Significance
Autophagy maintains cellular homoeostasis by bulk or selective degradation of cytoplasmic components including organelles or protein aggregates. Its role in axon regeneration remains speculative. Here, we found that boosting autophagy stabilized microtubules by degrading a microtubule destabilizing protein, SCG10 (superior cervical ganglia protein 10), in cultured CNS neurons and promoted axon growth. Furthermore, treatment with a specific autophagy-inducing peptide, Tat-beclin1, attenuated axon retraction, promoted axon regeneration, and improved locomotor functional recovery in mice with spinal cord injury. This study reveals a critical role of autophagy in stabilizing neuronal microtubules and a promising therapeutic effect of an autophagy-inducing reagent on CNS axons following injury.
Keywords: autophagy, microtubule stabilization, axon regeneration
Abstract
Remodeling of cytoskeleton structures, such as microtubule assembly, is believed to be crucial for growth cone initiation and regrowth of injured axons. Autophagy plays important roles in maintaining cellular homoeostasis, and its dysfunction causes neuronal degeneration. The role of autophagy in axon regeneration after injury remains speculative. Here we demonstrate a role of autophagy in regulating microtubule dynamics and axon regeneration. We found that autophagy induction promoted neurite outgrowth, attenuated the inhibitory effects of nonpermissive substrate myelin, and decreased the formation of retraction bulbs following axonal injury in cultured cortical neurons. Interestingly, autophagy induction stabilized microtubules by degrading SCG10, a microtubule disassembly protein in neurons. In mice with spinal cord injury, local administration of a specific autophagy-inducing peptide, Tat-beclin1, to lesion sites markedly attenuated axonal retraction of spinal dorsal column axons and cortical spinal tract and promoted regeneration of descending axons following long-term observation. Finally, administration of Tat-beclin1 improved the recovery of motor behaviors of injured mice. These results show a promising effect of an autophagy-inducing reagent on injured axons, providing direct evidence supporting a beneficial role of autophagy in axon regeneration.
It is generally believed that the inability of adult central nervous system (CNS) neurons to regenerate their axons following injury is due to the presence of abundant inhibitory factors in extrinsic milieu and the lack of intrinsic growth ability (1–5). A number of extrinsic growth-inhibitory factors have been identified, including oligodendrocyte-derived myelin-associated glycoprotein (MAG), Nogo, OMgp, or astrocyte-derived chondroitin sulfate proteoglycans (CSPGs), which act through their respective receptors to suppress axon growth and regeneration (6–11). However, genetic ablation or elimination of these inhibitory receptors does not promote axon regeneration (12, 13) or shows marginal effects (14, 15).
Many inhibitory factors act through signaling cascades to modulate cytoskeletal dynamics (16, 17). Indeed, it has been observed that CNS axons form numerous retraction bulbs (RBs) with a disorganized array of microtubules (MTs), whereas peripheral nervous system (PNS) axons rapidly form a growth cone with stable, well-organized bundling of MTs following injury (18). In line with this notion, pharmacological stabilization of MTs promotes axon regeneration after spinal cord injury (SCI) (19, 20). In addition, analyses of gene-targeted mice have led to identification of several intrinsic inhibitors of axon regeneration in the adult CNS, including phosphatase and tensin homolog (PTEN) and the suppressor of cytokine signaling 3 (SOCS3) (21, 22). However, manipulating individual proteins or in combinations allows limited axonal regeneration or sprouting, which is usually associated with temporary improvement in functional recovery. Moreover, achieving axonal regeneration through manipulating certain genes still remains therapeutically unattainable. Thus, development of other effective approaches to promote axonal regeneration is still a challenging task.
A lesion triggers drastic responses in soma and axon, including chromatolysis, axonal membrane sealing, growth cone RB formation, and Wallerian degeneration (23). The growth cone initiation and axon regeneration require an extensive remodeling of cytoplasmic compartment and axon structures, which involves the synthesis and degradation of local proteins (24, 25). Autophagy is one of the major pathways for bulk cytosolic degradation and efficient turnover under stress (26). It plays an important role in maintaining cellular homeostasis by degrading damaged organelles or pathological proteins through a lysosomal degradative process (27–29). In the nervous system, autophagosomes have been observed in cultured neurons or in vivo (30–35). Inhibition of autophagy by genetic elimination of autophagy-determination proteins has been shown to cause neuronal degeneration in mouse cerebellum Purkinje cells or other neurons in various brain regions (36, 37). By contrast, pharmacological inhibition of autophagy attenuates acute degeneration of retinal ganglion cell (RGC) axons (38, 39). Nevertheless, these seemingly controversial results suggest a critical role of autophagy in maintaining axonal homeostasis. It remains speculative that modulation of autophagy activity may promote axon regeneration in the adult CNS.
Augmenting autophagy to see the effect on injured axons is a direct approach, but most compounds have uncontrollable pleiotropic effects. In this study, we took advantage of Tat-beclin1 (Tat-Bec), a specific autophagy-inducing peptide (40), which has been used to enhance autophagy both in vitro and in vivo (41, 42). We found that treatment with Tat-Bec with optimized protocol caused an increase in autophagy activity, promoted axonal growth of CNS neurons cultured on a nonpermissive substrate, and prevented axonal degeneration following injury. Mechanistically, augmented autophagy caused a down-regulation of SCG10 (superior cervical ganglia protein 10), a MT-destabilizing protein, and stabilized MTs. Remarkably, local administration of Tat-Bec promoted axonal regeneration of descending serotonin neurons, which formed synaptic contact with spinal motor neurons, and improved functional recovery after SCI. Together, this study reveals a promising beneficial effect of an autophagy-inducing agent on injured axons.
Results
Autophagy Induction Following Nerve Injury and the Effects on Neurite Outgrowth.
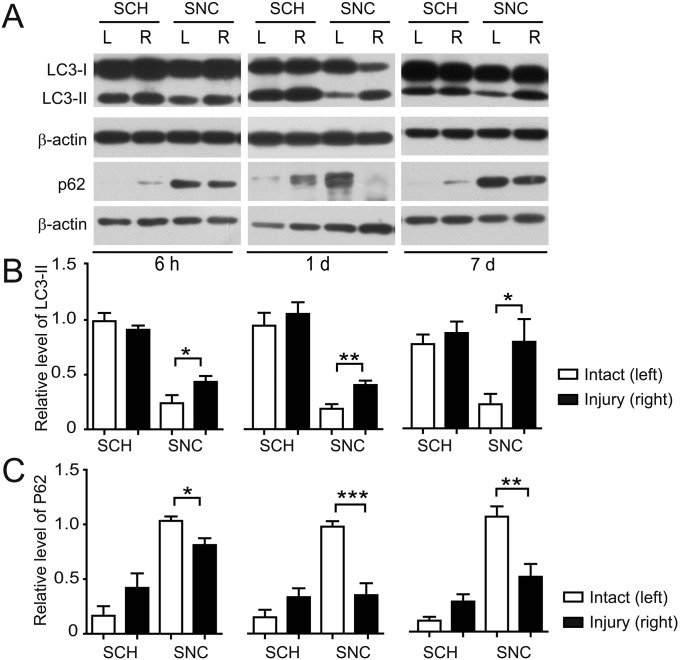
The disrupted autophagy flux after SCI (43) and the difference in regrowth capability between CNS and PNS axons prompted us to determine autophagy levels in mice subjected to spinal cord hemisection (SCH) and sciatic nerve crush (SNC), which serve as CNS and PNS axonal injury models, respectively. We found that in the SNC sample, the level of MT-associated protein (MAP) light chain-3B II (LC3-II), which is a lipidated form of LC3 associated with autophagosome membranes (44), increased compared with the sham sample (Fig. S1 A and B). This increase was observed at 6 h and was more pronounced 1 d or 7 d after SNC (Fig. S1 A and B). In correspondence, the level of autophagy substrate p62 decreased in SNC samples (Fig. S1 A and C). These results indicate that SNC-induced PNS axonal injury is accompanied by an increased activity of autophagy. By contrast, the increase in LC3-II was not significant and p62 gradually accumulated in SCH samples (Fig. S1 A–C). These results prompted us to investigate the role of autophagy in regulating CNS axons.
Fig. S1.
Induction of autophagy by nerve injury. (A) Immunoblots of LC3 and P62 in tissue samples of mice subjected to SCH or SNC for the indicated time. L, sham operation on the left; R, injured side on the right. β-actin was probed as the loading control. (B and C) Quantification for levels of LC3-II (B) or p62 (C) relative to β-actin. Data are presented as means ± SEM of three mice in each group. *P < 0.05, **P < 0.01, ***P < 0.001, injured vs. intact sample, Student’s t test.
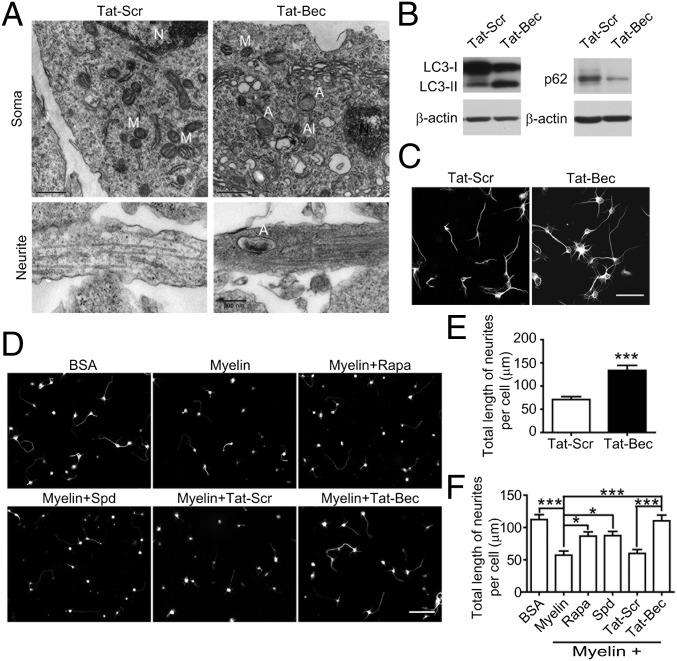
First, we determined effects of autophagy-inducing peptide Tat-Bec on cultured cortical neurons. To avoid the deleterious effect of the excessive extent of autophagy, we optimized concentrations of Tat-Bec. As shown in Fig. 1A, 5 µM Tat-Bec treatment for 3 h caused the appearance of a substantial numbers of autophagosome- or autolysosome-like structures in cultured cortical neurons at DIV1 (1 d in vitro) both in soma and neurites. The induction of autophagy activity was also reflected by an increase in LC3-II and a decrease in p62 in Tat-Bec–treated neurons compared with scrambled peptide (Tat-Scr)–treated neurons (Fig. 1B). Interestingly, treatment with Tat-Bec promoted neurite outgrowth (Fig. 1 C and E) and prevented the inhibitory effect of myelin on neurite growth (Fig. 1 D and F). In line with this notion, treatments with rapamycin (Rapa) or spermidine (Spd), two additional widely used autophagy inducers (45), also markedly ameliorated the inhibitory effect of myelin (Fig. 1 D and F). Thus, the induced autophagy activity is associated with the enhanced intrinsic growth potency of CNS axons.
Fig. 1.
Effects of autophagy induction on neurite outgrowth of cultured cortical neurons. (A) Representative electron microscopic images of cultured DIV1 cortical neurons treated with the indicated peptide (5 µM, 3 h). A, autophagosome; Al, autolysosome; M, mitochondria; N, nucleus. (Scale bars, 0.5 μm in soma and 0.2 μm in neurite.) (B) Immunoblots of LC3 and p62 in cultured DIV1 cortical neurons treated with Tat-Bec or Tat-Scr (5 µM, 3 h). β-actin was probed as a loading control. (C) Immunostaining for β-tubulin III (Tuj1) in DIV1 cortical neurons treated with Tat-Bec or Tat-Scr (5 µM, 3 h). (Scale bar, 50 μm.) (D) Immunostaining for Tuj1 in DIV2 cortical neurons cultured on BSA or myelin substrate with the indicated treatments (10 nM Rapa, 1 µM Spd, or 2.5 µM Tat-Scr or Tat-Bec) for 24 h. (Scale bar, 100 μm.) (E and F) Quantification of neurite length. Data are presented as means ± SEM from three experiments with 70–80 cells in each group. *P < 0.05, ***P < 0.001, Student’s t test (E) and ANOVA with Tukey’s post hoc tests (F).
Autophagy Induction Stabilizes Neuronal MTs by Down-Regulating SCG10.
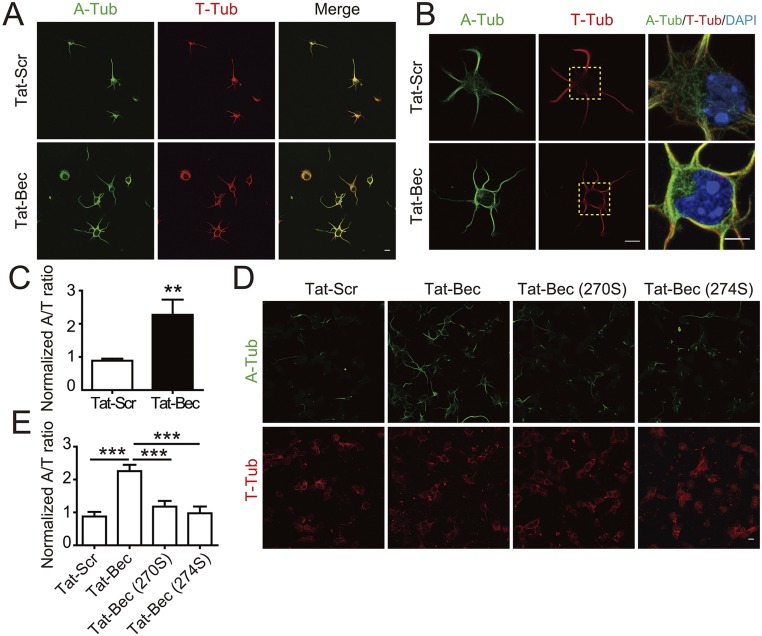
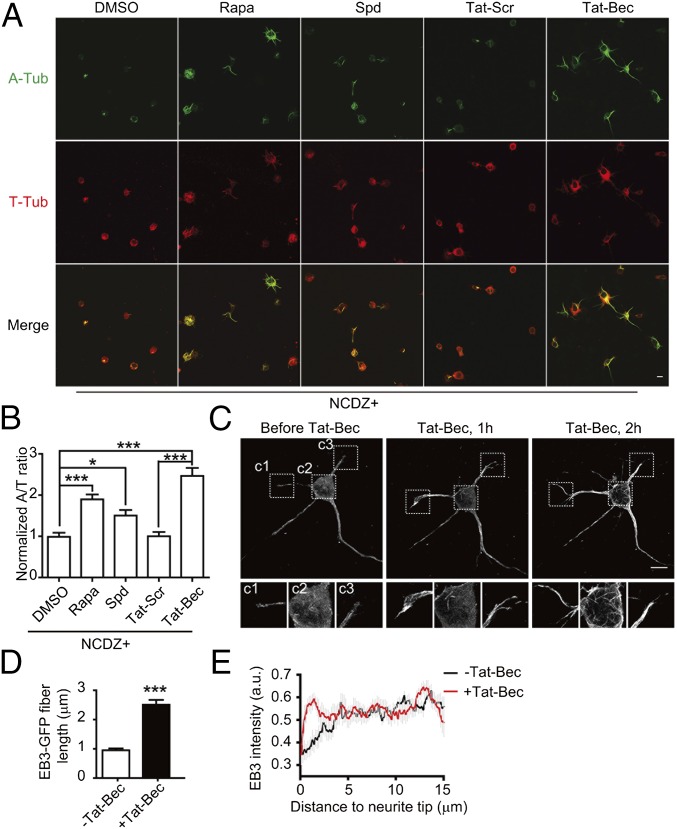
Cytoskeleton remodeling underlies axonal growth and repair (23, 46). Injured CNS axons have been found to contain many RBs, which are associated with disorganized MTs and autophagy-associated proteins (18, 47). We determined the relevance of autophagy with MT stability in neurons. The ratio of acetylated to tyrosinated α-tubulin (A/T ratio) was used to measure the relative ratio of stable to dynamic MTs (48). We found that treatment with Tat-Bec caused a marked increase in the A/T ratio compared with the Tat-Scr–treated group (Fig. S2 A and C), even under conditions of treatment with MT depolymerization agent Nocodazole (NCDZ) (Fig. 2 A and B). The A/T ratio was also increased in neurons treated with Rapa or Spd (Fig. 2 A and B). These results indicate that autophagy induction stabilizes MTs. In line with this notion, Tat-Bec–treated neurons exhibited more bundled MTs, which accumulated in the cell periphery and neurites (Fig. S2B). Time-lapse analysis for MT assembly in neurons expressing GFP-tagged MT plus-end binding protein EB3 (EB3-GFP) indicates that Tat-Bec treatment promoted MT polymerization toward the cell periphery and elongation into the distal regions of neurites (Fig. 2 C–E and Movies S1 and S2). The increased MT stabilization was not observed in neurons treated with Tat-Bec270S or Tat-Bec274S, two mutated forms of Tat-Bec with the phenylalanine changed to serine, which have been shown to lose autophagy-inducing activity (40) (Fig. S2 D and E). Together, autophagy induction increases MT stabilization in neurons.
Fig. S2.
Regulation of MT stability by Tat-Bec. (A and B) Immunostaining of A-Tub and T-Tub in DIV1 cortical neurons treated with Tat-Bec or Tat-Scr (5 μM, 3 h). DAPI was used to mark nuclei (B). [Scale bar, 10 μm (A) and 5 μm (B).] (C) Quantification of the A/T ratio. Shown are means ± SEM of 70–80 cells from three independent experiments. **P < 0.01, Student’s t test. (D) Immunostaining of A-Tub and T-Tub in DIV1 cortical neurons treated with Tat-Bec, mutated Tat-Bec, or Tat-Scr for 3 h. (Scale bar, 5 μm.) (E) Quantification of the A/T ratio. Shown are means ± SEM of 70–80 cells from three independent experiments. ***P < 0.001, ANOVA with Tukey’s post hoc tests.
Fig. 2.
Enhanced autophagy stabilizes MTs. (A) Immunostaining of acetylated α-tubulin (A-Tub) and tyrosinated α-tubulin (T-Tub) in DIV1 cortical neurons treated with NCDZ (1 µg/mL) plus the indicated reagents (40 nM Rapa, 2 µM Spd, or 5 µM Tat-Scr or Tat-Bec) for 3 h. Shown are the representative images. (Scale bar, 10 μm.) (B) Quantification for the A/T ratio. Data are shown as means ± SEM from three experiments with 70–80 cells in each group. *P < 0.05, ***P < 0.001, ANOVA with Tukey’s post hoc tests. (C) Time-lapse images of EB3-GFP expressed in DIV2 cortical neurons before and after 5 µM Tat-Bec treatment. Bottom images show EB3-GFP signals in neurites (c1 and c3) and soma (c2). (Scale bar, 10 μm.) (See also Movies S1 and S2.) (D) Quantification of EB3-GFP fiber length before and 2 h after Tat-Bec treatment, Data are shown as means ± SEM from three experiments with 18–20 cells in each group. ***P < 0.001. (E) EB3-GFP fluorescence intensity in neurites under the indicated conditions.
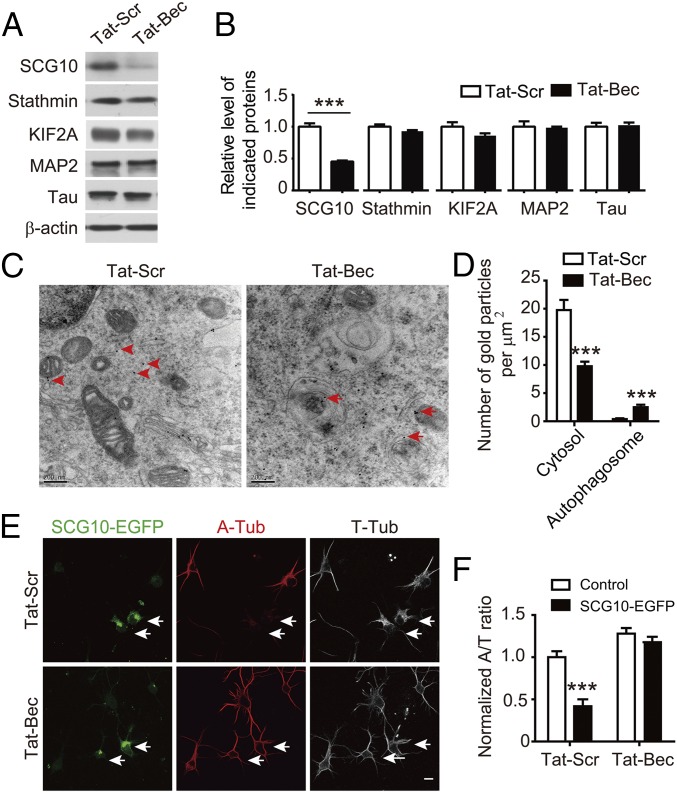
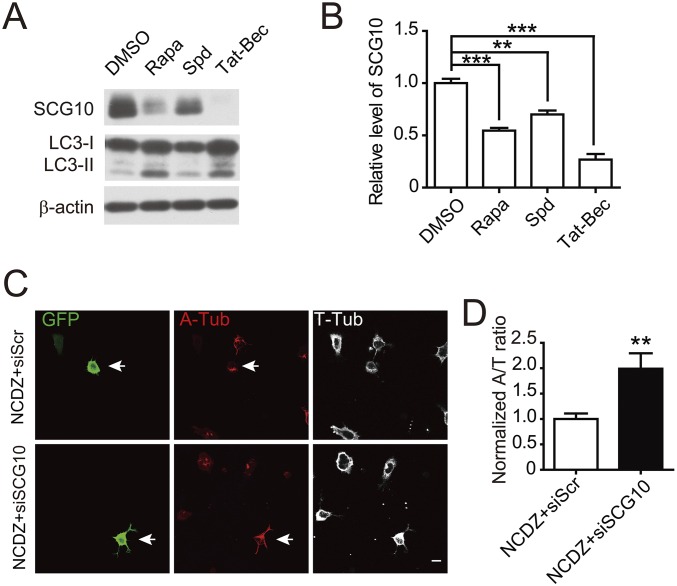
The MT stability and dynamics are regulated by combined actions of several classes of accessory proteins, including MAPs that bind along tubulin sides to stabilize MTs and proteins that destabilize MTs (49), including stathmin family proteins that bind free tubulin subunits to prevent MT assembly (50, 51) and kinesin-13 family proteins that insulate tubulin dimers from the ends of MT filaments (52). In a screening using mass spectra analysis for changed proteins upon Tat-Bec treatment of cultured DIV1 cortical neurons, we found that 219 among 2,232 proteins with validated peptide sequences exhibited a decrease in Tat-Bec–treated samples (Dataset S1). Among them, 19 proteins were found to be related with MT regulation after functional relevance analysis using the Gene Ontology database (53), including KIF2A, a member of the kinesin-13 family proteins, and SCG10, also named Stathmin-2, a neuron-specific member of the stathmin family proteins (49). Next, we verified these changes by immunoblot and found that SCG10 exhibited a marked decrease in Tat-Bec–treated cells (Fig. 3 A and B). However, there was no significant change in MAP2 and Tau, two major neuronal MAPs, as well as stathmin or KIF2A (Fig. 3 A and B), suggesting that SCG10 is prone to Tat-Bec–mediated autophagic degradation. In line with this notion, treatments with Rapa or Spd also caused down-regulation of SCG10 (Fig. S3 A and B). Analysis for the localization of SCG10 with transmission electronic microscopy (TEM) showed recruitment of SCG10 into autophagosomes or autolysosomes in Tat-Bec–treated neurons, accompanied by a decrease of SCG10 in cytoplasm (Fig. 3 C and D). These results suggest that SCG10 is a selective autophagy substrate in neurons. The role of SCG10 in regulating dynamics of neuronal MTs was determined by manipulating levels of SCG10. Down-regulation of SCG10 by small interference RNAs (54) increased the A/T ratio in DIV1 cortical neurons treated with NCDZ (Fig. S3 C and D), and overexpression of SCG10 caused the opposite effect (Fig. 3 E and F). These results suggest that SCG10 is a critical modulator for MT dynamics in neurons. Remarkably, the decreased MT stability in SCG10-overexpressing neurons was rescued by treatment with Tat-Bec (Fig. 3 E and F), suggesting the strong capability of induced autophagy in clearing exogenous obsolete proteins in neurons.
Fig. 3.
SCG10 as the substrate of autophagy. (A) Immunoblots of indicated proteins in DIV1 cortical neurons treated with 5 µM Tat-Scr or Tat-Bec for 3 h. Shown is a representative blot from three independent experiments with similar results. β-actin was probed as a loading control. (B) Quantification for levels of indicated proteins relative to β-actin. Data are presented as means ± SEM (n = 3). ***P < 0.001 (Tat-Bec vs. Tat-Scr), Student’s t test. (C) Immuno-EM analysis for the distribution of SCG10 in DIV1 cortical neurons treated with Tat-Scr or Tat-Bec. Note the SCG10 signals in the cytosol of the Tat-Scr group (red arrowheads) and autophagosomes in the Tat-Bec group (red arrows). (Scale bar, 200 nm.) (D) Quantification for numbers of SCG10-positive gold particles in cytosol and autophagosomes. Data are shown as means ± SEM of 30 cells from three experiments. (E) Immunostaining for A-Tub and T-Tub in SCG10-GFP–transfected cortical neurons treated with Tat-Scr or Tat-Bec (5 µM, 3 h) at DIV2. Note the difference in A-Tub signals in SCG10-EGFP–expressing neurons (arrows) between the two groups. (Scale bar, 10 μm.) (F) Quantification for the A/T ratio. Data are shown as means ± SEM of 70–80 cells from three experiments. ***P < 0.001, SCG10-EGFP vs. control, Student’s t test.
Fig. S3.
SCG10 as the substrate of autophagy and the role in regulating MT stability. (A) Immunoblots of SCG10 and LC3 in cultured DIV1 cortical neurons treated with DMSO or autophagy inducers (40 nM Rapa, 2 µM Spd, or 5 µM Tat-Bec) for 3 h. β-actin was probed as a loading control. (B) Quantification for levels of SCG10 relative to β-actin. Data are presented as means ± SEM from three independent experiments. **P < 0.01, ***P < 0.001, Student’s t test. (C) Immunostaining of A-Tub and T-Tub in DIV2 cortical neurons transfected with constructs encoding small interference RNA against SCG10 (siSCG10) or scrambled sequence (siScr) and treated with NCDZ (1 μg/mL, 3 h). Arrows indicate transfected cells. (Scale bar, 10 μm.) (D) Quantification of the A/T ratio. Shown are means ± SEM of 40–50 cells from three independent experiments. **P < 0.01, Student’s t test.
Tat-Bec Prevents Axons from Injury-Induced Degeneration.
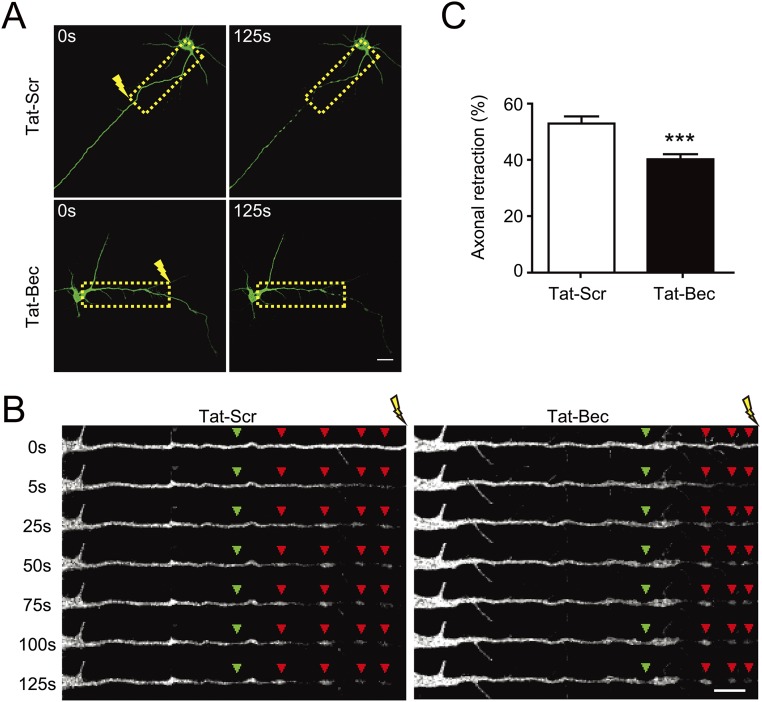
It has been shown previously that injured CNS axons form RBs that contain dispersed and disorganized MTs (18, 23), and autophagy-related proteins are associated with RBs (55). The regulation of MT stability by induced autophagy observed here prompted us to determine its effects on injured axons. First, cultured DIV3 cortical neurons pretreated with Tat peptides were subject to laser injury at a distance of 100 µm from the soma for 20 s (Fig. S4A), followed by live imaging for axonal behavior (Fig. S4B and Movies S3 and S4). As shown in time-lapse images of straightened axons, laser injury caused marked axonal retractions with intermittent bulbs formed in axonal shafts proximal to the injury site (Fig. S4B). Notably, pretreatment with Tat-Bec attenuated axonal retractions (Fig. S4 B and C and Movies S3 and S4), suggesting a protective role of autophagy for axons following injury.
Fig. S4.
Tat-Bec inhibits axonal retraction after laser injury. (A and B) DIV3 cortical neurons transfected with CAG-YFP were treated with 5 μM Tat-Bec or Tat-Scr for 1 h, and then axons were subject to laser injury at the site 100 µm from the soma for 20 s. Injured neurons were observed for 125 s under live-imaging conditions. Note the formation of RBs (red arrowheads) proximal to the injury site and swelling of the axonal shaft. Green arrowheads indicate the ends of intact axons, and yellow flashes indicate laser injury sites. Boxed areas in A were chosen for live-imaging (see kymographs in B). [Scale bars, 10 µm (A) and 5 µm (B).] (See also Movies S3 and S4.) (C) Percentage of retracted axon length. Shown are means ± SEM of 25–30 neurons in each group. ***P < 0.001, Student’s t test.
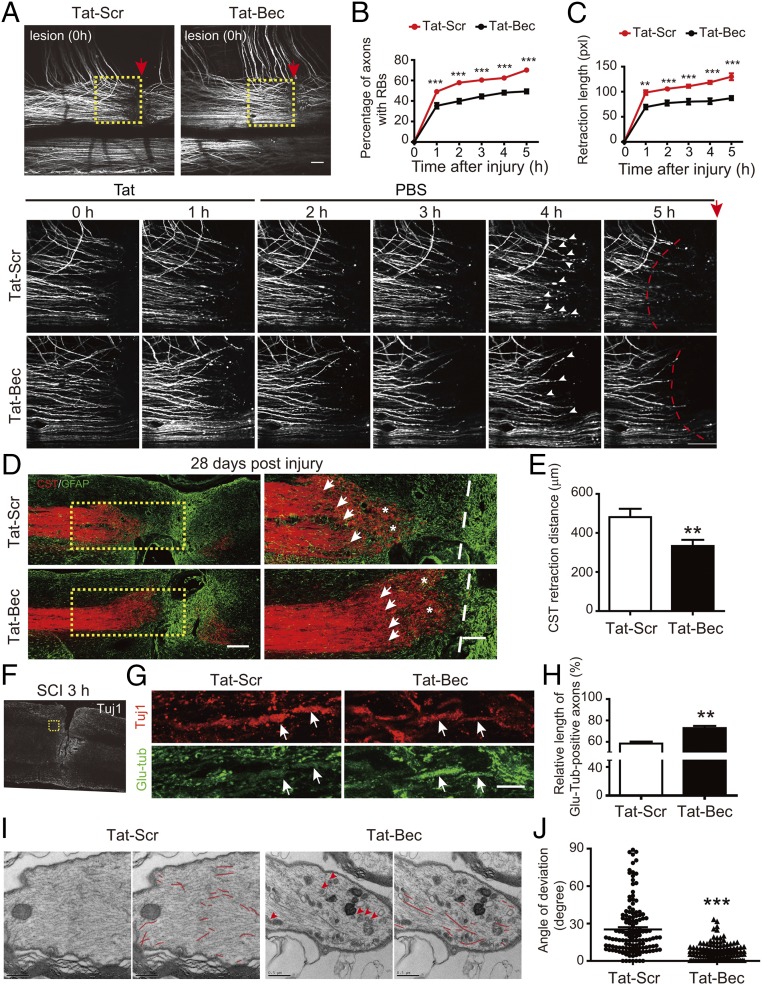
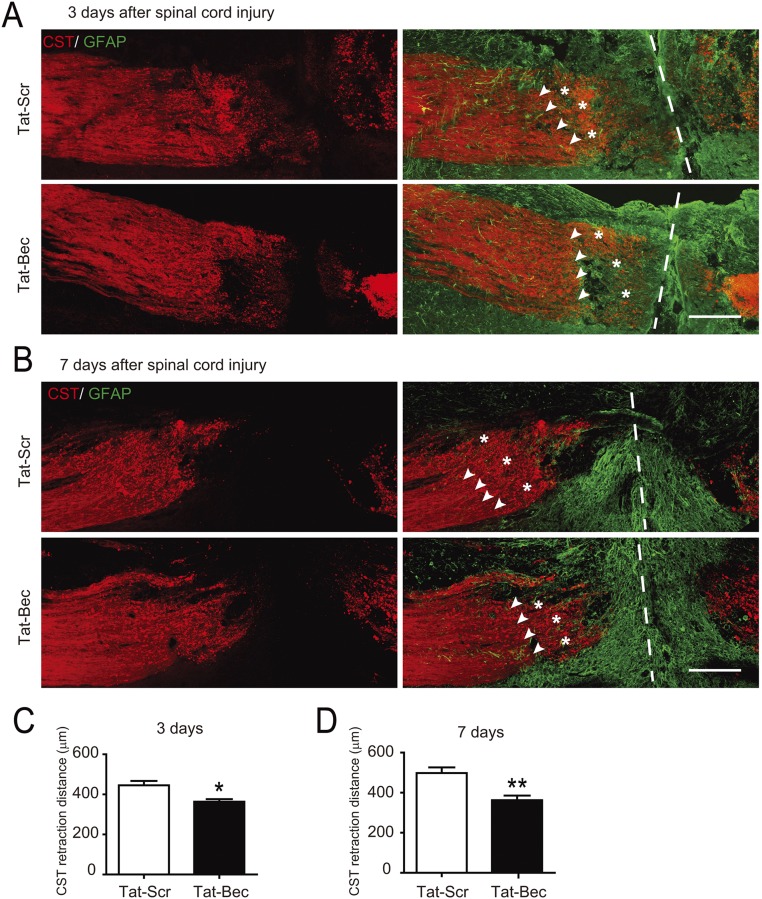
The effect of Tat-Bec was further investigated in a mouse model of SCI. First, we used adult Thy1-YFP-M transgenic mice (56) to visualize the dynamic behavior of the central axons of dorsal root ganglion (DRG) neurons coursing in the dorsal columns of the spinal cord following unilateral lesion at a segment between cervical 4 and 5 (C4–C5). The lesion sites were treated immediately with Tat peptides (3 µM in ACSF) for 1 h, washed with ACSF, and observed for 5 h after injury (Fig. 4A). In agreement with the previous observation (18), evident RBs were induced in many axons shortly after lesion, and subsequently the injured axons retracted gradually (see Fig. 4A, Tat-Scr). Interestingly, Tat-Bec treatment caused a marked decrease in the percentage of axons with RBs (Fig. 4B) and attenuated axon retraction distance (Fig. 4C) compared with the control peptides. Next, we analyzed the effects of Tat-Bec on cortical spinal tracts (CSTs) of Emx1-Cre; Ai9 mice, which expressed tdTomato in cortical neurons and the descending CST in the spinal cord (57), following SCH at C4–C5. We found that Tat-Bec also markedly attenuated axonal retraction at 3, 7, or 28 d postinjury (Fig. 4 D and E and Fig. S5 A–D;). Thus, Tat-Bec treatment markedly prevents axons from injury-induced degeneration.
Fig. 4.
Local administration of Tat-Bec protects axons and stabilizes MTs after nerve injury in mice. (A) Live-imaging observation of dorsal column axons in adult Thy1-YFP mice immediately after lesion (0 h) and 1–5 h after Tat peptide treatment. (Bottom) High magnification of boxed areas caudal to lesion sites in Top 0 to 5 h after injury. Red arrows indicate the injury sites; white arrowheads indicate RBs; dashed lines indicate the ends of intact axons. (Scale bar, 100 μm.) (B) The percentage of axons with RBs from 1 to 5 h after injury. Data are shown as means ± SEM from 10 mice in each group. (C) Quantification of axon retraction length 1 to 5 h after injury. (D) Adult Emx1-Cre; Ai9 mice were subjected to bilateral hemisection of the spinal cord at the C4/C5 level, followed by immediate application of Tat-Bec or Tat-Scr peptides. After 4 wk, longitudinal sections of injured regions were analyzed for tdTomato-labeled CST and GFAP-labeled glial scar. Dashed lines, incision border; arrows, ends of intact axons; asterisks, RBs. (Scale bar, 200 μm.) (E) Quantification of CST retraction distance from the incision border of the spinal cord. Data are shown as means ± SEM from 12 mice in each group. **P < 0.01. (F and G) Immunostaining for Glu-tub and Tuj1 in longitudinal sections of the spinal cord 3 h after SCI. Boxed area, regions rostral to the injury site; arrows, axonal tracts stained with Tuj1. (Scale bar, 10 μm.) (H) Quantification of the relative length of Glu-tub–positive fragments to Tuj1-positive axons in CST 3 h after SCI and peptide application. Data are shown as means ± SEM from six to eight mice in each group. **P < 0.01. (I) EM images of axonal stumps after injury with red lines tracing MTs manually. Samples were from Emx1-Cre; Ai9 mice treated with peptides 3 h after SCI. Arrowheads indicate autophagosomes. (Scale bar, 0.5 μm.) (J) Quantification for the MT deviation angles relative to axonal axis. Shown are distribution and means ± SEM of MT degrees in at least 15 fields from three mice in each group. ***P < 0.001.
Fig. S5.
Tat-Bec inhibits CST retraction in mice. (A and B) Longitudinal sections of injured regions with tdTomato-labeled CST and GFAP-labeled glial scar 3 (A) or 7 d (B) after bilateral hemisection of the spinal cord at the C4/C5 level. Dashed lines, incision border; arrows, ends of intact axons; asterisks, RBs. (Scale bar, 200 μm.) (C and D) Quantification of CST retraction distance from the incision border of the spinal cord. Data are shown as means ± SEM of four to six mice in each group. *P < 0.05, **P < 0.01, Student’s t test.
We also analyzed MTs of CST following lesion and peptide administration (Fig. 4 F and G). Immunostaining showed that the detyrosinated α-tubulin (Glu-tub), which marks polymerized MTs (58), was better preserved in lesioned CSTs of Tat-Bec–treated mice compared with the Tat-Scr control group (Fig. 4 G and H). TEM analysis showed that, in contrast to randomly arrayed MTs in the Tat-Scr group, MTs mostly aligned in parallel after Tat-Bec treatment (Fig. 4I), with much smaller deviation angles relative to the axonal axis (Fig. 4J). Notably, many autophagosomes accumulated in axons of Tat-Bec–treated animals (Fig. 4I). These results further support the conclusion that Tat-Bec–induced autophagy maintains the stability of MTs in injured axons.
Tat-Bec Promotes Axonal Regeneration of Monoaminergic Neurons and Improves Motor Behavior Recovery After SCI.
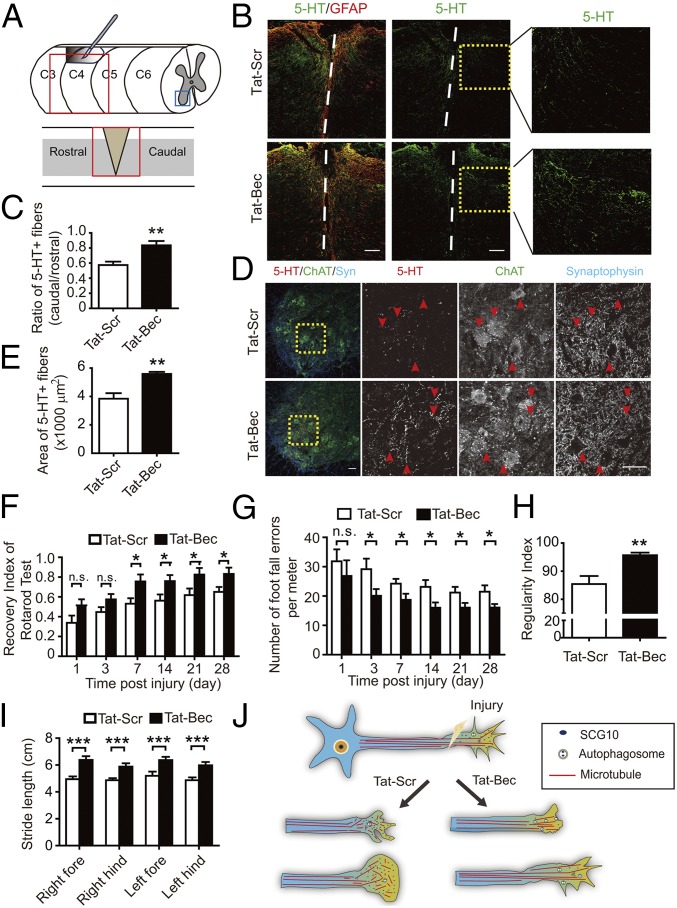
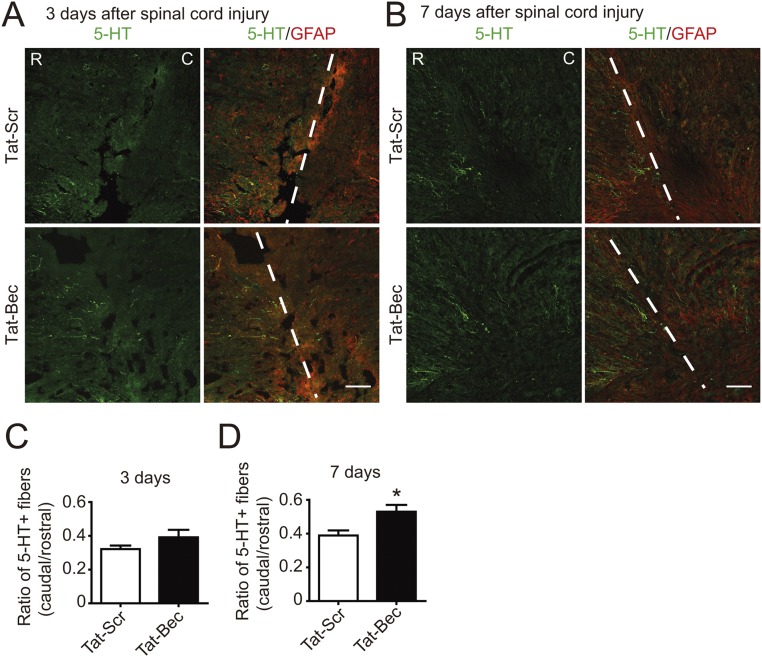
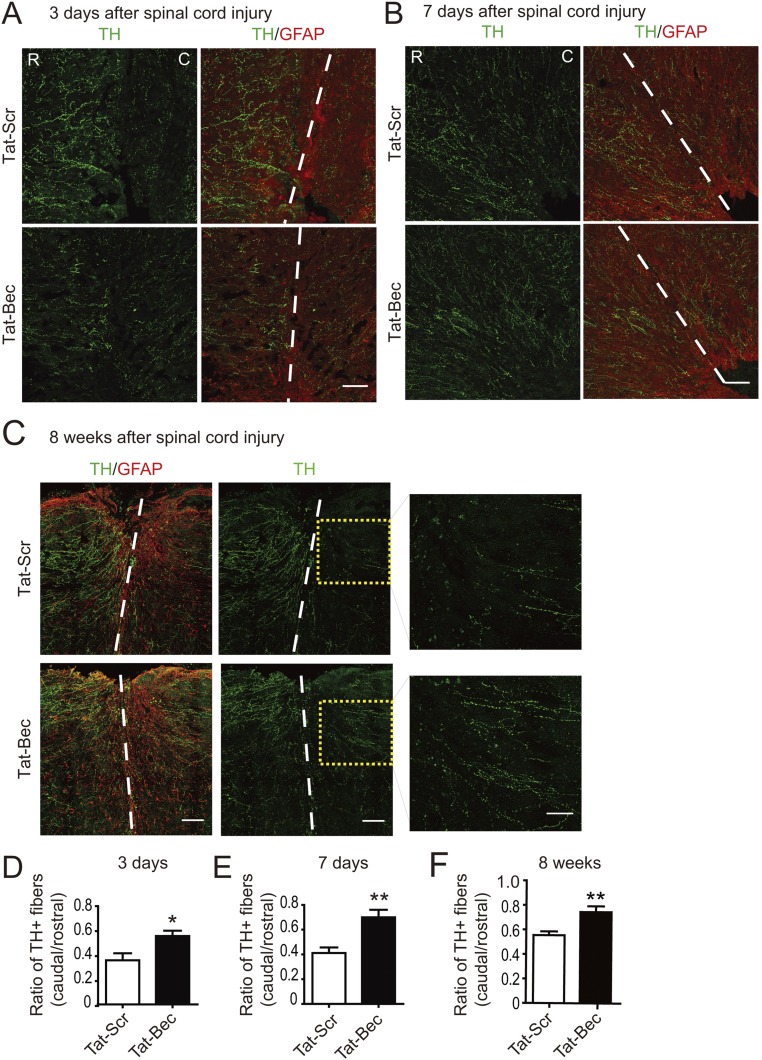
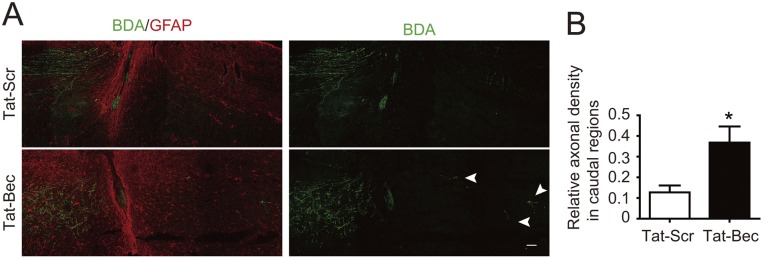
In addition to CSTs, other descending axonal pathways, including raphespinal and rubrospinal fiber tracts, also contribute to locomotor recovery after SCI (15, 59). Thus, we determined regeneration of serotonergic and dopaminergic axon fibers by staining serotonin (5-HT) and tyrosine hydroxylase (TH), respectively, in adult mice after dorsal bilateral SCH at C4–C5 for days to weeks (Fig. 5A). We found that Tat-Bec administration immediately after SCH significantly promoted regrowth of 5-HT or TH-positive fibers caudal to the injury sites, which were delimited by the GFAP-labeled glia scar (Fig. 5 B and E and Figs. S6 and S7). Remarkably, the regenerated 5-HT axons formed synaptic contacts with ventral horn motor neurons marked by choline acetyltransferase (ChAT) in the caudal spinal cord (Fig. 5D). Notably, the Tat-Bec–induced regeneration of 5-HT or TH-positive fibers was observed as early as 3 or 7 d after injury (Figs. S6 and S7). At 8 wk after injury, we also noticed subtle but significantly more regenerated CST axons, which were labeled by BDA (biotinylated dextran amine) injected into layer 4/5 of the mouse sensorimotor cortex, in Tat-Bec–treated animals following SCI (Fig. S8 A and B). Thus, Tat-Bec treatment promotes regrowth of descending spinal axons after injury.
Fig. 5.
Tat-Bec promotes regrowth of monoaminergic axons and improves motor function recovery after SCI. (A) Schematic representation of the spinal cord bilateral hemisection model. Boxed areas indicate the longitudinal (C4/C5) and transverse plane of the ventral horn in C6. (B) Immunostaining of serotonin (5-HT) and GFAP in the longitudinal section of level C4/5 spinal cord 8 wk after injury. Boxed areas indicate caudal regions to the injury sites (dashed lines). (Scale bar, 100 μm.) (C) Quantification for the ratio of 5-HT+ fibers caudal to rostral sides of the injury sites. Shown are means ± SEM from seven mice in each group. (D) Immunostaining of 5-HT, ChAT, and synaptophysin (Syn) in cross-sections of the spinal cord level C6. Arrowheads in boxed areas indicate ChAT-positive motor neurons innervated by 5-HT+ fibers. [Scale bars, 100 μm (Left) and 50 μm (boxed areas).] (E) Quantification for the area of 5-HT+ fibers in the ventral horn. Data are shown as means ± SEM from four mice in each group. (F) Quantification for the Recovery Index in the rotarod test at the indicated time postinjury and peptide administration (see Materials and Methods). Data are shown as means ± SEM from 14 mice in each group. (G) Number of foot falls on the grid in 5 min at the indicated time postinjury and peptide administration. Data are shown as means ± SEM from 12 mice in each group. (H and I) Quantification for RI (H) and stride length (I) in gait analysis. Data are shown as means ± SEM from 12–15 mice in each group. (J) Proposed model for a role of Tat-Bec–induced autophagy in axonal regrowth after injury.
Fig. S6.
Effect of Tat-Bec on 5-HT axon regeneration in mice. (A and B) Immunostaining of serotonin (5-HT) and GFAP in the longitudinal section of level C4/C5 spinal cord 3 d (A) or 7 d (B) after injury. Dashed lines, incision border. C, caudal; R, rostral. (Scale bar, 100 μm.) (C and D) Quantification for the ratio of 5-HT+ fibers caudal to rostral sides of injury sites at the indicated time after injury. Shown are means ± SEM of 4–6 mice in each group. *P < 0.05, Student’s t test.
Fig. S7.
Effect of Tat-Bec on TH axon regeneration in mice. (A–C) Immunostaining of hydroxylase (TH) and GFAP in the longitudinal section of level C4/5 spinal cord 3 d (A), 7 d (B), or 8 wk (C) after injury. C, caudal; R, rostral. Dashed lines, incision border; boxed areas in C indicate caudal regions to the injury sites. (Scale bar, 100 μm.) (D–F) Quantification for the ratio of TH+ fibers caudal to rostral sides of the injury sites at the indicated time after injury. Shown are means ± SEM of 4–7 mice in each group. *P < 0.05, **P < 0.01, Student’s t test.
Fig. S8.
Effect of Tat-Bec on CST regeneration. (A) BDA-labeled CST axons and immunolabeling of GFAP in the sagittal sections of the cervical spinal cord 8 wk after injury. Note the appearance of CST fibers caudal to the lesion site (arrowheads). (Scale bar, 100 μm.) (B) Quantification for relative intensity of BDA-traced CST (caudal BDA/rostral BDA). Data are shown as means ± SEM of six mice in each group. *P < 0.05, Student’s t test.
Finally, we determined the effects of Tat-Bec on locomotor behavior of lesioned mice. We found that Tat-Bec treatment markedly increased the time mice stayed on Rotarod treadmills beginning from 1 wk postinjury (Fig. 5F) and decreased the number of foot fall errors in the grid walk test as early as 3 d postinjury (Fig. 5G). In the CatWalk analysis for motor coordination of mice 8 wk after SCI, Tat-Bec treatment markedly elevated the Regularity Index (RI) and increased the stride length of both hind paws and fore paws (Fig. 5 H and I). Together, Tat-Bec administration promotes functional recovery after SCI.
Discussion
It has been speculated that autophagy-related proteins might act as promising therapeutic targets for curing axonal injuries following traumatic lesion. However, because of unavoidable pleiotropic effects of most autophagy-inducing substances, the role of autophagy in axonal homoeostasis still remains unclear. Furthermore, the extent and duration of autophagy are crucial to cell health, and acute or chronic manipulations of autophagy have led to, in several instances, controversial conclusions. Here, we report a marked therapeutic effect of a specific autophagy-inducing peptide Tat-Bec on SCI. We found that administration of Tat-Bec at optimized doses causes autophagy in neurons and promotes axonal growth in neurons exposed to inhibitory substrate myelin. Interestingly, induced autophagy stabilizes MTs by down-regulating SCG10. Notably, local and temporal Tat-Bec administration attenuates axonal retraction within the critical window that has been shown to be crucial for the regeneration potential of injured axons (60). In line with this notion, Tat-Bec administration also promotes axon regeneration and consequently improves locomotor ability after SCI.
The limited intrinsic axon growth capacity, the presence of extracellular inhibitory factors, and the lack of neurotrophic factors are major obstacles limiting regeneration of CNS axons after injury (61). Interestingly, several combinatory approaches have been shown to be able to promote axon regeneration after injury (62, 63). However, interventions for multiple targets make clinical applications difficult. Because several signaling pathways converge on the regulation of cytoskeleton dynamics (23, 46), it is probable to promote axon regeneration by manipulating axonal cytoskeleton directly. Interestingly, moderate MT stabilization by treatment with Taxol or epothilone B, two clinically approved drugs, promotes axon regeneration after SCI (19, 20). However, these reagents cause MT stabilization globally and affect many other cellular functions, such as cell proliferation, secretion, or migration. Thus, more precise control of cytoskeletal dynamics merits further investigation.
Accumulating evidence suggests that MTs play important roles in the regulation of autophagosome formation and transportation in axons (33, 64). Whether autophagy in turn modulates MT dynamics is not known. In this study, we found that induced autophagy increases MT stability through the degradation of SCG10, a MT-destabilization protein, and promotes axon regeneration after injury (Fig. 5J). These results are in agreement with previous findings that administration of MT stabilizers can promote axon regeneration and improve locomotor activity (19, 20). A drug design based on Tat-Bec regulation of SCG10 has great potential for precise control of MT dynamics that is crucial for axon repair. This study does not exclude the possibility for the effect of autophagy on other substrates that restrain the intrinsic axonal regrowth ability of CNS neurons.
Materials and Methods
Mice, Axon Injury, and Peptide Application.
The use of animals was approved by the Institutional Animal Care and Use Committee of the Institute of Neuroscience, Chinese Academy of Sciences. Adult mice (8–10 wk) expressing fluorescent proteins (Thy1-YFP and Emx1-Cre; Ai9) were subject to SCI, followed by observation at different times after lesion.
For live imaging of axons after injury in vivo, a small unilateral lesion was produced in Thy1-YFP mice by transecting only the superficial axons of the dorsal columns at the C4/C5 level with Vannas spring scissors after removing the lamina. Immediately after lesioning, injury sites were bathed in ACSF containing 3 μM Tat peptides for 1 h, followed by washes with ACSF. Axons before and after peptide treatment were observed under a Prairie two-photon microscopy every 1 h for a total of 5 h. The animals were kept on a heating pad under anesthesia status through i.p. injection with a mixture of tribromoethanol and tert-amyl alcohol every 2 h during the imaging session.
To determine the effects of peptides on regeneration of CSTs or descending, monoaminergic axons and the motor behavior of the animal, adult mice (8–10 wk) were subjected to bilateral spinal hemisection followed by peptide administration. Briefly, mice were intraperitoneally anesthetized with a mixture of tribromoethanol and tert-amyl alcohol, followed by a shear along the dorsal midline to expose the segments of spinal cord C4–C8 and a laminectomy to cut off the dura matter. The bilateral halves of the C4 segment were hemisected with straight cornea scissors at a depth of 2 mm from the dorsal surface. After hemostasis with a gelatin sponge, Tat peptides (500 µM, 5 µL) were injected into incision gaps with a microsyringe. After muscle and skin reposition and suture, mice were placed at a worm cage until recovered. Axonal repair and animal motor behavior were observed at different times after injury.
For SNC, the sciatic nerves below the ischial tuberosity were exposed and crushed with dissecting forceps three times, with each lasting for 15 s, until the crushed trunk became transparent with the myelin sheath.
Behavior Analysis.
Motor coordination was assessed using the rotarod test and grid walk test at different days after injury. The CatWalk system (Noldus) was used for gait analysis. See SI Materials and Methods for detailed information.
Statistical Analysis.
All results are expressed as means ± SEM. Analysis for significant differences between two groups was performed using two-tailed unpaired Student’s t test. One-way ANOVA followed by post hoc Tukey’s test was used for comparisons among multiple groups. All statistical analyses were conducted using GraphPad Prism5 for Windows.
SI Materials and Methods
Constructs, Reagents, and Antibodies.
The oligonucleotides (SCG10 siRNA sequence, 5′-GCAGATCAACAAGCGTGCTT; scrambled sequence used as the control, 5′-AGCTAGCGAGACACTCGATT) were annealed and cloned into pSUPER vector. The construct pEGFP-SCG10 was kindly provided by Jianguo Chen, Peking University, Beijing. The cDNA encoding for MT plus-end binding protein 3 (EB3) was subcloned into Adenovirus vector pAdeno-CMV-EGFP-3×FLAG. Tat peptides (Tat-Bec, YGRKKRRQRRRGGTNVFNATFEIWHDGEFGT; Tat-Scrambled, YGRKKRRQRRRGGVGNDFFINHETTGFATEW) were synthesized by GL Biochem (Shanghai) Ltd. Inhibitory CNS myelin extract (CME) was isolated from SD rat (200–300 g) brains as shown previously (65). NCDZ and Spd were from Sigma Aldrich. Rapa was from Selleck. Artificial cerebrospinal fluid (ACSF) contains 125 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 2 mM CaCl2, 25 mM NaHCO3, and 20 mM d-(+)-glucose (all from Sigma), pH 7.4, and 310 mOsm1−1. Antibodies were from Sigma (Acetylated tubulin, LC3b, β-actin, serotonin), Abcam (synaptophysin, fibronectin, Glu-tublin, tyrosinated tubulin), Millipore (TH, GFAP), Covance (Tuj1), BD (p62), Protech (SCG10), Chemicon (ChAT), and Invitrogen (Alexa Fluor 488, 555, or 647 fluorescence-labeled secondary antibodies).
Cell Culture, Transfection, and Treatments.
Neurons dissociated from E16.5 SD rats were cultured on poly–d-Lysine precoating glass coverslips in medium [80% (vol/vol) DMEM, 10% (vol/vol) FBS, and 10% (vol/vol) F12]. At 4 h after plating, culture medium was changed to Neuronal Basal medium containing 2% (vol/vol) B27 and 1% l-glutamine. Electroporation of dissociated neurons was performed with an Amaxa Nucleofector device before plating. Neurons at DIV1 or DIV2 were treated with the respective reagents. To test neurite growth in the presence of myelin, glass coverslips were coated with 1.67 µg/mL CME. NCDZ was added to the medium at a concentration of 1 µg/mL.
Live Imaging for Axonal Retraction After Laser Injury of Cultured Cortical Neurons.
Cortical neurons were transfected with pCAG-YFP plasmid to label neuronal morphology and treated at DIV3 with Tat peptides (5 μM) for 1 h. Microscopic laser (405 nm wave length) was applied to axons at the sites ∼100 μm away from the soma for 20 s. Axonal behavior was observed every 5 s for a total of 125 s in a 37 °C chamber saturated with 5% CO2. Images were taken under an inverted NIKON A1R laser scanning confocal microscope with a 60× oil immersion objective.
Live Imaging for EB3 Motion in Cultured Cortical Neurons.
Cortical neurons at DIV1 were infected with EB3-EGFP–expressing adenovirus. After 24 h, movement of EB3 puncta was observed every 5 s for a total of 30 s, before and after Tat-Bec (5 μM) treatment.
Immunoblotting, Immunostaining, and Axon Tracing.
To determine protein levels, tissues or cultured neurons were lysed and homogenized in RIPA buffer supplemented with protease inhibitor mixture, followed by standard SDS/PAGE and Western blotting with primary and HRP-conjugated secondary antibodies. Protein band signals were visualized with chemiluminescent substrate (ThermoFisher Scientific) and quantified by Multi Gauge software.
To analyze the status of MTs, cultured neurons transfected with the indicated constructs or treated with the indicated reagents were subjected to immunostaining with antibodies against acetylated, tyrosinated, or total tubulin integrated in MTs. Briefly, cultured cortical neurons were fixed in 4% PFA and simultaneously permeabilized in PHEM buffer (60 mM Pipes, 25 mM Hepes, 5 mM EGTA, and 1 mM MgCl2) containing 0.25% glutaraldehyde, 3.7% paraformaldehyde, 3.7% sucrose, and 0.1% Triton X-100 and quenched in 50 mM ammonium chloride. After washes and blockade with 10% goat serum in PBS, cells were incubated in primary antibodies at 4 °C overnight, and subsequently fluorescence-labeled secondary antibodies (1:1,000) at room temperature (RT) for 2 h. DAPI or fluorescent phalloidin was applied at RT for 20–30 min to label the nucleus and β-actin of cultured neurons. Images were obtained by NIKON A1R laser scanning confocal microscopy.
For histological analysis of axons after injury in vivo, 20-µm serial frozen sections were washed in PBS containing 0.1% Triton, blocked with 10% goat serum in PBS containing 0.3% Triton for 2 h, and incubated with primary antibodies at 4 °C overnight and subsequently fluorescence-labeled secondary antibodies (1:1,000) at RT for 2 h. Images were obtained by Nikon TiE-A1 plus laser scanning confocal microscopy.
To trace CSTs after injury, mice at 6 wk post-SCH were intraperitoneally anesthetized and fixed in a stereotaxic frame. A hole with a 1.5-mm diameter was produced on the mice skull by foramen drilling and received BDA (10%, 10,000 MW; Invitrogen) injection into layer 4/5 of the right sensorimotor cortex (0.1, 0.6, and 1.1 mm posterior to the Bregma; 1.0 and 1.4 mm lateral; 0.7 mm deep). Saggital sections of the left spinal cord were analyzed for CST regeneration after injury.
Mass Spectrometry and Data Analysis.
DIV1 cortical neurons were treated without (blank) or with peptides (15 μM Tat-Scr or 5 μM or 15 μM Tat-Bec) for 3 h. Total proteins were extracted in SDT buffer (2% SDS, 0.1 M DTT, 0.1 M Tris/HCl, pH 7.6). Filter-aided sample preparation procedure (FASP) was applied for protein digestion (66). Peptides in the digested mixture were separated through a nano-emitter column (15 cm length, 75 μM inner diameter) packed in-house with 3 μM C18 ReproSil particles and introduced into mass spectrometry via a nanoelectrospray ion source (source voltage, 1.7–2.2 kV). A linear gradient from 4% to 26% buffer B (buffer A, 0.1% formic acid in ddH2O; buffer B, 0.1% formic acid in acetonitrile) over 61 min was used for peptide separation at a flow rate of 250 nL/min. For the collision-induced dissociation (CID) mode performed on LTQ-Orbitrap Velos (Thermo Fisher Scientific), a full scan was acquired with resolution R = 60,000 at m/z 400. The 20 most intense ions were sequentially isolated for MS/MS sequencing. Raw data were processed using the MaxQuant software (Version 1.5.2.8), and peak lists were searched with the Uniprot database. Protein identification was performed using 20 ppm tolerance at the MS level (FT mass analyzer) and 0.5 Da at the MS/MS level (ion trap analyzer), with a posterior global FDR (false discovery rate) of 1% based on the reverse sequence of the rat FASTA (a format of amino acid sequence) file. Up to two missed trypsin cleavages were allowed, and oxidation of methionine and N-terminalacetylation were searched as variable posttranslational modification and cysteine carbamidomethylation as fixed modification. The minimal required peptide length was set to seven amino acids. All data were normalized by the levels of β-actin, and the following criteria were used to identify changed proteins: the value difference of Tat-Scr vs. blank was less than 1.2 fold, the value of Tat-Bec group was less than the Tat-Scr group, and the value of the 15 μM Tat-Bec group was less than the 5 μM Tat-Bec group.
Electron Microscopy Analysis.
Cultured neurons or the spinal cords from mice after SCI were fixed for 1 h with 2.5% glutaraldehyde in 0.1 M PBS, pH 7.4, followed by postfixation with 1% osmium tetroxide for 30 min, three washes with 0.1 M PB, and dehydration in ascending ethanol series and finally were embedded in araldite for over 2 d. Thin sections were stained with methanolic uranyl acetate and lead citrate. Organelles or MTs were observed by Joel JEM-1230 TEM.
To determine the subcellular distribution of SCG10, cultured neurons were fixed with 4% PFA, 0.1% picric acid, and 0.05% glutaraldehyde in 0.1 M PB for 2 h at 4 °C; then cut into 200-µm slices; incubated with 1% osmic oxide for 30 min; dehydrated; and embedded in Epon 812, followed by polymerization at 37 °C for 12 h, 45 °C for 12 h, and 60 °C for 24 h. Ultra-thin sections were cut and collected on nickel grids (200 mesh), followed by staining with primary antibodies against SCG10 (1:100) at 4 °C for 48 h, extensive washes with PB (10× 10 min), and then incubation with secondary antibodies conjugated with 12-nm gold particles (1:100, Jackson Immuno Research) at RT for 2 h. After extensive washes with PB (10× 10 min), sections were incubated with methanolic uranyl acetate and lead citrate and then observed with Joel JEM-1230 TEM.
Behavior Analysis.
Motor coordination was assessed using the rotarod test and grid walk test 1, 3, 7, 14, 21, and 28 d after SCI. The rotarod test was performed at a speed from 5 rpm to 80 rpm in 8 min. Mice were trained three times before surgery, and the time spent on the rod until falling off was recorded as preinjury normal control. The rotarod recovery index was set as the ratio of the time staying on the rod postinjury to the time staying on the rod preinjury.
For the grid walk test, mice were allowed to freely roam on a 52 cm × 32 cm raised grid (2.5 cm gaps between wires) for 2 min with video recording. Foot fall errors were counted manually in a single-blind manner, and total numbers of faults per meter were quantified.
For gait analysis, mice were tested 8 wk after SCI on the automated gait analysis system CatWalk (Noldus). Recorded footprints were processed automatically by using device-specific software quantifying the step distance of hindlimbs (stride length) and interpaw coordination (RI).
Supplementary Material
Acknowledgments
We thank Dr. Qian Hu of the ION Imaging Facility for microscope analysis, Dr. Yu Kong for EM analysis, and Dr. Jianguo Chen for the SCG10 construct. We are grateful to Drs. Zhigang He, Zhenyu Yue, and Fengfeng Bei for constructive suggestions during the course of this work. This study was partially supported by National Key Basic Research Program of China Grant 2014CB910203; National Natural Science Foundation of China Grants 31330032, 31490591, 31321091, and 61327902; and the Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDB02040003.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611282113/-/DCSupplemental.
References
- 1.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296(5574):1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 2.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore DL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326(5950):298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17(1):120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4(9):703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 6.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417(6892):941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 7.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420(6911):74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKerracher L, et al. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13(4):805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 10.Domeniconi M, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35(2):283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 11.Atwal JK, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322(5903):967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 12.Zheng B, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 2005;102(4):1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omoto S, Ueno M, Mochio S, Takai T, Yamashita T. Genetic deletion of paired immunoglobulin-like receptor B does not promote axonal plasticity or functional recovery after traumatic brain injury. J Neurosci. 2010;30(39):13045–13052. doi: 10.1523/JNEUROSCI.3228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JK, et al. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009;29(27):8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44(3):439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Mimura F, et al. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. J Biol Chem. 2006;281(23):15970–15979. doi: 10.1074/jbc.M510934200. [DOI] [PubMed] [Google Scholar]
- 17.Gordon-Weeks PR, Fournier AE. Neuronal cytoskeleton in synaptic plasticity and regeneration. J Neurochem. 2014;129(2):206–212. doi: 10.1111/jnc.12502. [DOI] [PubMed] [Google Scholar]
- 18.Ertürk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci. 2007;27(34):9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruschel J, et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348(6232):347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellal F, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331(6019):928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PD, et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64(5):617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KK, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat Rev Neurosci. 2012;13(3):183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- 24.Verma P, et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25(2):331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Exp Neurol. 2010;223(1):28–37. doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19(8):983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 28.Cuervo AM. Autophagy: In sickness and in health. Trends Cell Biol. 2004;14(2):70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Lim J, Yue Z. Neuronal aggregates: Formation, clearance, and spreading. Dev Cell. 2015;32(4):491–501. doi: 10.1016/j.devcel.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Coleman M, Zhang L, Zheng X, Yue Z. Autophagy in axonal and dendritic degeneration. Trends Neurosci. 2013;36(7):418–428. doi: 10.1016/j.tins.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, et al. Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice. Neuron. 2015;87(2):355–370. doi: 10.1016/j.neuron.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinsztein DC, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1(1):11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 33.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196(4):407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maday S, Holzbaur EL. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell. 2014;30(1):71–85. doi: 10.1016/j.devcel.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993;121(2):305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao YG, et al. The autophagy gene Wdr45/Wipi4 regulates learning and memory function and axonal homeostasis. Autophagy. 2015;11(6):881–890. doi: 10.1080/15548627.2015.1047127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu M, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci USA. 2007;104(36):14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch JC, et al. Acute axonal degeneration in vivo is attenuated by inhibition of autophagy in a calcium-dependent manner. Autophagy. 2010;6(5):658–659. doi: 10.4161/auto.6.5.12188. [DOI] [PubMed] [Google Scholar]
- 39.Knöferle J, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci USA. 2010;107(13):6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoji-Kawata S, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494(7436):201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013;110(51):20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cinque L, et al. FGF signalling regulates bone growth through autophagy. Nature. 2015;528(7581):272–275. doi: 10.1038/nature16063. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, et al. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6:e1582. doi: 10.1038/cddis.2014.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 45.Klionsky DJ, et al. 2016. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222.
- 46.Hur EM, Saijilafu, Zhou FQ. Growing the growth cone: Remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 2012;35(3):164–174. doi: 10.1016/j.tins.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribas VT, et al. Early and sustained activation of autophagy in degenerating axons after spinal cord injury. Brain Pathol. 2015;25(2):157–170. doi: 10.1111/bpa.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180(3):619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10(5):319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 50.Gavet O, et al. The stathmin phosphoprotein family: Intracellular localization and effects on the microtubule network. J Cell Sci. 1998;111(Pt 22):3333–3346. doi: 10.1242/jcs.111.22.3333. [DOI] [PubMed] [Google Scholar]
- 51.Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H. Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth. J Neurobiol. 2004;58(1):60–69. doi: 10.1002/neu.10279. [DOI] [PubMed] [Google Scholar]
- 52.Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: Structure, function, and dynamics. Physiol Rev. 2008;88(3):1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 53.Ashburner M, et al. The Gene Ontology Consortium Gene ontology: Tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morii H, Shiraishi-Yamaguchi Y, Mori N. SCG10, a microtubule destabilizing factor, stimulates the neurite outgrowth by modulating microtubule dynamics in rat hippocampal primary cultured neurons. J Neurobiol. 2006;66(10):1101–1114. doi: 10.1002/neu.20295. [DOI] [PubMed] [Google Scholar]
- 55.Ribas VT, Lingor P. Autophagy in degenerating axons following spinal cord injury: Evidence for autophagosome biogenesis in retraction bulbs. Neural Regen Res. 2015;10(2):198–200. doi: 10.4103/1673-5374.152367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 57.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11(12):1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 58.Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987;6(9):2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24(46):10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams PR, et al. A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat Commun. 2014;5:5683. doi: 10.1038/ncomms6683. [DOI] [PubMed] [Google Scholar]
- 61.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 62.Lu P, et al. Motor axonal regeneration after partial and complete spinal cord transection. J Neurosci. 2012;32(24):8208–8218. doi: 10.1523/JNEUROSCI.0308-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bei F, et al. Restoration of visual function by enhancing conduction in regenerated axons. Cell. 2016;164(1-2):219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu MM, Nirschl JJ, Holzbaur EL. LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell. 2014;29(5):577–590. doi: 10.1016/j.devcel.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larocca JN, Norton WT. 2007. Isolation of myelin. Curr Protoc Cell Biol Chapter 3:Unit3.25.
- 66.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6(5):359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.