Abstract
Background and Purpose
While the molecular pathways of baclofen toxicity are understood, the relationships between baclofen‐mediated perturbation of individual target organs and systems involved in cardiovascular regulation are not clear. Our aim was to use an integrative approach to measure multiple cardiovascular‐relevant parameters [CV: mean arterial pressure (MAP), systolic BP, diastolic BP, pulse pressure, heart rate (HR); CNS: EEG; renal: chemistries and biomarkers of injury] in tandem with the pharmacokinetic properties of baclofen to better elucidate the site(s) of baclofen activity.
Experimental Approach
Han‐Wistar rats were administered vehicle or ascending doses of baclofen (3, 10 and 30 mg·kg−1, p.o.) at 4 h intervals and baclofen‐mediated changes in parameters recorded. A pharmacokinetic–pharmacodynamic model was then built by implementing an existing mathematical model of BP in rats.
Key Results
Final model fits resulted in reasonable parameter estimates and showed that the drug acts on multiple homeostatic processes. In addition, the models testing a single effect on HR, total peripheral resistance or stroke volume alone did not describe the data. A final population model was constructed describing the magnitude and direction of the changes in MAP and HR.
Conclusions and Implications
The systems pharmacology model developed fits baclofen‐mediated changes in MAP and HR well. The findings correlate with known mechanisms of baclofen pharmacology and suggest that similar models using limited parameter sets may be useful to predict the cardiovascular effects of other pharmacologically active substances.
Abbreviations
- CO
cardiac output
- CBT
core body temperature
- DBP
diastolic BP
- HR
heart rate
- ka
absorption rate
- ke
elimination rate
- Kin_CO
zero‐order rate constant of cardiac output
- Kin_TPR
zero‐order rate constant of total peripheral resistance
- kout_CO
first‐order dissipation rate constant of cardiac output
- kout_TPR
first‐order dissipation rate constant of total peripheral resistance
- MAP
mean arterial pressure
- PKPD
pharmacokinetic–pharmacodynamic
- PP
pulse pressure
- SBP
systolic BP
- SV
stroke volume
- TPR
total peripheral resistance
Tables of Links
| TARGETS |
|---|
| GABAB1 receptor |
| GABAB2 receptor |
| LIGANDS |
|---|
| Baclofen |
| Formic acid |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Baclofen is a marketed drug approved for the treatment and alleviation of signs and symptoms associated with neuropathic pain, clonus, dystonia, insomnia, muscle spasticity, rigidity addiction and anxiety. The CNS depressant properties and availability of baclofen (Perry et al., 1998) has made it a drug of abuse for recreational users. However, baclofen misuse has resulted in a number of drug abusers presenting to emergency rooms and hospitalizations due to acute high dose baclofen toxicity (Leung et al., 2006). Patients presenting with acute baclofen toxicity exhibit multiple and varied symptoms including hypertension and/or hypotension, profound hypothermia, bradycardia and/or tachycardia and can lapse into prolonged coma (Leung et al., 2006).
Baclofen is an agonist at the inhibitory metabotropic GABAB receptor. The receptor consists of two major subunits, GABAB1 and GABAB2, combining as obligatory heterodimers to form a functional receptor (White et al., 1998). GABAB receptors are ubiquitously expressed at both pre and postsynaptic neurons in the CNS and in peripheral target organs including the heart, kidney and vasculature. Postsynaptic GABAB receptors mediate their effects via receptor activated G proteins Giα and Goα coupling to adenylyl cyclase and direct activation of potassium channels while presynaptic GABAB receptors modulate glutamatergic and GABA neurotransmitter release via increased calcium release.
While molecular signal transduction pathways involved in baclofen‐mediated activation of GABAB receptors within the CNS are relatively well understood, the relationships resulting from baclofen's perturbation of individual target organs involved in cardiovascular regulation are not clear. In particular, the cardiovascular system is composed of multiple organs and tissues whose combined functions contribute to overall cardiovascular homeostasis. Cardiovascular parameters such as BP, cardiac output and total peripheral resistance (TPR) are functional parameters that are tightly regulated by the CNS via the baroreflex arc, the autonomic nervous system, the renin‐angiotensin system and local mediators such as endothelin and nitric oxide. Modelling quantifiable parameters such as BP and heart rate (HR) responses can elucidate site‐ specific mechanisms of drug‐induced responses (Snelder et al., 2013, 2014). This information can facilitate translation of basic science to the clinical situation and promote the development of improved pharmaceutical therapies by informing the selection of better drug candidates for drug development (Collins et al., 2015).
This study evaluated the pharmacokinetic properties of baclofen in tandem with multiple physiological and biochemical endpoints that contribute to overall cardiovascular function. For this, the effects of baclofen on BP, HR, CNS, body temperature, plasma chemistries, urine chemistries and renal biomarkers of injury were simultaneously measured in the same group of rats using an integrative pharmacology approach (Kamendi et al., 2010) coupled with quantitative pharmacokinetic–pharmacodynamic (PKPD) modelling. In addition to detailed characterization of baclofen‐mediated effects in multiple organ systems, BP and HR response data were used in quantitative PKPD modelling to characterize site‐specific mechanisms of baclofen activation on overall cardiovascular function. Summary data of other salient treatment‐related changes in power spectra derived from cortical EEG, plasma clinical chemistries, urine chemistries and biomarkers of acute kidney injury that were measured concurrently in these studies are reported separately (see Figure 3 and Supporting Information Tables S1–S3 respectively).
Figure 3.
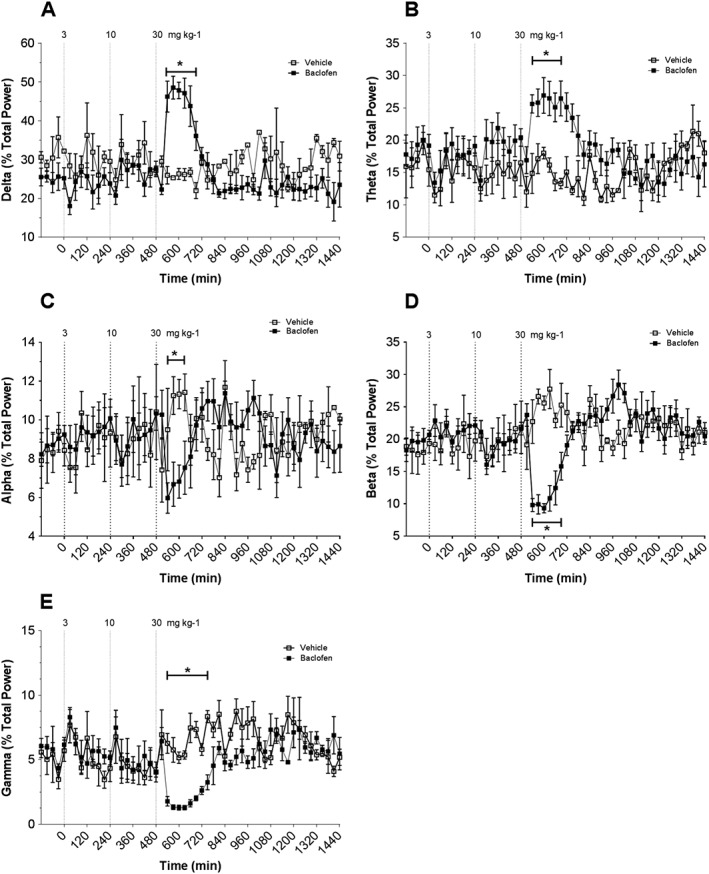
EEG measured continuously for 24 h using radiotelemetry in conscious rats. Rats were treated with vehicle and baclofen at 3, 10 and 30 mg·kg−1. Dashed line indicates time of compound administration. Panels show effects of vehicle and baclofen administration on: (A) EEG δ; (B) EEG θ; (C) EEG α; (D) EEG β; € EEG γ. Data points correspond to means ± SEM. Significant changes in responses are denoted by *P < 0.05.
Methods
Group sizes
Fifteen rats were dosed with baclofen, and PK was collected in five rats. Similarly, nine rats were used as controls dosed with saline.
Randomization
A simple randomization was carried out the day of dosing based upon quality of blood sampling.
Blinding
The operator was not blinded to treatment. Treatments were blinded to all other team members analysing specific parameters including necropsy, histopathology, clinical chemistries, biomarkers and data analysis (statistician). Treatments were labelled as Group 1 or Group 2, corresponding to vehicle and baclofen respectively.
Normalization
Drug induced kidney injury biomarkers, urine electrolytes and chemistries, and blood chemistries parameters were normalized to baseline (24 h urine collected before dosing) and log transformed.
For continuously sampled parameters [mean arterial pressure (MAP), systolic BP (SBP), diastolic BP (DBP), pulse pressure (PP), HR, core body temperature (CBT) and EEG], data were not normalized.
Validity of animal species or model selection
Rats are a commonly used rodent species for early safety evaluations and their cardiovascular response to drugs extensively studied. The present studies have used this species to extend the rat cardiovascular PKPD model of others (Snelder et al., 2013) to specifically model the cardiovascular response to baclofen.
Animals
Male Hans‐Wistar rats from Charles River Laboratories (Raleigh, NC, USA) were implanted with PhysioTel® multiplus radio transmitters (model TL11M2‐C50‐PXT). At time of surgery, animals were 10–12 weeks of age and their weights averaged about 300 g. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Surgical implantation of radio telemetry and catheters
Rats 10–12 weeks of age, weight averaging 300 g, were surgically implanted with PhysioTel multiplus radio transmitters (model TL11M2‐C50‐PXT) at Charles River Laboratories using aseptic surgical techniques (Kamendi et al., 2010). Briefly, rats were anaesthetized with isoflurane (5%). The chest, abdomen and right inner thigh were then shaved and disinfected. The transmitter body was placed in the abdomen and sutured to the abdominal wall. The BP catheter tip was inserted into the abdominal aorta just distal to the renal vessels and secured with Vetbond™ (3 M, St Paul, MN, USA). Two bio‐potential leads for EEG recordings were trochared s.c. to the cranium and attached with skull screw electrodes. The first screw was located over the right cerebral hemisphere at 2.5 mm posterior from bregma and +3.0 mm lateral from midline. The second screw was located over the left cerebral hemisphere at 5.5 mm posterior from bregma and +3.0 mm lateral from midline. Radio telemetry transmitters enabled measurement of BP, EEG and CBT. The ability to record these parameters was confirmed using Dataquest ART™ software v3.1 (DSI, St Paul, MN, USA) before completion of surgery. After 2 weeks of recovery, the rats were outfitted with an in‐dwelling jugular vein catheter (BASi, West Lafayette, IN, USA) for blood sampling. The catheter was filled with a 1:1 heparin–glycerol lock solution to maintain patency and the animals were shipped 4 days later to AstraZeneca Pharmaceuticals (AZ), Waltham, MA, USA.
Housing and husbandry
After arrival at the site, the surgically prepared animals were allowed to acclimatize in‐house for 7 days. The animals were housed in a pathogen free facility which has been accredited by Association for Assessment and Accreditation of Laboratory Care International. Each animal was individually housed in box‐shoe polycarbonate cages with corncob bedding material placed on racks. Animals were fed lab diet rodent chow and had free access to water.
On study Day −2, rats were removed from home cages and placed into the experimental home cage system for acclimatization. The system allowed for automated blood sampling, telemetry and acquisition of other parameters as previously described (Kamendi et al., 2010). Animals were housed in this system for the duration of the study.
Experimental design
On Day 1, baseline measures of all parameters were recorded for 24 h. On Day 1, at time = 0, rats were administered vehicle or ascending doses of baclofen (3, 10 and 30 mg·kg−1, p.o.) at 4 h intervals (n = 14). Timed blood samples were collected in tubes containing 10 μL EDTA at t = 0, 5, 10, 15, 20, 25, 30, 60, 120, 180, 240, 245, 250, 255, 260, 265, 270, 360, 480, 485, 490, 495, 500, 505, 510, 515, 520, 600, 720, 960, 1020 and 1440 min to determine the plasma concentration time profile of baclofen. In addition, the elimination profile was determined after the highest dose. For each sample, the blood volume removed was replaced immediately with an equal volume of isotonic saline. Total daily blood volume sampled from each animal was within acceptable sampling limits in accord with Institutional Animal Care and Use Committee guidelines and did not alter haemodynamic parameters of control animals as compared with non‐sampled animals (data not shown).
Telemetry data acquisition
Radio telemetry signals were captured at an acquisition frequency of 500 Hz. BPs, EEG and CBT were continuously recorded over the length of the study and processed using Dataquest A.R.T 4.2 Gold software (DSI). MAP, systolic pressure (SBP), diastolic pressure (DBP) and HR were derived from the BP wave using a built‐in software algorithm. CBT was derived from the CBT wave. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Determination of baclofen in plasma
Briefly, plasma samples of 50 μL were mixed with 250 μL of acetonitrile (containing 250 ng·mL−1 carbutamide as an internal standard) to precipitate proteins and centrifuged at 2500× g for 5 min. The supernatants (200 μL) were dried down to completeness under nitrogen and then reconstituted with 250 μL of 80:20 10 mM ammonium formate: acetonitrile +0.1% (v v‐1) formic acid prior to HPLC‐MS/MS analysis. An analytical standard was prepared by dissolving 2 mg·mL−1 baclofen in DMSO with serial dilution to various concentrations using blank rat plasma to generate a calibration curve.
Sample separation was carried out using a Discovery HS F5, 33 × 2.1 mm LC column (SUPLECO, Bellefonte, PA, USA) held at 50°C, connected to a Shimadzu HPLC system (Shimadzu Scientific Instruments, Columbia, MD, USA) with LC20ADvP pump, CBM20A controller and CTC PAL autosampler (CTC Analytics, Zwingen, Switzerland) held at 10°C. Samples (5 μL) were eluted at 500 μL·min−1 with a 2.3 min gradient elution method. Mobile phase A consisted of 10 mM ammonium formate +0.1% (v v‐1) formic acid. Mobile phase B consisted of 0.1% (v v‐1) formic acid in acetonitrile. The elution method was: 5% B for 0.5 min, then a linear increase from 5 to 95% in 1 min, hold at 95% B for 0.5 min, then a return to 5% B for 0.3 min. Eluted samples were analysed with a Sciex API 4000 MS (Applied Biosystems, Framingham, MA, USA) fitted with an electrospray ionization probe operated in positive ion mode with selected reaction monitoring of m/z 214.2–151.0 with a collision energy of 25 V for baclofen and m/z 272.1–156.1 with a collision energy of 15 V for carbutamide. Data were collected and analysed using Analyst software v1.4.1 (Applied Biosystems).
Materials: drugs, chemicals reagents and other materials (including sources)
Baclofen (Sigma, St Louis, MO, USA); ammonium formate (Sigma); formic acid (Sigma); acetonitrile (Sigma); carbutamide (Sigma).
PKPD modelling and simulation methods
Baclofen pharmacokinetics were fitted using a one‐compartment model with linear absorption (k a), elimination (k e) and volume of distribution (V). To account for nonlinearity in dose, an available fraction was fit with a first order Hill response and half‐max (K B) defined by
The model was fit to all PK measurements across the three doses simultaneously.
Cardiovascular physiological observations were fit based on the model from Snelder et al. (2014). The model structure was modified to include linear effect compartments linking the plasma concentration with drug effect. Specifically, the equations governing the model are as follows:
Where k outHR, k outSV and k outTPR reflect the rate of turnover in HR, stroke volume (SVe) and TPR respectively. The parameter FB governs the strength of the feedback from MAP onto HR, SV and TPR respectively. The parameters k inHR, k inSV and k inTPR are defined such that MAP, HR, SV and TPR are at baseline in the absence of drug. SV was corrected for HR as in Snelder et al. (2014) through the following equation:
The drug effect parameters EFF HR, EFF SV and EFF TPR represent drug effect on HR, SV and TPR respectively. These were modelled as both linear and Emax effects based on the concentration in an effect compartment:
Effect compartment concentrations are defined by C E, and equilibration rate was represented by keq. For linear drug effect, EFF parameters were fit with linear slope SHR, SSV and STPR:
For an Emax drug effect, half‐max drug effect was represented by IC50, and the individual drug effects were represented as
The simulations were initialized at the analytically determined steady state values of each parameter, MAP0, SV0, HR0 and TPR0, for MAP, SV, HR and TPR respectively. HR was related to cardiac output (CO) and SV through the relationship CO = SV*HR.
The cardiovascular physiology model was linked to the baclofen PK model with HR and MAP fit to observed data. The model was fit by population approach allowing for inter‐individual variability in the baseline parameters. Baseline values for HR0 and MAP0 were fit according to
Here θ HR0 and θ MAP0 represent the typical population values of baseline HR and MAP, while n HR0 and n MAP0 are assumed to be normally distributed with expectation value 0 and variance of ωHR 2 and ωMAP 2 respectively. Population fits were performed using the Lindstrom–Bates First‐Order Conditional Estimation algorithm, and all modelling and simulation were performed in Phoenix (Pharsight, Cary, NC, USA). The feedback parameter FB was set to depend on the MAP as in Snelder et al. (2014) through the following formula:
Results
Toxicokinetics
Baclofen was detected in the plasma of rats dosed with the drug (Figure 1 and Table 1).
Figure 1.
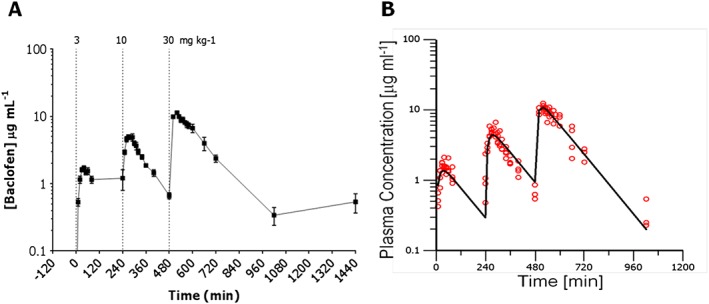
Linear plasma concentration‐time plots following three ascending doses of baclofen. Panels show: (A) measured levels of baclofen in rat plasma following administration of 3, 10 and 30 mg·kg−1, p.o.; (B) best fit for plasma concentrations following administration of 3, 10 and 30 mg·kg−1, p.o. baclofen.
Table 1.
Parameters determined from best fit of pharmacokinetic sub‐model
| Parameter | Estimate | Units | CV% |
|---|---|---|---|
| V | 1.57 | L·kg−1 | 8.8 |
| ke | 0.00817 | min−1 | 9.0 |
| ka | 0.0662 | min−1 | 13.3 |
| KB | 75.3 | mg·kg−1 | 28.1 |
Systolic blood pressure
Baclofen 3 mg·kg−1 induced a significant (19%, P < 0.05) transient increase in SBP with maximum changes occurring 60 min post‐dose (Figure 2A). Doses of 10 and 30 mg·kg−1 each significantly (P < 0.05) increased SBP by 45%. At all doses, SBP changes returned to baseline prior to the administration of the next dose and by the end of the study following the last dose.
Figure 2.
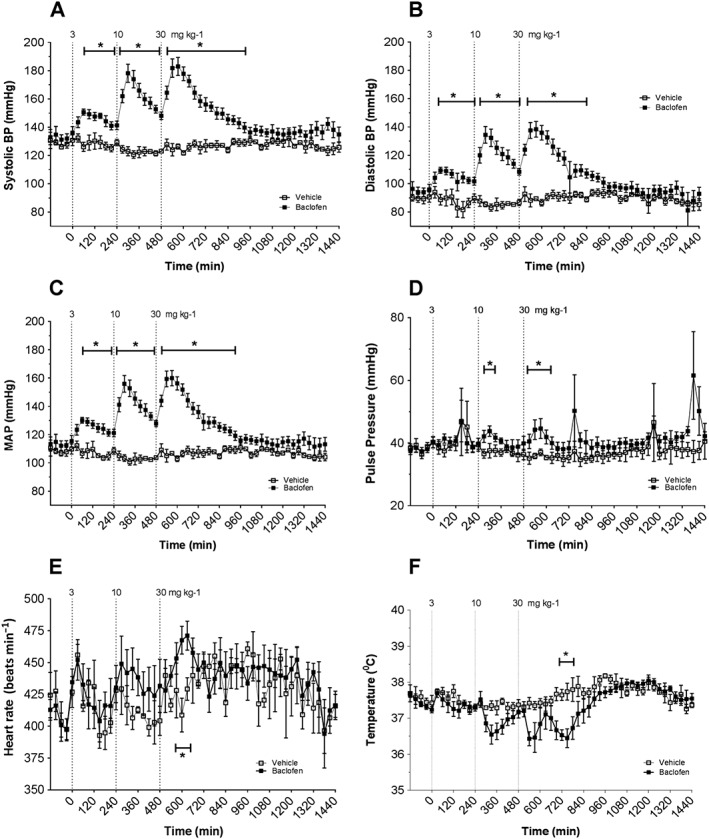
BPs, heart rate, QA interval and CBT measured continuously for 24 h using radiotelemetry in conscious rats. Rats were treated with vehicle or baclofen at 3, 10 and 30 mg·kg−1, p.o. in an ascending dose paradigm. Dashed line indicates time of compound administration at 0, 4 and 8 h. Panels show effects of vehicle, baclofen, p.o. on: (A) systolic BP; (B) diastolic BP; (C) mean arterial BP (MAP) (D) pulse pressure; (E) heart rate; (F) core body temperature. Data points correspond to means ± SEM. Significant changes in responses are denoted by * P < 0.05, baclofen from vehicle.
Diastolic blood pressure
Baclofen at 3, 10 and 30 mg·kg−1 significantly (P < 0.05 each) increased DBP to a maximum of 22%, 58% and 55%, respectively, when compared with time‐matched vehicle controls (Figure 2B). These changes were transient at each dose with a return to baseline DBP values prior to administration of subsequent doses and by the end of the study.
Mean arterial BP (MAP)
Baclofen at 3, 10 and 30 mg·kg−1 doses significantly (P < 0.05 each) increased MAP to a maximum of 22%, 51% and 50%, respectively, compared with time‐matched vehicle control responses (Figure 2C). MAP recordings returned to baseline before the next dose was administered and by the end of the study.
Pulse pressure (PP)
Consistent with the observed changes of SBP, DBP and MAP, baclofen at 3 mg·kg−1 increased PP 11% (P = N. S.; Figure 2D) compared with time‐matched vehicle control responses at 60 min. Baclofen at 10 and 30 mg·kg−1 dose‐dependently and significantly (P < 0.05 each) increased PP further to 16% and 20%, respectively, compared with time‐matched vehicle controls.
Heart rate
A general trend, albeit variable, for dose‐dependent increases in HR was observed. Baclofen at 3 mg·kg−1 caused a 4% increase in HR 60 min post‐dose compared with vehicle‐treated animals (Figure 2E). Baclofen at 10 mg·kg−1 increased HR further with a maximum 9% increase at 90 min following administration. Baclofen at 30 mg·kg−1 significantly (P < 0.05) increased HR by 14% 2 h post‐dose compared with time‐matched vehicle animals. This parameter returned to baseline before administration of each subsequent dose and by the end of the study following the final dose.
Core body temperature
Baclofen also produced a trend toward dose‐dependent decreases in CBT (Figure 2F). Baclofen at 30 mg·kg−1 only caused a significant (P < 0.05) 1°C decrease in CBT compared with time‐matched vehicle control responses. CBT returned to baseline values before administration of each dose and by the end of the study.
Electroencephalogram power
No biologically relevant changes in δ power were observed following baclofen administration of 3 or 10 mg·kg−1 doses. At 30 mg·kg−1, baclofen significantly (P < 0.05 each) increased δ and θ power (Figure 3A–B) by 96% and 62%, respectively, compared with timed‐matched vehicle‐treated animals. Baclofen also significantly decreased (P < 0.05 each) α, β and γ spectral power (Figures 3C–E) by 40%, 65% and 70%, respectively, compared with time‐matched controls. All spectral bands returned to baseline values by the end of the study.
Modelling of HR and MAP data
The mechanism of the effect of baclofen on CV endpoints could be driven by effect on HR, SV, TPR or some combination of these. To attempt to identify which observable measurements were reflecting primary effects and which were reflecting secondary effects driven by baroreflex mechanisms, we have applied a systems modelling approach. We utilized the model built by Snelder et al., 2014. PK was fit first (Figure 1; Table 1) and fixed for subsequent pharmacodynamic (PD) fitting to HR and MAP. Several potential PD model structures were then analysed for their ability to qualitatively and quantitatively describe the behaviour of the MAP and HR observations under treatment (Figure 4).
Figure 4.
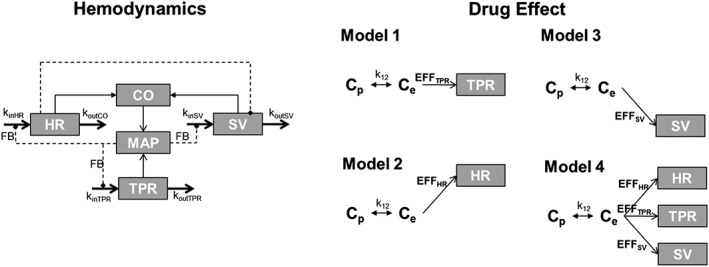
Schematic of different PKPD models used to fit the baclofen‐mediated cardiovascular response. Haemodynamic model utilized in analysis of the data. Model 1 assumes baclofen acts on TPR alone. Model 2 assumes site of action is HR alone. Model 3 assumes drug effect on SV alone. Model 4 allows for drug effect on all endpoints simultaneously. An effect compartment is included to account for differences from plasma exposure at each of the endpoints individually.
In Models 1–3, we explored whether a drug effect applied to each of three possible sites of action to determine if any single effect could account for the observation. In Model 1, drug effect is potentiated by TPR alone (fixed Eff TPR = 0 and Eff SV = 0). Here, the model does not fit well and results in poorly estimated parameters. The baroreflex mechanism predicts that any increase or decrease in MAP will be counteracted by an opposing change in HR, which is inconsistent with the data and leading to poor fit (Figure 5A). In Model 2, the effect is potentiated by HR alone (fixed S SV = 0 and S TPR = 0). Here, the model fits well to the HR data but does not capture the magnitude of observed change in MAP as the baroreflex response again is activated this time minimizing the change in MAP (Figure 5B). In Model 3, the effect is potentiated by SV alone (fixed S HR = 0 and S TPR = 0). Again, the model is not able to capture the change observed in the data or obtain good fit parameters (Figure 5C). Instead, the population model accounts for the change in HR and MAP by increasing the variability (Table 2).
Figure 5.
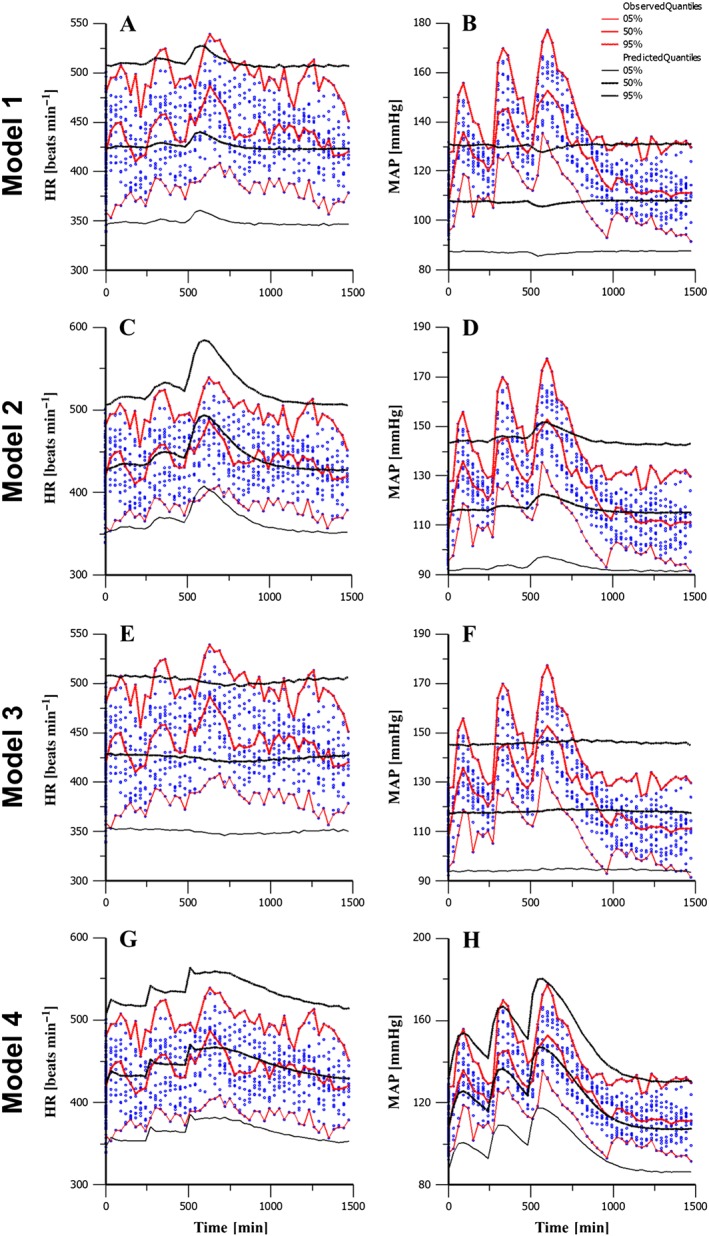
Best fits of the CV population models to HR and MAP observations. Panels (A–B) show HR and MAP best fits resulting from a fit to TPR alone. Panels (C–D) show HR and MAP fits for baclofen effect on HR alone. Panels (E–F) show HR and MAP from drug effect on SV alone. Panels (G–H) show HR and MAP resulting from simultaneous effect on HR, SV and TPR.
Table 2.
Parameters determined from best fit of pharmacodynamic sub‐model
| Parameter | Unit | Model 1 | CV% | Model 2 | CV% | Model 3 | CV% | Model 4 | CV% |
|---|---|---|---|---|---|---|---|---|---|
| koutHR | min−1 | 0.193 | (fix) | 0.193 | (fix) | 0.193 | (fix) | 0.193 | (fix) |
| koutTPR | min−1 | 0.06 | (fix) | 0.06 | (fix) | 0.06 | (fix) | 0.06 | (fix) |
| koutSV | min−1 | 0.0021 | (fix) | 0.0021 | (fix) | 0.0021 | (fix) | 0.0021 | (fix) |
| FB | mmHg−1 | 0.0029 | (fix) | 0.0029 | (fix) | 0.0029 | (fix) | 0.0029 | (fix) |
| HR_SV | – | 0.32 | (fix) | 0.32 | (fix) | 0.32 | (fix) | 0.32 | (fix) |
| FB_MAP | – | −1.98 | (fix) | −1.98 | (fix) | −1.98 | (fix) | −1.98 | (fix) |
| MAPSHR | mmHg | 155 | (fix) | 155 | (fix) | 155 | (fix) | 155 | (fix) |
| CO0 | mL·min−1 | 129 | (fix) | 129 | (fix) | 129 | (fix) | 129 | (fix) |
| TPR0 | mmHg·min·mL−1 | 0.84 | (calc) | 0.91 | (calc) | 0.90 | (calc) | 0.84 | (calc) |
| SV0 | mL | 0.30 | (calc) | 0.30 | (calc) | 0.30 | (calc) | 0.30 | (calc) |
| θHR0 | beats·min−1 | 424 | (fix) | 428 | 2.1 | 430 | 3.5 | 423 | 0.97 |
| θMAP0 | mmHg | 108 | (fix) | 118 | 1.5 | 116 | 2 | 109 | 1.1 |
| STPR | mL·mg−1 | −0.003 | 245 | 0 | (fix) | 0 | (fix) | – | – |
| SHR | mL·mg−1 | 0 | (fix) | 0.0088 | 6 | 0 | (fix) | – | – |
| SSV | mL·mg−1 | 0 | (fix) | 0 | (fix) | 0.0057 | 58 | – | – |
| EmaxTPR | – | – | – | – | – | – | – | 0.167 | 14 |
| EmaxHR | – | – | – | – | – | – | – | 0.111 | 16 |
| EmaxSV | – | – | – | – | – | – | – | 0.054 | 29 |
| IC50 | μg·mL−1 | – | – | – | – | – | – | 2.97 | 13 |
| keq | min−1 | 0.04 | 73 | 0.01 | 44 | 0.011 | 31 | 0.028 | 8.7 |
| ωHR 2 | – | 0.014 | – | 0.0055 | – | 0.007 | – | 0.0048 | – |
| ωMAP 2 | – | 0.003 | – | 0.0024 | – | 0.002 | – | 0.0029 | – |
In Model 4, we allow the drug effect on HR, TPR and SV simultaneously. In this case, the model captures both the magnitude and direction as well as the timing of the effect on HR and MAP (Figure 5D). The population model accounts the degree of variability on the treated animals through baseline differences between individuals. In Model 4, the drug effect on each parameter was incorporated as an Emax effect, which was found to fit significantly better than a linear drug effect (P < 0.001).
Model 4 also produced reasonable parameter estimates (Table 2). The parameters governing drug potency were significantly differentiated from zero, as measured by the 95% confidence intervals because both were greater than two standard deviations above zero. The effect was found to be strongest on TPR and HR, while the effect on SV was smaller. This suggests that the effect on HR and TPR is of greatest importance, with potential for some effect driven through SV. It is worth noting that Model 4 is similar to a model where drug effect is present on the FB parameter itself. We therefore also tried incorporating all three effects as a single drug effect on FB but ruled this model out based on Bayesian information criterion (BIC).
Goodness of fit
Figures 6 illustrates the outcome of best fit for Model 4. Each measurement and prediction of HR and MAP for each individual is included on the plots. The predicted values are plotted along the x‐axis, while the observed values are plotted along the y‐axis. The left panel is for HR, and the right panel is for MAP. The line represents the line of unity, which would occur for a perfect fit with zero error.
Figure 6.
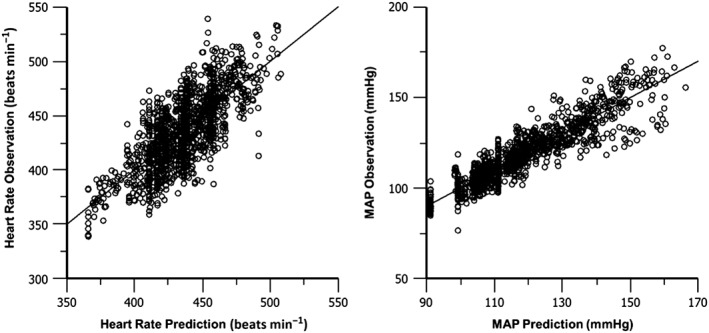
Individual predictions versus the observations of HR and MAP for best fit of Model 4. Each point represents the model prediction for that individual compared against the actual observed data that was measured at that time point.
Discussion and conclusions
This study evaluated mechanisms of baclofen‐induced changes in cardiovascular function using quantitative pharmacology. For this, multiple physiological and biochemical endpoints that contribute to overall cardiovascular function in tandem with pharmacokinetic properties of baclofen were measured. Baclofen increased all measured BPs as well as HR in a dose‐dependent manner. Baclofen also induced changes in body temperature and altered brain EEG function (Figure 3) at the highest dose tested consistent with the known pharmacological activities of this GABAB agonist. There were no physiologically relevant responses observed with plasma chemistries, urine chemistries and drug induced kidney injury biomarkers (see Supporting Information Tables S1–S3 respectively). A mathematical modelling approach supported the hypothesis that baclofen modulates cardiovascular activity by inducing effects on multiple cardiovascular systems.
Quantitative pharmacology approaches can help to define mechanisms of drug activation directly at end target organs or indirectly via feedback mechanisms. The PKPD models published by Snelder et al., 2014 successfully characterized mechanisms of BP regulation as a product of cardiac output and TPR working in parallel to maintain BP homeostasis. Both cardiac output and TPR are subject to modulation by secondary targets including the CNS and renal systems. In the present study, acute baclofen exposure dose‐dependently increased BP and HR. HR data served as a surrogate measure for cardiac output based on the general assumption that SV was relatively unchanged and only small magnitude changes were observed in PP (Figure 2D).
Fitting BP and HR simultaneously to the Snelder model supports the hypothesis that baclofen has activity on both HR and peripheral resistance. The BP response following each dose was rapid and rose sharply and in parallel to measured baclofen concentrations. On the other hand, baclofen induced increases in HR were associated with a delay observed following each dose. The model fits were capable of capturing the timing and magnitude of the effects. Our model demonstrated clear effect of baclofen on TPR, consistent with GABAB agonist mediated activity on the vasculature. Additionally, the model also indicated that baclofen has activity on increasing HR, as well as a much smaller magnitude effect on stroke volume. These two effects are consistent with centrally mediated baclofen activity.
High resolution PKPD data combined with mechanistic systems models provide the opportunity for both retrospective and prospective applications. For example, the present results suggest the ability to separate ‘system specific parameters’ from ‘drug specific parameters’, allowing for the development of testable hypotheses to further explore possible sites of action retrospectively after aggregate pharmacological effects have been observed and quantified. In effect, the model allows for indirect comparison with any previously studied compounds with well characterized effects and known sites of action. On the other hand, there is significant value in drug discovery using such models prospectively to understand what the effect could be in a new, untested, system. For example, predicting expected effects in higher mammalian species based on preclinical observations in the rodent is a frequent application of PKPD in safety pharmacology studies during drug development. In such prospective application, a model can be used to predict behaviour in a new species or setting by readjusting the parameters appropriately based on an understanding of how they differ between systems.
Our observations of increased HR and BP in rat are consistent with recorded clinical observations where patients exposed to the highest doses of baclofen greater than 200 mg presented with frequent hypertension and sometimes tachycardia or bradycardia (Persson and Henning, 1980; Leung et al., 2006). Although not observed in this study, hypotension has been observed in animals (Chahl and Walker 1980; Persson and Henning, 1980; García et al., 2013) and in less than 10% of patients (Leung et al., 2006). It is noteworthy, however, that the concentrations of baclofen measured in this rodent study were significantly higher than those reported to induce hypotension in other rat studies. Future work should be devoted to exploring the predicted effect in man, once a validated version of the Snelder et al. (2014) structure and parameter sets in man can be developed.
The modelling approach worked very well for suggesting possible drug actions of baclofen; however, several assumptions implicit in the model were necessary over the course of the analysis. We did not use a measure of direct cardiac output as these are difficult, costly and time consuming to implement in conscious animals. Instead, we relied on HR as a surrogate for cardiac output as the magnitude of changes in PP observed with baclofen was smaller than changes in MAP. This is consistent with the model fits showing baclofen‐mediated changes in SV may be small and may not contribute as much as effects on TPR and HR to the overall MAP. It should be noted that Snelder et al., 2014 have also used HR and MAP alone to similar effect. An additional assumption in the modelling was to use the previously published model parameters directly in the model fitting process rather than refitting the system parameters to a panel of drug induced responses. This is one of the strengths of a systems model as it allows you to keep the system parameters fixed from experiment to experiment, capturing the changes in drug specific parameters.
BP changes can be mediated centrally as well as peripherally. Central GABAB receptor activation in the spinal cord, brainstem and mid brain nuclei modulate changes in BP via multiple mechanisms including sympathetic activation, vagal stimulation and vasopressin release inducing varied responses in BP depending on the site of activation (Amano and Kubo, 1993; Takenaka et al., 1996; Callera et al., 1999; Zhang et al., 2007; Li and Pan, 2007; Kobuchi et al., 2009; Warner et al., 2011; Yamaguchi and Hama, 2011; Hanafusa et al., 2012; Li et al., 2013; Wang et al., 2013; Rasoulpanah et al., 2014). Activation of GABAB receptors in the brainstem increases BP, while activation in the rostral ventrolateral medulla and higher brain nuclei such as paraventricular, ventral tegmental area and ventral medial hypothalamus either decreases or has no effect on BP. Injecting baclofen in the intra lateral cerebral ventricles particularly in the depressor anterior hypothalamus increases BP via sympathetic nervous activation (Takenaka et al., 1996). Peripheral GABAB receptors directly induce vasodepression in the retina and in the pulmonary vascular bed (Kaye et al., 2004; Hinds et al., 2013). The mechanism underpinning these effects may be related to changes in calcium handling and/or nitric oxide production as evidenced by studies using cell culture systems (Wang et al., 2014). Additionally, GABAB receptors are associated with renal‐induced elevations in BP (Donato et al., 2013) tubular fibrosis and atrophy as well as attenuation of renal nerve‐induced noradrenaline release (Erdö, 1990; Fujimura et al., 1999; Sasaki et al., 2007). In this present study, at the concentrations tested, baclofen had no effect on urine chemistries or a battery of biomarkers of renal injury (see Supporting Information Tables S2 and S3 respectively), consistent with the observations of others showing that concentrations of baclofen higher than those achieved herein were required to induce changes in renal function (Fujimura et al., 1999). Taken together, these data suggest a peripheral mechanism of activation is unlikely and supports a secondary mechanism where baclofen may be acting at multiple central targets to increase BP.
HR can also be regulated at multiple targets of drug action. Stimulation of GABAB receptors in the nucleus of the tractus solitarius induces bradycardia (DiMicco and Monroe, 1998; Callera et al., 1999; Kim et al., 2000). Central injections of baclofen to the intra lateral cerebral ventricles or anterior hypothalamus increase HR. In contrast, direct activation of peripheral GABAB receptors on the heart myocardium and parasympathetic activation decrease HR (Varga and Kunos, 1992; Mendelowitz, 1996; Blackshaw et al., 2000; Lorente et al., 2000). Peripheral activation of GABAB receptors expressed on cardiac sarcolemmal membranes also activate G‐protein‐coupled inward rectifier potassium channels (Kir3 channels) that diminish cardiac excitation (Dirk, 1999; Lorente et al., 2000) supporting a central mechanism of activation. The modelling analysis in this study suggests indirect activation via a secondary site of action as the mechanism of HR increase. Although the study design does not allow for identification and confirmation of baclofen's secondary site of action responsible for HR changes, the paradigm allowed for assessment of multiple possible targets of drug action. In particular, central nervous activity was assessed using EEG. Baclofen at 30 mg·kg−1 induced massive excitation of GABAB receptors as measured using cortical EEG resulting in increased EEG δ and θ activity and diminished α, β and γ activity. Consistent with the EEG signature, animal activity was also diminished for approximately 8 h following administration of baclofen 30 mg·kg−1 (data not shown). The EEG patterns observed in the rat studies are consistent with clinical reports of CNS depression and deep coma‐like state observed in patients presenting in emergency rooms with confirmed baclofen intoxication (Pommier et al., 2014; Leung et al., 2006). Although no significant central nervous effects were observed at the lower doses in the present rat studies, we cannot rule out drug penetration to the brain or spinal pathways that are major sites of CNS modulation of cardiovascular function.
Baclofen also induced instantaneous and prolonged decreases in temperature at the middle and high doses. GABAB receptors inhibited amphetamine and methamphetamine induced hyperthermia as well as prolonged central hyperthermia in a patient with basilar artery occlusion (Bexis et al., 2004; Huang et al., 2009; van Nieuwenhuijzen and McGregor, 2009). Stimulation of GABAB receptors located in the hypothalamus, intermediolateral column and rostral raphe pallidus also alters CBT (Addae et al., 1986; Jackson and Nutt, 1991; Tupone et al., 2014; Quéva et al., 2003). Additionally, CBT and BP share a common sympathetic nervous system activation pathway (Bain et al., 2014). Thus, baclofen can activate thermoregulatory mechanisms through multiple targets in the CNS as well as in the periphery.
Conclusion
The results from the present study indicate that at the doses tested, baclofen induces cardiovascular, CNS and thermoregulatory changes without changes in urine chemistry or biomarkers of renal injury. The changes which we observed are consistent with the pharmacological action of baclofen as a GABAB receptor agonist. A mathematical model fits the baclofen‐mediated changes in MAP and HR to gauge effects on cardiac output and TPR. Results support the hypothesis that baclofen modulates cardiovascular activity via action at multiple sites of action in the cardiovascular system. In addition, this model, developed based on baclofen‐mediated responses in rat, may have predictive value in assessing cardiovascular events occurring in higher mammalian species. While the modelling analysis of the data presented here provides a method to integrate and draw insight from multiple physiological measurements simultaneously, it is only capable of providing insight into endpoints that we have examined directly. The model does not strictly identify the organ(s) mediating the effects per se. Taken together, the data on HR and MAP combined with effects on body temperature argue strongly for the presence of centrally mediated effects. However, without additional experimental intervention data, it is impossible to rule out that some of the effects may be mediated peripherally. This modelling approach represents an additional step toward ways of identifying possible cardiovascular adverse events earlier in preclinical testing phases. Information gleaned from these types of PKPD modelling studies may potentially be leveraged to develop credible and focused hypotheses to investigate possible underpinning mechanisms of action, foster better decision‐making and prepare mitigation plans where necessary before clinical testing, with the eventual aim of improving patient safety. Although additional and more rigorous translational validation studies are required with other cardiovascular agents, this information provides an initial conceptual proof that modelling systems developed using animal models may have predictive value for preclinical risk assessment.
Author contributions
H.K., H.B., D.L., M.‐E.B. and D.S. conducted the research. H.K., J.M. and R.B. designed the research study. H.K. and J.M. analysed the data. H.K., J.M. and R.B. wrote the paper.
Conflict of interest
The authors declare that the research herein was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. AstraZeneca does not sell any drug or device mentioned in this article.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Plasma Chemistries
Table S2 Urine analysis
Table S3 Biomarkers of Kidney Injury
Supporting info item
Acknowledgements
We would like to thank Laboratory Animal Services and Statistical Services for their support. This work was fully funded by AstraZeneca Pharmaceuticals.
Kamendi, H. , Barthlow, H. , Lengel, D. , Beaudoin, M. ‐E. , Snow, D. , Mettetal, J. T. , and Bialecki, R. A. (2016) Quantitative pharmacokinetic–pharmacodynamic modelling of baclofen‐mediated cardiovascular effects using BP and heart rate in rats. British Journal of Pharmacology, 173: 2845–2858. doi: 10.1111/bph.13561.
Contributor Information
Jerome T Mettetal, Email: jay.mettetal@astrazeneca.com.
Russell A Bialecki, Email: russell.bialecki@dupont.com.
References
- Addae JI, Rothwell NJ, Stock MJ, Stone TW (1986). Activation of thermogenesis of brown fat in rats by baclofen. Neuropharmacology 25: 627–631. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain AR, Morrison SA, Ainslie PN (2014). Cerebral oxygenation and hyperthermia. Front Physiol 5: 92. doi: 10.3389/fphys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Phillis BD, Ong J, White JM, Irvine RJ (2004). Baclofen prevents MDMA‐induced rise in core body temperature in rats. Drug Alcohol Depend 74: 89–96. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Smid SD, O'Donnell TA, Dent J (2000). GABA(B) receptor‐mediated effects on vagal pathways to the lower oesophageal sphincter and heart. Br J Pharmacol 130: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH (1999). Activation of GABAA but not GABAB receptors in the NTS blocked bradycardia of chemoreflex in awake rats. Am J Physiol 276: H1902–H1910. [DOI] [PubMed] [Google Scholar]
- Chahl LA, Walker SB (1980). The effect of baclofen on the cardiovascular system of the rat. Br J Pharmacol 69: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins TA, Bergenholm L, Abdulla T, Yates JWT, Evans N, Chappell MJ et al. (2015). Modeling and simulation approaches for cardiovascular function and their role in safety assessment. CPT: Pharmacometrics Syst Pharmacol 4: 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Monroe AJ (1998). GABAB receptors in the dorsomedial hypothalamus and heart rate in anesthetized rats. Brain Res 788: 245–250. [DOI] [PubMed] [Google Scholar]
- Donato V, Pisani GB, Trumper L, Monasterolo LA (2013). Effects of “in vivo” administration of baclofen on rat renal tubular function. Eur J Pharmacol 715: 117–122. [DOI] [PubMed] [Google Scholar]
- Erdö SL (1990). Baclofen binding sites in rat kidney. Eur J Pharmacol 184: 305–309. [DOI] [PubMed] [Google Scholar]
- Fujimura S, Shimakage H, Tanioka H, Yoshida M, Suzuki‐Kusaba M, Hisa H et al. (1999). Effects of GABA on noradrenaline release and vasoconstriction induced by renal nerve stimulation in isolated perfused rat kidney. Br J Pharmacol 127: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MC, Godoy YC, Celuch SM (2013). Impaired hypotensive responses induced by intrathecally injected drugs in fructose‐fed rats. Eur J Pharmacol 706: 17–24. [DOI] [PubMed] [Google Scholar]
- Hanafusa N, Okamoto K, Takatori S, Kawasaki H (2012). Involvement of hypothalamic periventricular GABAergic nerves in the central pressor response to clonidine in freely‐moving conscious rats. J Pharmacol Sci 118: 382–390. [DOI] [PubMed] [Google Scholar]
- Huang YS, Hsiao MC, Lee M, Huang YC, Lee JD (2009). Baclofen successfully abolished prolonged central hyperthermia in a patient with basilar artery occlusion. Acta Neurol Taiwan 18: 118–122. [PubMed] [Google Scholar]
- Jackson HC, Nutt DJ (1991). Inhibition of baclofen‐induced hypothermia in mice by the novel GABAB antagonist CGP 35348. Neuropharmacology 30: 535–538. [DOI] [PubMed] [Google Scholar]
- Kamendi HW, Brott DA, Chen Y, Litwin DC, Lengel DJ, Fonck C et al. (2010). Combining radiotelemetry and automated blood sampling: a novel approach for integrative pharmacology and toxicology studies. J Pharmacol Toxicol Methods 62: 30–39. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Koh HC, Kang JS, Lee H, Shin IC, Om SA et al. (2000). Mediation of the cardiovascular response to spinal gamma‐aminobutyric acid(B) receptor stimulation by adenosine A(1) receptors in anesthetized rats. Neurosci Lett 296: 153–157. [DOI] [PubMed] [Google Scholar]
- Kobuchi S, Shintani T, Sugiura T, Tanaka R, Suzuki R, Tsutsui H et al. (2009). Renoprotective effects of gamma‐aminobutyric acid on ischemia/reperfusion‐induced renal injury in rats. Eur J Pharmacol 623: 113–118. [DOI] [PubMed] [Google Scholar]
- Leung NY, Whyte IM, Isbister GK (2006). Baclofen overdose: defining the spectrum of toxicity. Emerg Med Australas 18: 77–82. [DOI] [PubMed] [Google Scholar]
- Li B, Liu Q, Xuan C, Guo LL, Shi R, Zhang Q et al. (2013). GABAB receptor gene transfer into the nucleus tractus solitarii induces Ccronic blood pressure elevation in normotensive rats. Circ J 77: 2558–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente P, Lacampagne A, Pouzeratte Y, Richards S, Malitschek B, Kuhn R et al. (2000). Gamma‐aminobutyric acid type B receptors are expressed and functional in mammalian cardiomyocytes. Proc Natl Acad Sci U S A 97: 8664–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D (1996). Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am J Physiol 271: H2609–H2614. [DOI] [PubMed] [Google Scholar]
- Perry HE, Wright RO, Shannon MW, Woolf AD (1998). Baclofen overdose: drug experimentation in a group of adolescents. Pediatrics 101: 1045–1048. [DOI] [PubMed] [Google Scholar]
- Persson B, Henning M (1980). Effect of GABA analogues on blood pressure and central GABA metabolism in the rat. Acta Pharmacol Toxicol (Copenh) 47: 135–143. [DOI] [PubMed] [Google Scholar]
- Pommier P, Debaty G, Bartoli M, Viglino D, Carpentier F, Danel V et al. (2014). Severity of deliberate acute baclofen poisoning: a nonconcurrent cohort study. Basic Clin Pharmacol Toxicol 114: 360–364. [DOI] [PubMed] [Google Scholar]
- Quéva C, Bremner‐Danielsen M, Edlund A, Ekstrand AJ, Elg S, Erickson S et al. (2003). Effects of GABA agonists on body temperature regulation in GABA(B(1))−/− mice. Br J Pharmacol 140: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Tohda C, Kim M, Yokozawa T (2007). Gamma‐aminobutyric acid specifically inhibits progression of tubular fibrosis and atrophy in nephrectomized rats. Biol Pharm Bull 30: 687–691. [DOI] [PubMed] [Google Scholar]
- Snelder N, Ploeger BA, Luttringer O, Rigel DF, Webb RL, Feldman D et al. (2013). PKPD modelling of the interrelationship between mean arterial BP, cardiac output and total peripheral resistance in conscious rats. Br J Pharmacol 169: 1510–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelder N, Ploeger BA, Luttringer O, Rigel DF, Fu F, Beil M et al. (2014). Drug effects on the CVS in conscious rats: separating cardiac output into heart rate and stroke volume using PKPD modeling. Br J Pharmacol 171: 5076–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF (2014). Autonomic regulation of brown adipose tissue thermogenesis in health & disease: Potential clinical applications for altering BAT thermogenesis. Front Neurosci 8: 14. doi: 10.3389/fnins.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhuijzen PS, McGregor IS (2009). Sedative and hypothermic effects of gamma‐hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry. Drug Alcohol Depend 103: 137–147. [DOI] [PubMed] [Google Scholar]
- Varga K, Kunos G (1992). Inhibition of baroreflex bradycardia by ethanol involves both GABAA and GABAB receptors in the brainstem of the rat. Eur J Pharmacol 214: 223–232. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Wang W, Patel KP, Rozanski GJ, Zucker IH (2013). Spinal cord GABA receptors modulate the exercise pressor reflex in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 305: R42–R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner L, Yin M, Glaser KJ, Woollard JA, Carrascal CA, Korsmo MJ et al. (2011). Noninvasive in vivo assessment of renal tissue elasticity during graded renal ischemia using MRelastography. Invest Radiol 46: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH et al. (1998). Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396: 679–682. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Hama H (2011). Changes in vasopressin release and autonomic function induced by manipulating forebrain GABAergic signaling under euvolemia and hypovolemia in conscious rats. Endocr J 58: 559–573. [DOI] [PubMed] [Google Scholar]
- Zhang W, Herrera‐Rosales M, Mifflin S (2007). Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius. Hypertension 49: 659–663. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Sasaki S, Uchida A, Fujita H, Nakamura K, Ichida T et al. (1996). GABAB‐ergic stimulation in hypothalamic pressor area induces larger sympathetic and cardiovascular depression in spontaneously hypertensive rats. Am J Hypertens 9 (10 Pt 1): 964–972. [DOI] [PubMed] [Google Scholar]
- Amano M, Kubo T (1993). Involvement of both GABAA and GABAB receptors in tonic inhibitory control of blood pressure at the rostral ventrolateral medulla of the rat. Naunyn Schmiedebergs Arch Pharmacol 348: 146–153. [DOI] [PubMed] [Google Scholar]
- Rasoulpanah M, Kharazmi F, Hatam M (2014). Evaluation of GABA receptors of ventral tegmental area in cardiovascular responses in rat. Iran J Med Sci 39: 374–381. [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL (2007). Role of gamma‐aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320: 615–626. [DOI] [PubMed] [Google Scholar]
- Dirk JS (1999). Structure and function of cardiac potassium channels. Cardiovasc Res 42: 377–390. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhen‐Ying Cheng Z, Schmid KL (2014). GABAB receptors expressed in human aortic endothelial cells mediate intracellular calcium concentration regulation and endothelial nitric oxide synthase translocation. BioMed Res Int Article ID 871735. doi: 10.1155/2014/871735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye AD, Hoover JM, Baber SR, Ibrahim IN, Fields AM (2004). Analysis of gamma‐aminobutyric acid‐mediated responses in the pulmonary vascular bed of the cat. Anesth Analg 99: 758–763. [DOI] [PubMed] [Google Scholar]
- Hinds K, Monaghan KP, Frolund BJ, McGeown G, Curtis TM (2013). GABAergic control of arteriolar diameter in the rat retina. Invest Ophthalmol Vis Sci 54: 6798–6805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Plasma Chemistries
Table S2 Urine analysis
Table S3 Biomarkers of Kidney Injury
Supporting info item


