Abstract
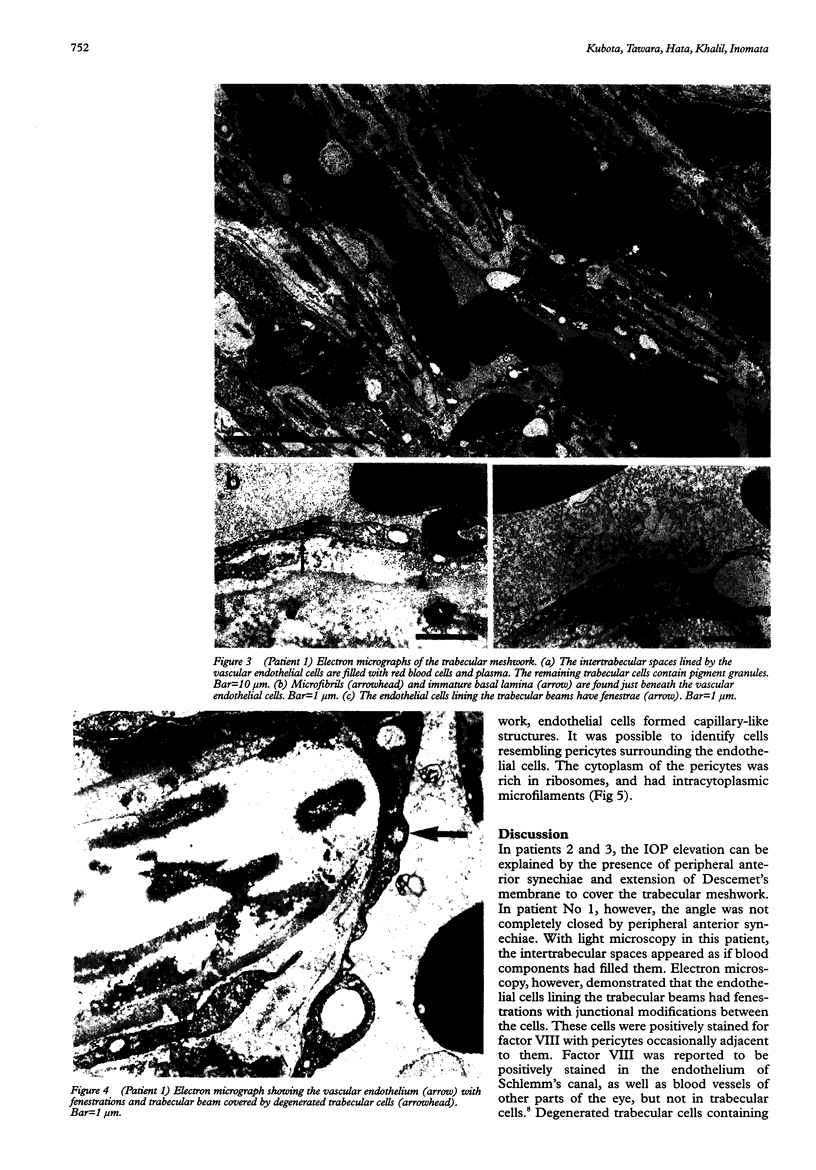
AIMS/BACKGROUND: To investigate histological changes in the trabecular meshwork in eyes with neovascular glaucoma. METHODS: Light and electron microscopic studies were carried out on the trabecular meshwork of three enucleated eyes with neovascular glaucoma. The presence and distribution of factor VIII in the trabecular meshwork was assessed using the ABC method. RESULTS: Peripheral anterior synechiae covering the trabecular meshwork were detected in two eyes, which would explain the rise in intraocular pressure. In the third the angle was not completely closed by peripheral anterior synechiae. The spaces between the trabecular beams were lined by a single layer of vascular endothelium, and were filled with red blood cells in this patient. Factor VIII was positively stained in the endothelial cells, lining both these spaces and Schlemm's canal. A basal lamina and microfibrils were detected just beneath the newly formed vascular endothelial cells. CONCLUSION: The neovascular tissue found in the trabecular spaces might be one of the factors responsible for intraocular pressure elevation in eyes with neovascular glaucoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtoy P. J., Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983 Jun;83(3):258–273. doi: 10.1016/s0022-5320(83)90133-8. [DOI] [PubMed] [Google Scholar]
- Gartner S., Henkind P. Neovascularization of the iris (rubeosis iridis). Surv Ophthalmol. 1978 Mar-Apr;22(5):291–312. doi: 10.1016/0039-6257(78)90175-3. [DOI] [PubMed] [Google Scholar]
- Gartner S., Taffet S., Friedman A. H. The association of rubeosis iridis with endothelialisation of the anterior chamber: report of a clinical case with histopathological review of 16 additional cases. Br J Ophthalmol. 1977 Apr;61(4):267–271. doi: 10.1136/bjo.61.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka T., Bill A., Ichinohasama R., Ishida T. Aspects of the development of Schlemm's canal. Exp Eye Res. 1992 Sep;55(3):479–488. doi: 10.1016/0014-4835(92)90121-8. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Inomata H., Sakamoto T., Ryan S. J. Pericytes of newly formed vessels in experimental subretinal neovascularization. Arch Ophthalmol. 1995 Feb;113(2):227–231. doi: 10.1001/archopht.1995.01100020111041. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Miller H., Orr G., Sorgente N., Ryan S. J. Morphologic observations on experimental subretinal neovascularization in the monkey. Invest Ophthalmol Vis Sci. 1987 Jul;28(7):1116–1130. [PubMed] [Google Scholar]
- Lee W. R. Doyne Lecture. The pathology of the outflow system in primary and secondary glaucoma. Eye (Lond) 1995;9(Pt 1):1–23. doi: 10.1038/eye.1995.2. [DOI] [PubMed] [Google Scholar]
- Miller H., Miller B., Ryan S. J. Newly-formed subretinal vessels. Fine structure and fluorescein leakage. Invest Ophthalmol Vis Sci. 1986 Feb;27(2):204–213. [PubMed] [Google Scholar]
- Murata T., Ishibashi T., Inomata H., Sueishi K. Media conditioned by coculture of pericytes and endothelial cells under a hypoxic state stimulate in vitro angiogenesis. Ophthalmic Res. 1994;26(1):23–31. doi: 10.1159/000267370. [DOI] [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]