Abstract
Background
Despite cannabis being the most widely used illicit substance in the United States, individuals diagnosed with cannabis use disorder have few well-researched, affordable treatment options available to them. Though found to be effective for improving treatment outcomes in other drug populations, exercise is an affordable and highly accessible treatment approach that has not been routinely investigated in cannabis users.
Objectives
The aim of this paper is to inform the topic regarding exercise’s potential as an adjunctive treatment for individuals with cannabis use disorder.
Methods
We reviewed the evidence surrounding cannabis use and its current treatment in the United States, explored the rationale for including exercise in the treatment of substance use disorders, and in particular, proposed a biological mechanism (i.e., endocannabinoids) that should be examined when utilizing exercise for the treatment of cannabis use disorder.
Results
Cannabis use is widespread and increasing in the United States. Chronic, heavy cannabis use may dysregulate the endogenous cannabinoid system, which has implications for several psychobiological processes that interact with the endocannabinoid system such as reward processing and the stress response. Given that exercise is a potent activator of the endocannabinoid system, it is mechanistically plausible that exercise could be an optimal method to supplement cessation efforts by reducing psychophysical withdrawal, managing stress, and attenuating drug cravings.
Conclusion
We suggest there is a strong behavioral and physiological rationale to design studies which specifically assess the efficacy of exercise, in combination with other therapies, in treating cannabis use disorder. Moreover, it will be especially important to include the investigation of psychobiological mechanisms (e.g., endocannabinoids, hippocampal volume) which have been associated with both exercise and substance use disorders to examine the broader impact of exercise on behavioral and physiological responses to treatment.
1. Cannabis Use in the United States
At present, cannabis is the most commonly used illicit drug in the United States (1). Unlike many other illicit substances, its use is rapidly increasing, which has contributed to cannabis use disorder (CUD) diagnoses doubling in the past decade (2). This may be a result of relaxing public opinion surrounding cannabis, increased legalization, or the greater availability and relatively low cost of synthetic cannabis derivatives (e.g., K2, “Spice”) (3). Since synthetic cannabinoids are fairly new and frequently evolving drugs, their long-term effects on the brain have yet to be established; however, some preliminary reports indicate that their psychoactive potency and potential for abuse is much greater than that of naturally grown cannabis (3). Keeping these trends in mind, it is likely that there will be a further increase in individuals who develop CUD in the future.
In 2014, an estimated 4.2 million people had CUD, but less than a quarter of these individuals received treatment for their condition (1). Reasons for not receiving treatment include an unwillingness to give up cannabis use, inadequate healthcare cost coverage, fear of stigmatization, loss of reputation, perceived criminal and socioeconomic consequences, and limited access to treatment facilities (1). Of the small percentage of individuals who do receive treatment, the most effective approaches (e.g., behavioral and motivational therapy) yield a modest 50 percent 2-week abstinence rate, and less than half of patients will continue to remain abstinent by 12 months (4). Thus, there is a need for efficacious treatment options which have the potential to promote both immediate and long-term abstinence.
The National Institute on Drug Abuse (NIDA) states that in order to be effective, substance use treatment needs to be readily accessible, attend to multiple needs of the individual, address comorbid psychological concerns (e.g., stress reactivity, depression), and be multi-faceted, including group and individual therapy in combination with other approaches such as pharmacotherapy (5). Because it is low cost, readily accessible, and does not come with a surrounding stigma or adverse side effects, exercise could be a promising adjunctive treatment approach to promote sustained abstinence among CUD patients.
2. General Benefits of Exercise
Because there are several physical and psychological benefits associated with regular physical activity participation, there is a strong rationale for including exercise in substance use treatment programs. Individuals with substance use disorders (SUDs) report that a major contributor to their protracted drug use is avoidance of withdrawal (6), which is characterized by a cluster of physical and psychological symptoms including craving, negative affect, anhedonia, irritability, difficulty sleeping, headaches, appetite changes, nausea, and sweating (7,8). SUD patients also report that a combination of stress, anxiety, drug-relevant cues, boredom, and lack of positive engagement in relationships and the work environment are additional factors contributing to relapse (9–11). In non-substance use populations, exercise has been shown to address many of the aforementioned psychological, physical, and behavioral factors that contribute to relapse without introducing unwanted side effects, so it is possible that exercise could have positive effects on treatment outcomes for SUD patients (12).
For instance, exercise has been shown to mitigate many of the psychiatric disorders, such as clinical depression and anxiety, which are comorbid with continued drug use as well as drug abstinence (13). Exercise can also improve sleep quality (14), reduce drug craving (15), and manage physical (e.g., cortisol and catecholamines) and psychological (e.g., tension and anxiety) manifestations of stress (16). Behaviorally, regular exercise participation has also been shown to increase self-efficacy (17), increase social engagement (18), and may serve as a behavioral replacement, reducing exposure to former environmental and drug-related cues (12).
3. Exercise in the Treatment of Cannabis Use Disorder
To date, most of the research investigating exercise as a treatment for SUDs has been conducted in alcohol and tobacco use populations. Though there is substantial variability in the types of studies (e.g., survey, cross-sectional, randomized controlled trials (RCTs)) and populations (e.g., adolescents, overweight or obese individuals, women only) investigated in this area, there is a broad general consensus that physical activity participation and/or exercise interventions are associated with reduced rates of substance use, attenuated withdrawal symptoms, enhanced mood outcomes, improved treatment retention, and increased rates of short- and long-term abstinence (for reviews, see (12,19–22)). However, investigations that have included or specifically recruited CUD patients have been notably sparse, which is unfortunate because CUD comprises 17% of inpatient admissions, third behind alcohol (41%) and opiate (20%) admissions (23). Only four studies, just one of which was an RCT, have included (or reported on) individuals with CUD. Flemmen et al. (2014) found that depressive symptoms decreased in a small sample of SUD inpatients (75 percent of the sample reported smoking cannabis as either their primary or secondary use disorder) after 8 weeks of high intensity interval training compared to a usual care control group (24). Other than this initial study, no other RCTs have included or reported on the results regarding CUD patients in their studies. There are, however, a handful of non-RCTs that include CUD patients, and their preliminary results suggest that exercise can enhance treatment outcomes for this population.
Roessler (2010) conducted a 2-month long exercise training pilot study on 38 outpatient SUD patients. The participants reported using a variety of drugs, and 51 percent of the sample reported cannabis use. Participants exercised in small groups under the supervision of a trainer for two hours, three times per week. Exact adherence rates were not reported, but only 20 of the initial 38 participants (52 percent) completed the 2 months of training. At the end of the program, the remaining 20 participants reported decreases in craving, decreases in feeling a lack of control over substance use, reduced drug tolerance, and fewer depressive periods (25).
Brown et al. (2010) also conducted a pilot study to determine the feasibility of a 12-week moderate intensity aerobic exercise program in an outpatient setting among individuals with SUDs (31 percent cannabis). A total of 16 participants, who were currently inactive and in ongoing substance use treatment, were required to attend once weekly, moderate intensity group exercise sessions, and were prescribed 2–3 additional moderate intensity sessions to be completed at home. Participants attended approximately 9 of the 12 supervised sessions and averaged 4 days of exercise per week. Overall, there was a significant increase in percent days abstinent by the end of the trial, and they also found that non-attenders (people who completed less than 75 percent of the supervised sessions) were 80 percent likely to relapse by the 3-month follow-up compared to a 20 percent relapse rate among attenders (>75 percent attendance) (26). Though pilot studies, both Roessler (2010) and Brown et al. (2010) lacked control groups and substance-specific analyses (e.g., assessed outcomes specific to cannabis), so it is impossible to estimate the treatment potential of exercise among CUD patients in these trials.
Finally, only one small study has investigated the effects of a brief aerobic exercise trial among individuals who consumed solely cannabis. Buchowski and colleagues (2011) found that 10 days of moderate intensity aerobic exercise for 30 minutes resulted in significantly reduced levels of cannabis consumption and daily cravings in 12 non-treatment-seeking individuals (15). Furthermore, cannabis use remained significantly lower at a 2-week follow-up compared to baseline use. Without a control group, it is impossible to attribute the results to exercise alone, but this study provides a basis for further investigation of exercise’s effects in this population.
In sum, there is accumulating evidence that exercise may be an effective adjunctive approach when treating CUD; however, much more research is needed to match the level and the scientific degree of the work that has been conducted in tobacco and alcohol use populations. Based on the data provided by numerous RCTs in the SUD area, a recent meta-analysis found that exercise was effective in increasing abstinence rates and attenuating anxiety and depression symptoms in tobacco, alcohol, and illicit substance use (mostly heroin/opioid) populations. Notably, exercise’s effects on abstinence were significantly greater in the opioid studies compared to the alcohol and tobacco studies. The authors speculated that activation of the endogenous opioid system by exercise may have alleviated withdrawal and provided a superior psychobiological substitute for those who used heroin compared to those who used tobacco or alcohol, which primarily affect non-opioid (e.g., acetylcholinergic and GABAergic) pathways (19). Along a similar vein, exercise interventions conducted with CUD patients and which include investigation of a psychobiological mechanism are an appropriate and intriguing next area of research, as exercise has been shown to repeatedly and robustly activate the endocannabinoid (eCB) system in healthy individuals (27–32).
4. The Endocannabinoid System
The endocannabinoid (eCB) system is an expansive neuromodulatory network found throughout the central and peripheral nervous systems as well as other tissues (e.g., adipose, skeletal muscle). It derives its name from Cannabis sativa (marijuana) because cannabis’s main psychoactive constituent, Δ9-tetrahydrocannabinol (THC), led to the discovery of the cannabinoid-1 receptor (CB1) (33). In addition, scientists have isolated and routinely investigated two main eCBs, N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) (34,35). ECBs and CB1 receptors are heavily expressed in areas that have been implicated in reward processing, behavioral reinforcement, and emotional regulation (e.g., the limbic system including the striatum) (36). In particular, CB1 receptors are densely populated on GABAergic neurons that are co-localized with dopamine neurons in these brain regions (37). In general, eCB transmission suppresses synaptic activity, but the net effects of eCB signaling can either be inhibitory or excitatory due to its actions on either glutamatergic (excitatory) or GABAergic (inhibitory) neurons (38). For instance, CB1 agonism either by AEA, 2-AG, or THC (a partial agonist) reduces inhibitory GABAergic output to dopamine neurons in the ventral tegmental area and substantia nigra pars compacta, thereby acutely increasing dopaminergic activity in reward-related pathways (37).
In animals, the eCB system has been found to regulate voluntary wheel running, which many researchers consider to be a reinforcing behavior in rodents (39–41). Rodents that are lacking CB1 receptors or have their CB1 receptors blocked engage in 30–40% less wheel running than control animals (42). This reduction has been found to be specifically related to the motivational aspects of wheel running (43) and is likely mediated through eCB-induced inhibition of GABA, which, in turn, permits a net increase of dopamine transmission in reward-processing brain regions (44). In addition, blocking or genetically mutating CB1 receptors located on GABAergic neurons in mice also appears to negate the anxiolytic effects of an acute bout of exercise, suggesting that the eCB system could also affect running behaviors by influencing the psychological rewards that accompany exercise (45).
Initial evidence indicates that the eCB system also responds to exercise in humans. In particular, just 30–45 minutes of aerobic exercise performed at 70–80% of one’s maximum, which is a commonly prescribed dose for a single exercise bout, has been found to increase circulating plasma eCB concentrations, particularly AEA, anywhere from two- to threefold from baseline levels (27–29). Moreover, increases in plasma AEA after exercise have been found to correlate with improvements in positive affect in a small sample of recreationally fit humans, though more research needs to be conducted on mood outcomes associated with eCB increases during and after exercise (28). To date, no acute aerobic exercise studies have reported significant increases in 2-AG. However, Koltyn and colleagues (2014) found that 3 minutes of submaximal, isometric exercise resulted in significant elevations of both plasma AEA and 2-AG, suggesting that the type of exercise stimulus may have differential impacts on eCB signaling (32).
Almost no research has been conducted to directly examine the effects of chronic exercise training on the eCB system in humans, and the few studies that have examined eCB levels as a secondary aim of their projects have found conflicting results (46–49). Relevant to its presence in reward-processing brain regions, though, one study found that plasma AEA concentrations were significantly lower at baseline, after acute exercise, and after 2 weeks of inactivity in runners categorized as “exercise dependent” (based on the Exercise Dependence Scale, which incorporates the DSM-IV criteria for substance abuse and dependence) compared to runners who did not meet the criteria for exercise dependence (50). Similarly, individuals with substance use disorders have been found to have lower baseline levels of AEA than healthy controls (51,52).
In addition to its presence in reward-processing brain regions, the eCB system has also been located on the hippocampus, amygdala, and the hypothalamic-pituitary-adrenal (HPA) axis, implicating its involvement in the stress response. Multiple lines of evidence suggest that the eCB system is involved in acute stress responses and chronic stress adaptations (53). Acutely, diminished concentrations of AEA or administration of a CB1 antagonist increases downstream HPA axis activity and cortisol concentrations; i.e., the suppression of eCB activity releases the inhibitory restraint on the HPA axis (54–56). Conversely, increasing AEA levels or administering a CB1 agonist generally suppresses HPA axis activity and reduces cortisol concentrations (57). Unlike the fast actions of AEA in regulating the stress response, 2-AG may have a larger role in long-term stress responses and adaptations. For example, chronic, unpredictable stress increases 2-AG tissue content in the amygdala (55,58) and reduces 2-AG content and CB1 expression in the hippocampus (59), both of which are associated with blunted corticosterone responses to acute stress (58). Because the amygdala typically excites the HPA axis while the hippocampus suppresses it, both of these 2-AG adaptations would serve to dampen the HPA axis in response to chronic stress.
In humans, maladaptive stress responses are observed in several stress-related psychological disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD). Likewise, eCB dysfunction has also been observed in many of these patient populations (60). Another patient population which experiences considerable amounts of stress are individuals going through drug withdrawal and sustained drug abstinence, and they cite stress as a major cause of relapse (9,61).
5. The Endocannabinoid System in CUD
CUD is a chronic, recurring disorder, with more than half of patients relapsing within a year of achieving initial abstinence (4). Koob and LeMoal (2008) posit that drug relapse is the result of both a decline in normal reward functioning, leading to depression and anhedonia, and an increase in “anti-reward” processes, leading to heightened stress, anxiety, and physical symptoms. In particular, they suggest that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis is partially responsible for aversive and prolonged withdrawal states (8). In alignment with this theory, it is commonly reported that heightened levels of perceived stress and anxiety contribute to relapse (9,61). Newly-abstaining individuals have higher basal levels of adrenocorticotropic hormone (ACTH) and cortisol as well as blunted ACTH/cortisol responses to stress-inducing laboratory procedures compared to controls (62). While the main contributors to HPA axis dysfunction among SUD patients remain elusive, an impaired eCB system in these individuals is a possible candidate mechanism due to its influence over the stress response.
With long-term, heavy cannabis use, the eCB system becomes dysregulated in both animals and humans. In animals, it has been found repeatedly that chronic exposure to cannabis downregulates CB1 receptors (63); however, only a handful of studies have investigated the effects of substance use on the eCB system in humans. One investigation found that basal plasma concentrations of AEA were significantly reduced in alcohol use disorder (AUD) patients compared to moderate, social drinkers and that higher AEA concentrations were associated with less alcohol craving (51). Similarly, Morgan et al. (2013) found that AEA levels were significantly reduced in the cerebrospinal fluid of heavy cannabis users (approx. 27 times per month) compared to light users (approx. 4 times per month) (52). Muhl et al. (2014) found that AEA was increased in the serum of individuals who had used cannabis at least 20 times in their lifetimes compared to those who had consumed it less than 5 times; however, all of the study participants were abstinent from cannabis use for at least 6 months, and the cannabis use grouping cutoffs (> 20 times or < 5 times) were based on the increased likelihood of developing schizophrenia with heavier cannabis use. Other than lifetime occurrences, no other details concerning the amount of cannabis consumption prior to abstinence were provided, so it is not clear whether this group division represents a clinically meaningful difference in cannabis use (outside of examining its relation to schizophrenia), especially after extended abstinence (64). Finally, Goodwin et al. (2012) found that in individuals with heavy cannabis use there was a positive correlation between SR141716 (a CB1 inverse agonist) plasma concentrations and increases in plasma cortisol from baseline levels. There was also a significant negative correlation between plasma cortisol and plasma THC (an exogenous CB1 partial agonist) levels (65). Collectively, these studies suggest that the eCB system may be downregulated with substance use which may explain, in part, the findings of elevated ACTH and/or blunted cortisol reactivity in those with SUDs (62).
Outside of measuring circulating eCB levels, brain-imaging studies suggest that CB1 receptors are also affected by long-term drug use. Both post-mortem (66) and in-vivo studies (67,68) have found that there are significant decreases in the density and binding potential of CB1 receptors in the hippocampus, ventral tegmental area, and the substantia nigra among chronic consumers of cannabis compared to age-matched controls. It has also been found that four weeks of inpatient abstinence results in CB1 receptor density returning to control levels in all areas except the hippocampus (68). In AUD patients, Hirvonen et al. (2013) and Ceccarini et al. (2014) also found that CB1 receptors were downregulated in several brain regions (e.g., hippocampus, ventral striatum, caudate, putamen, hypothalamus) (69,70). Thus, brain imaging studies suggest that CB1 receptors are aberrant in SUD patients in several brain regions associated with reward processing and the stress response. The consistent finding of altered CB1 receptors in the hippocampus is of particular concern because of its input to the HPA axis. There is evidence that chronic stress conditions and SUDs are associated with decreased hippocampal volume, sometimes in an additive manner (71–73). Aerobic exercise is one stimulus that has been shown to promote CB1 receptor-mediated neurogenesis in the hippocampus of animals (74,75); however, this is only one potential mechanism out of many purported mechanisms (e.g., brain-derived neurotrophic factor changes, histological changes [increase in DCX newborn neurons, glial cells]), for exercise’s effects on the maintenance of hippocampal gray matter volume (30,76). It is unknown whether these findings will eventually translate to human SUD patients, and if so, whether any observed changes in the hippocampus will meaningfully contribute to relevant behavioral outcomes such as long-term stress management and relapse prevention.
Through its activation of the eCB system, exercise may also aid cessation efforts by acting as a form of agonist therapy. Agonist therapies, such as methadone or buprenorphine therapy for opioid use disorder or nicotine-replacement therapy for tobacco use disorder, aid SUD patients through the withdrawal period so that other forms of treatment (e.g., CBT) can be more effective. Compared to a placebo, CB1 agonists have been found to dose-dependently reduce cannabis withdrawal symptoms in the laboratory in small samples of non-treatment seeking cannabis consumers (77,78). Extending these laboratory findings, RCTs have investigated the effects of CB1 agonist therapy on treatment outcomes for cannabis and have found that CB1 agonists result in higher treatment retention rates and reduced severity and duration of cannabis withdrawal symptoms (79,80).
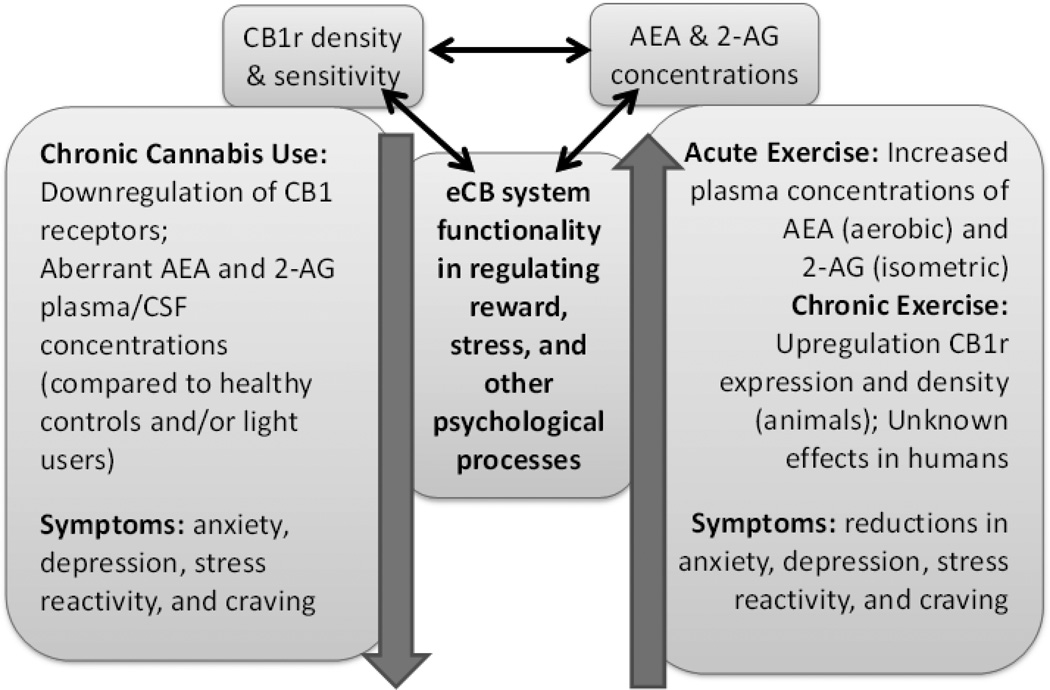
Outside of exogenous therapies, no human studies have examined the effects of endogenous cannabinoid (i.e., AEA, 2-AG) enhancement on substance use outcomes. The results from animal studies, though, provide evidence to suggest that eCB augmentation may be useful. These studies typically use a fatty acid amide hydrolase (FAAH) inhibitor, which metabolizes AEA, to increase circulating AEA concentrations. Chauvet et al. (2014) found that in rats experiencing withdrawal, administration of an FAAH inhibitor significantly reduced drug-seeking behaviors and cue-induced reinstatement (a laboratory measure of relapse) compared to a vehicle solution. It was also reported that stress-induced reinstatement through administration of yohimbine, a pharmacological substance which is anxiogenic in rodents, was significantly lower in the FAAH inhibitor-treated animals compared to the vehicle-treated animals (81). Justinova et al. (2015) observed similar results in non-human primates in that an FAAH inhibitor reduced levels of drug-primed and cue-primed reinstatement after an extinction period (82). Conversely, Cippitelli et al. (2008) found that an FAAH inhibitor had no effects compared to a vehicle on cue or stress-induced (yohimbine) ethanol reinstatement. However, they did find that the FAAH inhibitor eliminated the anxiogenic effects of acute ethanol administration during the withdrawal period, suggesting that FAAH inhibitors may still confer positive psychological outcomes during abstinence despite having no effect on reinstatement (83). In sum, emerging animal evidence indicates that endogenous cannabinoid enhancement may reduce cue- and stress-induced reinstatement, which is an animal model for relapse in humans. Therefore, augmenting AEA levels through exercise in humans, wherein the pharmacological manipulation of endogenous cannabinoids is not currently possible, could have similar benefits on preventing relapse in newly-abstaining SUD patients. See Figure 1 for a schematic of the hypothetical relationships between the eCB system, CUD, and exercise.
Figure 1. Schematic of the relationships between the endocannabinoid system, chronic cannabis use, and exercise.
It is proposed that chronic, heavy cannabis use dysregulates the endocannabinoid (eCB) system, which is highlighted by a downregulation of CB1 receptors (CB1r) in several brain regions including the hippocampus, substantia nigra, and ventral striatum, which are regions implicated in reward and stress processing. Changes in eCB physiology as a result of cannabis use could contribute to psychological outcomes including heightened anxiety, depression, stress reactivity, and craving. Conversely, acute exercise in humans has been shown to increase circulating levels of anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and chronic exercise in animals has been found to upregulate CB1 receptors, most notably in the hippocampus. ECB changes have been associated with beneficial psychological outcomes including reductions in anxiety and increases in positive affect. Exercise has also been shown to relieve anxiety, depression, and craving in a variety of substance use populations. More research needs to be conducted to further develop these proposed relationships.
6. Conclusion
Cannabis use is rapidly increasing in the United States, and treatment options for the growing number of individuals who will go on to develop CUD are severely understudied. The results from several RCTs suggest that regular exercise may be efficacious in combination with other treatment approaches to promote sustained abstinence in tobacco and alcohol use populations. Unfortunately, very few studies have investigated the effects of exercise in other prominent SUD populations, such as CUD patients. Along with its low cost, low stigma, and high accessibility, exercise may be a useful treatment option for cannabis users due to its effects on the eCB system. The eCB system appears to be impaired in CUD patients, and this may contribute to their heightened experience of stress, aberrant cortisol patterns, and prolonged relapse vulnerability. ECBs released during exercise may not only help restore eCB function in brain regions that regulate reward and stress processes but also act as a form of agonist therapy which could dampen residual withdrawal symptoms, increase treatment retention, and promote long-term abstinence.
References
- 1.Center for Behavior Health Statistics and Quality. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50) [Google Scholar]
- 2.Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72:1. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills B, Yepes A, Nugent K. Synthetic Cannabinoids. Am. J. Med. Sci. 2015;350:59–62. doi: 10.1097/MAJ.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 4.Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute on Drug Abuse. National Institutes of Health; U.S. Department of Health and Human Services. Treatment Approaches for Drug Addiction. [Revised January 2016]; Retrieved from http://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
- 6.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorelick DA, Levin KH, Copersino ML, Heishman SJ, Liu F, Boggs DL, Kelly DL. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend. 2012;123:141–147. doi: 10.1016/j.drugalcdep.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 9.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 10.Hendershot CS, Witkiewitz K, George WH, Marlatt GA. Relapse prevention for addictive behaviors. Subst Abuse Treat Prev Policy. 2011;6:17. doi: 10.1186/1747-597X-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smit ES, Hoving C, Schelleman-Offermans K, West R, de Vries H. Predictors of successful and unsuccessful quit attempts among smokers motivated to quit. Addict Behav. 2014;39:1318–1324. doi: 10.1016/j.addbeh.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Linke SE, Ussher M. Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am J Drug Alcohol Abuse. 2015;41:7–15. doi: 10.3109/00952990.2014.976708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn AL, Jewell JS. The effect of exercise on mental health. Curr Sports Med Rep. 2010;9:202–207. doi: 10.1249/JSR.0b013e3181e7d9af. [DOI] [PubMed] [Google Scholar]
- 14.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38:427–449. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 15.Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6:e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsatoulis A, Fountoulakis S. The protective role of exercise on stress system dysregulation and comorbidities. Ann N.Y. Acad Sci. 2006;1083:196. doi: 10.1196/annals.1367.020. [DOI] [PubMed] [Google Scholar]
- 17.McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci Rev. 2000;28:85–88. [PubMed] [Google Scholar]
- 18.Kirkcaldy BD, Shephard RJ, Siefen RG. The relationship between physical activity and self-image and problem behaviour among adolescents. Soc Psychiatry Psychiatr Epidemiol. 2002;37:544–550. doi: 10.1007/s00127-002-0554-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Wang Y, Wang Y, Li R, Zhou C. Impact of physical exercise on substance use disorders: a meta-analysis. PLoS One. 2014;9:e110728. doi: 10.1371/journal.pone.0110728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zschucke E, Heinz A, Ströhle A. Exercise and physical activity in the therapy of substance use disorders. Scientific World Journal. 2012;2012:901741. doi: 10.1100/2012/901741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2012;1:CD002295. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: 2010. Treatment Episode Data Set (TEDS). 1998 – 2008. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-50, HHS Publication No. (SMA) 09-4471. [Google Scholar]
- 24.Flemmen G, Unhjem R, Wang E. High-intensity interval training in patients with substance use disorder. Biomed Res Int. 2014;2014:616935. doi: 10.1155/2014/616935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roessler KK. Exercise treatment for drug abuse--a Danish pilot study. Scand J Public Health. 2010;38:664–669. doi: 10.1177/1403494810371249. [DOI] [PubMed] [Google Scholar]
- 26.Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart GG, Dubreuil ME, Gordon AA. A Pilot Study of Aerobic Exercise as an Adjunctive Treatment for Drug Dependence. Ment Health Phys Act. 2010;3:27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- 28.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner”s high’. J Exp Biol. 2012;215:1331–1336. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- 29.Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2013;113:869–875. doi: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- 30.Heyman E, Gamelin F-X, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Feuerecker M, Hauer D, Toth R, Demetz F, Hölzl J, Thiel M, Kaufmann I, Schelling G, Choukèr A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur J Appl Physiol. 2012;112:2777–2781. doi: 10.1007/s00421-011-2237-0. [DOI] [PubMed] [Google Scholar]
- 32.Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15:1294–1304. doi: 10.1016/j.jpain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 34.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 35.Sugiura T, Kodaka T, Kondo S, Tonegawa T, Nakane S, Kishimoto S, Yamashita A, Waku K. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma × glioma hybrid NG108-15 cells. Biochem Biophys Res Commun. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- 36.Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- 39.Fuss J, Gass P. Endocannabinoids and voluntary activity in mice: runner’s high and long-term consequences in emotional behaviors. Exp Neurol. 2010;224:103–105. doi: 10.1016/j.expneurol.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011;61:1109–1122. doi: 10.1016/j.neuropharm.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brené S, Bjørnebekk A, Aberg E, Mathé AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen EB, Hillman C. Naloxone and rimonabant reduce the reinforcing properties of exercise in rats. Exp Clin Psychopharmacol. 2011;19:389–400. doi: 10.1037/a0024142. [DOI] [PubMed] [Google Scholar]
- 44.De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G, Usiello A, Centonze D. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, et al. A runner’s high depends on cannabinoid receptors in mice. Proc Natl Acad Sci U S A. 2015;112(42):13105–13108. doi: 10.1073/pnas.1514996112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You T, Disanzo BL, Wang X, Yang R, Gong D. Adipose tissue endocannabinoid system gene expression: depot differences and effects of diet and exercise. Lipids Health Dis. 2011;10:194. doi: 10.1186/1476-511X-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-Aranda F, Sauchelli S, Pastor A, Gonzalez ML, de la Torre R, Granero R, Jiménez-Murcia S, Baños R, Botella C, Fernández-Real JM, Fernández-García JC, Frühbeck G, Gómez-Ambrosi J, Rodríguez R, Tinahones FJ, Arcelus J, Fagundo AB, Agüera Z, Miró J, Casanueva FF. Moderate-vigorous physical activity across body mass index in females: moderating effect of endocannabinoids and temperament. PLoS One. 2014;9:e104534. doi: 10.1371/journal.pone.0104534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Marzo V, Côté M, Matias I, Lemieux I, Arsenault BJ, Cartier A, Piscitelli F, Petrosino S, Alméras N, Després J-P. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217. doi: 10.1007/s00125-008-1178-6. [DOI] [PubMed] [Google Scholar]
- 49.Gasperi V, Ceci R, Tantimonaco M, Talamonti E, Battista N, Parisi A, Florio R, Sabatini S, Rossi A, Maccarrone M. The fatty acid amide hydrolase in lymphocytes from sedentary and active subjects. Med Sci Sports Exerc. 2014;46:24–32. doi: 10.1249/MSS.0b013e3182a10ce6. [DOI] [PubMed] [Google Scholar]
- 50.Antunes HKM, Leite GSF, Lee KS, Barreto AT, Santos RVT, Dos Souza H de S, Tufik S, de Mello MT. Exercise deprivation increases negative mood in exercise-addicted subjects and modifies their biochemical markers. Physiol Behav. 2016;156:182–190. doi: 10.1016/j.physbeh.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 51.Mangieri RA, Hong K-IA, Piomelli D, Sinha R. An endocannabinoid signal associated with desire for alcohol is suppressed in recently abstinent alcoholics. Psychopharmacology. 2009;205:63–72. doi: 10.1007/s00213-009-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan CJA, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, Curran HV, Brandner B, Leweke FM. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202:381–382. doi: 10.1192/bjp.bp.112.121178. [DOI] [PubMed] [Google Scholar]
- 53.Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Hill MN, Carrier EJ, Ho W-SV, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- 55.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 56.Rademacher DJ, Meier SE, Shi L, Ho W-SV, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–1262. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U. S.A. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- 60.Hill MN, Patel S. Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol Mood Anxiety Disord. 2013;3:19. doi: 10.1186/2045-5380-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P. Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study. Br J Psychiatry. 2013;202:62–67. doi: 10.1192/bjp.bp.112.114389. [DOI] [PubMed] [Google Scholar]
- 62.Sinha R. New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 64.Muhl D, Kathmann M, Hoyer C, Kranaster L, Hellmich M, Gerth CW, Faulhaber J, Schlicker E, Leweke FM. Increased CB2 mRNA and anandamide in human blood after cessation of cannabis abuse. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:691–695. doi: 10.1007/s00210-014-0984-2. [DOI] [PubMed] [Google Scholar]
- 65.Goodwin RS, Baumann MH, Gorelick DA, Schwilke E, Schwope DM, Darwin WD, Kelly DL, Schroeder JR, Ortemann-Renon C, Bonnet D, Huestis MA. CB1 - cannabinoid receptor antagonist effects on cortisol in cannabis-dependent men. Am J Drug Alcohol Abuse. 2012;38:114–119. doi: 10.3109/00952990.2011.600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 67.Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. [(18) F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol. 2015;20:357–367. doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- 68.Hirvonen J, Goodwin RS, Li C-T, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, Van Laere K. Changes in Cerebral CB1 Receptor Availability after Acute and Chronic Alcohol Abuse and Monitored Abstinence. J Neurosci. 2014;34:2822–2831. doi: 10.1523/JNEUROSCI.0849-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, Li C-T, Hines CS, Sun H, Terry GE, Morse C, Zoghbi SS, Pike VW, Innis RB, Heilig M. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hedges DW, Woon FL. Alcohol use and hippocampal volume deficits in adults with posttraumatic stress disorder: A meta-analysis. Biol Psychol. 2010;84:163–168. doi: 10.1016/j.biopsycho.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 73.Filbey FM, McQueeny T, Kadamangudi S, Bice C, Ketcherside A. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res. 2015;293:46–53. doi: 10.1016/j.bbr.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira-Vieira TH, Bastos CP, Pereira GS, Moreira FA, Massensini AR. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus. 2014;24:79–88. doi: 10.1002/hipo.22206. [DOI] [PubMed] [Google Scholar]
- 75.Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biedermann SV, Fuss J, Steinle J, Auer MK, Dormann C, Falfán-Melgoza C, et al. The hippocampus and exercise: histological correlates of MR-detected volume changes. Brain Struct Funct. 2016;221(3):1353–1363. doi: 10.1007/s00429-014-0976-5. [DOI] [PubMed] [Google Scholar]
- 77.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone Decreases Marijuana Withdrawal and a Laboratory Measure of Marijuana Relapse. Neuropsychopharmacology. 2013;38:1557–1565. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milman G, Bergamaschi MM, Lee D, Mendu DR, Barnes AJ, Vandrey R, Huestis MA. Plasma cannabinoid concentrations during dronabinol pharmacotherapy for cannabis dependence. Ther Drug Monit. 2014;36:218–224. doi: 10.1097/FTD.0b013e3182a5c446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, Booth J, McGregor IS. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA psychiatry. 2014;71:281–291. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- 80.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chauvet C, Nicolas C, Thiriet N, Lardeux MV, Duranti A, Solinas M. Chronic stimulation of the tone of endogenous anandamide reduces cue- and stress-induced relapse in rats. Int. J. Neuropsychopharmacol. 2014;18(1):pyu025. doi: 10.1093/ijnp/pyu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, Chefer SI, Barnes C, Yasar S, Piomelli D, Goldberg SR. Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse. Neuropsychopharmacology. 2015;40(9):2185–2197. doi: 10.1038/npp.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]