Abstract
OBJECTIVE
To examine recurrent preterm birth and early term birth in women’s initial and immediately subsequent pregnancies.
METHODS
This retrospective cohort study included 163,889 women who delivered their first and second liveborn singleton neonates between 20 and 44 weeks of gestation in California from 2005 through 2011. Data from hospital discharge records and birth certificates were used for analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression models adjusted for risk factors.
RESULTS
Shorter gestational duration in the first pregnancy increased the risk of subsequent preterm birth (both early, before 32 weeks of gestation, and later, from 32 to 36 weeks of gestation) as well as early term birth (37–38 weeks of gestation). Compared with women with a prior term birth, women with a prior early preterm birth (before 32 weeks of gestation) were at the highest risk for a subsequent early preterm birth (58/935 [6.2%] compared with 367/118,505 [0.3%], adjusted OR 23.3, 95% CI 17.2–31.7). Women with a prior early term birth had more than a twofold increased risk for subsequent preterm birth (before 32 weeks of gestation: 171/36,017 [0.5%], adjusted OR 2.0, 95% CI 1.6–2.3; from 32 to 36 weeks of gestation: 2,086/36,017 [6.8%], adjusted OR 3.0, 95% CI 2.9–3.2) or early term birth (13,582/36,017 [37.7%], adjusted OR 2.2, 95% CI 2.2–2.3).
CONCLUSION
Both preterm birth and early term birth are associated with these outcomes in a subsequent pregnancy. Increased clinical attention and research efforts may benefit from a focus on women with a prior early term birth as well as those with prior preterm birth.
Preterm birth (before 37 weeks of gestation) affects more than 1 in 10 newborns in some populations and is a leading cause of neonatal death and long-term morbidity.1,2 Early preterm birth (before 32 weeks of gestation) is associated with the highest risk for short-and long-term morbidity and mortality.3,4
Prior preterm birth, overall or by delivery subtypes, increases the risk for a subsequent preterm birth.5,6 Other risk factors for recurrent preterm birth include black race, underweight maternal body mass index, maternal smoking, short interpregnancy interval, and short cervical length.7,8
Increasingly, data have shown that the risk for recurrent preterm birth is influenced by prior gestational duration, wherein the earlier the prior birth, the higher the risk for a subsequent preterm birth.6 Whether this risk extends to “early term birth” (37 and 38 weeks of gestation) is not clear. Those born at 37 and 38 weeks of gestation have been shown to have poorer mortality and morbidity outcomes9,10 and pregnancies delivering at these time points have been shown to have a greater likelihood of pathologic placental conditions closely tied to preterm birth (ie, chronic uteroplacental malperfusion).3
This study sought to gain a better understanding of the relationship between gestational durations in two pregnancies by examining risks for preterm birth and early term birth in women with their first two singleton deliveries in California from 2005 through 2011. We specifically tested the hypothesis that women with an early term birth in the first pregnancy would be at increased risk for a subsequent preterm birth in their next pregnancy.
MATERIALS AND METHODS
This retrospective cohort study included 163,889 mother–first child–second child triads drawn from a 7-year birth cohort of 3,767,337 singleton live births in California from 2005 through 2011. Sample selection proceeded by first identifying 700,634 women who delivered two or more singleton live births between 2005 and 2011 based on birth certificate files using linkage algorithms that leveraged identifiers and other data including first and last name (married or maiden), date and place of maternal birth, address, phone number, and reported month and year of delivery of the first pregnancy as reported in the second.
For this study, we focused specifically on women in the cohort who delivered two consecutive singleton live births in their first and second pregnancies between 20 and 44 weeks of gestation during the study period and had linked hospital discharge records available through the California Office of Statewide Health Planning and Development. Gravidity and parity reported on the birth certificate were used to exclude women who had a pregnancy or pregnancies before the study period and who had miscarriages, elective abortions, stillbirths, or deliveries outside of California between the first and second births. Gestational age at delivery was classified based on “best obstetric estimate” reported on the birth certificate, which relies on ultrasound dating where present along with last menstrual period.11,12 Sample selection steps are listed in Appendix 1, available online at http://links.lww.com/AOG/A824.
Participants were categorized into four outcome groups based on the length of gestation in the second pregnancy (before 32, 32–36, 37–38, and 39 weeks of gestation or more). Length of gestation in the first pregnancy was divided into these same four subgroups and further categorized into before 32, 32, 33, 34, 35, 36, 37, 38, and 39 weeks of gestation or more. For this analysis, each gestational age included completed weeks such that, for example, 32 weeks of gestation included women delivered at 32 0/7 to 32 6/7 weeks of gestation.
Demographic characteristics derived from birth certificate data included maternal age, race or ethnicity, education, country of birth, interpregnancy interval, paternity change between pregnancies, and Medi-Cal (California Medicaid) payment for the delivery. Interpregnancy interval was estimated as months from the birth date of the first pregnancy to conception of the second pregnancy (calculated by birth date and gestational length of the second pregnancy). Paternity change was determined by comparing the identifiers for the father (name and date of birth) on the birth certificate in the first pregnancy with those in the second. Smoking and cesarean delivery were coded as yes if either was reported on the hospital discharge record or the birth certificate. Coding for hypertension (any, pre-existing with and without preeclampsia, gestational with and without preeclampsia), diabetes (any, pre-existing, and gestational), urinary tract infection (UTI) without an indwelling catheter, other non-UTI infection, maternal anemia, illicit drug abuse, mental disorder, and delivery subtypes (spontaneous and medically indicated) was based on the International Classification of Diseases, 9th Revision, Clinical Modification13 four-digit codes contained in the hospital discharge files.
Pregnancies resulting in spontaneous preterm birth were considered to be those in which hospital discharge records or birth certificate indicated premature rupture of membranes, premature labor, or those in which tocolytic medications were administered. Pregnancies resulting in medically indicated birth were considered to be those without premature rupture of membranes, premature labor, or tocolytic administration for which there was a code for “medical induction” or “artificial rupture of membranes” or for which there was a cesarean delivery without any of the aforementioned codes.
We first examined the relationship between maternal characteristics and obstetric factors across pregnancies with outcomes in the second pregnancy to identify variables associated with our outcomes of interest. Initially, crude odds ratios (ORs) and their 95% confidence intervals (CIs) derived from logistic regression were used to measure the relationship between each of the maternal characteristics and obstetric factors across pregnancies and gestational length in the second pregnancy (before 32, 32–36, and 37–38 weeks of gestation compared with 39 weeks of gestation or more). We then examined the relationship between gestational age in the second pregnancy and gestational age in the first pregnancy (by before 32, 32–36, and 37–38 weeks of gestation compared with 39 weeks of gestation or more groupings and then by before 32, 32, 33, 34, 35, 36, 37, 38 weeks of gestation compared with 39 weeks of gestation or more groupings) adjusting for factors in the crude analyses that were shown to be associated with preterm birth at P<.05. Subanalyses were also conducted that examined patterns by preterm birth subtypes of spontaneous and medically indicated.
All analyses were performed using SAS 9.3 and were based on data received by the Genetic Disease Screening Program at the California Department of Public Health as of February 1, 2015. Methods and protocols for the study were approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California.
RESULTS
A number of maternal characteristics (eg, maternal age younger than 18 years), obstetric factors (eg, pre-existing hypertension), and diagnoses (eg, UTI) were associated with preterm birth and early term birth (Table 1 Appendices 2–4 [available online at http://links.lww.com/AOG/A824]). Women of black race and women with an interpregnancy interval less than 6 months had more than three times the odds of delivering the second child before 32 weeks of gestation (OR 3.1, 95% CI 2.3–4.2 and OR 3.3, 95% CI 2.8–4.0, respectively). Pre-existing and gestational hypertension with and without preeclampsia were associated with elevated and often substantial risks for preterm birth and early term birth with the highest risks observed for birth before 32 weeks of gestation among women with pre-existing hypertension and preeclampsia in both pregnancies (OR 88.2, 95% CI 28.8–269.9).
Table 1.
Significant Associations of Obstetric Factors and Diagnoses With Preterm Birth (Before 32 Weeks of Gestation and 32–36 Weeks of Gestation) and Early Term Birth (37–38 Weeks of Gestation) in the Second Pregnancy
| Obstetric Factors and Diagnoses | Gestational Week at Delivery of the 2nd Pregnancy | ||
|---|---|---|---|
| Before 32* | 32–36* | 37–38* | |
| Pre-existing hypertension without preeclampsia | |||
| Both pregnancies | † | 1.9 (1.2–3.0) | 1.5 (1.2–1.9) |
| 2nd pregnancy only | † | 2.0 (1.4–2.9) | 1.3 (1.1–1.6) |
| Pre-existing hypertension with preeclampsia | |||
| Both pregnancies | 88.2 (28.8–269.9) | 27.6 (11.4–66.6) | 4.6 (2.0–10.9) |
| 1st pregnancy only | † | 3.4 (2.0–6.0) | 2.0 (1.4–2.8) |
| 2nd pregnancy only | † | 5.5 (2.8–10.8) | 2.1 (1.3–3.5) |
| Gestational hypertension without preeclampsia | |||
| Both pregnancies | † | 3.7 (2.7–5.2) | 2.3 (1.9–2.8) |
| 1st pregnancy only | 0.8 (0.5–1.2) | 1.3 (1.1–1.5) | 1.1 (1.0–1.1) |
| 2nd pregnancy only | 1.2 (0.5–2.7) | 2.2 (1.8–2.8) | 1.5 (1.4–1.7) |
| Gestational hypertension with preeclampsia | |||
| Both pregnancies | 20.3 (13.8–30.0) | 13.9 (11.3–17.0) | 3.7 (3.1–4.4) |
| 1st pregnancy only | 0.9 (0.6–1.3) | 1.4 (1.3–1.6) | 1.2 (1.1–1.3) |
| 2nd pregnancy only | 13.9 (10.4–18.6) | 9.7 (8.4–11.1) | 2.5 (2.2–2.8) |
| Pre-existing diabetes | |||
| Both pregnancies | 4.3 (1.9–9.7) | 3.6 (2.5–5.0) | 2.8 (2.3–3.5) |
| 1st pregnancy only | 2.0 (0.9–4.2) | 1.7 (1.2–2.3) | 1.3 (1.1–1.5) |
| 2nd pregnancy only | 3.5 (1.6–7.3) | 2.3 (1.7–3.3) | 1.5 (1.2–1.8) |
| Gestational diabetes | |||
| Both pregnancies | 1.2 (0.8–1.8) | 1.9 (1.7–2.1) | 1.6 (1.5–1.7) |
| 1st pregnancy only | 2.0 (1.5–2.6) | 1.4 (1.2–1.6) | 1.2 (1.1–1.3) |
| 2nd pregnancy only | 1.3 (1.0–1.8)‡ | 1.6 (1.5–1.8) | 1.4 (1.3–1.4) |
| Urinary tract infection | |||
| 1st pregnancy only | 2.3 (1.5–3.5) | 1.3 (1.1–1.6) | 1.1 (1.0–1.2) |
| 2nd pregnancy only | 9.0 (6.4–12.7) | 2.9 (2.4–3.6) | 1.4 (1.2–1.6) |
| Anemia, 2nd pregnancy only | 1.6 (1.3–2.0) | 1.1 (1.0–1.2)‡ | 1.0 (0.9–1.0)‡ |
| Drug abuse | |||
| Both pregnancies | † | 2.9 (1.5–5.5) | 1.6 (1.1–2.4) |
| 1st pregnancy only | 1.4 (0.6–3.1) | 1.4 (1.0–1.8)‡ | 0.8 (0.7–1.0) |
| 2nd pregnancy only | 7.1 (4.4–11.5) | 3.3 (2.6–4.3) | 1.0 (0.8–1.2)‡ |
| Mental disorder | |||
| Both pregnancies | 2.8 (1.3–6.4) | 1.2 (0.8–1.8) | 1.3 (1.0–1.5) |
| 2nd pregnancy only | 2.6 (1.8–3.8) | 1.5 (1.2–1.8) | 0.9 (0.9–1.0) |
| Cesarean delivery, 1st pregnancy | 1.5 (1.3–1.7) | 1.3 (1.2–1.4) | 1.4 (1.4–1.5) |
Data are odds ratio (95% confidence interval).
Odds ratio was calculated by the crude logistic regression analyses; obstetric factor or diagnosis in neither pregnancy was the reference except for cesarean delivery at the first pregnancy with no history of cesarean delivery as the reference.
Not computed as a result of frequency less than 5.
95% confidence interval does not include 1.0.
Other notable associations included an increased risk for preterm birth and early term birth in women with pre-existing diabetes across both pregnancies (OR 4.3, 95% CI 1.9–9.7, OR 3.6, 95% CI 2.5–5.0, and OR 2.8, 95% CI 2.3–3.5, respectively, for birth before 32 weeks of gestation, 32–36 weeks of gestation, and early term birth). Women with a UTI (without catheter insertion) at discharge for the second pregnancy and those who reported illicit drug “addiction or abuse” (eg, marijuana, methamphetamines, or cocaine) in the second pregnancy only were also at substantially increased risk for birth before 32 weeks of gestation (OR 9.0, 95% CI 6.4–12.7 and OR 7.1, 95% CI 4.4–11.5, respectively).
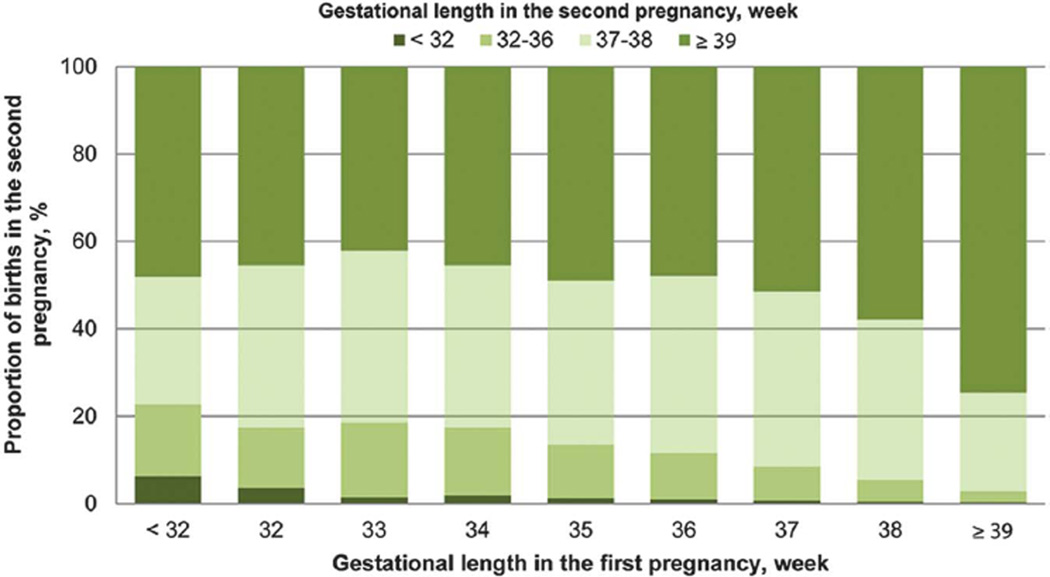
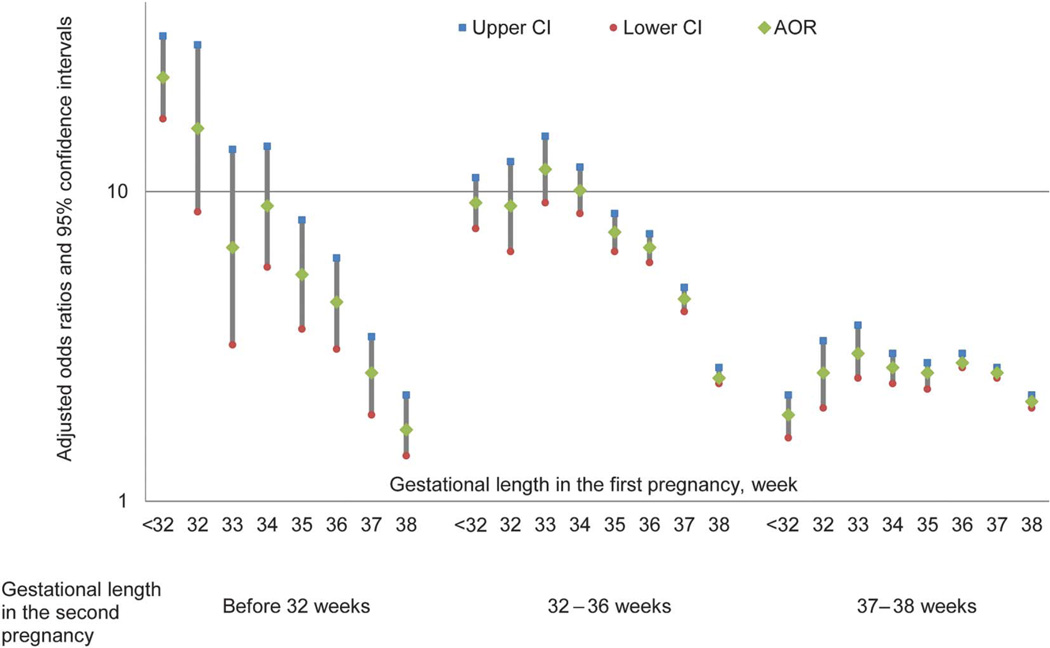
Gestational age (by preterm birth subgroup and by specific week) at delivery in the first pregnancy was found to be associated with preterm birth (before 32, 32–36 weeks of gestation) and early term birth in the second pregnancy in both crude and adjusted analyses (Tables 2 and 3; Figs. 1 and 2; Appendix 5 [available online at http://links.lww.com/AOG/A824]). Women with the first pregnancy before 32 weeks of gestation were at the highest risk for subsequent birth before 32 weeks of gestation (58/935 [6.2%], adjusted OR 23.3, 95% CI 17.2–31.7), 32–36 weeks of gestation (154/935 [16.5%], adjusted OR 9.2, 95% CI 7.6–11.1), and early term birth (273/935 [29.2%], adjusted OR 1.9, 95% CI 1.6–2.2) when compared with women with the first pregnancy gestation 39 weeks of gestation or more (367/118,505 [0.3%], 2,928/118,505 [2.5%], 26,744/118,505 [22.6%] for before 32 weeks of gestation, 32–36 weeks of gestation, and early term birth in the second pregnancy, respectively). An increased risk for preterm birth and early term birth was also observed among women with an early term birth in the first pregnancy. Women with the first pregnancy outcome of early term birth had higher adjusted odds of the second pregnancy gestation before 32 weeks of gestation (171/36,017 [0.5%], adjusted OR 2.0, 95% CI 1.6–2.3), 32–36 weeks of gestation (2,086/36,017 [6.8%], adjusted OR 3.0, 95% CI 2.9–3.2), and 37–38 weeks of gestation (13,582/36,017 [37.7%], adjusted OR 2.2, 95% CI 2.2–2.3) when compared with women with the first pregnancy birth at 39 weeks of gestation or more. Similar associations were found when delivery subtypes of spontaneous and medically indicated in both pregnancies were examined with some indication of stronger associations when both pregnancies resulted in spontaneous deliveries (Table 4).
Table 2.
Risk of Preterm Birth (Before 32 Weeks of Gestation and 32–36 Weeks of Gestation) and Early Term Birth (37–38 Weeks of Gestation) in the Second Pregnancy by Gestational Week at Birth Grouping in the First Pregnancy
| Gestational Week at Delivery of the 1st Pregnancy |
Gestational Week at Delivery of the 2nd Pregnancy | |||
|---|---|---|---|---|
| Before 32 | 32–36 | 37–38 | 39 or More | |
| Sample (n) | 702 | 6,199 | 43,901 | 113,087 |
| Before 32 | 58 (8.3) | 154 (2.5) | 273 (0.6) | 450 (0.4) |
| Crude OR (95% CI) | 31.1 (23.2–41.6) | 10.4 (8.6–12.5) | 2.0 (1.7–2.3) | |
| Adjusted OR (95% CI)* | 23.3 (17.2–31.7) | 9.2 (7.6–11.1) | 1.9 (1.6–2.2) | |
| 32–36 | 106 (15.1) | 1,031 (10.6) | 3,302 (3.5) | 3,993 (3.5) |
| Crude OR (95% CI) | 6.4 (5.1–8.0) | 7.8 (7.2–8.4) | 2.7 (2.6–2.9) | |
| Adjusted OR (95% CI)* | 5.8 (4.7–7.3) | 7.6 (7.0–8.2) | 2.8 (2.6–2.9) | |
| 37–38 | 171 (24.5) | 2,086 (33.6) | 13,582 (17.8) | 20,178 (17.8) |
| Crude OR (95% CI) | 2.1 (1.7–2.5) | 3.1 (3.0–3.3) | 2.2 (2.2–2.3) | |
| Adjusted OR (95% CI)* | 2.0 (1.6–2.3) | 3.0 (2.9–3.2) | 2.2 (2.2–2.3) | |
| 39 or more | 367 (52.2) | 2,928 (47.2) | 26,744 (60.9) | 88,466 (78.2) Reference |
OR, odds ratio; CI, confidence interval.
Data are n (%) unless otherwise specified.
Adjusted for maternal and obstetric factors in both pregnancies (maternal age, education, mother’s birthplace, Medi-Cal for delivery, smoking, pre-existing and gestational hypertension with or without preeclampsia, pre-existing and gestational diabetes, urinary tract infection, anemia, drug abuse, and mental disorder), maternal race or ethnicity, cesarean delivery at the first pregnancy, interpregnancy interval, and paternity change between pregnancies.
Table 3.
Risk of Preterm Birth (Before 32 Weeks of Gestation and 32–36 Weeks of Gestation) and Early Term Birth (37–38 Weeks of Gestation) in the Second Pregnancy by Gestational Week at Birth in the First Pregnancy
| Gestational Week at Delivery of the 2nd Pregnancy |
Gestational Week at Delivery of the 1st Pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before 32 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 or More |
|
| Sample (n) | 935 | 334 | 556 | 1,166 | 2,130 | 4,246 | 10,180 | 25,837 | 118,505 |
| Before 32 | 58 (6.2) | 12 (3.6) | 8 (1.4) | 22 (1.9) | 25 (1.2) | 39 (0.9) | 59 (0.6) | 112 (0.4) | 367 (0.3) |
| 23.4 (17.2–31.8) | 16.0 (8.6–29.8) | 6.6 (3.2–13.7) | 9.0 (5.7–14.0) | 5.4 (3.6–8.1) | 4.4 (3.1–6.1) | 2.6 (1.9–3.4) | 1.7 (1.4–2.1) | ||
| 32–36 | 154 (16.5) | 46 (13.8) | 95 (17.1) | 181 (15.5) | 260 (12.2) | 449 (10.6) | 799 (7.8) | 1,287 (5.0) | 2,928 (2.5) |
| 9.2 (7.6–11.2) | 9.0 (6.4–12.5) | 11.8 (9.2–15.0) | 10.1 (8.5–12.0) | 7.4 (6.4–8.5) | 6.5 (5.9–7.3) | 4.5 (4.1–4.9) | 2.5 (2.4–2.7) | ||
| 37–38 | 273 (29.2) | 124 (37.1) | 219 (39.4) | 432 (37.0) | 801 (37.6) | 1,726 (40.7) | 4,092 (40.2) | 9,490 (36.7) | 26,744 (22.6) |
| 1.9 (1.6–2.2) | 2.6 (2.1–3.3) | 3.1 (2.5–3.7) | 2.7 (2.4–3.1) | 2.6 (2.3–2.8) | 2.8 (2.7–3.0) | 2.6 (2.5–2.7) | 2.1 (2.0–2.2) | ||
| 39 or more | 450 (48.1) | 152 (45.5) | 234 (42.1) | 531 (45.5) | 1,044 (49.0) | 2,032 (47.9) | 5,230 (51.4) | 14,948 (57.9) | 88,466 (74.7) Reference |
Data are n (%) or adjusted odds ratio (95% confidence interval) unless otherwise specified.
Adjusted for maternal and obstetric factors in both pregnancies (maternal age, education, mother’s birth place, Medi-Cal for delivery, smoking, pre-existing and gestational hypertension with or without preeclampsia, pre-existing and gestational diabetes, urinary tract infection, anemia, drug abuse, and mental disorder), maternal race or ethnicity, cesarean delivery at the first pregnancy, interpregnancy interval, and paternity change between.
Fig. 1.
Distribution of gestational length in the second pregnancy by gestational length in the first pregnancy.
Yang. Recurrent Preterm Birth and Early Term Birth. Obstet Gynecol 2016.
Fig. 2.
Risk of preterm birth (before 32, 32–36 weeks of gestation) and early term birth (37–38 weeks of gestation) in the second pregnancy by gestational week at birth in the first pregnancy.
Yang. Recurrent Preterm Birth and Early Term Birth. Obstet Gynecol 2016.
Table 4.
Crude and Adjusted Odds Ratios and 95% Confidence Intervals of Gestational Week at Delivery of the Second Pregnancy in Association With Gestational Week at Delivery of the First Pregnancy by Delivery Subtypes
| Gestational Week at Delivery of the 1st Pregnancy |
Gestational Week at Delivery of the 2nd Pregnancy | |||
|---|---|---|---|---|
| Spontaneous | ||||
| Before 32 | 32–36 | 37–38 | 39 or More | |
| Spontaneous | ||||
| Before 32 | 43 (28.3) | 97 (10.8) | 23 (4.2) | 14 (3.1) |
| Crude OR (95% CI) | 41.8 (19.4–90.3) | 15.2 (8.3–27.7) | 2.5 (1.3–5.0) | |
| Adjusted OR (95% CI)† | 61.2 (24.1–155.3) | 12.0 (6.4–22.4) | 2.2 (1.1–4.6) | |
| 32–36 | 71 (46.7) | 517 (57.5) | 218 (40.1) | 85 (18.7) |
| Crude OR (95% CI) | 11.4 (6.4–20.2) | 13.3 (9.7–18.3) | 3.9 (2.8–5.4) | |
| Adjusted OR (95% CI)† | 11.6 (6.2–21.8) | 14.4 (10.3–20.0) | 4.1 (3.0–5.8) | |
| 37–38 | 20 (13.2) | 173 (19.2) | 141 (26) | 111 (24.4) |
| Crude OR (95% CI) | 2.5 (1.3–4.8) | 3.4 (2.5–4.7) | 1.9 (1.4–2.7) | |
| Adjusted OR (95% CI)† | 1.9 (0.9–4.1) | 3.3 (2.3–4.6) | 2.0 (1.4–2.7) | |
| 39 or more | 18 (11.8) | 112 (12.5) | 161 (29.7) | 245 (53.8) Reference |
| Medically indicated | ||||
| Before 32 | 0 (0) | 5 (0.3) | 2 (0.2) | 4 (0.3) |
| Crude OR (95% CI) | * | 1.4 (0.4–5.4) | * | |
| Adjusted OR (95% CI)† | * | 1.0 (0.3–3.9) | * | |
| 32–36 | 8 (3.5) | 83 (5.1) | 32 (2.9) | 9 (0.7) |
| Crude OR (95% CI) | 6.0 (2.3–15.6) | 10.7 (5.3–21.3) | 5.4 (2.6–11.3) | |
| Adjusted OR (95% CI)† | 5.3 (1.9–14.8) | 10.4 (5.2–21.0) | 5.4 (2.5–11.5) | |
| 37–38 | 50 (22.1) | 551 (34.2) | 315 (28.8) | 217 (16) |
| Crude OR (95% CI) | 1.5 (1.1–2.2) | 2.9 (2.5–3.5) | 2.2 (1.8–2.7) | |
| Adjusted OR (95% CI)† | 1.3 (0.9–1.9) | 2.8 (2.4–3.4) | 2.1 (1.7–2.6) | |
| 39 or more | 168 (74.3) | 974 (60.4) | 744 (68.1) | 1,125 (83) Reference |
| Gestational Week at Delivery of the 2nd Pregnancy | |||
|---|---|---|---|
| Medically Indicated | |||
| Before 32 | 32–36 | 37–38 | 39 or More |
| 3 (30) | 21 (14.4) | 96 (4.4) | 152 (3.2) |
| * | 10.2 (5.8–18.0) | 2.3 (1.8–3.1) | |
| 9.8 (5.4–17.7) | 2.3 (1.7–3.0) | ||
| 3 (30) | 64 (43.8) | 790 (35.9) | 995 (20.9) |
| * | 4.8 (3.1–7.3) | 2.9 (2.6–3.3) | |
| 4.7 (3.0–7.1) | 3.0 (2.7–3.4) | ||
| 2 (20) | 26 (17.8) | 609 (27.7) | 1,013 (21.3) |
| * | 1.9 (1.1–3.2) | 2.2 (2.0–2.5) | |
| 1.9 (1.1–3.2) | 2.2 (2.0–2.6) | ||
| 2 (20) | 35 (24) | 703 (32) | 2,595 (54.6) Reference |
| 1 (2.1) | 5 (0.9) | 30 (0.2) | 61 (0.2) |
| * | 7.16 (2.86–17.91) | 1.5 (1.0–2.3) | |
| * | 5.76 (2.28–14.55) | 1.4 (0.9–2.1) | |
| 2 (4.3) | 31 (5.4) | 428 (2.9) | 596 (1.6) |
| * | 4.5 (3.1–6.6) | 2.2 (1.9–2.5) | |
| * | 4.2 (2.9–6.1) | 2.1 (1.9–2.4) | |
| 10 (21.3) | 174 (30.5) | 3,897 (26.5) | 5,696 (15) |
| 1.6 (0.8–3.3) | 2.7 (2.2–3.2) | 2.1 (2.0–2.2) | |
| 1.6 (0.8–3.2) | 2.5 (2.1–3.0) | 2.0 (1.9–2.1) | |
| 34 (72.3) | 361 (63.2) | 10,345 (70.4) | 31,512 (83.2) Reference |
OR, odds ratio; CI, confidence interval.
Data are n (%) unless otherwise specified.
Not computed as a result of frequency less than 5.
Adjusted for maternal and obstetric factors in both pregnancies (maternal age, education, mother’s birthplace, Medi-Cal for delivery, smoking, pre-existing and gestational hypertension with or without preeclampsia, pre-existing and gestational diabetes, urinary tract infection, anemia, drug abuse, and mental disorder), maternal race or ethnicity, cesarean delivery at the first pregnancy, interpregnancy interval, and paternity change between.
When the second pregnancy outcomes were examined by specific gestational week at delivery in the first pregnancy, an increase in risk for preterm birth by each decrease in gestational week at the first birth was observed. This pattern extended through 37 and 38 weeks of delivery, wherein women with a delivery in their first pregnancy at 37 or 38 weeks of gestation were at increased risk for delivering preterm or early term in their subsequent pregnancy. The rates and adjusted ORs were 0.6% and 2.6 (95% CI 1.9–3.4), 7.8% and 4.5 (95% CI 4.1–4.9), and 40.2% and 2.6 (95% CI 2.5–2.7), respectively, for birth before 32 weeks of gestation, 32–36 weeks of gestation, and early term birth in those with a delivery at 37 weeks of gestation in the first pregnancy compared with 0.3%, 0.5%, and 22.6%, respectively, in those with a delivery at 39 weeks of gestation or more in the first pregnancy. For those with a delivery at 38 weeks of gestation in their first pregnancy, the rates and adjusted ORs were 0.4% and 1.7 (95% CI 1.4–2.2), 5.0% and 2.5 (95% CI 2.4–2.7), and 36.7% and 2.1 (95% CI 2.0–2.2), respectively, for birth before 32 weeks of gestation, 32–36 weeks of gestation, and early term birth in subsequent pregnancy.
DISCUSSION
This study confirms that a prior preterm birth increases the risk for a subsequent preterm birth with higher odds among those with a prior early preterm birth.6,14,15 Risks extend to both spontaneous and indicated prior preterm birth.5,16 Women with a prior early term birth are also at increased risk for subsequent preterm birth.
Although early term birth has been associated with a higher risk for mortality and morbidities9,10,17 and with pathologic placental conditions, namely chronic uteroplacental malperfusion,3 this is the first study to demonstrate preterm birth and early term birth in association with subsequent preterm birth and early term birth based on a systemic literature search in PubMed undertaken on April 5, 2016, and using terms of (early term birth) AND (preterm birth) AND (recurren* OR subsequent) with no date and language restrictions.
This study confirms a number of risk factors associated with recurrent preterm birth including black race, short interpregnancy interval, illicit drug use, pre-existing and gestational hypertension, pre-existing diabetes, and UTI.8,18–20 Like others, the risk of recurrent preterm birth is stronger for women with a birth before 32 weeks of gestation compared with 32–36 weeks of gestation.5,6,16,20,21 The risk increases by each week of earlier gestation of a prior preterm birth.
Although the association between prior and recurrent preterm birth is well established, mechanisms underlying this association are not well understood. Genetic, environmental, and behavioral risk factors contribute to recurrence risks both independently and in combination.22 Women with recurrent preterm birth (spontaneous and indicated) are more likely to have recurrent intrauterine infection, repeated placental complications, and chronic maternal diseases (eg, hypertension) than women with one preterm birth.6,23 Our observation that 1) preterm birth and early term birth in the first pregnancy were associated with subsequent preterm birth and early term birth and that 2) their recurrence shared common risk factors (eg, pre-existing hypertension, diabetes) suggests that preterm birth and early term birth share common etiologies and may reflect a continuum of risk related to gestational duration of less than 39 weeks of gestation.
Our findings provide a better understanding of early term birth and the importance of continuation of gestation beyond 38 weeks of gestation.24 Although the association of prior early term birth with subsequent preterm birth is less strong than prior preterm birth (Table 2), many more women fall in this category, making this an important risk factor to the overall societal burden of preterm birth.
Study strengths include the population-based design and the broad range of data including maternal, obstetric, and behavioral factors. The large sample size also allowed for stratified analysis by gestational weeks and delivery subtypes of spontaneous and medically indicated.
Several study limitations should be considered. First, the study cohort was limited to the first two consecutive live singleton births delivered in California between 2005 and 2011. The observed patterns need to be further investigated in women with higher gravidities and parities, in women with history of miscarriage, elective abortion, and stillbirth, in spontaneous preterm birth subcategories (eg, preterm premature rupture of membranes), and by placental abnormalities (eg, placental previa or abruption). The study sample was composed of a small percentage of black women and a large proportion of women with some college education (Appendix 6, available online at http://links.lww.com/AOG/A824). Follow-up in other populations is needed to determine if patterns are similar. This study relied on data from hospital discharge records and birth certificates for analysis. Data were not available to examine other important risk factors for preterm birth (eg, maternal body mass index,7 income,25 employment,26 tocolysis,27 assisted reproduction,28 and cervical surgery29). The existing data could not identify elective delivery at 37 and 38 weeks of gestation, which are important points for investigation as this area of work progresses. Hospital discharge records and birth certificates also had limited information for classifying deliveries at 37 and 38 weeks of gestation as spontaneous or medically indicated. Furthermore, approximately 30% of mothers with two or more singleton births during the study period were excluded as a result of no linkage to hospital discharge data (Appendix 1, available online at http://links.lww.com/AOG/A824). The effect of this exclusion is unknown. Another quarter of the remaining eligible patients had to be further excluded as a result of missing information on maternal and obstetric factors (Appendix 1, available online at http://links.lww.com/AOG/A824) although a differential effect (bias) is unlikely because those included and excluded had similar rates in preterm birth and early term (4.2% and 26.8% compared with 4.9% and 27.8%, respectively).
In summary, prior preterm birth and early term birth are associated with these outcomes in a subsequent pregnancy. Recurrent preterm birth and early term birth share common risk factors. More attention to prior early term birth may be warranted.
Supplementary Material
Acknowledgments
This study was supported by funding from the Preterm Birth Initiative—California, University of California San Francisco School of Medicine, the March of Dimes Prematurity Research Center at Stanford University, March of Dimes Prematurity Research Center Ohio Collaborative, Bill and Melinda Gates Foundation, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant R00-HD65786).
Footnotes
Presented as a poster at the Society for Maternal-Fetal Medicine’s 36th Annual Pregnancy Meeting, February 1–6, 2016, Atlanta, Georgia.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Esplin MS. Overview of spontaneous preterm birth: a complex and multifactorial phenotype. Clin Obstet Gynecol. 2014;57:518–530. doi: 10.1097/GRF.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Stanek J. Comparison of placental pathology in preterm, late-preterm, near-term, and term births. Am J Obstet Gynecol. 2014;210:234.e1–234.e6. doi: 10.1016/j.ajog.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Kramer MR, Hogue CR. What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiol Rev. 2009;31:84–98. doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. Am J Obstet Gynecol. 2014;210:131.e1–131.e8. doi: 10.1016/j.ajog.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McManemy J, Cooke E, Amon E, Leet T. Recurrence risk for preterm delivery. Am J Obstet Gynecol. 2007;196:576.e1–576.e6. doi: 10.1016/j.ajog.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 8.Simonsen SE, Lyon JL, Stanford JB, Porucznik CA, Esplin MS, Varner MW. Risk factors for recurrent preterm birth in multiparous Utah women: a historical cohort study. BJOG. 2013;120:863–872. doi: 10.1111/1471-0528.12182. [DOI] [PubMed] [Google Scholar]
- 9.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. 2011;117:1279–1287. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailit JL, Gregory KD, Reddy UM, Gonzalez-Quintero VH, Hibbard JU, Ramirez MM, et al. Maternal and neonatal outcomes by labor onset type and gestational age. Am J Obstet Gynecol. 2010;202:245.e1–245.e12. doi: 10.1016/j.ajog.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep. 2015;64:1–20. [PubMed] [Google Scholar]
- 12.Mustafa G, David RJ. Comparative accuracy of clinical estimate versus menstrual gestational age in computerized birth certificates. Public Health Rep. 2001;116:15–21. doi: 10.1093/phr/116.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lydon-Rochelle MT, Holt VL, Cárdenas V, Nelson JC, Easterling TR, Gardella C, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193:125–134. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 14.Petrini JR, Callaghan WM, Klebanoff M, Green NS, Lackritz EM, Howse JL, et al. Estimated effect of 17 alpha-hydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 15.Bloom SL, Yost NP, McIntire DD, Leveno KJ. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol. 2001;98:379–385. doi: 10.1016/s0029-7844(01)01466-1. [DOI] [PubMed] [Google Scholar]
- 16.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195:643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Raju TN. Moderately preterm, late preterm and early term infants: research needs. Clin Perinatol. 2013;40:791–797. doi: 10.1016/j.clp.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelliffe-Pawlowski LL, Baer RJ, Blumenfeld YJ, Ryckman KK, O’Brodovich HM, Gould JB, et al. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG. 2015;122:1484–1493. doi: 10.1111/1471-0528.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study [published erratum appears in BMJ 2003;327:851] BMJ. 2003;327:313. doi: 10.1136/bmj.327.7410.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, Cole FS, et al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol. 2007;196:131, e1–e6. doi: 10.1016/j.ajog.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 21.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283:1591–1596. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 22.Ananth CV. Epidemiologic approaches for studying recurrent pregnancy outcomes: challenges and implications for research. Semin Perinatol. 2007;31:196–201. doi: 10.1053/j.semperi.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh TT, Chen SF, Shau WY, Hsieh CC, Hsu JJ, Hung TH. The impact of interpregnancy interval and previous preterm birth on the subsequent risk of preterm birth. J Soc Gynecol Investig. 2005;12:202–207. doi: 10.1016/j.jsgi.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Ananth CV, Friedman AM, Gyamfi-Bannerman C. Epidemiology of moderate preterm, late preterm and early term delivery. Clin Perinatol. 2013;40:601–610. doi: 10.1016/j.clp.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Bibby E, Stewart A. The epidemiology of preterm birth. Neuro Endocrinol Lett. 2004;25(suppl 1):43–47. [PubMed] [Google Scholar]
- 26.Brett KM, Strogatz DS, Savitz DA. Employment, job strain, and preterm delivery among women in North Carolina. Am J Public Health. 1997;87:199–204. doi: 10.2105/ajph.87.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackney DN, Olson-Chen C, Thornburg LL. What do we know about the natural outcomes of preterm labour? A systematic review and meta-analysis of women without tocolysis in preterm labour. Paediatr Perinat Epidemiol. 2013;27:452–460. doi: 10.1111/ppe.12070. [DOI] [PubMed] [Google Scholar]
- 28.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007;109:967–977. doi: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- 29.Jakobsson M, Gissler M, Sainio S, Paavonen J, Tapper AM. Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2007;109:309–313. doi: 10.1097/01.AOG.0000253239.87040.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.