Abstract
Background
A growing number of studies suggest that maternal stress during pregnancy promotes atopic disorders in the offspring. This is the first systematic review to address prenatal maternal stress (PNMS) and the subsequent risk of atopy‐related outcomes in the child.
Methods
The review was performed in accordance to the PRISMA criteria. We searched and selected studies in PubMed, Scopus, Embase and PsychINFO until November 2014.
Results
Sixteen (with 25 analyses) of 426 identified articles met the review criteria. Five main PNMS exposures (negative life events, anxiety/depression, bereavement, distress and job strain) and five main atopic outcomes (asthma, wheeze, atopic dermatitis, allergic rhinitis and IgE) were assessed across the studies. Overall, 21 of the 25 analyses suggested a positive association between PNMS and atopic outcomes. Of the 11 exposure–response analyses reported, six found statistically significant trends.
Conclusion
This systematic review suggests a relationship between maternal stress during pregnancy and atopic disorders in the child. However, the existing studies are of diverse quality. The wide definitions of often self‐reported stress exposures imply a substantial risk for information bias and false‐positive results. Research comparing objective and subjective measures of PNMS exposure as well as objective measures for atopic outcome is needed.
Keywords: asthma, atopic disorders, child allergy, prenatal maternal stress, systematic review
Atopic disorders including asthma, rhinoconjunctivitis and dermatitis are common global health problems with increasing prevalence 1. Atopy is considered to result from a genetically determined predisposition combined with environmental exposures, where altered immune expression leads to clinical presentation of atopic disorders. Many host and environmental factors that may alter immune expression have been proposed, and recently, there has been a growing interest in the mechanisms linking psychosocial factors to atopic disorders 2. As atopic predisposition is evident already at birth, prenatal factors are thought to have great impact on the development of atopy 3, and it is becoming clear that the in utero environment influences immune development independently of genetic susceptibility 4, 5.
There is a growing interest in the consequences of early life stress for health later in life 6. Experience of stress by the mother during pregnancy has been linked to adversities in the child, especially preterm birth and low birthweight 7. In addition, a number of biological pathways by which maternal stress during pregnancy can promote immune dysregulation have been proposed 8, 9. The exact functional consequences hereof remain to be clarified, but it is suggested that gestational exposure to maternal stress alters the development of humoral immune competence in the foetus and the hormonal and immunological responses to stress in postnatal life, thus implicating a risk of developing atopic disorders 10, 11.
The stress concept was introduced in the 1920s and 1930s when Cannon's and Selye's path‐breaking experimental work showed that external threats may have physiological and pathological effects 12, 13. McEwen more recently, in a seminal paper, reviewed the contemporary understanding of stress 14. The stress model includes an environmental factor (stressor), recognition of the stressor (perceived stress), a pathophysiological response (activation of the autonomic nervous system and hypothalamo–pituitary–adrenal (HPA) axis, the stress response) and a health effect (stress‐related disorder). In every day speech as well as in science, stress has been used not only to refer to the complete model but often denotes one or more of these constituent elements. Due to the complexity of the concept, best practice of how to measure stress in research has been discussed much. Schreier et al. 15 point to in‐depth interviews as the best measure in their review of different methods; however, it is rarely used compared to less time‐consuming measures such as self‐reporting.
A number of observational studies in humans have addressed the association between PNMS and atopic disorders. The objective of this systematic review was therefore to examine whether maternal stress during pregnancy increases the risk of atopic diseases in the children, and further to address whether observational human studies support the experimental findings and to clarify where future research contributions are needed.
Materials and methods
Search strategy
The search was performed according to the PRISMA criteria, a widely recommended method for systematic reviews of observational studies 16. The search strategy and inclusion and exclusion criteria were developed among the total group of authors after which the two‐first authors individually conducted the literature search and identified the relevant studies based on the described inclusion and exclusion criteria. The available literature was identified by searching four online databases (NCBI PubMed, Scopus, Embase and PsycINFO) including publications prior to 1 November 2014. Search terms were selected with reference to relevant index terms (MeSH, Emtree or Thesaurus), and free text searches were used in each database. Our full electronic search strategy is provided in Table 1, and Figure S1 shows a full search example of the search performed in NCBI PubMed.
Table 1.
Overview of full electronic search strategy
| Overall'term' | PubMed' | Scopus' | Embase' | PsycINFO' |
|---|---|---|---|---|
| Stress” | Stress,'Psychological' | Stress*'OR'Psychological'Stress' | Mental'Stress'OR'Life' | Psychological'Stress'OR' |
| OR'Life'Change'Events' | OR'Psychosocial'Stress'OR' | Event'OR'Anxiety'OR' | Life'Changes'OR'Anxiety' | |
| OR'Anxiety'OR' | Early'Life'Stress'OR'Depress*' | Depression'OR' | OR'Depression'(Emotion)' | |
| Depression'OR' | OR'Abuse'OR'Anxiety*'OR' | Bereavement'OR' | OR'Bereavement'OR' | |
| Bereavement'OR' | Demoralization'OR'Death'Of' | Parental'Deprivation' | Parental'Death'OR'Abuse' | |
| Parental'Death'OR' | Relative'OR'Bereavement'OR' | Abuse'OR' | OR'Demoralization'OR' | |
| Abuse'OR' | Stressful'Life'Event'OR' | Demoralization'OR' | Daily'Hassles'OR'Job''Strain' | |
| Demoralization'OR' | Distress*'OR'Daily'Hassles'OR' | Daily'Hassles'OR'Job' | ||
| Daily'Hassles'OR'Job' | Advers*' | Strain' | ||
| Strain' | ‘ | |||
| Prenatal* | Maternal'Exposure'OR' | Prenatal'OR'Pregnancy'OR' | Maternal'Behavior' | Prenatal'Exposure'OR' |
| Prenatal'Exposure' | Perinatal'OR'Matenal*'OR' | OR'Pregnancy'OR' | Pregnancy'OR'Pregnancy' | |
| Delayed'Effects'OR' | Antenatal' | Prenatal'Exposure'OR' | Outcomes'OR'Maternal' | |
| Pregnancy'OR' | Pregnancy' | Exposure' | ||
| Pregnancy' | Complication' | |||
| Complications' |
‘ ‘ |
|||
|
Atopic' disease' |
Asthma'OR'Dermatitis,' | Hypersenstivity*'OR'Asthma' | Asthma'OR' | Asthma'OR'Dermatitis'OR' |
| Atopic'OR'Rhinitis'OR' | OR'Allergy*'OR'Allergi*'OR' | Dermatitis'OR' | Immunoglobulins'OR' | |
| Immunoglobubn'E'OR' | Eczema'OR'Rhinitis'OR' | Rhintis'OR' | Rhinitis'OR'Immunologic' | |
| Hypersensitivity' | Conjunctivitis'OR'Rhino' | Immunoglobulin'OR' | Disorders' | |
| Conjunctivitis'OR'Dermatitis' | Hypersensitivity' | |||
| OR'Atop*'OR'Wheeze'' | ||||
| Results' | 94' | 54' | 199' | ‘100’ |
Study criteria
The inclusion criteria were as follows: (i) language: English language full‐length, original publications in peer‐reviewed journals, (ii) design: cross‐sectional and case–control studies as well as prospective cohort studies, (iii) exposure: stressors (negative life events, bereavement, abuse, daily hassles and job strain) and negative emotions (distress, demoralization, anxiety and depressive symptoms) experienced by the mother during pregnancy 17 and (iv) outcome: atopic disorders defined as either unspecific conditions (hypersensitivity, atopy, allergy and immunological disorder) or specific disorders (dermatitis, rhinitis, asthma, conjunctivitis, eczema, increased IgE and wheeze). In consequence hereof, the included studies were grouped into the following five outcomes: asthma, atopic dermatitis (AD), allergic rhinitis, wheeze and IgE.
We excluded studies (i) with endogenous/systemic stressor exposures (e.g. oxidative stress) or pharmacological interventions (e.g. injection of glucocorticoids), (ii) where the stress exposure was not restricted to pregnancy and (iii) where the atopic outcome was related to anaphylaxis and urticaria. We did not perform a meta‐analysis, as exposures, outcomes and study designs were too heterogenic across studies.
Results
Study selection
All manuscripts that met the selection criteria were assessed. After the removal of duplicates, we identified 426 potentially relevant publications (Fig. 1). After screening, 390 studies were excluded based on title, keywords and abstract. Generally, the exclusions were due to lack of suitability in terms of study aim, study design or exposure or outcome variables. From a snowball search among references in the included papers, we included one additional paper not retrieved in the systematic search. Of the resulting 36 studies, 16 met our inclusion criteria.
Figure 1.
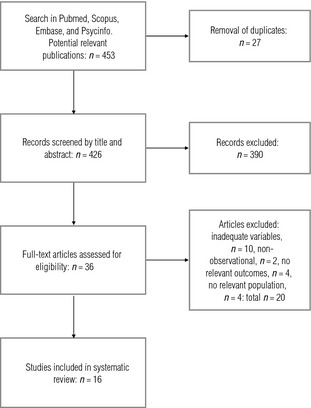
Flow diagram of study selection for the systematic review.
Characteristics of studies
Tables 2 and 3 summarize the characteristics of the 16 studies included. Fourteen 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 were prospective cohort studies and two 32, 33 were cross‐sectional studies using retrospective data. Sample sizes ranged from 147 to 3.2 million individuals, with a mean of 221,117 (median 994). Prenatal stress measures were assessed and categorized as (i) negative life events apart from bereavement (five studies 18, 22, 26, 29, 32), (ii) anxiety or depression (three studies 19, 21, 33), (iii) bereavement (two studies 20, 23), (iv) distress (four studies 25, 27, 28, 31) and (v) job strain (two studies 24, 30). Five main outcomes were assessed across the 16 studies, namely (i) asthma (seven studies 19, 20, 21, 22, 23, 24, 32), (ii) wheezing (five studies 18, 21, 27, 32, 33), (iii) atopic dermatitis (six studies 22, 24, 28, 30, 31, 32), (iv) allergic rhinitis (two studies 22, 32) and (v) IgE in cord blood at birth and/or in a blood sample drawn in infancy (five studies 22, 25, 26, 27, 29). Quality assessment of the included studies is provided as supplemental material (see Table S1).
Table 2.
The effect of prenatal maternal stress factors on atopic disorders in children, studies included
| References | Design | Study population (Country) | Prenatal stress factor (Measurement, time of assessment) | Outcome atopic disorder (Measurement, age of child) | Covariates accounted for | |
|---|---|---|---|---|---|---|
| 1a | de Marco et al. 32, a | Cross‐sectional retrospective population‐based study |
3854 mother–child pairs (Italy) |
Negative life events (Self‐reported, any time during pregnancy) |
Asthma |
Maternal: Ag, SES, Et, Sm, FA Child: Sx, BF, sharing room with sibling Other: EE, APC |
| 1b | Wheezing | |||||
| 1c | Atopic dermatitis | |||||
| 1d |
Allergic rhinitis (Maternal reported, 3‐14 years (mean 8.5 SD = ±3.2) |
|||||
| 2a | Hartwig et al. 22, b | Prospective cohort study |
994 mother–child pairs (Australia) |
Negative life events (Self‐reported, 2nd and 3rd trimester |
Asthma (doctor diagnosis, 6 and 14 years) |
Maternal: Ag, SES, Sm, FA, postnatal maternal life events Child: Sx, PB, BW number of siblings, multiple birth, BF, second‐hand smoking, Other: EE, APC, parity |
| 2b |
Atopic dermatitis (doctor diagnosis or self‐reported, 6 and 14 years) |
|||||
| 2c |
Allergic Rhinitis (doctor diagnosis, 6 and 14 years) |
|||||
| 2d |
Blood IgE (Blood sample, 6 and 14 years) |
|||||
| 3 | Chiu et al. 18 | Prospective cohort study |
653 mother–child pairs (USA) |
Negative life events (Self‐reported, any time during pregnancy) |
Wheezing (Maternal reported, 0–2 years) |
Maternal: Ag, SES, Et, Sm, FA, pre‐pregnancy BMI Child: Sx, BW, season of birth Other: EE |
| 4 | Peters et al. 26 | Prospective cohort study |
403 mother–child pairs (USA) |
Negative life events (Self‐reported, 2nd to 3rd trimester) |
Cord blood IgE (Blood sample, 0 years) |
Maternal: Ag, SES, Et, Sm Child: Sx, BW, PB, season of birth |
| 5 | Sternthal et al. 29 | Prospective cohort study |
478 mother–child pairs (USA) |
Negative life events (Self‐reported, 2nd trimester) |
Cord blood IgE (Blood sample, 0 years) |
Maternal: Ag, SES, Et, Sm, FA Child: Sx, BW, season of birth |
| 6 | Cookson et al. 19 | Prospective population‐based cohort study |
5810 mother–child pairs (UK) |
Anxiety (Self‐reported, 2nd and 3rd trimester) |
Asthma (Maternal‐reported doctor diagnosis, 7½ years) |
Maternal: Ag, SES, Sm, FA, Child: Sx, PB, BF, number of siblings, multiple birth Other: APC |
| 7a | Guxens et al. 21, a | Prospective population‐based cohort study |
4848 mother–child pairs (Netherlands) |
Depression, Anxiety (Self‐reported, 2nd trimester) |
Asthma (Maternal‐reported doctor diagnosis, 6 years) |
Maternal: Ag, SES, Et, Sm, FA, pre‐pregnancy BMI Child: Sx, BW, PB, BF, day care attendance, eczema, lower respiratory tract infections Other: parity, pet keeping |
| 7b |
Wheezing (Maternal reported, 1, 2, 3, and 4 years) |
|||||
| 8 | Lefevre et al. 33 |
Cross‐sectional retrospective, case‐control |
147 mother–child pairs (France) |
Depression, Anxiety (Self‐reported, any time during pregnancy) |
Wheezing (Maternal reported, mean = 15.4 months (SD ± 6.7 months) |
Maternal: Sm, FA Child: Sx, Ag, place of birth |
| 9 | Fang et al. 20 | Prospective register‐based, nationwide study |
920 147 mother–child pairs (Sweden) |
Bereavement (Register, from one year before pregnancy to child birth) |
Asthma (Register, doctor diagnosed, or use of asthma medication, 1–4 and 7–12 years) |
Maternal: Ag, SES, Sm, perinatal BMI Child: Sx, Ag |
| 10 | Khashan et al. 23 | Prospective register‐based, nationwide cohort study |
3 019 003 mother–child pairs (Sweden) |
Bereavement (Register, from half a year before pregnancy to child birth) |
Asthma (Register, doctor diagnosed, 0‐9 years) |
Maternal: Ag, SES, Sm, FA Child: Sx, place of birth Other: calendar year |
| 11a | Reyes et al. 27, a | Prospective cohort study |
279 mother–child pairs (USA) |
Psychological distress (Self‐reported, 3rd trimester) |
Wheezing (Maternal reported, 0–5 years ‐14 times) |
Maternal: Ag, SES, Et, FA Child: Sx, wheeze reported in flu season, second‐hand smoking |
| 11b |
Blood IgE (Blood sample, 0, 2, 3, and 5 years) |
|||||
| 12 | Sausenthaler et al. 28 | Prospective cohort study |
3004 mother–child pairs (Germany) |
Stress‐related factors (Medical records, any time during pregnancy) |
Atopic dermatitis (Parental‐reported doctor diagnosis, 0–6 years – 6 times) |
Maternal: Ag, SES, Sm, FA |
| 13 | Wen et al. 31 | Prospective cohort study |
1159 mother–child pairs (Taiwan) |
Psychological distress (Self‐report, 3rd trimester) |
Atopic dermatitis (Maternal‐reported doctor diagnosis, 6 months and 2 years) |
Maternal: SES, FA, cord blood IgE, susceptible genotype Child: Sx |
| 14 | Lin et al. 25 | Prospective cohort study |
334 mother–child pairs (Taiwan) |
Psychological distress (Self‐reported, 3rd trimester) |
Cord blood IgE (Blood sample, 0 years) |
Maternal: Ag, SES, Sm, FA, Other: EE |
| 15a | Larsen et al. 24, a | Prospective cohort Study | 32 771 mother–child pairs (Denmark) | Job strain (Self‐reported, 2nd trimester) |
Asthma (maternal‐reported and/or maternal reported doctor diagnosis, 7 years) |
Maternal: Ag, BMI, Sm, alcohol, gestational age at interview, FA, medicine Child: Sx, Other: parity, EE, APC |
| 15b |
Atopic Dermatitis (parental‐reported, 7 years) |
|||||
| 16 | Wang et al. 30 | Prospective cohort study with retrospective stress assessment |
19 381 mother–child pairs (Taiwan) |
Job strain (Self‐reported, 6 months and 36 months after delivery) |
Atopic dermatitis (Maternal‐reported doctor diagnosis, 3 years) |
Maternal: SES, Sm, FA Child: Sx, number of siblings |
Maternal: Ag, age; SES, socio‐economic status; Et, ethnicity; Sm, smoking; FA, family history of relevant atopy. Child: Sx, sex; BW, birthweight; PB, preterm birth; BF, breast feeding. Other: EE, environmental exposure; APC, adverse pregnancy conditions.
Studies separated into several analyses.
Table 3.
The effect of PNMS on atopic disorders in children, study results
| References | Exposure PNMS | Results: crude odds ratiosa | Results: adjusted odds ratiosa , b | Exposure‐responsec | |
|---|---|---|---|---|---|
| Asthma | |||||
| 1a | de Marco et al. 32, d | Negative life events | N/A | 1.71 (1.02–2.89) | N/A |
| 2a | Hartwig et al. 22, d | Negative life events, | |||
| 1st–2nd trimester | 2.38 (1.33–4.26), 6 years | 1.73 (0.87–3.44), 6 years | + | ||
| 2.24 (1.10–4.54), 14 years | 1.26 (0.54–2.91), 14 years | + | |||
| 2nd–3rd trimester | 1.65 (0.88–3.08), 6 years | 0.99 (0.47–2.08), 6 years | − | ||
| 2.50 (1.17–5.35), 14 years | 1.81 (0.74–4.46), 14 years | − | |||
| 6 | Cookson et al. 19 | Anxiety, | +* | ||
| 2nd trimester | 1.64 (1.31–2.05) | 1.53 (1.22–1.93) | |||
| 3rd trimester | 1.76 (1.40–2.20) | 1.65 (1.30–2.08) | |||
| 7a | Guxens et al. 21, d | Depression, Anxiety | N/A | 1.45 (0.91–2.31) | + |
| 9 | Fang et al. 20 | Bereavement | N/A | 1.06 (0.97–1.15)e, 1–4 years | N/A |
| N/A | 1.06 (0.95–1.17), 7–12 years | ||||
| 10 | Khashan et al. 23 | Bereavement | 1.22 (1.05–1.41) f | 1.43 (1.06–1.92) g | N/A |
| 15a | Larsen et al. 24, d | Job strain | 1.16 (0.99–1.35) | 1.08 (0.92–1.27) | N/A |
| Wheezing | |||||
| 1b | de Marco et al. 32, d | Negative life events | N/A | 1.41 (1.03–1.94) | N/A |
| 3 | Chiu et al. 18 | Negative life events | 4.13 (1.58–10.8) 1.9 (1.7–2.2) | 3.79 (1.39–10.3) | +* |
| 7b | Guxens et al. 21, d | Depression, Anxiety | 2.15 (1.47–3.13) | +* | |
| 8 | Lefevre et al. 33 | Depression | N/A | 1.55 (0.12–19.8) | N/A |
| Anxiety | N/A | 1.98 (0.69–5.68) | N/A | ||
| 11a | Reyes et al. 27, d | Psychological distress | 1.60 (1.24–2.06) | 1.66 (1.29–2.14) | N/A |
| Atopic dermatitis | |||||
| 1c | de Marco et al. 32, d | Negative life events | N/A | 1.53 (1.11–2.10) | N/A |
| 2b | Hartwig et al. 22, d | Negative life events | |||
| 1st–2nd trimester | 1.43 (0.72–2.84), 6 years | 1.41 (0.61–3.29), 6 years | − | ||
| 1.43 (0.76–2.69), 14 years years | 1.18 (0.54–2.60), 14 years | + | |||
| 2nd–3rd trimester | 2.34 (1.23–4.47), 6 years | 2.38 (0.63–2.19), 6 years | + | ||
| 3.23 (1.79–5.83), 14 years | 4.19 (1.97–8.89), 14 years | +* | |||
| 12 | Sausenthaler et al., 2009 28 | Stress‐related factors | 1.13 (0.72–1.77) | 1.13 (0.71–1.79) | N/A |
| 13 | Wen et al. 31 | Psychological distress | 2.3 (1.1–5.2) | 2.3 (1.1–5.3) | N/A |
| 15b | Larsen et al. 24, d | Job strain | 1.14 (1.01–1.29) | 1.15 (1.02–1.31) | N/A |
| 16 | Wang et al. 30 | Job strain | N/A | 1.34 (1.16–1.54) | +* |
| Allergic rhinitis | |||||
| 1d | de Marco et al. 32, d | Negative life events | N/A | 1.75 (1.08–2.84) | N/A |
| 2c | Hartwig et al.22, d | Negative life events | |||
| 1st–2nd trimester | 2.18 (1.24–3.82), 6 years | 1.62 (0.81–3.26), 6 years | + | ||
| 1.90 (1.10–3.27), 14 years | 0.96 (0.49–1.88), 14 years | + | |||
| 2nd–3rd trimester | 1.69 (0.92–3.11), 6 years | 1.24 (0.58–2.62), 6 years | + | ||
| 2.65 (1.52–4.62), 14 years | 2.38 (1.21–4.70), 14 years | +* | |||
| IgE | |||||
| 2d | Hartwig et al. 22, d | Negative life events | |||
| 1st–2nd trimester | 0.83 (0.44–1.60), 6 years | 0.70 (0.41–1.21), 6 years | − | ||
| 0.85 (0.45–1.62), 14 years | 0.72 (0.33–1.57), 14 years | − | |||
| 2nd–3rd trimester | 0.80 (0.33–1.91), 6 years | 0.73 (0.29–1.84), 6 years | − | ||
| 1.45 (0.63–3.32), 14 years | 1.71 (0.64–4.61), 14 years | + | |||
| 4 | Peters et al. 26 | Negative life events | N/A | N/A | +* |
| 5 | Sternthal et al. 29 | Negative life events | 2.23 (0.97–5.13) | 2.19 (0.89–5.38) | N/A |
| 11b | Reyes et al. 27, d | Psychological distress | 1.04 (0.74–1.45) | N/A | N/A |
| 14 | Lin et al. 25 | Psychological distress | N/A | 4.0 (1.3–12.8) | +* |
Bold indicates significant reported results.
Table 2 describes each studies respective adjustments for covariates.
Dose–response symbols: +, positive dose response; −, no dose response; *, significant reported results.
Studies separated into several analyses.
Hazard ratio.
Odds ratio calculated by current authors of the systematic review.
Relative risk.
N/A, nothing reported.
Findings
The 16 studies analysed a total of 25 relevant associations, as several studies assessed more than one relevant outcome (e.g. multiple atopic outcomes and subanalyses for trimester and age of the children). Overall, 21 analyses showed a positive association between PNMS and risk of atopic disorders in the children; of which 14 were statistically significant. In some studies, odds ratios (ORs) were reported for more than one exposure level. In these cases, we state the OR from the group with the highest exposure.
In the seven studies on asthma, adjusted ORs ranged between 0.99 and 1.81, whereof three were statistically significant 19, 23, 32. Of the remaining four studies, three found significant associations in subanalyses 20, 21, 22, 24. Thus, Fang et al. reported no significant association for the overall analysis, but observed boys exposed to PNMS during the second trimester to be of higher risk of asthma (age group 1–4 years: HR = 1.55; 95% CI = 1.19–2.02 and 7–12 years: HR = 1.58; 95% CI = 1.11–2.25).
All five studies that assessed the risk of wheeze found positive associations with adjusted ORs between 1.41 and 3.79, four were statistically significant 18, 21, 27, 32.
Children of mothers who experienced stress during pregnancy had significantly higher risk of AD in four of six studies, with adjusted ORs between 1.13 and 4.19 24, 30, 31, 32. The remaining two studies reported nonsignificant positive associations 22, 28, except for one statistically significant finding on stress exposure during 2nd to 3rd trimester and increased risk of AD at 14 years of age 22.
In the two studies dealing with allergic rhinitis, three of four analyses found positive associations, one significantly so 22, 32. The adjusted ORs ranged between 0.96 and 2.38.
Increased levels of blood IgE in children with mothers who experienced stress during pregnancy were found in three of five studies, with a significant OR of 4 in one study 25. For all studies, adjusted ORs ranged between 0.70 and 4. Peters et al. 26 reported a statistically significant positive exposure–response association.
Exposure–response analyses were performed in 11 of the 25 analyses (i.e. seven studies) 18, 19, 21, 22, 25, 26, 30. All seven studies reported statistically significant exposure–response relations for at least one atopic outcome.
Discussion
To our knowledge, this is the first systematic review of a growing epidemiological literature addressing the possible link between stress experienced by the pregnant women and the risk of atopic disorders in the child. Of the 25 included analyses, 21 suggested a positive association between PNMS and atopic outcomes in children, whereof 14 findings were statistically significant. The risk of asthma, wheeze, AD, allergic rhinitis and increased IgE in blood was generally associated with PNMS across the reviewed studies. In addition, of the 11 exposure–response analyses reported, six found significant positive trends between PNMS and an atopic outcome in the children.
Strengths and limitations of the studies
PNMS assessment
Within the reviewed studies, PNMS was studied for the two main domains: (i) stressors (negative life events or stressful conditions) and (ii) negative emotions (distress, anxiety or depressive symptoms).
Negative life events or stressful conditions
Eight of the reviewed articles assessed negative life events or adverse social conditions 18, 20, 22, 23, 26, 28, 29, 32. Negative life events are the most commonly used exposure measure in epidemiological prenatal stress research and have been considered to be quite objective as they often are not influenced by subjective evaluation and information bias 34. Surely, the objectivity relies on the chosen conditions/events as well as the assessment methodology. The more severe events are more likely to be reported as stressful by all exposed individuals. Furthermore, objective assessments based on data from registers (e.g. death of a close relative) are less influenced by information and reporting bias.
In the two register‐based studies 20, 23, the negative life events were confined to bereavement, defined as death of a close relative, (spouse or child). Both studies suggested an association between maternal bereavement and asthma in the children, one study with statistically significance. Sausenthaler et al. 28 examined a number of stress‐related factors specifically related to the pregnancy including objective measures (pregnancy complications reported in maternity certificates) as well as subjective measures (perceived psychological or social stress reported in questionnaires).
In five of the cohort studies 18, 22, 26, 29, 32, the women reported on negative events relating to different aspects of life during pregnancy, obtained through questionnaires. Typically, the questionnaires assessed a combination of objective situations (e.g. ‘Did you lose your job?’) and more subjective evaluations (e.g. ‘Did you experience mourning?’ or ‘Did you experience uneasiness?’). Thus, these studies combined external stressors with internal experiences. On one hand, this introduces a subjective appraisal of an event, which can be regarded an asset in stress research 35. On the other hand, the objectivity of the assessment is lost, and the results are prone to bias as discussed later. None of the authors made separate analyses of the subjective and objective measures. A potential difference between the effects of the two types of stressors could therefore not be inferred from their results.
Distress, anxiety or depressive symptoms
The mothers self‐rated their mental state on anxiety and depression scales in three studies 19, 21, 33, and depression or anxiety or a combination hereof was used as measure of stress. Anxiety and depression are often used as a proxy for stress 17. All studies reported positive associations, strongest for anxiety, but only the findings of Cookson et al., who examined the largest population, were statistically significant.
Self‐report versus objective measurements of stress exposure
It has been debated whether a subjective self‐report or objective assessment of stressful events and conditions represents the most reliable measure of PNMS.
In most studies within the field of PNMS research, exposure assessments include self‐reported stress, although a few incorporate objective measures in terms of biomarkers of stress in relation to offspring illnesses, including atopic outcomes 10, 36, as called for by Mutambudzi et al. 37.
Ideally, a valid subjective stress measure would be preferable to avoid bias – reporting or recall. This is, however, not possible as maternal disease status or personality likely will influence the probability of reporting stress and a diagnosis of atopic disorders in her child. Objective measurement would therefore make a stronger case. Objective measures may, however, be difficult to interpret, especially in pregnancy. A Danish study measured cortisol levels in pregnant women and related this to stressful life events. Women experiencing more than one stressful life event had 27% higher evening cortisol concentration in plasma compared to women not experiencing stress. In early pregnancy, the women experiencing stressful life events did, however, not present with higher evening cortisol levels 38. Utilization of an integrated index where biomarkers are measured across multiple physiological systems may be a more reliable predictor, but this approach lacks consensus in the literature so far and has several limitations 10. More procedural problems, such as timing of assessment and reproducibility would also become related challenges in this context.
Randomized controlled trials would represent a strong design in delineation of the impact of maternal stress on atopic disorders in the offspring. For ethical reasons, very few experiments with humans have been carried out within this research field. Monk and colleagues found that when pregnant women solved demanding computer tasks, foetal heart rate was elevated only when the woman rated herself as anxious, indicating the need of a subjective assessment as well 39. Interestingly, Lobel concludes that subjective measures of stress seem to be the best predictor of adverse birth outcomes, for example, relative to birthweight 40.
The applied external exposures ranged from job strain to loss of a close relative. It is therefore not surprising that the reviewed studies report different odds ratios. Rare, sudden and severe life events, such as loss of a close relative, are found to be associated with atopy in the child. Bereavement implicates sharp contrast in exposure, and it might therefore be easier to detect differences. Bereavement is also a massive stressor with an acute and long‐term effect. It can therefore be debated whether findings for bereavement may extrapolate to less severe and more common experiences, such as the more chronic mild stress related to job strain. Common stressors such as job stress and daily hassles are, however, experienced by a larger proportion of pregnant women. As the experiences may be interpreted as natural parts of life rather than genuine stressors, the lesser contrast in exposure may make it more difficult to observe differences in outcome.
Timing of exposure during pregnancy
There was no specific time‐point during pregnancy where exposure to PNMS seemed to be more critical. Fang et al. 20 included bereavement for up to one year before pregnancy compared to half a year before pregnancy in Khashan et al. 23. The latter found a stronger and significant effect (OR 1.43) compared to Fang et al. (OR 1.06). It comes to mind that the impact of bereavement mainly occur when bereavement occurs close to conception. Although the two studies used almost the same population and measures, these register studies present with several dissimilarities; Fang et al. used more recent data and defined bereavement as the death of a parent, sibling, child or spouse while Khashan et al. restricted the definition of bereavement to death of a child or spouse; Fang et al. defined asthma based on medication use, while Khashan et al., used inpatient hospitalization. These methodological differences likely partake in the differences in findings and illustrate the complexity in comparison and interpretation of results between even closely related studies. Cookson et al. 19 measured exposure to stress at week 18 and 32 during pregnancy, and the associations to the risk of asthma were of same strengths at the two time intervals. Hartwig et al. 22 did not observe differences in the strength of the associations between child asthma and PNMS in early compared to late pregnancy. This was contrary to their findings for allergic rhinitis, AD and IgE, where only exposure in late pregnancy was associated with increased ORs. One may speculate whether these discrepancies could be due to differences in critical windows for different atopic diseases. Organogenesis primarily takes place in early pregnancy whereas immune functionality, such as immune competence and Th1/Th2 balance, is acquisitioned later in pregnancy 41. The effect of PNMS on asthma development might rely on both incomplete development of the respiratory tract and immune dysregulation, whereas the functional aspects of the immune system seem to play the most important role in the other atopic disorders 41. Timing of PNMS exposure has been found to be relevant for a number of immunological outcomes in animal studies, but this has only been sparsely investigated in humans 41.
Atopic outcome assessments
Asthma and wheeze
Most of the reviewed studies show a positive association between PNMS and the risk of childhood asthma, significantly so in seven of 12 analyses. The nonsignificant OR found in 3 of the 12 analyses could be due to relative few cases (see Table 2) 21, 22, 33. Five of the studies 19, 21, 22, 24, 32 obtained information on asthma diagnoses through questionnaires, which introduces a risk of information bias compared to information on outcomes from health registers or other independent sources, that to some extend avoids such information bias. However, these sources most often contain information from hospitalized patients and not from primary care, and therefore, only more severe asthma cases are included. It is not certain how this influences the association with PNMS.
Recurrent wheeze in early childhood has previously been used as a proxy for the development of asthma later in life 11. Recurrent wheeze is, however, common in children below 4 years of age while persistent wheeze by the end of the first decade of life usually is associated with a diagnosis of asthma 42. The two studies 18, 33 that followed the children until 2 years of age might underestimate the effect of stress due to random misclassification of the outcome. Guxens et al. 21 assessed symptoms of wheeze each year after birth and found that the association between PNMS and asthma increased with age. Of note, it is likely that mothers experiencing stress, anxiety or depression over‐report symptoms in their children which consequently will lead to an overestimation of the true association 43. Ideally, an objective measure of asthma, for example, test of bronchial hyper‐responsiveness, with follow‐up at older ages, should be used as outcome measure of asthma. This would increase the specificity of the outcome and reduce bias. Another approach could be to use different definitions of asthma to explore the consistency of the results, for example use of asthma medication, asthma hospitalization and parental report of asthma 44.
Atopic dermatitis, allergic rhinitis and IgE
Although all but one of the analyses showed increased OR of AD and allergic rhinitis after exposure to PNMS, the ORs vary between 1.13 and 4.19. Among others, this might represent regional differences in diagnostic practice. The only opposing result was reported in the study by Sausenthaler et al. 28. However, many of their stress measures (pregnancy‐related complications) are debatable. The atopic outcomes were assessed using doctors' diagnoses in five studies and maternally reported symptoms in one study. No studies used register‐based information for these conditions.
It is not clear whether PNMS increases the risk of certain atopic disorders more than others. De Marco et al. 32 found similar ORs for PNMS and asthma, AD and allergic rhinitis. The cross‐sectional design might have increased the risk of recall bias as it is likely that mothers of atopic children remember their pregnancies as more complicated and also that distressed mothers over‐report symptoms. Hartwig et al. 22 assessed all four outcomes, with divergent results. Larsen et al. 24 found that self‐reported high job strain was associated with AD at age 7, whereas the association with asthma did not achieve statistical significance. The differences between disorders might, in part, be due to differences in the child age, when the outcome is recorded. For AD and allergic rhinitis, higher ORs were evident at age 14 compared to age six in child 22. As especially allergic rhinitis often first appear later in life (in contrast to for example nonallergic asthma), this could partly play an impact on the herein reported associations.
Although all the included atopic disorders are often accompanied by increased IgE levels, all cases are not characterized by an underlying atopy with IgE sensitization. In particular, for asthma, many cases are nonatopic. However, in the studies with very young children, the asthmatic symptoms are more likely to be associated to atopic predisposition 45. The overall positive findings reported by the studies examining blood IgE as outcome measure may support the importance of the IgE mediated mechanism. Only one study 27 did not find a significant association between PNMS and increased IgE levels in child, possibly due to limited power (n = 279). Conversely, the study by Lin et al. 25, with an almost similar sample size (n = 334), reported OR of 4. This outcome may therefore be interpreted with caution. In two studies, IgE was measured at different ages, where Hartwig et al. 22 found an association only at age 14. This may imply that PNMS increases the risk of IgE sensitization at birth, but that the effect might diminish, as the children grow older. Of note, these differences in timing of assessments of IgE levels in child blood may also contribute to the indefinite results across the included studies. Future studies may capitalize from assessing several time periods throughout infancy and childhood to further elucidate the relationship. Still, the IgE studies are methodologically strong, as in addition to the objective nature of the IgE measure, the risk of recall bias is minimized because the mother is not familiar with the IgE status of their newborn.
Methodological issues
Reporting bias
In an article by Schreier HM and colleagues, different ways of measuring stress are reviewed. As a conclusion, they propose interview‐based assessments of stress, where there is more flexibility to get in‐depth information as well as information on duration and severity of the experienced stress 15. This is a resource intensive method, which is probably why it has not been used in any studies on prenatal stress and atopy. In addition, the selection of the method used in studies may be carried out relative to practical issues as well as the question being used. On the other hand, self‐reported data can introduce reporting bias related to reporting style or personality traits, even in follow‐up studies with prospective data collection. This represents a challenge in studies, where the mother reports on both her own stress exposure and atopic disease and symptoms (including physician diagnosed atopic disease) in her child. This may also be the case in studies solely relying on physician's reports or health register data because the child may often be brought to the physician for examination by the mother's initiative, and a more anxious mother may be more likely to bring her child to the physician and thereby getting her child a diagnosis of atopic disease. As neither stress (ranging from the death of a close relative to daily hassles or discomfort) nor atopy (ranging from treatment requiring daily medication to discrete skin rash) are strictly well‐defined items, reporting bias in the reviewed studies is possible, especially in the two studies using retrospective interviews 32, 33. Life‐changing events, such as mourning, divorce and loss of job, have, however, been shown to be recalled very accurately, even after several years 46. Although one should be aware of the associated bias to the specific chosen survey measures of PNMS, it is also important to highlight that there now exists extensive literature validating this approach for many of these tools 17.
Confounding
All studies adjusted the results for the most relevant confounders. The number of included confounders varied from five 28 to 27 19. Despite the overall adequate adjustment for potential confounders, results may not fully be averting other possible mediators on the association between PNMS and risk of atopic diseases in children. For example, diet, exercise and drinking and smoking habits may be difficult to sufficiently capture and may also likely differ between PNMS exposed and unexposed mothers. Moreover, the vast heterogeneity of included confounders across the studies makes it difficult to compare, why reporting of both unadjusted and adjusted results is also relevant and should be recommended.
Biological mechanisms potentially mediating the effects of PNMS
A number of biological pathways by which PNMS can promote immune dysregulation and lead to atopy in the foetus have been proposed though not completely understood 8, 9. Atopic diseases are characterized by an inappropriate inflammatory response of the immune system. It has been suggested that the effects on postnatal immune function largely rely on a promotion of Th2 responses and accordingly enhanced recruitment and activation of IgE‐producing cells, a characteristic for atopy 41. Important domains of the immune system, including t‐cell differentiation, develop prenatally and are susceptible to foetal programming 9, 47, 48. The prenatal HPA axis seems particularly susceptible to modulation by maternal stress, which can lead to long‐term regulatory alterations of immune function 49. Due to a mutual regulatory relationship between the HPA‐axis and the immune system, effects on the HPA system have consequences for immune function as well, as the glucocorticoid hormones are essential in immune development. Therefore, alterations in maternal stress hormones level such as cortisol might influence foetal immune modulation. Our understanding of the transition of the maternal stress response to the foetus still lacks causality, although several explanatory mechanisms have been suggested, including transplacental transfer of stress mediators and maternal stress‐response activation of placental release of neuroendocrine stress transmitters to the foetus 50, 51, 52, 53, 54, 55, 56, 57. Further knowledge of the biological transduction, including the potential modulatory involvement of the placenta, is needed and could be important in future intervention and management of PNMS. Furthermore, there has been recent interest in changes in infant microbiota related to PNMS. An infant's intestinal microbiota starts forming already in utero and is known to have a critical impact on the maturation and development of the gastro‐intestinal tract, metabolism, immunity and the HPA axis. As PNMS have shown to affect the composition of offspring bacterial microbiota, this pathway may potentially alter the immune development and increase this risk of allergies in child 4.
Interpretation of the results
The literature examining the correlation between PNMS and atopic disorders in humans is very sparse. For this reason, we included studies using quite different exposure and outcome measures as well as methodology. Although the suggested biological mechanisms points towards an underlying atopic disposition in children exposed to PNMS, it might be misleading to look at the various atopic disorders as a collective group of diseases. There may be differences in the pathways to immunopathology between PNMS and each specific atopic disorder, which could interfere with the found associations. The studies with the strongest methodology in terms of outcome measures were the two register‐based asthma studies 20, 23 and the five studies on IgE blood levels in infants 22, 25, 26, 27, 29. The register‐based study by Khashan et al. also used a very large cohort and points to an association between asthma and PNMS. The IgE studies are more vague and characterized by small populations. However, in two of three studies that looked at exposure–response relations, significant association was found.
The herein reviewed literature advocates for an association, but methodological challenges and diverse study designs make it difficult to quantify a specific risk. Furthermore, the relationship between PNMS and the risk of atopic disorders in children might also be influenced by the timing and type of stressor. To further explore this field of research and to provide more conclusive results, there is a need for well‐powered studies with objective measures for atopic disease with as little risk of bias as possible. Moreover, studies comparing objective and subjective measures of PNMS exposure could help advance this body of research. Lastly, studies on certain life events that are more time specific, such as bereavement, would enable a closer look into the importance of timing during pregnancy.
In conclusion, this systematic literature review indicates that PNMS increases the risk of developing allergic diseases in childhood. However, the reviewed studies are heterogeneous and of diverse quality, and only a few studies applied both high‐quality exposure and outcome measures. Research comparing objective and subjective measures of PNMS exposure as well as objective measures for atopic outcome is needed to help advance this field of research. This would also enable more knowledge on whether certain outcomes are more susceptible to PNMS as well as the influence of exposure timing.
Author contributions
Vivi Schlünssen, Niklas Andersson and Mette Vinther Hansen took responsibility for the integrity of the reported results and the accuracy of the review. VS, NWA, MVH and HAK developed the aims of the study; NWA, MVH and VS obtained the reviewed studies; NWA, MVH, HAK, VS, ADL and KSH interpreted the included studies and their results; NWA and MVH drafted the manuscript with assistance and critical feedback from VS, HAK, KSH and ADL.
Funding
Nothing declared.
Conflict of interest
The authors (NWA, MVH, ADL and KSH) have no conflict of interest to declare. HAK performed patient evaluations for the Danish National Board of Industrial Injuries. From 2012 to March 2015, VS performed patient evaluations for the Danish National Board of Industrial Injuries. From 2008 to 2014, VS performed patient evaluations for the Danish Labour Union HK Denmark.
Supporting information
Table S1. Quality assessment and ranked list of studies included.
Figure S1. Example of full search strategy from NCBI Pubmed search.
Andersson NAW, Hansen MV, Larsen AD, Hougaard SK, Kolstad HA, Schlünssen V. Prenatal maternal stress and atopic diseases in the child: a systematic review of observational human studies. Allergy 2016; 71: 15–26.
Edited by: Bodo Niggemann
References
- 1. Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: a major global health concern. Curr Opin Allergy Clin Immunol 2012;12:39–41. [DOI] [PubMed] [Google Scholar]
- 2. Mathews HL, Janusek LW. Epigenetics and psychoneuroimmunology: mechanisms and models. Brain Behav Immun 2011;25:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warner JA, Warner JO. Early life events in allergic sensitisation. Br Med Bull 2000;56:883–893. [DOI] [PubMed] [Google Scholar]
- 4. Zijlmans MA, Korpela K, Riksen‐Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015;53:233–245. [DOI] [PubMed] [Google Scholar]
- 5. Simmons JP, Nelson LD, Simonsohn U. False‐positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci 2011;22:1359–1366. [DOI] [PubMed] [Google Scholar]
- 6. Fagundes CP, Glaser R, Kiecolt‐Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun 2013;27:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Littleton HL, Bye K, Buck K, Amacker A. Psychosocial stress during pregnancy and perinatal outcomes: a meta‐analytic review. J Psychosom Obstet Gynaecol 2010;31:219–228. [DOI] [PubMed] [Google Scholar]
- 8. Pincus‐Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF et al. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol 2006;177:8484–8492. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 2001;13:419–449. [DOI] [PubMed] [Google Scholar]
- 10. Wright RJ, Schreier HM. Seeking an integrated approach to assessing stress mechanisms related to asthma: is the allostatic load framework useful? Am J Respir Crit Care Med 2013;187:115–116. [DOI] [PubMed] [Google Scholar]
- 11. Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol 2007;21(Suppl 3):8–14. [DOI] [PubMed] [Google Scholar]
- 12. Cannon WB. Bodily changes in pain, hunger, fear, and rage: An account of recent researches into the function of emotional excitement: New York and London D. Appleton; 1916.
- 13. Selye H. A syndrome produced by diverse nocuous agents. Nature 1936;138:32. [DOI] [PubMed] [Google Scholar]
- 14. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171–179. [DOI] [PubMed] [Google Scholar]
- 15. Schreier HMC, Miller GE, Chen E. Clinical potentials for measuring stress in youth with asthma. Immunol Allergy Clin North Am 2011;31:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:65–94. [DOI] [PubMed] [Google Scholar]
- 17. Nast I, Bolten M, Meinlschmidt G, Hellhammer DH. How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatr Perinat Epidemiol 2013;27:313–322. [DOI] [PubMed] [Google Scholar]
- 18. Chiu YHM, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med 2012;186:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cookson H, Granell R, Joinson C, Ben‐Shlomo Y, Henderson AJ. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol 2009;123:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang F, Hoglund CO, Arck P, Lundholm C, Langstrom N, Lichtenstein P et al. Maternal bereavement and childhood asthma‐analyses in two large samples of Swedish children. PLoS ONE 2011;6:e27202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guxens M, Sonnenschein‐van der Voort AM, Tiemeier H, Hofman A, Sunyer J, de Jongste JC et al. Parental psychological distress during pregnancy and wheezing in preschool children: the Generation R Study. J Allergy Clin Immunol 2014;133:59–67. [DOI] [PubMed] [Google Scholar]
- 22. Hartwig IR, Sly PD, Schmidt LA, van Lieshout RJ, Bienenstock J, Holt PG et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol 2014;134:160–169. [DOI] [PubMed] [Google Scholar]
- 23. Khashan AS, Wicks S, Dalman C, Henriksen TB, Li J, Mortensen PB et al. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population‐based study. Psychosom Med 2012;74:635–641. [DOI] [PubMed] [Google Scholar]
- 24. Larsen AD, Schlunssen V, Christensen BH, Bonde JP, Obel C, Thulstrup AM et al. Exposure to psychosocial job strain during pregnancy and odds of asthma and atopic dermatitis among 7‐year old children ‐ a prospective cohort study. Scand J Work Environ Health 2014;40:639–648. [DOI] [PubMed] [Google Scholar]
- 25. Lin YC, Wen HJ, Lee YL, Guo YL. Are maternal psychosocial factors associated with cord immunoglobulin E in addition to family atopic history and mother immunoglobulin E? Clin Exp Allergy 2004;34:548–554. [DOI] [PubMed] [Google Scholar]
- 26. Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts‐Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy 2012;67:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reyes M, Perzanowski MS, Whyatt RM, Kelvin EA, Rundle AG, Diaz DM et al. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner‐city children. Ann Allergy Asthma Immunol 2011;107:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sausenthaler S, Rzehak P, Chen CM, Arck P, Bockelbrink A, Schafer T et al. Stress‐related maternal factors during pregnancy in relation to childhood eczema: results from the LISA Study. J Investig Allergol Clin Immunol 2009;19:481–487. [PubMed] [Google Scholar]
- 29. Sternthal MJ, Enlow MB, Cohen S, Canner MJ, Staudenmayer J, Tsang K et al. Maternal interpersonal trauma and cord blood IgE levels in an inner‐city cohort: a life‐course perspective. J Allergy Clin Immunol 2009;124:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang IJ, Wen HJ, Chiang TL, Lin SJ, Chen PC, Guo YL. Maternal employment and atopic dermatitis in children: a prospective cohort study. Br J Dermatol 2013;168:794–801. [DOI] [PubMed] [Google Scholar]
- 31. Wen HJ, Wang YJ, Lin YC, Chang CC, Shieh CC, Lung FW et al. Prediction of atopic dermatitis in 2‐yr‐old children by cord blood IgE, genetic polymorphisms in cytokine genes, and maternal mentality during pregnancy. Pediatr Allergy Immunol 2011;22:695–703. [DOI] [PubMed] [Google Scholar]
- 32. de Marco R, Pesce G, Girardi P, Marchetti P, Rava M, Ricci P et al. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatr Allergy Immunol 2012;23:724–729. [DOI] [PubMed] [Google Scholar]
- 33. Lefevre F, Moreau D, Semon E, Kalaboka S, Annesi‐Maesano I, Just J. Maternal depression related to infant's wheezing. Pediatr Allergy Immunol 2011;22:608–613. [DOI] [PubMed] [Google Scholar]
- 34. Graignic‐Philippe R, Dayan J, Chokron S, Jacquet AY, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev 2014;43:137–162. [DOI] [PubMed] [Google Scholar]
- 35. Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci 2012;15:669–674. [DOI] [PubMed] [Google Scholar]
- 36. Beijers R, Jansen J, Riksen‐Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics 2010;126:e401–e409. [DOI] [PubMed] [Google Scholar]
- 37. Mutambudzi M, Meyer JD, Warren N, Reisine S. Effects of psychosocial characteristics of work on pregnancy outcomes: a critical review. Women Health 2011;51:279–297. [DOI] [PubMed] [Google Scholar]
- 38. Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J, Levine S. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology 2005;30:647–656. [DOI] [PubMed] [Google Scholar]
- 39. Monk C, Myers MM, Sloan RP, Ellman LM, Fifer WP. Effects of women's stress‐elicited physiological activity and chronic anxiety on fetal heart rate. J Dev Behav Pediatr 2003;24:32–38. [DOI] [PubMed] [Google Scholar]
- 40. Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. J Behav Med 1994;17:225–272. [DOI] [PubMed] [Google Scholar]
- 41. Veru F, Laplante DP, Luheshi G, King S. Prenatal maternal stress exposure and immune function in the offspring. Stress 2014;17:133–148. [DOI] [PubMed] [Google Scholar]
- 42. Wright AL. Epidemiology of asthma and recurrent wheeze in childhood. Clin Rev Allergy Immunol 2002;22:33–44. [DOI] [PubMed] [Google Scholar]
- 43. Vernon SW, Roberts RE. Measuring nonspecific psychological distress and other dimensions of psychopathology. Further observations on the problem. Arch Gen Psychiatry 1981;38:1239–1247. [DOI] [PubMed] [Google Scholar]
- 44. Hansen S, Strom M, Maslova E, Mortensen EL, Granstrom C, Olsen SF. A comparison of three methods to measure asthma in epidemiologic studies: results from the Danish National Birth Cohort. PLoS ONE 2012;7:e36328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832–836. [DOI] [PubMed] [Google Scholar]
- 46. Ferree NK, Cahill L. Post‐event spontaneous intrusive recollections and strength of memory for emotional events in men and women. Conscious Cogn 2009;18:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meaney M, Szyf M. Maternal care as a model for experiencedependent chromatin plasticity? Trends Neurosci 2005;28:456–463. [DOI] [PubMed] [Google Scholar]
- 48. Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav 2006;50:623–631. [DOI] [PubMed] [Google Scholar]
- 49. Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 2001;13:113–128. [DOI] [PubMed] [Google Scholar]
- 50. Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet 1998;352:707–708. [DOI] [PubMed] [Google Scholar]
- 51. Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta‐hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–166. [DOI] [PubMed] [Google Scholar]
- 52. Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev 2005;29:237–258. [DOI] [PubMed] [Google Scholar]
- 53. Majzoub JA, Karalis KP. Placental corticotropin‐releasing hormone: function and regulation. Am J Obstet Gynecol 1999;180:S242–S246. [DOI] [PubMed] [Google Scholar]
- 54. Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ 1999;318:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Glover V. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms. Adv Neurobiol 2015;10:269–283. [DOI] [PubMed] [Google Scholar]
- 56. O'Donnell KJ, Bugge JA, Freeman L, Khalife N, O'Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta‐HSD2. Psychoneuroendocrinology 2012;37:818–826. [DOI] [PubMed] [Google Scholar]
- 57. Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic‐pituitary‐adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab 2001;86:104–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quality assessment and ranked list of studies included.
Figure S1. Example of full search strategy from NCBI Pubmed search.


