Abstract
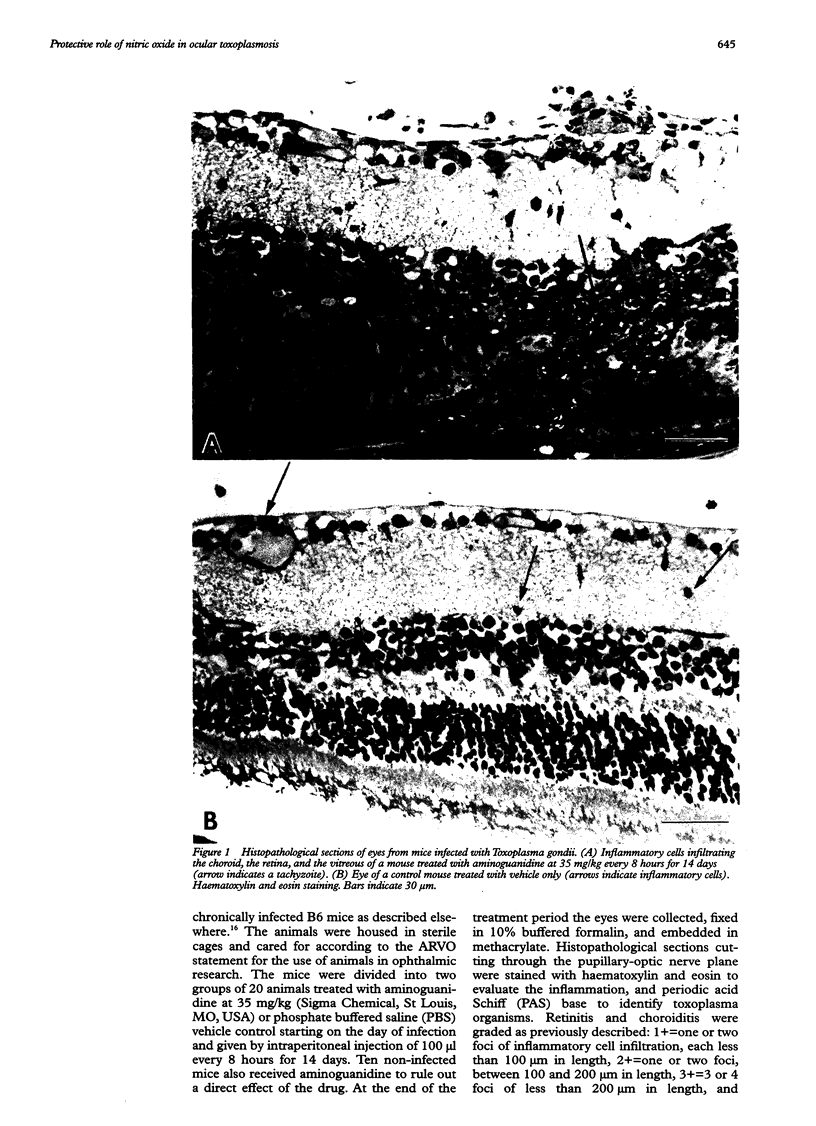
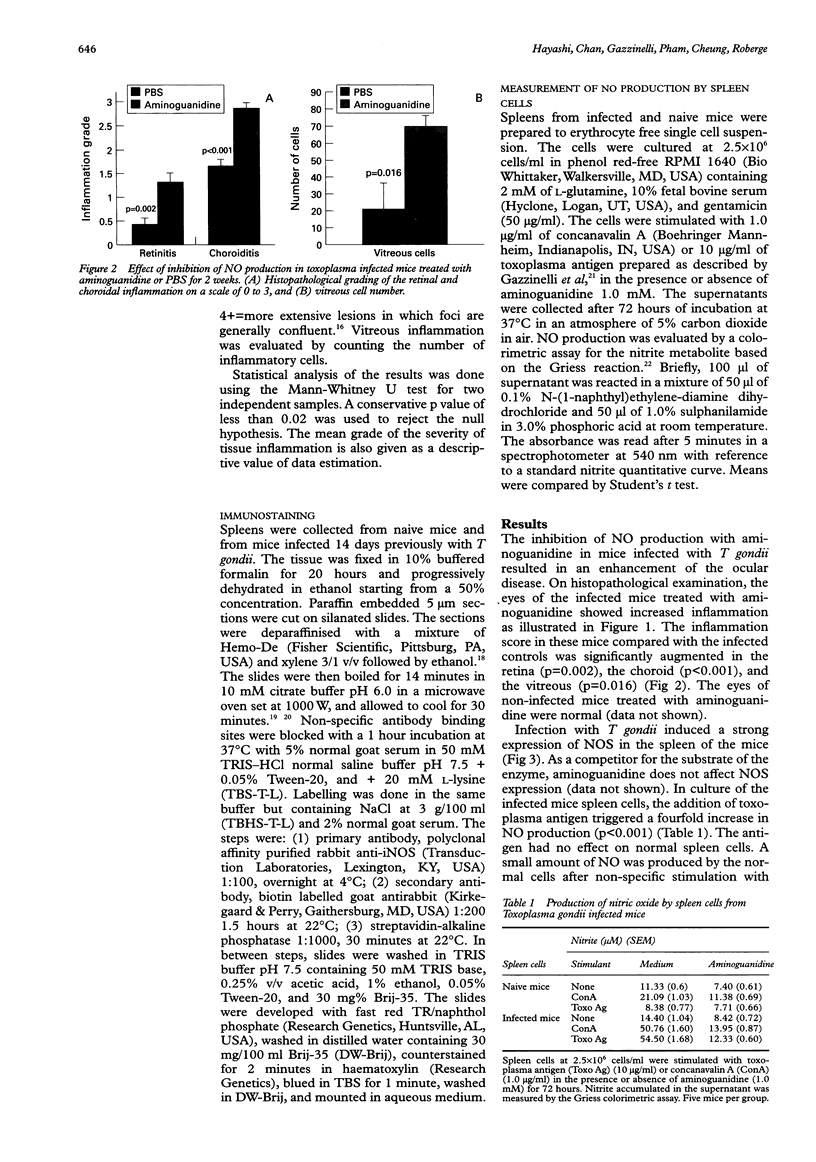
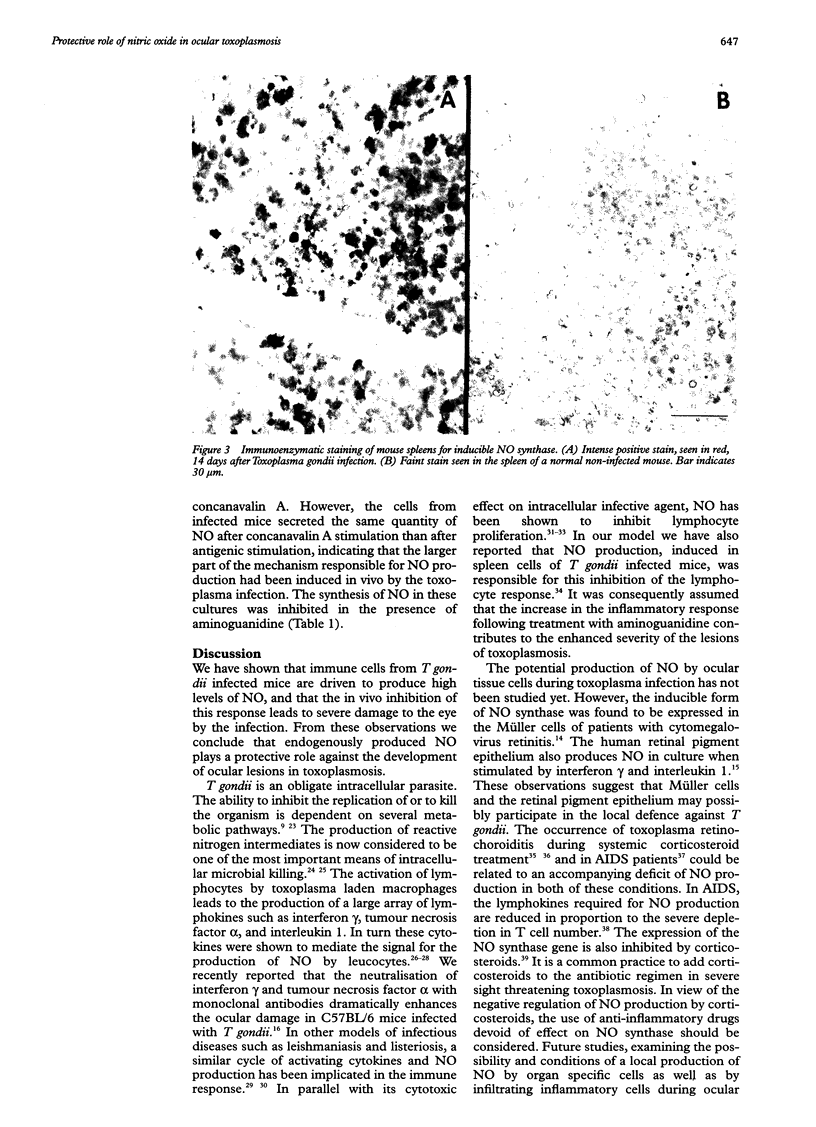
AIMS: To evaluate the role of nitric oxide (NO) in ocular involvement during systemic toxoplasmosis. METHODS: C57B1/6 mice were infected with Toxoplasma gondii strain ME49. The synthesis of NO was inhibited by an intraperitoneal injection of aminoguanidine every 8 hours, starting on the day of infection. Control infected mice received phosphate buffered saline vehicle alone. After 14 days, the ocular lesions were evaluated by histopathological examination. The expression of NO synthase induced in the spleen by toxoplasma infection was evaluated by immunostaining. The production of NO by the spleen cells of infected mice was measured by the colorimetric assay of Griess in the supernatant of cultures stimulated with toxoplasma antigen or concanavalin A. RESULTS: The inhibition of NO production in T gondii infected mice resulted in a marked increase in the symptoms of ocular inflammation. We observed a strong induction of NO synthase expression in the spleen of infected animals. In culture, the spleen cells from these mice produced high levels of NO in response to T gondii antigens. This elevation of NO synthesis was suppressed in the presence of aminoguanidine. CONCLUSION: This study indicates that NO plays a crucial role in the protection against T gondii infection as reflected by the severity of the ocular involvement.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Beaman M. H., Wong S. Y., Remington J. S. Cytokines, Toxoplasma and intracellular parasitism. Immunol Rev. 1992 Jun;127:97–117. doi: 10.1111/j.1600-065x.1992.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Boockvar K. S., Granger D. L., Poston R. M., Maybodi M., Washington M. K., Hibbs J. B., Jr, Kurlander R. L. Nitric oxide produced during murine listeriosis is protective. Infect Immun. 1994 Mar;62(3):1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUVREUR J., DESMONTS G. Congenital and maternal toxoplasmosis. A review of 300 congenital cases. Dev Med Child Neurol. 1962 Oct;4:519–530. [PubMed] [Google Scholar]
- Candolfi E., Hunter C. A., Remington J. S. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun. 1994 May;62(5):1995–2001. doi: 10.1128/iai.62.5.1995-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Denham S., Rowland I. J. Inhibition of the reactive proliferation of lymphocytes by activated macrophages: the role of nitric oxide. Clin Exp Immunol. 1992 Jan;87(1):157–162. doi: 10.1111/j.1365-2249.1992.tb06430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighiero P., Reux I., Hauw J. J., Fillet A. M., Courtois Y., Goureau O. Expression of inducible nitric oxide synthase in cytomegalovirus-infected glial cells of retinas from AIDS patients. Neurosci Lett. 1994 Jan 17;166(1):31–34. doi: 10.1016/0304-3940(94)90833-8. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Evans T. G., Thai L., Granger D. L., Hibbs J. B., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993 Jul 15;151(2):907–915. [PubMed] [Google Scholar]
- Gazzinelli R. T., Brézin A., Li Q., Nussenblatt R. B., Chan C. C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp Parasitol. 1994 Mar;78(2):217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Eltoum I., Wynn T. A., Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993 Oct 1;151(7):3672–3681. [PubMed] [Google Scholar]
- Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991 Jan 1;146(1):286–292. [PubMed] [Google Scholar]
- Geller D. A., Nussler A. K., Di Silvio M., Lowenstein C. J., Shapiro R. A., Wang S. C., Simmons R. L., Billiar T. R. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner P. D., Silveira C., Kruszon-Moran D., Martins M. C., Burnier Júnior M., Silveira S., Camargo M. E., Nussenblatt R. B., Kaslow R. A., Belfort Júnior R. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992 Aug 15;114(2):136–144. doi: 10.1016/s0002-9394(14)73976-5. [DOI] [PubMed] [Google Scholar]
- Goureau O., Hicks D., Courtois Y. Human retinal pigmented epithelial cells produce nitric oxide in response to cytokines. Biochem Biophys Res Commun. 1994 Jan 14;198(1):120–126. doi: 10.1006/bbrc.1994.1017. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim F. T., Gazzinelli R. T., Denkers E., Hieny S., Shearer G. M., Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991 Oct 1;147(7):2310–2316. [PubMed] [Google Scholar]
- Hayashi S., Chan C. C., Gazzinelli R., Roberge F. G. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996 Feb 15;156(4):1476–1481. [PubMed] [Google Scholar]
- Krick J. A., Remington J. S. Toxoplasmosis in the adult--an overview. N Engl J Med. 1978 Mar 9;298(10):550–553. doi: 10.1056/NEJM197803092981006. [DOI] [PubMed] [Google Scholar]
- Munakata S., Hendricks J. B. Effect of fixation time and microwave oven heating time on retrieval of the Ki-67 antigen from paraffin-embedded tissue. J Histochem Cytochem. 1993 Aug;41(8):1241–1246. doi: 10.1177/41.8.8331288. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fernández M. A., Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992 Jun;33(1):35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nicholson D. H., Wolchok E. B. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch Ophthalmol. 1976 Feb;94(2):248–254. doi: 10.1001/archopht.1976.03910030120009. [DOI] [PubMed] [Google Scholar]
- O'Connor G. R., Frenkel J. K. Editorial: Dangers of steroid treatment in toxoplasmosis. Periocular injections and systemic therapy. Arch Ophthalmol. 1976 Feb;94(2):213–213. doi: 10.1001/archopht.1976.03910030093001. [DOI] [PubMed] [Google Scholar]
- Parke D. W., 2nd, Font R. L. Diffuse toxoplasmic retinochoroiditis in a patient with AIDS. Arch Ophthalmol. 1986 Apr;104(4):571–575. doi: 10.1001/archopht.1986.01050160127028. [DOI] [PubMed] [Google Scholar]
- Rao N. A., Font R. L. Toxoplasmic retinochoroiditis: electron-microscopic and immunofluorescence studies of formalin-fixed tissue. Arch Ophthalmol. 1977 Feb;95(2):273–277. doi: 10.1001/archopht.1977.04450020074012. [DOI] [PubMed] [Google Scholar]
- Reed J. A., Manahan L. J., Park C. S., Brigati D. J. Complete one-hour immunocytochemistry based on capillary action. Biotechniques. 1992 Sep;13(3):434–443. [PubMed] [Google Scholar]
- Schleifer K. W., Mansfield J. M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993 Nov 15;151(10):5492–5503. [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Silveira C., Belfort R., Jr, Burnier M., Jr, Nussenblatt R. Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am J Ophthalmol. 1988 Sep 15;106(3):362–364. doi: 10.1016/0002-9394(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]




