Abstract
Background and aims Plants are able to grow under phosphorus (P)-deficient conditions by coordinating Pi acquisition, translocation from roots to shoots and remobilization within the plant. Previous reports have demonstrated that cell-wall pectin contributes greatly to rice cell-wall Pi re-utilization under P-deficient conditions, but whether other factors such as ethylene also affect the pectin-remobilizing capacity remains unclear.
Methods Two rice cultivars, ‘Nipponbare’ (Nip) and ‘Kasalath’ (Kas) were cultured in the +P (complete nutrient solution), −P (withdrawing P from the complete nutrient solution), +P+ACC (1-amino-cyclopropane-1-carboxylic acid, an ethylene precursor, adding 1 μm ACC to the complete nutrient solution) and −P+ACC (adding 1 μm ACC to −P nutrient solution) nutrient solutions for 7 d.
Key Results After 7 d −P treatment, there was clearly more soluble P in Nip root and shoot, accompanied by additional production of ethylene in Nip root compared with Kas. Under P-deficient conditions, addition of ACC significantly increased the cell-wall pectin content and decreased cell-wall retained P, and thus more soluble P was released to the root and translocated to the shoot, which was mediated by the expression of the P deficiency-responsive gene OsPT2, which also strongly induced by ACC treatment under both P-sufficient and P-deficient conditions.
Conclusions Ethylene positively regulates pectin content and expression of OsPT2, which ultimately makes more P available by facilitating the solubilization of P fixed in the cell wall and its translocation to the shoot.
Keywords: Rice, phosphorus, ethylene, cell-wall polysaccharides, pectin, transport, remobilization, gene expression
INTRODUCTION
Phosphorous (P) is a macro-element that is essential for plant growth and development. It not only provides the backbone for the biosynthesis of nucleic acids, phospholipids and the energy-carrying molecule ATP, but also has a regulatory role in metabolism and signal transduction through phosphoryl group transfer and protein activation (Marschner, 1995). However, its high chemical fixation rate, slow diffusion rate and substantial fraction of organically bound P render Pi (the form of P available to plants) one of the least available nutrients for crops (Vance et al., 2003; Wu et al., 2013). For this reason, crops are often supplied with inorganic P fertilizers (Hammond et al., 2004). However, the non-renewable nature of inorganic P fertilizers means that cheap sources of P, such as phosphate rocks, will be exhausted within the next 60–90 years (Runge-Metzger, 1995). In addition, excessive P added to crops can pollute local watercourses, contributing to the process of eutrophication (Withers et al., 2001). Therefore, there is a need to develop more P-efficient crops.
To cope with P deficiency, many plant species have developed two strategies (Zhu et al., 2014). One is based on maximizing P uptake from the soil. Production of more lateral roots, root hairs and root biomass has been defined as one of the most valuable P uptake mechanisms (Watanabe et al., 2006), while accumulating evidence has also demonstrated the pivotal role of the secretion of organic acids (Ae et al., 1990; Otani et al., 1996; Zhu et al., 2012), phosphatases (Hall, 1969; Pammenter and Woolhouse, 1975) and other substances (Otani et al., 1996) in plant P-deficiency resistance. For instance, organic acids secreted from radish (Raghanus satiuvs L.) and rape (Brassica napus L.) can release P from insoluble P sources in soil (Zhang et al., 1997). A second strategy depends on the plant’s capacity to utilize previously assimilated P. Phosphohydrolases, acid phosphatase (APase), ribonuclease (RNase) can remobilize P in established tissues, and this mobilized P can be translocated to younger, growing tissues (Yun and Kaeppler, 2001; Gong et al., 2011). Recently, Zhu et al. (2014) found that cell-wall pectin, which has been shown to be a cation absorber, has a particularly high affinity for Al3+, Fe3+ and Cd2+ (Blamey et al., 1990; Chang et al., 1999; Zhu et al., 2012) and can facilitate the remobilization of P deposited in the cell wall. Nevertheless, whether other factors also affect the pectin remobilizing capacity remains unclear.
Ethylene, a well-known phytohormone, is produced from methionine through S-adenosyl-l-methionine and 1-aminocyclopropane-1-carboxylic acid (ACC), via reactions catalysed by ACC synthase (ACS) and ACC oxidase (ACO), respectively (Kende, 1993). ACS and ACO are encoded by multigene families and regulated by many biotic and abiotic factors (Wang et al., 2002). Ethylene controls many physiological and developmental processes in higher plants, including ripening of fruit, abscission and senescence. Ethylene production in plants is very low under normal conditions, but increases under a variety of stresses (Lieberman, 1979). For instance, ethylene production is induced in roots by P deficiency in bean (Phaseolus vulgaris L.) (Li et al., 2009) and Medicago (Medicago falcata L.) (Borch et al., 1999). The application of ACC (an ethylene precursor) to roots of several P-sufficient plants promoted root hair formation (Tanimoto et al., 1995), indicating a role for ethylene in mediating this P-deficiency response. However, the mechanism underlying ethylene biosynthesis in response to P deficiency remains elusive.
Rice is one of the most important cereal crops. It accounts for approx. 21 % of the caloric supply for the world’s population, and almost 80 % in some South-East Asian countries (Wu et al., 2011). We used two typical rice cultivars, the more efficient P utilization cultivar ‘Nipponbare’ (Nip) and the less efficient P utilization cultivar ‘Kasalath’ (Kas) (Zhu et al., 2014), which showed an opposite trend in their P uptake efficiency in the soil due to the existence of the PHOSPHORUS STARVATION TOLERANCE 1 (PSTOL1) gene in Kas, which can improve root growth and increase yield by as much as 20 % in phosphorus-deficient soils (Gamuyao et al., 2012). Thus, to exclude the advantage of PSTOL1, all experiments were performed hydroponically, and we demonstrated a correlation between ethylene production and cell-wall P re-utilization. We then further verified this relationship by studying their pectin content, which served as an indicator of cell-wall P reutilization efficiency. This study therefore represents the first evidence of a novel regulatory mechanism for remobilization of the P deposited in the cell wall by pectin under P-deficient conditions.
MATERIALS AND METHODS
Plant materials and growth conditions
Rice (Oryza sativa) subsp. japonica ‘Nipponbare’ (Nip) and subsp. indica ‘Kasalath’ (Kas) were used in this study, with subsequent study of 11 additional rice cultivars: ‘Shuitianhuagu’, ‘Luopinglanhangu’, ‘Zhonghua8’, ‘Xiangjiangnuo’, ‘Jinyuan45’, ‘Haoyaowen’, ‘BaiXXzigu’, ‘Wuyunjing’, ‘Lindao 4’, ‘Wubie’ and ‘Yangdao6’. Seeds were transferred to an incubator at 25 °C for germination. Germinated seeds were transferred to the following treatments: +P (complete nutrient solution), −P (withdrawing P from the complete nutrient solution), +P+ACC (adding 1 μm ACC to the complete nutrient solution) and −P+ACC (adding 1 μm ACC to −P nutrient solution). The nutrient solution contained 114·25 mg L−1 NH4NO3, 50·375 mg L−1 NaH2PO4·2H2O, 89·25 mg L−1 K2SO4, 110·75 mg L−1 CaCl2, 405 mg L−1 MgSO4·7H2O, 1·875 mg L−1 MnCl2·4H2O, 0·0925 mg L−1 (NH4)6Mo7O24, 1·1675 mg L−1 H3BO3, 0·04375 mg L−1 ZnSO4·7H2O, 0·03875 mg L−1 CuSO4, 34·75 mg L−1 FeSO4·7H2O and 46·53 mg L−1 EDTA-Na2. The solution was renewed every 3 d. All experiments were conducted in a growth chamber with a 14-h/26 °C day and a 10-h/23 °C night regime, light intensity of 400 μmol m−2 s−1 and relative humidity of 60 %. All experiments in this study were conducted independently at least twice.
Determination of soluble Pi concentrations
Roots and leaves of rice were weighed and homogenized with a mortar and pestle in liquid nitrogen. The inorganic phosphate (Pi) was extracted with 4 mL of 5 % (v/v) sulphuric acid (5 m) solution. After centrifugation at 16 800 g, 400 μL supernatant was transferred and mixed with a 200- μL aliquot of 15 % (w/v) fresh ascorbic acid (pH 5·0) dissolved in ammonium molybdate. The mixture was incubated at 37 °C for 30 min and the absorbance at 650 nm was recorded. Pi concentration was calculated by normalization to the fresh weight (Zheng et al., 2009).
Cell-wall extraction and fractionation
Extraction of crude cell-wall materials and subsequent fractionation of cell-wall components were carried out according to Zhong and Läuchli (1993) with minor modifications according to Yang et al. (2011). Roots were ground with a mortar and pestle in liquid nitrogen, homogenized with 75 % ethanol for 20 min in ice-cold water, and then centrifuged at 6200 g for 10 min. The supernatant was removed, and the pellets were homogenized and washed with acetone, methanol/chloroform with a ratio of 1 : 1, and methanol for 20 min each, with all supernatant was removed after each centrifugation. The remaining pellet, i.e. the cell-wall material, was dried and stored at 4 °C for further use.
Pectin was extracted from the cell-wall material (about 2 mg) by three rounds of extraction with 1 mL hot water at 100 °C for 1 h each, and the supernatants were combined in a 5-mL tube after centrifugation at 13 800 g for 10 min.
Uronic acid and total polysaccharide measurement
The uronic acid content in pectin was assayed according to Blumenkrantz and Asboe-Hansen (1973) using galacturonic acid (Sigma, St Louis, MO, USA) as a standard. Briefly, 200 μL pectin extracts were incubated with 1 mL of 98 % H2SO4 (containing 0·0125 m Na2B4O7·10H2O) at 100 °C for 5 min. After chilling, 20 μL M-hydro-dipheny (0·15 %) was added to the solution, which was incubated for 20 min at room temperature before the absorbance was measured spectrophotometrically at 520 nm.
P retention in the cell-wall materials
To determine the P retention in the cell wall under P deficiency, a total of 5 mg of cell wall material was placed into a 2-mL Eppendorf tube with 2 m HCl. The solution was shaken on a rotary shaker for 3 d. After treatment, samples were centrifuged at 16 800 g and the supernatant was collected for concentration determination by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Fisons ARL Accuris, Ecublens, Switzerland). The P adsorption ability of cell-wall material was calculated according to the ratio of P concentration in the −P plants to that in the +P plants.
Gene expression analysis
Plants were grown under the same conditions used for soluble Pi content measurements. Root tissues were collected 24 h after treatments and immediately frozen in liquid nitrogen before total RNA extraction. Total RNA was isolated from roots using TRIzol (Invitrogen, Carlsbad, CA, USA). The cDNA was prepared from 1 μg total RNA using the PrimeScript RT reagent kit (Takara, Shiga, Japan). For the quantitative analysis of gene expression, 1 μL 10-fold-diluted cDNA was used for real-time PCR performed with SYBR Premix ExTaq (Takara) and OsPT2 gene-specific primers (forward: 5′-GACGAGACCGCCCAAGAAG-3′; reverse: 5′-TTTTCAGTCACTCACGTCGAGAC-3′), OsPT6 gene-specific primers (forward: 5′-TATAACTGATCGATCGAGACCAGAG-3′; reverse: 5′-TGGATAGCCAGGCCAGTTATATATC-3′) and OsPT8 gene-specific primers (forward: 5′-AGAAGGCAAAAGAAATGTGTGTTAAAT-3′; reverse: 5′-AAAATGTATTCGTGCCAAATTGCT-3′). Each cDNA sample was run in triplicate. Expression data were normalized to the expression level of the actin gene (forward: 5′-AAGTTCTGGGAAGTGGTT-3′; reverse: 5′-CTCCCAATGAGTGACAAA-3′) (Jia et al., 2011; Wu et al., 2011).
Measurement of ethylene production
Ethylene production in the roots of treated seedlings was analysed by placing detached roots into 15-mL glass vials containing 1 mL of water and rapidly sealing them with a gas-proof septum in accordance with Wu et al. (2011). The sealed vials were incubated in a dark growth chamber for 4 h at 30 °C. Gas (1 mL) was withdrawn from the airspace of each vial using a gas-tight syringe (Focus GC, Thermo, Waltham, MA, USA) and injected into a gas chromatograph (Focus GC, Thermo) equipped with a capillary column (CP-carboPLOT P7, Varian, Palo Alto, CA, USA) and flame-ionization detector for ethylene determination. Ethylene production was calculated on the basis of fresh weight (f. wt) of root samples.
Statistical analysis
Each experiment was repeated at least three times. Data were analysed by one-way analysis of variance (ANOVA) and the means were compared by Duncan’s multiple range test. Different letters on the histograms indicate that the means were statistically different at the P < 0·05 level.
RESULTS
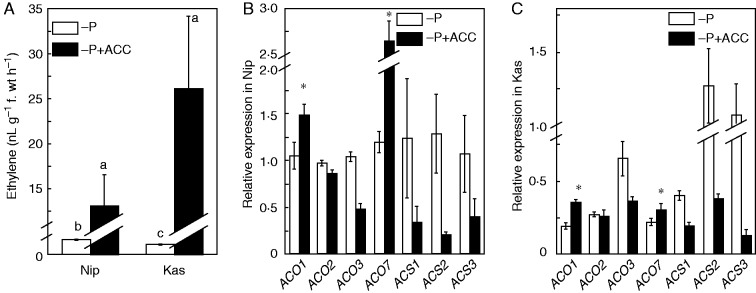
To investigate the effect of ethylene on the re-utilization of P in the rice roots, we tested a typical P utilization efficient japonica variety, ‘Nipponbare’ (Nip), and a typical P utilization less efficient indica variety, ‘Kasalath’ (Kas) (Zhu et al., 2014), which was further confirmed by the relatively better performance of the Nip in the hydroponics for 5 weeks (Supplementary Data Fig. S1). One-week-old rice seedlings were transferred to −P nutrient solution, and root and shoot samples were collected after 1 week for the analysis of soluble Pi content and ethylene production. There was clearly more soluble P in Nip root and shoot compared with Kas under −P conditions (Fig. 1A, B), which is in accordance with our previous study (Zhu et al., 2014). Interestingly, the P-starved Nip plants showed greater ethylene production in the roots compared with P-starved Kas plants (Fig. 1C), implying that ethylene may be involved in the different P re-utilization efficiency in these two rice cultivars.
Fig. 1.
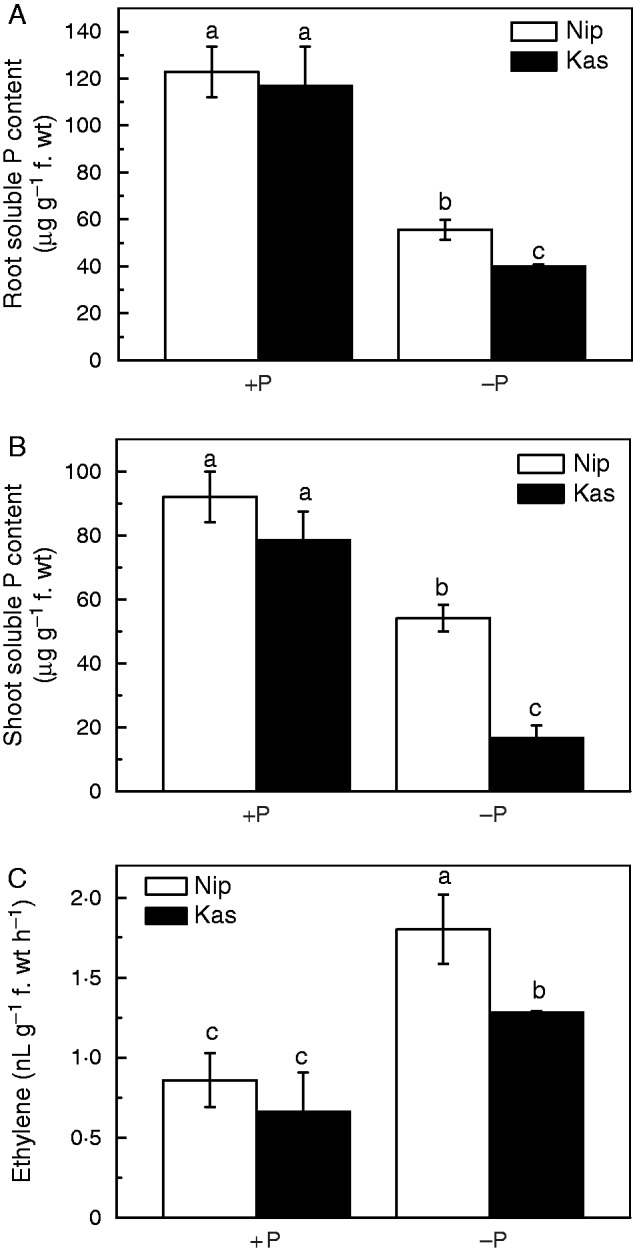
Soluble Pi content and root ethylene production in rice cultivars Nip and Kas. Seedlings (after germination) were subjected to P-deficient/sufficient nutrient solution for 1 week, and the root (A) and shoot (B) soluble Pi contents and root ethylene production (C) were measured. Data are means ± s.d. (n = 4). Columns with different letters are significantly different at P < 0·05.
We next examined whether the observed enhanced ethylene production under P deficiency in Nip was due to regulation of the expression of members of the ethylene-biosynthesis gene families ACS and ACO. There are six putative ACS genes and seven putative ACO genes in the rice genome (Rzewuski and Sauter, 2008; Wu et al., 2011). However, OsACS6 and OsACO6 were excluded from this analysis because OsACS6 is the closest orthologue of AtACS10 and AtACS12, which display aminotransferase activity instead of ACS activity (Yamagami et al., 2003; Iwai et al., 2006; Wu et al., 2011) while OsACO6 is a pseudogene (Rzewuski and Sauter, 2008). Quantitative reverse transcription PCR (qRT-PCR) analysis showed that P deficiency resulted in down-regulation of OsACS1, OsACS2, OsACS3, OsACO1, OsACO3 and OsACO7 in Kas compared with Nip (Fig. 2). This down-regulation mirrored changes in ethylene levels (Fig. 1C), suggesting that these ACS and ACO genes may be responsible for the decreased ethylene biosynthesis in roots under P deficiency in Kas.
Fig. 2.
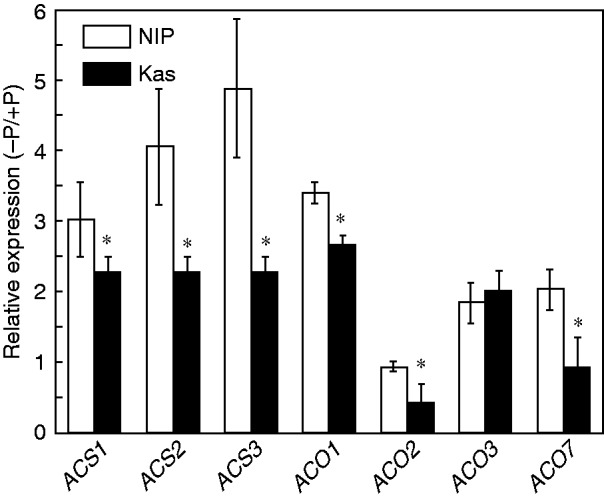
Effect of P deficiency on expression of genes for ethylene biosynthesis enzymes. Seedlings were transferred to P-depleted (−P) or P-supplied (+P) nutrient solution for 1 week. After treatment, total root RNA was subjected to qRT-PCR using gene-specific primers. Data are means ± s.d. (n = 4). Asterisks indicate a significant difference from Nip at P < 0·05.
To evaluate the role of ethylene in P homeostasis in rice plants, 1-week-old seedlings were transferred to P-deficient nutrient solution supplemented with 1 μm ACC. The resulting increased ethylene level was accompanied by elevated transcription of OsACO1 and OsACO7 in both Nip and Kas (Fig. 3), suggesting that OsACO1 and OsACO7 may play pivotal roles in the conversion of exogenously applied ACC to ethylene under P deficiency.
Fig. 3.
Effect of ACC on root ethylene production (A) and the expression of genes for ethylene biosynthesis enzymes (B, C). Seedlings were transferred to P-depleted (−P) nutrient solution in the presence or absence of ACC for 1 week. After treatment, total root RNA was subjected to qRT-PCR analysis using gene-specific primers. Data are means ± s.d. (n = 4). Asterisks indicate a significant difference from the absence of ACC at P < 0·05.
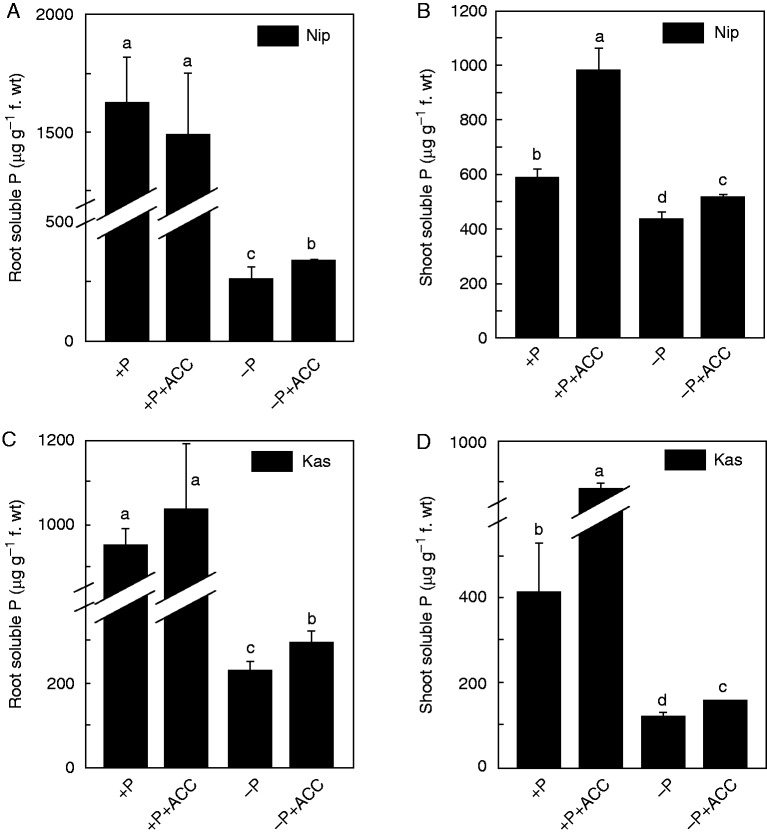
Interestingly, under P-sufficient conditions, the root soluble P contents with or without ACC supply were similarly high (Fig. 4A, C) although the shoot soluble P content was higher under the +P+ACC treatment compared with the +P treatment, indicating that ACC applied exogenously resulted in transfer of more P to the shoot under P-sufficient conditions (Fig. 4B, D). By contrast, under P-deficient conditions, both the root and the shoot soluble P contents in the seedlings of the −P+ACC treatment were significantly higher than that of the −P treatment (Fig. 4). These findings indicate that ethylene is involved in the re-utilization of P when there is no available P in the nutrient solution. As ethylene has a similar effect on the root and shoot soluble P content in Nip and Kas, we performed all of the following experiments in Nip.
Fig. 4.
Effect of ACC on the root (A, C) and shoot soluble P content (B, D). Seedlings were transferred to P-depleted (−P) nutrient solution in the presence or absence of ACC for 1 week. Data are means ± s.d. (n = 4). Columns with different letters are significantly different at P < 0·05.
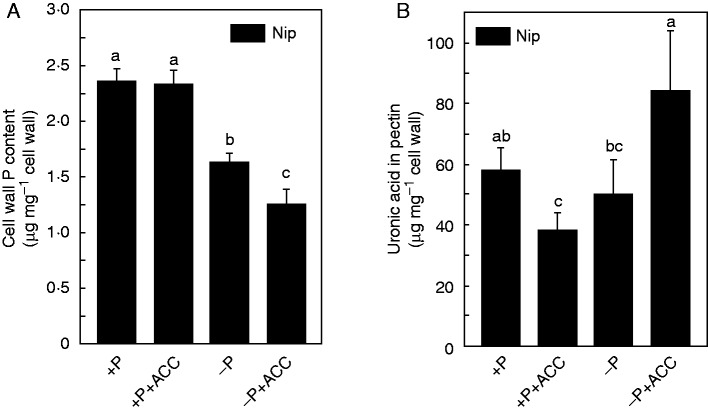
We next set out to determine the source of the available P under the −P+ACC treatment compared with −P treatment. As nearly 50 % of the total P is accumulated in the cell wall of rice and the high cation attracting/holding capacity of pectin may allow P in insoluble forms with cations to be released as the cations are bound by pectin (Zhu et al., 2014), we extracted the cell wall and measured the P retained in it. Notably, less P accumulated in the cell wall under −P+ACC treatment (Fig. 5A), implying that ACC facilitates the release of P from the cell wall. Although the cell wall is composed of a polysaccharide matrix mainly including cellulose, hemicellulose and pectin, only pectin was found to contribute greatly to the pool of P released from the cell wall (Zhu et al., 2014). In the present study, we found more pectin in the root cell wall under −P+ACC treatment compared with the −P treatment (Fig. 5B), and the higher pectin content allows more P to be released from the root cell wall. Thus, more soluble P is available under the −P+ACC treatment, which is in accordance with the results of our previous study.
Fig. 5.
Effect of ACC on the retention of the P in the cell wall (A) and the pectin content (B). Seedlings were transferred to P-depleted (−P) nutrient solution in the presence or absence of ACC for 1 week. Data are means ± s.d. (n = 4). Columns with different letters are significantly different at P < 0·05.
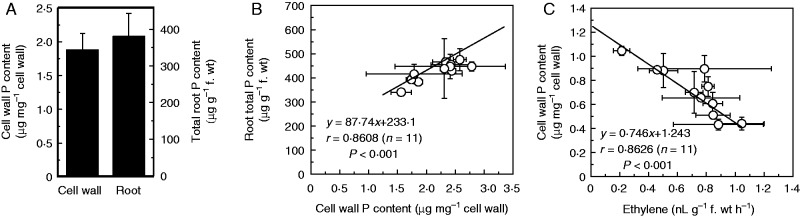
The above described changes in ethylene and cell-wall P content upon P starvation suggest a possible relationship between the two. To test this possibility, first, to confirm the contribution of P in the cell wall to whole plant P including that in the main organic fractions (ribosomal RNA, phospholipids, free nucleotides, phosphorylated intermediates), cell-wall P and root total P content in Nip when under P-sufficient conditions were measured. As shown in Fig. 6A, there was about 1·88 μg P in the Nip cell wall (1 mg) extracted from the fresh roots (1 g of fresh root can provide about 100 mg cell wall), and thus about 188 μg P in the cell wall of 1 g of fresh root. However, there was about 380 μg total P in this 1 g of fresh root (Fig. 6A), thus indicating that about 49 % of the total P was stored in the cell wall, which is in accordance with our previous study (Zhu et al., 2014). Second, an array of 11 different rice cultivars were used, and a general positive correlation between root cell-wall P content and root total P content was found (Fig. 6B). Last, a relationship was also observed between ethylene and cell-wall P content (Fig. 6C), suggesting that for more total P in the roots, more is stored in the cell wall and ethylene can release the P retained in the cell wall.
Fig. 6.
Identification of a correlation among root total P, cell-wall P content and root ethylene production. (A) Cell-wall P content and root total P content. Nip was transferred to P-supplied (+P) nutrient solution for 1 week. After treatment, total roots were subjected to P content analysis and cell-wall extraction. (B) Root cell-wall P content correlates with root total P content under P-sufficient conditions. Eleven different rice cultivars were transferred to P-supplied (+P) nutrient solution for 1 week. After treatment, total roots were subjected to P content analysis and cell-wall extraction. (C) Root ethylene production correlates with root cell-wall P content. Eleven different rice cultivars were transferred to P-depleted (−P) nutrient solution for 1 week. After treatment, total roots were subjected to cell-wall extraction and ethylene production. Data are means ± s.d. (n = 4).
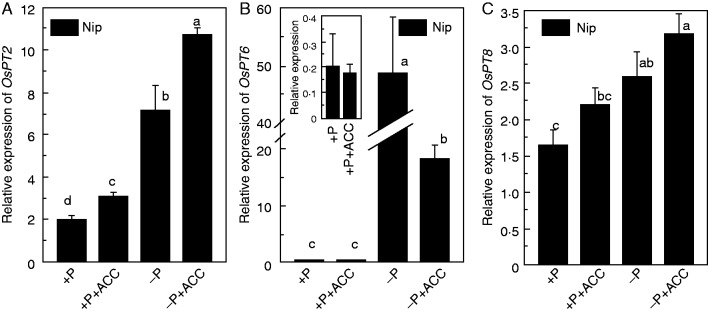
To determine whether ethylene participates in P translocation from root to shoot under P-deficient conditions, the expression of genes typically induced in response to P deficiency and involved in P translocation from root to shoot were analysed by qRT-PCR in Nip roots grown in normal or P-deficient medium supplemented with 1 μm ACC. Under P-sufficient conditions, expression of the P deficiency-responsive gene OsPT2 was strongly induced by ACC treatment (Fig. 7). Although P deficiency induced the transcription of OsPT2, OsPT6 and OsPT8, the addition of ACC further increased the expression of OsPT2 (Fig. 7). However, there was down-regulation of OsPT6 under −P+ACC treatment compared with −P (Fig. 7B). This inconsistency may be attributable to the dual role of OsPT6, which not only acts as the P uptake transporter, but also can translocate P from the root to shoot.
Fig. 7.
Effect of ACC on the expression of Pi translocation-related genes in Nip. Seedlings were transferred to P-depleted (−P) or P-supplied (+P) nutrient solution in the presence or absence of ACC for 1 week. The inset of B amplified the effect of ACC on the expression of OSPT6 under +P nutrient solution. After treatment, total root RNA was subjected to qRT-PCR analysis using gene-specific primers. Data are means ± s.d. (n = 4). Columns with different letters are significantly different at P < 0·05.
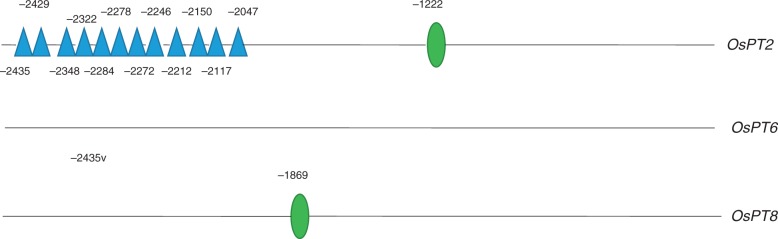
The occurrence of ethylene-responsive elements (EREs) in the promoters of the selected OsPT2, OsPT6 and OsPT8 genes was investigated to provide additional indirect evidence of their ethylene regulation. An 8-bp AWTTCAAA motif has been identified as an ERE in the promoter region of several ethylene-induced genes (Montgomery et al., 1993; Itzhaki et al., 1994; Tapia et al., 2005; García et al., 2010). Although no predicted ERE was identified in the promoter of OsPT6, the promoters of OsPT2 and OsPT8 each contained one AWTTCAAA motif, located 1222 and 1869 bp, respectively, upstream of the transcription start site (Fig. 8). In addition, the OsPT2 promoter contains 12 GCCGCC motifs located in the region 2435–2043 bp upstream of the transcription start site (Fig. 8). The GCCGCC motif is a target sequence for members of the ethylene-responsive element-binding factor (ERF) transcription factor family (Chakravarthy et al., 2003), implying that ethylene may be involved in the transport of P from root to shoot by regulating the expression of OsPT2, which was further confirmed in Kas (Supplementary Data Fig. S2)
Fig. 8.
Occurrence of ethylene transcription factor-responsive elements in the promoter regions of OsPT2, OsPT6 and OsPT8. A stretch of 2500 bp upstream of the transcription initiation site was examined. The predicted ethylene-responsive elements AWTTCAAA and GCCGCC are represented by triangles and oval symbols, respectively. The numbering below each symbol indicates the position of each motif relative to the site of initiation of transcription.
DISCUSSION
Marked ethylene production has been widely observed in various plant species in response to nutrient deficiency in general (Lynch and Brown, 1997) and P deficiency in particular (Borch et al., 1999; Li et al., 2009). Root cell-wall polysaccharides, especially pectin, possess the ability to remobilize the insoluble P during P starvation (Nagarajah et al., 1970; Ae et al., 1996; Gessa et al., 1997; Zhu et al., 2014). However, the relationship between ethylene production and cell-wall P re-utilization under P deficiency remains unclear. In the present study, by using two rice cultivars differing in their cell-wall P remobilization capacity and by exogenous application of ACC, we not only confirmed the positive effect of ethylene on rice cell-wall P re-utilization under P deficiency, but also uncovered the underlying physiological and molecular mechanism.
P deficiency has detrimental effects on plant growth, development and reproduction; thus, any approaches leading to increased P uptake and translocation or re-utilization of the soluble/insoluble P from the soil or the plant itself are beneficial to the yield of crop plants. When there was no soluble/insoluble P in the hydroponic solution in our present study, the plants were rendered more resistant to P deficiency by increased re-utilization of P stored in root cell walls. This is particularly relevant as nearly 50 % of total P is present in the cell walls of Nip (Fig. 6A; Zhu et al., 2014) and root total P content correlates positively with root cell-wall P content through a further analysis of 11 rice cultivars (Fig. 6B). Cell walls are composed of a matrix of polysaccharides such as cellulose, hemicellulose and pectin. Among them, pectin is the main source of the negative charge of the cell wall, and is generally considered to be responsible for the binding of cations such as Al (Eticha et al., 2005; Yang et al., 2008). In a previous study, we found that pectin can remobilize the cell-wall-deposited P, but its upstream regulatory mechanism was unknown.
Ethylene has been demonstrated to be a key intermediate signal molecule in P deficiency (Borch et al., 1999; Li et al., 2009). Here, our results further confirmed that ethylene is involved in the P-deficiency response. More ethylene was produced in the Nip cultivar, which showed much better performance in terms of cell-wall P re-utilization (Fig. 1). The higher ethylene concentration in Nip was accompanied by higher root and shoot soluble P content (Fig. 4) and lower P retention in the cell wall (Fig. 5A), indicating that ethylene can enhance cell-wall P reutilization, which is further confirmed through analysis of the another 11 rice cultivars (Fig. 6C). Furthermore, the pattern of ethylene production was consistent with the expression pattern of OsACS1, OsACS2, OsACS3, OsACO1, OsACO3 and OsACO7, indicating that lower expression of these ACS and ACO genes may underlie the lower ethylene biosynthesis in Kas roots under P depletion (Fig. 2). Moreover, ACC treatment induced the ethylene production concomitantly with the up-regulated expression of OsACO1 and OsACO7 in both Nip and Kas (Fig. 3), suggesting that OsACO1 and OsACO7 may play pivotal roles in the conversion of exogenously applied ACC into ethylene.
These findings bring up the question of whether ethylene is the closest signal molecule to affect the cell-wall P re-utilization. Pectin has been demonstrated to be a key intermediate material in cell-wall P re-utilization, and it responds to multiple environmental stimuli. For instance, Yang et al. (2008) and Xiong et al. (2009) found that exposure of rice to Al and Cd led to an increment of pectin content in root. Yang et al. (2010) reported that osmotic stress inhibited Al accumulation in root apices of the Al-sensitive genotype VAX1, an effect that was attributed to a lower cell-wall pectin content and its degree of methylation. Zhu et al. (2012, 2014) demonstrated that exposure of Arabidopsis and rice cultivar Kas to P deficiency conditions led to a decrement of pectin content while this effect was diminished in rice cultivar Nip. Furthermore, they found that pectin contributed to the cell-wall P re-utilization in Arabidopsis and rice during P deficiency, which means that a higher pectin content allows for more cell-wall P re-utilization. Changes in the pectin content might depend on plant cultivar, culture conditions and physiological stress. Here, our results further confirmed that P deficiency has no effect on the pectin content in Nip (Fig. 5B), which is in agreement with previous reports, but we found that exogenous application of ACC increased the pectin level under P deficiency, increased the competition of pectin to the Fe/Al, which existed as FePO4 or AlPO4, and as consequence, reduced P retention in the cell wall (Fig. 5A). This allowed more P to be re-utilized, and thus there were higher root and shoot soluble P contents in Nip (Fig. 4). Together, these findings demonstrate that ethylene involvement in the regulation of root phosphorus remobilization is associated with cell-wall pectin and that pectin acts downstream of ethylene in this regulatory pathway.
Up-regulation of multiple Pi transporters that mediate Pi translocation acts as another effective way for plants to cope with P deficiency. As expected, ACC treatment in the presence or absence of P significantly enhanced the expression of OsPT2 (Fig. 7 and Supplementary Data Fig. S1), which is expressed mainly in the stele of primary and lateral roots under Pi-deficient conditions (Ai et al., 2009). These results indicated that ethylene might be involved in the transport of P from root to shoot by regulating the expression of OsPT2. This hypothesis was further supported by the indirect bioinformatic analysis of the OsPT2 promoter, which was found to harbour the ethylene-responsive elements AWTTCAAA and GCCGCC (Fig. 8). However, although P deficiency also induced the expression of OsPT6 (expressed in the epidermis, cortex and stelar tissue under Pi-deficient conditions) and OsPT8 (expressed constitutively and functioning in P haemostasis) (Wang et al., 2014), ACC applied exogenously did not further increase their expression, which is in accordance with our promoter analysis (Figs 7 and 8 and Supplemental Data Fig. S2). These findings rule out the possibility that OsPT6 and OsPT8 are transcriptionally regulated by ethylene under P-starvation conditions.
In conclusion, we identified the physiological and molecular pathway underlying ethylene-induced cell-wall P remobilization in rice under P deficiency. Our results support a model in which increased production of ethylene results in the increment of pectin content and up-regulation of OsPT2, which ultimately makes more P available by facilitating the solubilization of P fixed in the cell wall and its translocation to the shoot.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: phenotype of rice cultivars Nip and Kas. Figure S2: effect of ACC on the expression of Pi translocation-related genes in Kas.
ACKNOWLEDGMENTS
This work was funded by the National Key Basic Research Program of China (No. 2014CB441000), Natural Science Foundation of China (31501825) and the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (Nos. XDB15030302 and XDB15030202).
LITERATURE CITED
- Ae N, Arihara J, Okada K, Yoshihara T, Johansen C. 1990. Phosphorus uptake by pigeonpea and its role in cropping systems of Indian subcontinent. Science 248: 477–480. [DOI] [PubMed] [Google Scholar]
- Ae N, Otani T, Makino T, Tazawa J. 1996. Role of cell wall of groundnut roots in solubilizing sparingly soluble phosphorus in soil. Plant and Soil 186: 197–204. [Google Scholar]
- Ai PH, Sun SB, Zhao JN, et al. 2009. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. The Plant Journal 57: 798–809. [DOI] [PubMed] [Google Scholar]
- Blamey FPC, Edmeades DC, Wheeler DM. 1990. Role of root cation-exchange capacity in differential aluminum tolerance of Lotus species. Journal of Plant Nutrition 13: 729–744. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. 1973. New method for quantitative determination of uronic acids. Analytical Biochemistry 54: 484–489. [DOI] [PubMed] [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. 1999. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell and Environment 22: 425–431. [Google Scholar]
- Chakravarthy S, Tuori RP, D’Ascenzo MD, Fobert PR, Despres C, Martin GB. 2003. The tomato transcription factor Pti4 regulates defense-related gene expression via GCC box and non-GCC box cis elements. The Plant Cell 15: 3033–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. 1999. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell & Environment 22: 1009–1017. [Google Scholar]
- Eticha D, Stass A, Horst WJ. 2005. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell & Environment 28: 1410–1420. [Google Scholar]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, et al. 2012. The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488: 535–539. [DOI] [PubMed] [Google Scholar]
- García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R. 2010. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. Journal of Experimental Botany 61: 3885–3899. [DOI] [PubMed] [Google Scholar]
- Gessa C, Deiana S, Premoli A, Ciurli A. 1997. Redox activity of caffeic acid towards iron (III) complexed in a polygalacturonate network. Plant and Soil 190: 289–299. [Google Scholar]
- Gong YM, Guo ZH, He LY, Li JS. 2011. Identification of maize genotypes with high tolerance or sensitivity to phosphorus deficiency. Journal of Plant Nutrition 34: 1290–1302. [Google Scholar]
- Hall JL. 1969. Histochemical localization of β-glycerophosphatase activity in young root tips. Annals of Botany 33: 399–406. [Google Scholar]
- Hammond JP, Broadley MR, White PJ. 2004. Genetic responses to phosphorus deficiency. Annals of Botany 94: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki H, Maxson JM, Woodson WR. 1994. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GSTI) gene. Proceedings of the National Academy of Sciences, USA 91: 8925–8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y. 2006. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiology 142: 1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HF, Ren HY, Gu M, et al. 2011. The phosphate transporter gene OsPht1; 8 is involved in phosphate homeostasis in rice. Plant Physiology 156: 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. 1993. Ethylene biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 44: 283–307. [Google Scholar]
- Li YS, Mao XT, Tian QY, Li LH, Zhang WH. 2009. Phosphorus deficiency-induced reduction in root hydraulic conductivity in Medicago falcata is associated with ethylene production. Environmental and Experimental Botany 67: 172–177. [Google Scholar]
- Lieberman M. 1979. Biosynthesis and action of ethylene. Annual Review of Plant Physiology 30: 533–591. [Google Scholar]
- Lynch JP, Brown KM. 1997. Ethylene and plant responses to nutritional stress. Physiologia Plantarum 100: 613–619. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants. San Diego: Academic Press. [Google Scholar]
- Montgomery J, Goldman S, Deikman J, Margossian L, Fischer RL. 1993. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proceedings of the National Academy of Sciences, USA 90: 5939–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajah S, Posner AM, Quirk JP. 1970. Competitive adsorption of phosphate with polygalacturonate and other organic anions on kaolinate and oxide surface. Nature 228: 83–84. [DOI] [PubMed] [Google Scholar]
- Otani T, Ae N, Tanaka H. 1996. P uptake mechanisms of crop grown in soils with low P status. II. Significance of organic acids in root exudates of pigeonpea. Soil Science and Plant Nutrition 42: 533–560. [Google Scholar]
- Pammenter NW, Woolhouse HW. 1975. The utilization of P-N compounds by plants. II. The role of extracellular root phosphatases. Annals of Botany 39: 347–361. [Google Scholar]
- Runge-Metzger A. 1995. Closing the cycle: obstacles to efficient P management for improved global security In: H Tiessen, ed. Phosphorus in the global environment: transfers, cycles and management. Chichester: John Wiley and Sons, 27–42. [Google Scholar]
- Rzewuski G, Sauter M. 2008. Ethylene biosynthesis and signalling in rice. Plant Science 175: 32–42. [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. 1995. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. The Plant Journal 8: 943–948. [DOI] [PubMed] [Google Scholar]
- Tapia G, Verdugo I, Yanez M, et al. 2005. Involvement of ethylene in stress-induced expression of the TLC1.1 retrotransposon from Lycopersicon chilense Dun. Plant Physiology 138: 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157: 423–447. [DOI] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR. 2002. Ethylene biosynthesis and signaling networks. The Plant Cell 14: S131–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y, Piñeros MA, et al. 2014. Phosphate transporters OsPHT1; 9 and OsPHT1; 10 are involved in phosphate uptake in rice. Plant, Cell and Environment 37: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Osaki M, Yano H, Rao I.M. 2006. Internal mechanisms of plant adaptation to aluminum toxicity and phosphorus starvation in three tropical forages. Journal of Plant Nutrition 29: 1243–1255. [Google Scholar]
- Withers PJA, Edwards AC, Foy RH. 2001. Phosphorus cycling in UK agriculture and implications for phosphorus loss from soil. Soil Use and Management 17: 139–149. [Google Scholar]
- Wu JJ, Wang C, Zheng LQ, et al. 2011. Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. Journal of Experimental Botany 62: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Shou HX, Xu GH, Lian XM. 2013. Improvement of phosphorus efficiency in rice on the basis of understanding phosphate signaling and homeostasis. Current Opinion in Plant Biology 16: 205–212. [DOI] [PubMed] [Google Scholar]
- Xiong J, An LY, Lu H, Zhu C. 2009. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230: 755–765. [DOI] [PubMed] [Google Scholar]
- Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A. 2003. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. Journal of Biological Chemistry 278: 49102–49112. [DOI] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, et al. 2008. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology 146: 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhu XF, Peng YX, et al. 2011. Cell wall hemicellulose contributes significantly to Al adsorption and root growth in Arabidopsis. Plant Physiology 155: 1885–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Eticha D, Rao IM, Horst WJ. 2010. Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.). Journal of Experimental Botany 61: 3245–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SJ, Kaeppler SM. 2001. Induction of maize acid phosphatase activities under phosphorus starvation. Plant and Soil 237: 109–115. [Google Scholar]
- Zhang FS, Ma J, Cao YP. 1997. Phosphorus deficiency enhances root exudation of low-molecular weight organic acids and utilization of sparingly soluble inorganic phosphates by radish (Raghanus satiuvs L.) and rape (Brassica napus L.) plants. Plant and Soil 196: 261–264. [Google Scholar]
- Zheng LQ, Huang FL, Narsai R, et al. 2009. Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiology 151: 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Läuchli A. 1993. Changes of cell wall component and polymer size in primary roots of cotton seedlings under high salinity. Journal of Experimental Botany 44: 773–778. [Google Scholar]
- Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ. 2012. Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236: 989–997. [DOI] [PubMed] [Google Scholar]
- Zhu XF, Wang ZW, Wan JX, et al. 2014. Pectin enhances rice (Oryza sativa) root phosphorus remobilization. Journal of Experimental Botany 66: 1017–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.