Abstract
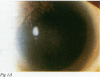
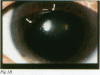
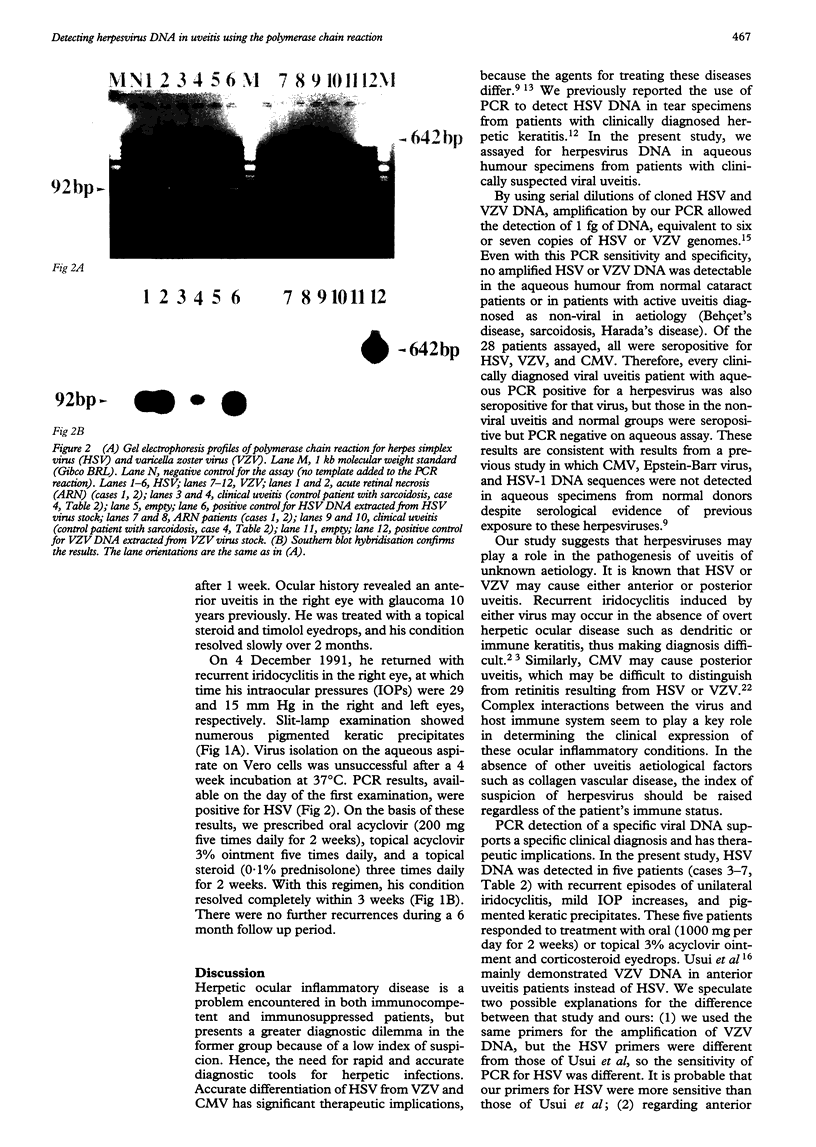
BACKGROUND: Herpesviruses are involved in the pathogenesis of many ocular diseases including keratitis, iridocyclitis, and acute retinal necrosis syndrome. The rapid and accurate diagnosis of herpetic infections has become increasingly important with the rising incidence of immunosuppressive diseases. The purpose of this study was to evaluate the use of the polymerase chain reaction (PCR) to detect herpesvirus DNA in uveitis patients. METHODS: Aqueous samples were aspirated from 11 patients with active uveitis of suspected viral origin. Using PCR, masked samples were assayed for herpes simplex virus (HSV), varicella zoster virus (VZV), and cytomegalovirus (CMV) to assist in supporting the clinical diagnosis of viral aetiology. Masked controls included 10 aqueous humour specimens from normal patients undergoing cataract surgery and specimens from seven patients diagnosed with active non-viral uveitis--Behçet's disease, sarcoidosis, Fuchs' heterochromic iridocyclitis, or Harada's disease. RESULTS: Ten of 11 cases clinically diagnosed as being of possible viral aetiology yielded aqueous PCR positive for a herpesvirus. Eight patients were PCR positive for amplified HSV DNA, of whom two had acute retinal necrosis, one had corneal endotheliitis, and five had recurrent iridocyclitis. VZV DNA was detected in one case of iridocyclitis, and CMV DNA in one case of chorioretinitis. Successful therapy was based on the PCR results. Ten normal aqueous specimens and the seven uveitis samples from cases not suspected of a viral aetiology were PCR negative for HSV, VZV, and CMV. CONCLUSION: These results demonstrate that detecting herpesvirus DNA in the aqueous humour is useful to support a clinical diagnosis of viral uveitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado J. A., Underwood J. L., Green W. R., Wu S., Murphy C. G., Hwang D. G., Moore T. E., O'Day D. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1994 Dec;112(12):1601–1609. doi: 10.1001/archopht.1994.01090240107034. [DOI] [PubMed] [Google Scholar]
- Biswas J., Mayr A. J., Martin W. J., Rao N. A. Detection of human cytomegalovirus in ocular tissue by polymerase chain reaction and in situ DNA hybridization. Graefes Arch Clin Exp Ophthalmol. 1993 Feb;231(2):66–70. doi: 10.1007/BF00920214. [DOI] [PubMed] [Google Scholar]
- Cao M., Xiao X., Egbert B., Darragh T. M., Yen T. S. Rapid detection of cutaneous herpes simplex virus infection with the polymerase chain reaction. J Invest Dermatol. 1989 Mar;92(3):391–392. doi: 10.1111/1523-1747.ep12277232. [DOI] [PubMed] [Google Scholar]
- Culbertson W. W., Blumenkranz M. S., Pepose J. S., Stewart J. A., Curtin V. T. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology. 1986 May;93(5):559–569. doi: 10.1016/s0161-6420(86)33701-1. [DOI] [PubMed] [Google Scholar]
- Demmler G. J., Buffone G. J., Schimbor C. M., May R. A. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis. 1988 Dec;158(6):1177–1184. doi: 10.1093/infdis/158.6.1177. [DOI] [PubMed] [Google Scholar]
- Easton H. G. Zoster sine herpete causing acute trigeminal neuralgia. Lancet. 1970 Nov 21;2(7682):1065–1066. doi: 10.1016/s0140-6736(70)90291-6. [DOI] [PubMed] [Google Scholar]
- Fox G. M., Crouse C. A., Chuang E. L., Pflugfelder S. C., Cleary T. J., Nelson S. J., Atherton S. S. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol. 1991 Feb;109(2):266–271. doi: 10.1001/archopht.1991.01080020112054. [DOI] [PubMed] [Google Scholar]
- Freeman W. R., Thomas E. L., Rao N. A., Pepose J. S., Trousdale M. D., Howes E. L., Nadel A. J., Mines J. A., Bowe B. Demonstration of herpes group virus in acute retinal necrosis syndrome. Am J Ophthalmol. 1986 Dec 15;102(6):701–709. doi: 10.1016/0002-9394(86)90396-x. [DOI] [PubMed] [Google Scholar]
- Gartry D. S., Spalton D. J., Tilzey A., Hykin P. G. Acute retinal necrosis syndrome. Br J Ophthalmol. 1991 May;75(5):292–297. doi: 10.1136/bjo.75.5.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang T. J. Diagnosis and therapy of herpes zoster ophthalmicus. Ophthalmology. 1991 Aug;98(8):1216–1229. doi: 10.1016/s0161-6420(91)32163-8. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Nishi M., Hanashiro R., Mori S., Masuda K., Mochizuki M., Hondo R. Polymerase chain reaction for the detection of the varicella-zoster genome in ocular samples from patients with acute retinal necrosis. Am J Ophthalmol. 1992 Nov 15;114(5):603–609. doi: 10.1016/s0002-9394(14)74491-5. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Yamamoto S., Nishida K., Okamoto S., Kinoshita S., Hayashi K., Manabe R. Demonstration of herpes simplex virus DNA in idiopathic corneal endotheliopathy. Am J Ophthalmol. 1991 Oct 15;112(4):419–423. doi: 10.1016/s0002-9394(14)76251-8. [DOI] [PubMed] [Google Scholar]
- Pavan-Langston D., Brockhurst R. J. Herpes simplex panuveitis. A clinical report. Arch Ophthalmol. 1969 Jun;81(6):783–787. doi: 10.1001/archopht.1969.00990010785005. [DOI] [PubMed] [Google Scholar]
- Pepose J. S., Flowers B., Stewart J. A., Grose C., Levy D. S., Culbertson W. W., Kreiger A. E. Herpesvirus antibody levels in the etiologic diagnosis of the acute retinal necrosis syndrome. Am J Ophthalmol. 1992 Mar 15;113(3):248–256. doi: 10.1016/s0002-9394(14)71575-2. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Pavan-Langston D., Tada R., Yamamoto R., Kinoshita S., Nishida K., Shimomura Y., Tano Y. Possible role of herpes simplex virus in the origin of Posner-Schlossman syndrome. Am J Ophthalmol. 1995 Jun;119(6):796–798. doi: 10.1016/s0002-9394(14)72788-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Shimomura Y., Kinoshita S., Nishida K., Yamamoto R., Tano Y. Detection of herpes simplex virus DNA in human tear film by the polymerase chain reaction. Am J Ophthalmol. 1994 Feb 15;117(2):160–163. doi: 10.1016/s0002-9394(14)73071-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Shimomura Y., Kinoshita S., Tano Y. Differentiating zosteriform herpes simplex from ophthalmic zoster. Arch Ophthalmol. 1994 Dec;112(12):1515–1516. doi: 10.1001/archopht.1994.01090240021012. [DOI] [PubMed] [Google Scholar]
- de Boer J. H., Luyendijk L., Rothova A., Baarsma G. S., de Jong P. T., Bollemeijer J. G., Rademakers A. J., Van der Lelij A., Zaal M. J., Kijlstra A. Detection of intraocular antibody production to herpesviruses in acute retinal necrosis syndrome. Am J Ophthalmol. 1994 Feb 15;117(2):201–210. doi: 10.1016/s0002-9394(14)73077-6. [DOI] [PubMed] [Google Scholar]