transporter genes from rice and sorghum, respectively. A large number of cis-regulatory elements were detected when all transporter genes were subjected to search against PlantCARE database (27). Distribution of the cis-regulatory elements among these transporter genes was shown in Figure 4. These elements were mainly associated with five important physiological processes, including light responsiveness, stress responsiveness, hormone responsiveness, meristem-specific expression and seed-specific gene expression. In addition, elements associated with other functions are placed in another category.
Figure 4.
Cis-regulatory elements in the upstream region of calcium transporter genes from sorghum and rice. Different elements were indicated using different colors. Elements responsive to light, hormone and stress were indicated in red, blue and yellow. Elements for meristem and seed specific expression were indicated in green and brown while other elements were indicated in light blue.
Many light-responsive elements were revealed in the promoter regions of all transporter genes from rice and sorghum, including LAMP, ACA motif, ATCT, AT1, TCCC, AAAC, GAG, GT1, TCT, GATA motif, Sp1, G Box, I Box and AE Box. These elements suggest that the calcium transporter proteins might be involved in regulation of photoperiodic control during flowering. The frequency of light-responsive elements in transporter gene promoter region ranges from 7 to 12 and the highest number was found in the promoter region of Os01g48680. In sorghum, calcium channel contains all four seed-specific elements, which were also detected in one ATPase and one exchanger (Sb07g028160 and Sb04g003135), respectively. In addition, 10 calcium ATPases and 8 calcium exchangers contain one or more seed-specific elements in their promoter region, while no seed-specific expression was detected in 5 ATPases (Sb08g001260, Sb01g038990, Sb06g027770, Sb09g001850 and Sb02g028935) and 3 exchangers (Sb06g031080, Sb03g008600 and Sb05g026100). Hormone-specific expression, i.e., TGA-element, GARE, ERE, TGACG, ABRE, IIb and CGTCA-motif, has also been observed in all calcium transporters. Meristem-specific regulation, i.e., presence of dOCT box, CCGTCC-box and CAT-box, were present only in some transporters while the presence of cis-regulatory elements like Skn-1 motif, GCN4 motif, O2-site and RY element confers endosperm-specific gene expression. Finally, the involvement of calcium transporter in regulation of abiotic stress has been identified according to the presence of different stress-responsive elements like HSE, MBS, although transporters Sb08g001260, Sb09g001850 and Sb06g031080 do not contain any stress-related elements.
Conclusion
In this study, a complete analysis of the calcium transporter gene family in rice and sorghum is presented, including multiple sequence alignment, domain analysis of Ca2+ transporters, phylogeny, chromosomal locations and analysis of their cis-regulatory elements. In silico analysis has revealed the existence of 28 and 31 members of transporter genes encoding channel, ATPase and exchanger in genomes of sorghum and rice, respectively. Multiple sequence alignment of these transporter proteins of sorghum and rice showed certain conservedness. Phylogenetic analysis further segregated these proteins into four clusters each representing calcium channel, IIA & IIB type calcium ATPases and calcium exchanger. Motif analysis revealed some conserved motifs between channel, ATPase and exchanger in rice and sorghum. The analysis of cis-regulatory elements for the predicted calcium transporters revealed that the major putative functions of these genes are associated with gene regulation of seed storage proteins, abiotic and biotic stress, photoperiod, growth hormone and meristem. Although the function of calcium transporters for calcium accumulation in seed has not been reported so far, the presence of seed-specific motif in upstream region suggests that these proteins might be involved in calcium accumulation.
Characterization of these proteins at physiological, molecular and structural level might shed some light on their functionality. The cause of gene movement and the erosion of gene colinearity between rice and sorghum chromosomes has been an unsolved task. However, advent of comparative genomics will provide insights for such changes in chromosomal organization. The identification of syntenic relationship between rice and sorghum in the present study showed that calcium transporters are not uniformly distributed but rather clustered on certain chromosomes. The present in silico analysis of the predicted transporter gene family has provided significant clues for exploring the expression and function of these calcium transporters under different environmental conditions. These studies would further help in understanding the molecular basis of many agriculturally important traits such as calcium accumulation in developing grains and their roles in development and defense against biotic and abiotic stresses. Furthermore, deciphering the diversity, organization and phylogeny of calcium transporter gene family in sorghum would also facilitate future annotation of transporter genes within genomes of other cereals.
Materials and Methods
Search of databases for the identification of calcium transporter family members
In order to perform comparative analysis, the sequences of Ca2+ transporters from rice (Oryza sativa) and sorghum (Sorghum bicolor) were downloaded from two different sources, TIGR (The Institute for Genomic Research; http://www.tigr.org/) pseudomolecule database (version 4) for rice and MIPS (Munich information center for protein sequence; http://mips.helholtz-muenchen.de/plant/sorghum/) for sorghum, respectively. For the identification of Ca2+ transporters in rice and sorghum, the homology search of the Ca2+ transporter proteins was performed by BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) using blastp and tblastn algorithm. Detailed analysis of Ca2+ transporters in whole genomes of rice and sorghum, including their identification, classification, sequence analysis, domain analysis, chromosomal locations and phylogenetic relationships were carried out as described below.
Phylogenetic analysis
Amino acid sequences of all the identified Ca2+ transporters from sorghum and rice were aligned separately using ClustalW (28) and the phylogenetic tree was constructed using UPGMA method of MEGA version 4.0.02 (29). Each node was tested using the bootstrap approach by taking 100 replications to ascertain the reliability of nodes. The number indicated percentages against each node.
Chromosomal distribution of calcium transporters and synteny between rice and sorghum
The sorghum genome is approximately two-fold larger in size but has two fewer chromosomes relative to rice. The difference in chromosome number between sorghum and rice is due to two chromosome fusion events that occurred prior to the divergence of sorghum from Pennisetum (30). The chromosomal position of each calcium transporter gene in the rice and sorghum was determined by blastn search with NCBI genome (chromosome) database against genomic sequence of each chromosome for the rice (Accession No. NC008394-NC008405) and sorghum (Accession No. CM000760-CM000769) and then manually marked. The chromosomal distribution was visualized by NCBI Map Viewer (http://www.ncbi.nlm.nih.gov/mapview/).
Analysis of conserved motifs
To identify the conserved motifs within the protein sequences of calcium transporter family, the deduced protein sequences of all the calcium transporters in rice and sorghum were analyzed using online MEME (Multiple Expectation Maximization for Motif Elicitation) tool version 3.5.7 (31). For motif analysis, maximum number of motifs was set to 10 and optimal motif width was set as 20 to 50 amino acids while other factors were set as default.
Domain analysis of calcium transporters
The putative protein sequences of calcium transporters from rice and sorghum were subjected to protein functional analysis using Pfam version 23.0 (32) and SMART (Simple Modular Architecture Research Tool) version 5.1 (33).
Analysis of cis-regulatory elements
For promoter analysis, sequences 1,000 bp upstream of the initiation codon of the putative calcium transporter genes were retrieved and subjected to search using CARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) of Plant CARE database to identify cis-regulatory elements.
Authors’ contributions
AK conceived the idea, designed the project and interpreted the results. AG performed most of the data analysis and drafted the manuscript. GT, DP and SG provided advice for upstream promoter analysis and revised the manuscript. All the authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Acknowledgements
This work was financially supported by Department of Biotechnology, Govt. of India as Programme Support for research and development in Agricultural Biotechnology at G.B. Pant University of Agriculture and Technology, Pantnagar (Grant No. BT/PR7849/AGR/02/2006). The facilities provided by DBT-funded SUB-DIC Bioinformatics Centre, Pantnagar for carrying out this in silico investigation is also acknowledged.
Supplementary Material
Figure S1
References
- 1.Blaustein M.P., Lederer W.J. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 2.Carafoli E. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth J.T. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim. Biophys. Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M.J. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 5.Santella L. The cell cycle: a new entry in the field of Ca2+ signaling. Cell. Mol. Life Sci. 2005;62:2405–2413. doi: 10.1007/s00018-005-5083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipskaia L., Lompre A.M. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol. Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Strehler E.E., Zacharias D.A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Monteith G.R., Roufogalis B.D. The plasma membrane calcium pump—a physiological perspective on its regulation. Cell Calcium. 1995;18:459–470. doi: 10.1016/0143-4160(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 9.Sze H. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- 10.Monteith G.R. Calcium and cancer: targeting Ca2+ transport. Nature Rev. Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 11.Nagata T. Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Mol. Biol. Evol. 2004;21:1855–1870. doi: 10.1093/molbev/msh197. [DOI] [PubMed] [Google Scholar]
- 12.Palmgren M.G., Harper J.F. Pumping with plant P-type ATPases. J. Exp. Bot. 1999;50:883–893. [Google Scholar]
- 13.Axelsen K.B., Palmgren M.G. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 2001;126:696–706. doi: 10.1104/pp.126.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuteja N., Mahajan S. Calcium signaling network in plants: an overview. Plant Signal Behav. 2007;2:79–85. doi: 10.4161/psb.2.2.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White P.J. Calcium channels in higher plants. Biochim. Biophys. Acta. 2000;1465:171–189. doi: 10.1016/s0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 16.White P.J. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim. Biophys. Acta. 2002;1564:299–309. doi: 10.1016/s0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 17.Schantz H.L. The place of grasslands in the earth’s cover of vegetation. Ecology. 1954;35:143–145. [Google Scholar]
- 18.Evans L.T. Cambridge University Press; Cambridge, UK: 1998. Feeding the Ten Billion: Plants and Population Growth. [Google Scholar]
- 19.Paterson A.H. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 20.Bowers J.E. A high-density genetic recombination map of sequence-tagged sites for sorghum, as a framework for comparative structural and evolutionary genomics of tropical grains and grasses. Genetics. 2003;165:367–386. doi: 10.1093/genetics/165.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altschul S.F. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 22.Altschul S.F. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Y. Comparative genome mapping of Sorghum bicolor (L.) Moench using an RFLP map constructed in a population of recombinant inbred lines. Plant Breed. 1999;118:225–235. [Google Scholar]
- 24.Klein P.E. A high-throughput AFLP-based method for constructing integrated genetic and physical maps: progress toward a sorghum genome map. Genome Res. 2000;10:789–807. doi: 10.1101/gr.10.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paterson A.H. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc. Natl. Acad. Sci. USA. 2004;101:9903–9908. doi: 10.1073/pnas.0307901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devos K.M. Updating the “Crop circle.”. Curr. Opin. Plant Biol. 2005;8:155–162. doi: 10.1016/j.pbi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Lescot M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson J.D. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 30.Kellogg E.A. Evolutionary history of the grasses. Plant Physiol. 2001;125:1198–1205. doi: 10.1104/pp.125.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey T.L. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006;34:W369–W373. doi: 10.1093/nar/gkl198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn R.D. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:247–251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz J. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1