Abstract
Tissue injury, whether by trauma, surgical intervention, metabolic dysfunction, ischemia, or infection, evokes a complex cellular response (inflammation) that is associated with painful hyperalgesic states. Although in the acute stages it is necessary for protective reflexes and wound healing, inflammation may persist well beyond the need for tissue repair or survival. Prolonged inflammation may well represent the greatest challenge mammalian organisms face, as it can lead to chronic painful conditions, organ dysfunction, morbidity, and death. The complexity of the inflammatory response reflects not only the inciting event (infection, trauma, surgery, cancer, or autoimmune) but also the involvement of heterogeneous cell types including neuronal (primary afferents, sensory ganglion, and spinal cord), non-neuronal (endothelial, keratinocytes, epithelial, and fibroblasts), and immune cells. In this commentary, we will examine 1.) the expression and regulation of two members of the transient receptor potential family in primary afferent nociceptors and their activation/regulation by products of inflammation, 2.) the role of innate immune pathways that drive inflammation, and 3.) the central nervous system’s response to injury with a focus on the activation of spinal microglia driving painful hyperalgesic states.
Keywords: inflammation, pain, innate immunity, microglia, nociceptors
Primary afferent nociceptors and inflammatory pain
Specialized primary afferent neurons that function to detect noxious chemical, thermal, and mechanical stimuli are referred to as nociceptors 1. Their cell bodies, found primarily in the trigeminal and dorsal root ganglion (DRG), provide sensory innervation to virtually all tissues – except the brain parenchyma. Specialized receptors, channels, and synthetic pathways help define the specificity of particular nociceptor subtypes, allowing the detection and signaling of both acute and persistent (chronic) noxious stimuli. We will focus on two principle receptors/channels that have been identified and characterized on nociceptors that detect noxious inflammatory stimuli. The first, transient receptor potential cation channel subfamily V member 1 (TRPV1 – previously known as vanilloid receptor 1 [VR1]), was initially reported to function as an integrator of multiple noxious stimuli through the demonstration that diverse products of inflammation, such as protons, anandamide, bradykinin, and nerve growth factor (NGF), functioned as positive modulators or full agonists at TRPV1 2, 3. Products of the lipoxygenase pathway of arachidonic acid, 12-(S)-hydroperoxyeicosatetraenoic acid and leukotriene B4, have also been found to activate TRPV1 in vitro, and activated protein kinase C can directly activate or lower the activation threshold of TRPV1 to thermal stimuli 2, 4– 8. Two derivatives of dopamine (N-arachidonoyl dopamine and N-oleoyl dopamine) have also been found to activate TRPV1 and are associated with experimental hyperalgesia 9, 10 (for review, see Figure one and also 11, 12).
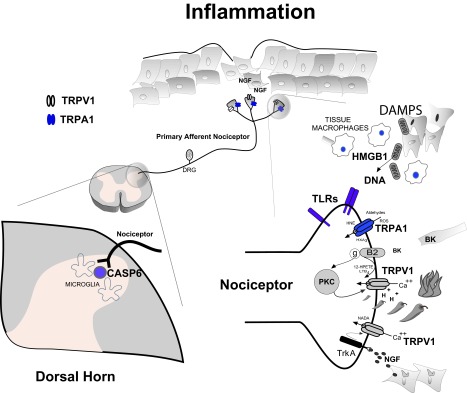
Figure 1. Inflammatory Pain.
Tissue injury evokes a complex series of cellular responses that together is proposed to drive painful hyperalgesic states. Specialized primary afferent nociceptors (top center) innervate tissues and signal potential or actual cellular injury through detection of noxious chemical, thermal and mechanical stimuli. Electrochemical transduction of noxious stimuli at nociceptor terminals include activation of transient receptor potential (TRP) ion channel family members. As a result of the synthesis and/or release of injury – induced inflammatory products, nociceptor transducing elements may be positively modulated or directly activated driving painful and hyperalgesic states. A number of these products (eg: peptides [BK], activation of PKC, TrkA activation by NGF, acid [H +], lipoxygenase products - 12-HPETE, LTB 4, NADA, as well as reactive oxygen species [ROS], aldehydes, HNE and HXA 3) have been shown to either modulate or activate TRPV1 and TRPA1 respectively (bottom right). Certain products of inflammation (eg: nerve growth factor [NGF], ROS, aldehydes) modulate multiple pain transducing receptors/elements. Depending on the mechanism and severity of tissue injury, innate immune cell responses will be recruited. Damage-associated molecular patterns (DAMPs) such as HMGB1 and mitochondrial derived DNA bind and activate toll-like receptors (TLRs) expressed on nociceptor terminals further driving hyperalgesia. Monocyte derived macrophages invade injured tissue and release a complex array of cytokines, chemokines and growth factors such as NGF. Together, they conspire to transform nociceptor phenotype to pathophysiologic states of persistent nociceptor activation, lowered firing thresholds and/or exaggerated response properties. Tissue inflammation also influences the central processing of nociceptive input in the dorsal horn of the spinal cord (bottom left). As a result, central nociceptor terminals upregulate and release signaling molecules such as CASP6 that activates microglia – dependent inflammatory hyperalgesia.
Taken together, it is proposed that the development of thermal hyperalgesic states, and in part spontaneous inflammatory pain, arises from the activation of TRPV1 expressed on C-type nociceptors. Moreover, the trophic factor NGF, derived from inflamed non-neuronal cells, has been found to drive both early and long-term pain behaviors 13– 17. In fact, long-term (days to weeks) development of thermal hyperalgesia appears to be dependent on increased expression of TRPV1 in nociceptors 18– 22. More recently, overexpression of TRPV1 has also been implicated in the persistent NGF-dependent inflammatory pain of oral cancer 23. Interestingly, links between TRPV1 and mechanical hypersensitivity pain have continued to emerge in the context of inflammation arising from pathophysiologic models of visceral/colorectal distension 24– 26, bone cancer pain 27– 29, sickle cell disease 30, and UVB-induced skin inflammation 31. Taken together, these findings also illustrate the limitations of certain models of inflammation. Notably, the experimental use of complete Freund’s adjuvant (CFA) or other agents may not necessarily induce inflammatory conditions observed in human disease.
A second transient receptor potential-related channel expressed on nociceptors, transient receptor potential cation channel subfamily A member 1 (TRPA1), was subsequently identified and has been considered by some investigators as a “gatekeeper for inflammation” 32. TRPA1 is now considered to play an important and possibly complementary role to TRPV1 in the development and maintenance of inflammatory pain states. This is supported by reports that TRPA1 is activated by both exogenous (allyl isothiocyanate [mustard oil], acrolein, and aldehydes) and endogenous (methylglyoxal, 4-hydroxynonenal, 12-lipoxygenase-derived hepoxilin A3, 5,6-epoxyeicosatrienoic acid, and reactive oxygen species [ROS]) inflammatory mediators 33. Increasingly, TRPA1 has been linked to persistent models of inflammatory pain, mechanical and cold hypersensitivity 34, inflammatory muscle pain 35, and pancreatitis pain driven by multiple inflammatory pathways 36– 39.
Given TRPV1 and TRPA1’s seminal roles in the signaling of inflammatory pain, there has been considerable interest in the development of high-affinity antagonists against them 40, 41. Indeed, there are endogenous inhibitors of TRPV1 and TRPA1, including resolvins and maresins, which are among the group of lipid mediators that are involved in resolving inflammation 42– 44. Preliminary reports suggest that resolvins may help to prevent or reduce inflammatory pain via transient receptor potential channels 42, 43, 45, 46. Although many of these compounds have been shown in preclinical studies to reduce inflammatory pain, there is concern that, owing to a broader pattern of expression of TRPV1 and TRPA1 in neuronal and non-neuronal cell types 47, complete inhibition of one or both channels may result in unwanted side effects such as hypothermia or inhibition of acute protective heat pain 41. These concerns may be heightened given reports that TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome 48, 49. Paradoxically, TRPV1 activation may be protective and anti-inflammatory in certain conditions, despite its peripheral activation producing neuropeptide release and neuroinflammation. Research is ongoing to devise transient receptor potential agonist/antagonist strategies that selectively block inflammatory pain without disrupting its homeostatic or acute pain protective roles. Given these challenges, perhaps a better understanding of our innate immune system’s response to injury and its subsequent role in driving inflammatory pain may provide complementary therapeutic approaches to our understanding of spontaneous and mechanical pain mediated by TRPV1 and TRPA1 35, 50.
Role of innate immune pathways
The innate immune system initiates and directs the acute inflammatory response to microbial infections and to sterile tissue injury in a multitude of disorders including sepsis, trauma, hemorrhage, cardiac arrest, vascular occlusion, organ transplantation, and injurious chemicals. Innate immune responses are triggered through the engagement of pattern recognition receptors (PRRs) by components of microorganisms known as pathogen-associated molecular patterns (PAMPs) and/or by factors released by stressed or injured host cells that are collectively known as damage-associated molecular patterns (DAMPs) 51– 53. The binding of PAMPs or DAMPs to their cognate PRR triggers early inflammatory responses via complex intracellular pathways involving multiple adapter proteins, interleukin-1 receptor-associated kinases (IRAKs), mitogen-activated protein kinases (MAPKs), and NFκB, which ultimately lead to the expression and/or activation of numerous inflammatory mediators, including cytokines (e.g. TNFα, IL-1β, IL-6, and IL-10), chemokines (e.g. IL-8), ROS, and adhesion molecules, and to leukocyte trafficking and activation within organs and other tissues. These responses help to acutely contain and eliminate the infection or endogenous threat, promote the development of adaptive specific immunity, and initiate the repair of injured tissues. However, in contrast to these benefits, dysregulated inflammatory responses can lead to deleterious outcomes via excessive pro-inflammatory products, the failure to resolve inflammation and restore immune homeostasis, and/or the development of immunosuppression.
PRRs have been most extensively studied in leukocytes, but they are expressed by multiple non-leukocyte cell populations including endothelial cells, cardiomyocytes, epithelial cells, and neurons 54– 60. Notably, PRRs expressed in cells of the nervous system, including glial cells and neurons, are postulated to contribute to a number of acute and chronic neurologic processes including, but not limited to, ischemic brain damage, Alzheimer’s disease, neuropathic pain, and other pain syndromes such as sickle cell disease 51, 61– 73. A number of DAMPs induce acute inflammation via PRRs and have been implicated in chronic neuropathic pain. Analogous to PRRs’ dualistic roles in systemic inflammatory conditions such as sepsis, their activation in cells of the nervous system can have beneficial effects, such as promoting neuronal repair, but, conversely, dysregulated inflammation can also have pathologic effects on the nervous system that lead to the development chronic pain.
Members of the Toll-like receptor (TLR) family and the receptor for advanced glycation end products (RAGE) are emerging as significant contributors to the pathogenesis of neuropathic pain 72, 74– 79. By far the most extensively studied PRRs are the TLRs, mammalian homologs of Drosophila Toll which participate in dorsoventral development and in antimicrobial defences 80– 82. TLRs are transmembrane proteins that are expressed at the cell surface and in endosomes and endolysosomes 53, 81, 82. Common microbial TLR agonists include LPS, bacterial lipoproteins, lipoteichoic acid, peptidoglycan, flagellin, and nucleic acids 81, 83– 90. Endogenous agonists of the TLRs include HMGB1 (TLR2, TLR4, and TLR9), heparan sulfate (TLR4), heat shock proteins (TLR2 and TLR4), hyaluronan (TLR2 and TLR4), versican (TLR2), RNA (TLR3), mitochondrial DNA (TLR9), and β-amyloid (TLR2 and TLR4) 61, 91– 101. TLRs and downstream signaling intermediaries, such as the adapter proteins MyD88 and TRIF, have also been reported to contribute to neuropathic pain syndromes 74– 76, 102, 103. RAGE is a multi-ligand member of the immunoglobulin superfamily that is expressed at the cell surface and in a secreted form 104. There are numerous endogenous RAGE agonists, including, but not limited to, β-amyloid, HMGB1, and S100 proteins, and there is accumulating evidence that RAGE is important in neuropathic pain 99, 101, 104– 109. Notably, HMGB1 has been reported by a number of groups to be released by stressed and injured tissues and to facilitate the development of neuropathic pain 63, 77, 78, 110– 112. In addition to the TLRs and RAGE, other PRRs may also contribute to inflammatory pain. For example, the NLRP3 inflammasome, a multiprotein cytosolic complex responsible for the production of active IL-1β and IL-18, has been implicated in chronic pain and has been reported to contribute to opioid-induced hyperalgesia in animal models 113– 116. Multiple factors stimulate the NLRP3 inflammasome, including microbial components such as LPS, nigericin, zymosan, and malarial hemozoin, and several endogenous factors, including β-amyloid, uric acid, ATP, and calcium pyrophosphate dehydrate 52, 117– 121.
Over the last decade and a half, strong links have been identified between the nervous system and the immune system. Multiple cell lineages in the central and peripheral nervous system express PRRs, including neurons, microglia, astrocytes, Schwann cells, and oligodendrocytes 72, 73, 122– 125. The links between the immune system and nervous system are bidirectional – the immune system is able to modulate neuronal function and vice versa. There is strong evidence that a neuroimmune response that is mediated through the vagus nerve, spleen, and cholinergic receptors modulates host responses to endotoxemia and infection 126, 127. Furthermore, several studies suggest that TRPV1 modulates the outcomes of bacterial sepsis 128– 131. There is also accumulating evidence that the activation of innate immune pathways, particularly TLR- and RAGE-dependent pathways, contributes to the development of chronic pain following nerve injury 62– 64, 67, 69, 79, 109, 132. From a mechanistic standpoint, leukocyte-derived factors released in response to DAMP-mediated activation of PRRs expressed by microglia and peripheral monocytes are believed to induce pain through their actions on sensory neurons.
Intriguingly, the direct activation of neuronally expressed PRRs may also be involved in the development of acute and chronic pain. TLR agonists have been reported to directly activate DRG neurons and to increase levels of TRPV1 expression in DRG neurons 73. Furthermore, TRPV1-expressing nociceptive neurons have also been reported to express TLR4 125. While the focus of this discussion has been on innate immune pathways in the pathogenesis of pain, recent reports also point to a role for the adaptive immune system in chronic pain 102, 133– 137. For example, modulating T lymphocyte cell responses pharmacologically has been reported to reduce chronic neuropathic allodynia and chronic constriction injury-induced neuropathic pain in rats 133, 134. Similarly, the downregulation of IL-12p70 (a proinflammatory cytokine that promotes the proliferation of T lymphocytes and natural killer cells), the deletion of the adapter protein MyD88, or the downregulation or neutralization of IL-17A (which links innate and adaptive immunity) have all been reported to attenuate chronic neuropathic pain in rodents 102, 134, 137, 138. The fact that diverse conditions, including chronic pain, sepsis, trauma, and ischemia reperfusion injury, have shared pathways raises the intriguing but complex possibility of developing therapeutics that can reverse inflammatory pain without compromising immune function.
The central nervous system’s response to injury
The spinal cord microglia, the tissue-resident immune-like macrophages of the central nervous system 139, can respond to peripheral injuries that are distant from the spinal cord to produce neuroinflammation in the central nervous system 140. Indeed, traumatic injuries to the peripheral nerves activate microglia, both in the dorsal horn where sensory nerve endings from the DRG terminate and in the ventral horn where activated microglia wrap around the injured motoneurons 141. In fact, neuroinflammation in the spinal cord, presented as microglia activation, is well known to contribute to the development of neuropathic pain after nerve injury 140– 143.
One of the first clues that microglia might contribute to inflammatory pain came from the report that spinal cord microglia are activated in the formalin inflammatory pain model 144. In this widely used inflammatory pain model, 5% formalin is injected subcutaneously into the hind paw of a rat or mouse. Fu et al. observed spinal cord microglia activation, defined as enhanced immunoreactive signaling of microglia markers, after formalin injection in male rats, starting on day 1 and peaking on day 7 post injection 143. Interestingly, pre-treatment of local anesthetic bupivacaine does not block formalin-induced spinal cord microglia activation, even though it successfully blocks formalin-evoked pain behaviors 145, indicating that the nociceptive input from the acute inflammatory response of formalin is not required for spinal cord microglia activation.
Subsequently, it was reported that p38 MAPK is activated in the spinal cord microglia after formalin injection in male rats 146, and this activation of p38 MAPK occurs in 2 phases 147. The first phase of microglial p38 activation starts quickly, just a few minutes after formalin injection, and lasts for 1 hour, the time course that correlates with early acute spontaneous nociceptive behavior 146, 147. Indeed, intrathecal inhibition of microglia with minocycline greatly attenuates formalin-evoked acute flinching behavior 148. The second phase of microglial p38 activation starts 1 day after formalin injection and lasts for 7 days, the time course that correlates with persistent mechanical hypersensitivity induced by formalin injection 147. Inhibition of p38 kinase attenuates both acute nociceptive behavior and persistent mechanical hypersensitivity induced by formalin injection 146, 147. In fact, there are two p38 isoforms in the spinal cord, with p38α expressed in neurons and p38β expressed in microglia 149. Downregulation of microglial p38β, rather than neuronal p38α, attenuates formalin injection-induced acute nociceptive behavior 149. In addition to p38 MAPK, Src family kinase (SFK) is also activated in spinal cord microglia, starting 1 day after formalin injection and lasting for 7 days 150. Unlike p38 MAPK, SFK is necessary for persistent mechanical hypersensitivity after formalin injection, although it is not required for formalin-induced acute spontaneous nociceptive behavior 150.
Recent evidence further supports the idea that formalin injection produces early microglial activation 151. Berta et al. demonstrated that within 30 minutes of formalin injection, caspase-6 (CASP6) is upregulated in the central terminals of primary afferents and is released in the spinal cord 151. The resultant CASP6-mediated cascade activates spinal cord microglia and stimulates microglial TNF-α synthesis and release through p38 and ERK kinases. In fact, formalin-induced second-phase inflammatory pain is CASP6 dependent, and intrathecal injection of CASP6 or CASP6-treated microglia produces pain behavior mediated in part through stimulation of spinal cord lamina II neurons. Moreover, CASP6 is also required for capsaicin-elicited secondary mechanical hypersensitivity as well as bradykinin, carrageenan, and CFA-induced inflammatory pain. As TRPA1 is one of the receptors targeted by formalin 152, it is likely that in the formalin inflammatory pain model, formalin activates DRG neurons through TRPA1 to induce CASP6 and subsequently activates spinal cord microglia shortly after formalin injection.
Although spinal cord microglia are clearly activated shortly after the formalin injection in the hind paw, whether the long-term microglia activation days after formalin injection is caused by tissue inflammation itself is controversial. Importantly, in addition to tissue inflammation, hind paw formalin injection also produces damage to peripheral nerve endings, as transcription factor ATF3, a marker for peripheral nerve injury 153, is induced in DRG neurons after formalin hind paw injection 154. Given that peripheral nerve injury is a well-known factor that activates spinal cord microglia to produce pain behaviors 140– 143, it is likely that peripheral nerve injury and tissue inflammation, together, are responsible for the spinal cord microglia activation after formalin hind paw injection.
Summary
Inflammatory pain constitutes an ongoing enigma for the development of novel analgesic agents. Despite the robust characterization of peripheral nociceptive channels (e.g. TRPV1 and TRPA1) capable of detecting a wide range of inflammatory stimuli, clinically relevant antagonists may surreptitiously disrupt essential homeostatic and protective functions such as TRPV1-dependent core temperature regulation or the detection of warmth. Time will tell if antagonists to TRPA1 will encounter similar sensory physiologic limitations surrounding their role in cold detection, mechanosensation, or cellular signaling. If systemic administration of transient receptor potential antagonists continues to be problematic, perhaps restricting these agents to peripheral and/or spinal targets could still provide the desired effect. Detailed examination of innate immune response elements holds additional promise for novel analgesic development in the treatment of inflammatory pain. For example, the role of the endogenous TLR4 and RAGE agonist HMGB1, a molecule previously associated with sepsis, now has emerged as an important participant in mediating inflammatory and neuroinflammatory pain states. Developing strategies around the blockade of HMGB1 and/or dampening overexpression of TLR4 or RAGE are plausible directions. Central spinal processing of nociceptive signaling can be modulated by microglia, the immune-like macrophage of the central nervous system, and recent evidence suggests that activated microglia also contribute to the pain produced by tissue inflammation. Further studies on the blockade of spinal CASP6 under painful pathophysiologic conditions such as bone cancer pain, sickle cell disease, or inflammatory bowel disease may represent another important therapeutic opportunity in analgesic development.
Abbreviations
CASP6, caspase 6; CFA, complete Freund’s adjuvant; DAMP, damage-associated molecular pattern; DRG, dorsal root ganglion; IRAK, interleukin-1 receptor-associated kinase, MAPK, mitogen-activated protein kinase; NGF, nerve growth factor; PAMP, pathogen-associated molecular patterns; PRR, pattern recognition receptor; RAGE, receptor for advanced glycation endproducts; ROS, reactive oxygen species; SFK, Src family kinase; TLR, Toll-like receptor; TRPA1, transient receptor potential cation channel subfamily A member 1; TRPV1, transient receptor potential cation channel subfamily V member 1.
Acknowledgements
The authors would like to thank Morgen Ahearn for her expert editorial assistance.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Cheryl Stucky, Department of Cell Biology, Neurobiology and Anatomy, Medical College of Wisconsin, Milwaukee, WI, USA
Thiago Cunha, Department of Pharmacology, University of São Paulo, São Paulo, Brazil
Ru-Rong Ji, Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Levine JD, Fields HL, Basbaum AI: Peptides and the primary afferent nociceptor. J Neurosci. 1993;13(6):2273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tominaga M, Caterina MJ, Malmberg AB, et al. : The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43. 10.1016/S0896-6273(00)80564-4 [DOI] [PubMed] [Google Scholar]
- 3. Schumacher MA: Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 2010;10(3):185–200. 10.1111/j.1533-2500.2010.00358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caterina MJ, Leffler A, Malmberg AB, et al. : Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- 5. Zygmunt PM, Petersson J, Andersson DA, et al. : Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–7. 10.1038/22761 [DOI] [PubMed] [Google Scholar]
- 6. Chuang HH, Prescott ED, Kong H, et al. : Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P 2-mediated inhibition. Nature. 2001;411(6840):957–62. 10.1038/35082088 [DOI] [PubMed] [Google Scholar]
- 7. Cho H, Shin J, Shin CY, et al. : Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci. 2002;22(4):1238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Premkumar LS, Ahern GP: Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408(6815):985–90. 10.1038/35050121 [DOI] [PubMed] [Google Scholar]
- 9. Huang SM, Bisogno T, Trevisani M, et al. : An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99(12):8400–5. 10.1073/pnas.122196999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Petrocellis L, Chu CJ, Moriello AS, et al. : Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143(2):251–6. 10.1038/sj.bjp.0705924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caterina MJ, Julius D: The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. 10.1146/annurev.neuro.24.1.487 [DOI] [PubMed] [Google Scholar]
- 12. Clapham DE: TRP channels as cellular sensors. Nature. 2003;426(6966):517–24. 10.1038/nature02196 [DOI] [PubMed] [Google Scholar]
- 13. Winter J, Forbes CA, Sternberg J, et al. : Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1(10):973–81. 10.1016/0896-6273(88)90154-7 [DOI] [PubMed] [Google Scholar]
- 14. Woolf CJ, Safieh-Garabedian B, Ma QP, et al. : Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62(2):327–31. 10.1016/0306-4522(94)90366-2 [DOI] [PubMed] [Google Scholar]
- 15. McMahon SB, Bennett DL, Priestley JV, et al. : The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1(8):774–80. 10.1038/nm0895-774 [DOI] [PubMed] [Google Scholar]
- 16. Nicholas RS, Winter J, Wren P, et al. : Peripheral inflammation increases the capsaicin sensitivity of dorsal root ganglion neurons in a nerve growth factor-dependent manner. Neuroscience. 1999;91(4):1425–33. 10.1016/S0306-4522(98)00706-4 [DOI] [PubMed] [Google Scholar]
- 17. Shu XQ, Mendell LM: Neurotrophins and hyperalgesia. Proc Natl Acad Sci U S A. 1999;96(14):7693–6. 10.1073/pnas.96.14.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hudson LJ, Bevan S, Wotherspoon G, et al. : VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13(11):2105–14. 10.1046/j.0953-816x.2001.01591.x [DOI] [PubMed] [Google Scholar]
- 19. Fukuoka T, Tokunaga A, Tachibana T, et al. : VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99(1–2):111–20. 10.1016/S0304-3959(02)00067-2 [DOI] [PubMed] [Google Scholar]
- 20. Amaya F, Oh-hashi K, Naruse Y, et al. : Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963(1–2):190–6. 10.1016/S0006-8993(02)03972-0 [DOI] [PubMed] [Google Scholar]
- 21. Luo H, Cheng J, Han JS, et al. : Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund's adjuvant in rats. Neuroreport. 2004;15(4):655–8. 10.1097/00001756-200403220-00016 [DOI] [PubMed] [Google Scholar]
- 22. Amaya F, Shimosato G, Nagano M, et al. : NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20(9):2303–10. 10.1111/j.1460-9568.2004.03701.x [DOI] [PubMed] [Google Scholar]
- 23. Ye Y, Dang D, Zhang J, et al. : Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther. 2011;10(9):1667–76. 10.1158/1535-7163.MCT-11-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones RC, 3rd, Xu L, Gebhart GF, et al. : The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25(47):10981–9. 10.1523/JNEUROSCI.0703-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miranda A, Nordstrom E, Mannem A, et al. : The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148(4):1021–32. 10.1016/j.neuroscience.2007.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vermeulen W, De Man JG, De Schepper HU, et al. : Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur J Pharmacol. 2013;698(1–3):404–12. 10.1016/j.ejphar.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 27. Ghilardi JR, Röhrich H, Lindsay TH, et al. : Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–31. 10.1523/JNEUROSCI.3815-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niiyama Y, Kawamata T, Yamamoto J, et al. : Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007;148(2):560–72. 10.1016/j.neuroscience.2007.05.049 [DOI] [PubMed] [Google Scholar]
- 29. Tong Z, Luo W, Wang Y, et al. : Tumor tissue-derived formaldehyde and acidic microenvironment synergistically induce bone cancer pain. PLoS One. 2010;5(4):e10234. 10.1371/journal.pone.0010234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hillery CA, Kerstein PC, Vilceanu D, et al. : Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–83. 10.1182/blood-2010-12-327429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sisignano M, Angioni C, Ferreiros N, et al. : Synthesis of lipid mediators during UVB-induced inflammatory hyperalgesia in rats and mice. PLoS One. 2013;8(12):e81228. 10.1371/journal.pone.0081228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bautista DM, Pellegrino M, Tsunozaki M: TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. 10.1146/annurev-physiol-030212-183811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koivisto A, Chapman H, Jalava N, et al. : TRPA1: a transducer and amplifier of pain and inflammation. Basic Clin Pharmacol Toxicol. 2014;114(1):50–5. 10.1111/bcpt.12138 [DOI] [PubMed] [Google Scholar]
- 34. Garrison SR, Stucky CL: Contribution of transient receptor potential ankyrin 1 to chronic pain in aged mice with complete Freund's adjuvant-induced arthritis. Arthritis Rheumatol. 2014;66(9):2380–90. 10.1002/art.38724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Asgar J, Zhang Y, Saloman JL, et al. : The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience. 2015;310:206–15. 10.1016/j.neuroscience.2015.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz ES, La JH, Scheff NN, et al. : TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33(13):5603–11. 10.1523/JNEUROSCI.1806-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cattaruzza F, Johnson C, Leggit A, et al. : Transient receptor potential ankyrin 1 mediates chronic pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304(11):G1002–12. 10.1152/ajpgi.00005.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sidhapuriwala JN, Hegde A, Ang AD, et al. : Effects of S-propargyl-cysteine (SPRC) in caerulein-induced acute pancreatitis in mice. PLoS One. 2012;7(3):e32574. 10.1371/journal.pone.0032574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terada Y, Fujimura M, Nishimura S, et al. : Roles of Ca v3.2 and TRPA1 channels targeted by hydrogen sulfide in pancreatic nociceptive processing in mice with or without acute pancreatitis. J Neurosci Res. 2015;93(2):361–9. 10.1002/jnr.23490 [DOI] [PubMed] [Google Scholar]
- 40. da Costa DS, Meotti FC, Andrade EL, et al. : The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148(3):431–7. 10.1016/j.pain.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 41. Nash MS, McIntyre P, Groarke A, et al. : 7- tert-Butyl-6-(4-chloro-phenyl)-2-thioxo-2,3-dihydro-1 H-pyrido[2,3- d]pyrimidin-4-one, a classic polymodal inhibitor of transient receptor potential vanilloid type 1 with a reduced liability for hyperthermia, is analgesic and ameliorates visceral hypersensitivity. J Pharmacol Exp Ther. 2012;342(2):389–98. 10.1124/jpet.112.191932 [DOI] [PubMed] [Google Scholar]
- 42. Xu ZZ, Zhang L, Liu T, et al. : Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16(5):592–7, 1p following 597. 10.1038/nm.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park CK, Xu ZZ, Liu T, et al. : Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31(50):18433–8. 10.1523/JNEUROSCI.4192-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serhan CN, Dalli J, Karamnov S, et al. : Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–65. 10.1096/fj.11-201442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim JY, Park CK, Hwang SW: Biological Roles of Resolvins and Related Substances in the Resolution of Pain. Biomed Res Int. 2015;2015: 830930. 10.1155/2015/830930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bang S, Yoo S, Yang TJ, et al. : 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. 2012;165(3):683–92. 10.1111/j.1476-5381.2011.01568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandes ES, Fernandes MA, Keeble JE: The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol. 2012;166(2):510–21. 10.1111/j.1476-5381.2012.01851.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fernandes ES, Liang L, Smillie SJ, et al. : TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J Immunol. 2012;188(11):5741–51. 10.4049/jimmunol.1102147 [DOI] [PubMed] [Google Scholar]
- 49. Tsuji F, Aono H: Role of transient receptor potential vanilloid 1 in inflammation and autoimmune diseases. Pharmaceuticals (Basel). 2012;5(8):837–52. 10.3390/ph5080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamaguchi K, Ono K, Hitomi S, et al. : Distinct TRPV1- and TRPA1-based mechanisms underlying enhancement of oral ulcerative mucositis-induced pain by 5-fluorouracil. Pain. 2016;157(5):1004–20. 10.1097/j.pain.0000000000000498 [DOI] [PubMed] [Google Scholar]
- 51. Chen GY, Nuñez G: Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37. 10.1038/nri2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iyer SS, Pulskens WP, Sadler JJ, et al. : Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106(48):20388–93. 10.1073/pnas.0908698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takeuchi O, Akira S: Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. 10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- 54. Ma Y, Haynes RL, Sidman RL, et al. : TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6(23):2859–68. 10.4161/cc.6.23.5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mukherjee P, Winkler CW, Taylor KG, et al. : SARM1, Not MyD88, Mediates TLR7/TLR9-Induced Apoptosis in Neurons. J Immunol. 2015;195(10):4913–21. 10.4049/jimmunol.1500953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khakpour S, Wilhelmsen K, Hellman J: Vascular endothelial cell Toll-like receptor pathways in sepsis. Innate Immun. 2015;21(8):827–46. 10.1177/1753425915606525 [DOI] [PubMed] [Google Scholar]
- 57. Droemann D, Goldmann T, Branscheid D, et al. : Toll-like receptor 2 is expressed by alveolar epithelial cells type II and macrophages in the human lung. Histochem Cell Biol. 2003;119(2):103–8. [DOI] [PubMed] [Google Scholar]
- 58. Armstrong L, Medford AR, Uppington KM, et al. : Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31(2):241–5. 10.1165/rcmb.2004-0078OC [DOI] [PubMed] [Google Scholar]
- 59. Zhu X, Bagchi A, Zhao H, et al. : Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med. 2007;35(3):886–92. 10.1097/01.CCM.0000256723.37586.A2 [DOI] [PubMed] [Google Scholar]
- 60. Feng Y, Chen H, Cai J, et al. : Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem. 2015;290(44):26688–98. 10.1074/jbc.M115.661835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cavassani KA, Ishii M, Wen H, et al. : TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205(11):2609–21. 10.1084/jem.20081370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Calvo M, Dawes JM, Bennett DL: The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11(7):629–42. 10.1016/S1474-4422(12)70134-5 [DOI] [PubMed] [Google Scholar]
- 63. Feldman P, Due MR, Ripsch MS, et al. : The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. 10.1186/1742-2094-9-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim D, Kim MA, Cho IH, et al. : A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282(20):14975–83. 10.1074/jbc.M607277200 [DOI] [PubMed] [Google Scholar]
- 65. Liesz A, Dalpke A, Mracsko E, et al. : DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–98. 10.1523/JNEUROSCI.2439-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Muhammad S, Barakat W, Stoyanov S, et al. : The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28(46):12023–31. 10.1523/JNEUROSCI.2435-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Obata K, Katsura H, Miyoshi K, et al. : Toll-like receptor 3 contributes to spinal glial activation and tactile allodynia after nerve injury. J Neurochem. 2008;105(6):2249–59. 10.1111/j.1471-4159.2008.05353.x [DOI] [PubMed] [Google Scholar]
- 68. Tanga FY, Nutile-McMenemy N, DeLeo JA: The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102(16):5856–61. 10.1073/pnas.0501634102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu ZZ, Kim YH, Bang S, et al. : Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med. 2015;21(11):1326–31. 10.1038/nm.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kohli DR, Li Y, Khasabov SG, et al. : Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–65. 10.1182/blood-2010-01-260372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nicotra L, Loram LC, Watkins LR, et al. : Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2):316–29. 10.1016/j.expneurol.2011.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kato J, Agalave NM, Svensson CI: Pattern recognition receptors in chronic pain: Mechanisms and therapeutic implications. Eur J Pharmacol. 2016;788:261–73. 10.1016/j.ejphar.2016.06.039 [DOI] [PubMed] [Google Scholar]
- 73. Qi J, Buzas K, Fan H, et al. : Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186(11):6417–26. 10.4049/jimmunol.1001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu T, Gao YJ, Ji RR: Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28(2):131–44. 10.1007/s12264-012-1219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stokes JA, Cheung J, Eddinger K, et al. : Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J Neuroinflammation. 2013;10:148. 10.1186/1742-2094-10-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shi XQ, Zekki H, Zhang J: The role of TLR2 in nerve injury-induced neuropathic pain is essentially mediated through macrophages in peripheral inflammatory response. Glia. 2011;59(2):231–41. 10.1002/glia.21093 [DOI] [PubMed] [Google Scholar]
- 77. Shibasaki M, Sasaki M, Miura M, et al. : Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149(3):514–21. 10.1016/j.pain.2010.03.023 [DOI] [PubMed] [Google Scholar]
- 78. Allette YM, Due MR, Wilson SM, et al. : Identification of a functional interaction of HMGB1 with Receptor for Advanced Glycation End-products in a model of neuropathic pain. Brain Behav Immun. 2014;42:169–77. 10.1016/j.bbi.2014.06.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brederson JD, Strakhova M, Mills C, et al. : A monoclonal antibody against the receptor for advanced glycation end products attenuates inflammatory and neuropathic pain in the mouse. Eur J Pain. 2016;20(4):607–14. 10.1002/ejp.775 [DOI] [PubMed] [Google Scholar]
- 80. Lemaitre B, Nicolas E, Michaut L, et al. : The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–83. 10.1016/S0092-8674(00)80172-5 [DOI] [PubMed] [Google Scholar]
- 81. Poltorak A, He X, Smirnova I, et al. : Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8. 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- 82. Rock FL, Hardiman G, Timans JC, et al. : A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95(2):588–93. 10.1073/pnas.95.2.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tabeta K, Georgel P, Janssen E, et al. : Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101(10):3516–21. 10.1073/pnas.0400525101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aliprantis AO, Yang RB, Mark MR, et al. : Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285(5428):736–9. 10.1126/science.285.5428.736 [DOI] [PubMed] [Google Scholar]
- 85. Hemmi H, Takeuchi O, Kawai T, et al. : A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. 10.1038/35047123 [DOI] [PubMed] [Google Scholar]
- 86. Hayashi F, Smith KD, Ozinsky A, et al. : The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–103. 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- 87. Takeuchi O, Kawai T, Mühlradt PF, et al. : Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13(7):933–40. 10.1093/intimm/13.7.933 [DOI] [PubMed] [Google Scholar]
- 88. Alexopoulou L, Holt AC, Medzhitov R, et al. : Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. 10.1038/35099560 [DOI] [PubMed] [Google Scholar]
- 89. Diebold SS, Kaisho T, Hemmi H, et al. : Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–31. 10.1126/science.1093616 [DOI] [PubMed] [Google Scholar]
- 90. Heil F, Hemmi H, Hochrein H, et al. : Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–9. 10.1126/science.1093620 [DOI] [PubMed] [Google Scholar]
- 91. Yu M, Wang H, Ding A, et al. : HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–9. 10.1097/01.shk.0000225404.51320.82 [DOI] [PubMed] [Google Scholar]
- 92. Vabulas RM, Ahmad-Nejad P, da Costa C, et al. : Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276(33):31332–9. 10.1074/jbc.M103217200 [DOI] [PubMed] [Google Scholar]
- 93. Jiang D, Liang J, Fan J, et al. : Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–9. 10.1038/nm1315 [DOI] [PubMed] [Google Scholar]
- 94. Johnson GB, Brunn GJ, Platt JL: Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172(1):20–4. 10.4049/jimmunol.172.1.20 [DOI] [PubMed] [Google Scholar]
- 95. Kim S, Takahashi H, Lin WW, et al. : Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–6. 10.1038/nature07623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reed-Geaghan EG, Savage JC, Hise AG, et al. : CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29(38):11982–92. 10.1523/JNEUROSCI.3158-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Scheibner KA, Lutz MA, Boodoo S, et al. : Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177(2):1272–81. 10.4049/jimmunol.177.2.1272 [DOI] [PubMed] [Google Scholar]
- 98. Taylor KR, Yamasaki K, Radek KA, et al. : Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282(25):18265–75. 10.1074/jbc.M606352200 [DOI] [PubMed] [Google Scholar]
- 99. Tian J, Avalos AM, Mao SY, et al. : Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–96. 10.1038/ni1457 [DOI] [PubMed] [Google Scholar]
- 100. Tükel C, Wilson RP, Nishimori JH, et al. : Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe. 2009;6(1):45–53. 10.1016/j.chom.2009.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Zoelen MA, Yang H, Florquin S, et al. : Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31(3):280–4. 10.1097/SHK.0b013e318186262d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu XJ, Zhang Y, Liu T, et al. : Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 2014;24(11):1374–7. 10.1038/cr.2014.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu XJ, Liu T, Chen G, et al. : TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep. 2016;6: 28188. 10.1038/srep28188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fritz G: RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36(12):625–32. 10.1016/j.tibs.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 105. Hori O, Brett J, Slattery T, et al. : The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270(43):25752–61. 10.1074/jbc.270.43.25752 [DOI] [PubMed] [Google Scholar]
- 106. Yan SD, Chen X, Fu J, et al. : RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–91. 10.1038/382685a0 [DOI] [PubMed] [Google Scholar]
- 107. Hofmann MA, Drury S, Fu C, et al. : RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. 10.1016/S0092-8674(00)80801-6 [DOI] [PubMed] [Google Scholar]
- 108. Bianchi R, Giambanco I, Donato R: S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 2010;31(4):665–77. 10.1016/j.neurobiolaging.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 109. Yamasoba D, Tsubota M, Domoto R, et al. : Peripheral HMGB1-induced hyperalgesia in mice: Redox state-dependent distinct roles of RAGE and TLR4. J Pharmacol Sci. 2016;130(2):139–42. 10.1016/j.jphs.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 110. Otoshi K, Kikuchi S, Kato K, et al. : Anti-HMGB1 neutralization antibody improves pain-related behavior induced by application of autologous nucleus pulposus onto nerve roots in rats. Spine (Phila Pa 1976). 2011;36(11):E692–8. 10.1097/BRS.0b013e3181ecd675 [DOI] [PubMed] [Google Scholar]
- 111. Nakamura Y, Morioka N, Abe H, et al. : Neuropathic pain in rats with a partial sciatic nerve ligation is alleviated by intravenous injection of monoclonal antibody to high mobility group box-1. PLoS One. 2013;8(8):e73640. 10.1371/journal.pone.0073640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang FF, Morioka N, Harano S, et al. : Perineural expression of high-mobility group box-1 contributes to long-lasting mechanical hypersensitivity via matrix metalloproteinase-9 upregulation in mice with painful peripheral neuropathy. J Neurochem. 2015;136(4):837–850. 10.1111/jnc.13434 [DOI] [PubMed] [Google Scholar]
- 113. Pelegrin P, Surprenant A: Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282(4):2386–94. 10.1074/jbc.M610351200 [DOI] [PubMed] [Google Scholar]
- 114. Franchi L, Warner N, Viani K, et al. : Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227(1):106–28. 10.1111/j.1600-065X.2008.00734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Guo H, Callaway JB, Ting JP: Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Grace PM, Strand KA, Galer EL, et al. : Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2016;113(24):E3441–50. 10.1073/pnas.1602070113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lamkanfi M, Malireddi RK, Kanneganti TD: Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284(31):20574–81. 10.1074/jbc.M109.023689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dostert C, Guarda G, Romero JF, et al. : Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4(8):e6510. 10.1371/journal.pone.0006510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Halle A, Hornung V, Petzold GC, et al. : The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–65. 10.1038/ni.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mariathasan S, Weiss DS, Newton K, et al. : Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–32. 10.1038/nature04515 [DOI] [PubMed] [Google Scholar]
- 121. Martinon F, Pétrilli V, Mayor A, et al. : Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 122. Bsibsi M, Ravid R, Gveric D, et al. : Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61(11):1013–21. 10.1093/jnen/61.11.1013 [DOI] [PubMed] [Google Scholar]
- 123. Olson JK, Miller SD: Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–24. 10.4049/jimmunol.173.6.3916 [DOI] [PubMed] [Google Scholar]
- 124. Bowman CC, Rasley A, Tranguch SL, et al. : Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43(3):281–91. 10.1002/glia.10256 [DOI] [PubMed] [Google Scholar]
- 125. Wadachi R, Hargreaves KM: Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85(1):49–53. 10.1177/154405910608500108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Borovikova LV, Ivanova S, Zhang M, et al. : Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- 127. Rosas-Ballina M, Tracey KJ: The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64(1):28–32. 10.1016/j.neuron.2009.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Clark N, Keeble J, Fernandes ES, et al. : The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 2007;21(13):3747–55. 10.1096/fj.06-7460com [DOI] [PubMed] [Google Scholar]
- 129. Alawi K, Keeble J: The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125(2):181–95. 10.1016/j.pharmthera.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 130. Guptill V, Cui X, Khaibullina A, et al. : Disruption of the transient receptor potential vanilloid 1 can affect survival, bacterial clearance, and cytokine gene expression during murine sepsis. Anesthesiology. 2011;114(5):1190–9. 10.1097/ALN.0b013e318212515b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wang Y, Wang DH: TRPV1 ablation aggravates inflammatory responses and organ damage during endotoxic shock. Clin Vaccine Immunol. 2013;20(7):1008–15. 10.1128/CVI.00674-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Guan Z, Kuhn JA, Wang X, et al. : Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19(1):94–101. 10.1038/nn.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Leger T, Grist J, D'Acquisto F, et al. : Glatiramer acetate attenuates neuropathic allodynia through modulation of adaptive immune cells. J Neuroimmunol. 2011;234(1–2):19–26. 10.1016/j.jneuroim.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 134. Hu JY, Li CL, Wang YW: Intrathecal administration of triptolide, a T lymphocyte inhibitor, attenuates chronic constriction injury-induced neuropathic pain in rats. Brain Res. 2012;1436:122–9. 10.1016/j.brainres.2011.11.051 [DOI] [PubMed] [Google Scholar]
- 135. Chen IF, Khan J, Noma N, et al. : Anti-nociceptive effect of IL-12p40 in a rat model of neuropathic pain. Cytokine. 2013;62(3):401–6. 10.1016/j.cyto.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 136. Kobayashi Y, Kiguchi N, Fukazawa Y, et al. : Macrophage-T cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. J Biol Chem. 2015;290(20):12603–13. 10.1074/jbc.M115.636506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yao CY, Weng ZL, Zhang JC, et al. : Interleukin-17A Acts to Maintain Neuropathic Pain Through Activation of CaMKII/CREB Signaling in Spinal Neurons. Mol Neurobiol. 2016;53(6):3914–26. 10.1007/s12035-015-9322-z [DOI] [PubMed] [Google Scholar]
- 138. Wan RQ, Diamant M, De Jong W, et al. : Changes in heart rate and body temperature during passive avoidance behavior in rats. Physiol Behav. 1990;47(3):493–9. 10.1016/0031-9384(90)90115-k [DOI] [PubMed] [Google Scholar]
- 139. Sieweke MH, Allen JE: Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342(6161):1242974. 10.1126/science.1242974 [DOI] [PubMed] [Google Scholar]
- 140. Ji RR, Berta T, Nedergaard M: Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. 10.1016/j.pain.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Beggs S, Trang T, Salter MW: P2X4R + microglia drive neuropathic pain. Nat Neurosci. 2012;15(8):1068–73. 10.1038/nn.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Clark AK, Malcangio M: Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. 10.3389/fncel.2014.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fu KY, Light AR, Matsushima GK, et al. : Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825(1–2):59–67. 10.1016/S0006-8993(99)01186-5 [DOI] [PubMed] [Google Scholar]
- 144. Fu KY, Light AR, Maixner W: Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101(4):1127–35. 10.1016/S0306-4522(00)00376-6 [DOI] [PubMed] [Google Scholar]
- 145. Svensson CI, Marsala M, Westerlund A, et al. : Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86(6):1534–44. 10.1046/j.1471-4159.2003.01969.x [DOI] [PubMed] [Google Scholar]
- 146. Li K, Lin T, Cao Y, et al. : Peripheral formalin injury induces 2 stages of microglial activation in the spinal cord. J Pain. 2010;11(11):1056–65. 10.1016/j.jpain.2010.01.268 [DOI] [PubMed] [Google Scholar]
- 147. Hua XY, Svensson CI, Matsui T, et al. : Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22(10):2431–40. 10.1111/j.1460-9568.2005.04451.x [DOI] [PubMed] [Google Scholar]
- 148. Svensson CI, Fitzsimmons B, Azizi S, et al. : Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005;92(6):1508–20. 10.1111/j.1471-4159.2004.02996.x [DOI] [PubMed] [Google Scholar]
- 149. Tan YH, Li K, Chen XY, et al. : Activation of Src family kinases in spinal microglia contributes to formalin-induced persistent pain state through p38 pathway. J Pain. 2012;13(10):1008–15. 10.1016/j.jpain.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 150. Tsujino H, Kondo E, Fukuoka T, et al. : Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15(2):170–82. 10.1006/mcne.1999.0814 [DOI] [PubMed] [Google Scholar]
- 151. Berta T, Park CK, Xu ZZ, et al. : Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-α secretion. J Clin Invest. 2014;124(3):1173–86. 10.1172/JCI72230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. McNamara CR, Mandel-Brehm J, Bautista DM, et al. : TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104(33):13525–30. 10.1073/pnas.0705924104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bráz JM, Basbaum AI: Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain. 2010;150(2):290–301. 10.1016/j.pain.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lin T, Li K, Zhang FY, et al. : Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J Neuroimmunol. 2007;192(1–2):40–8. 10.1016/j.jneuroim.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]