Significance
Macropinocytosis is a way for cells to engulf large volumes of their extracellular fluid. This process allows immune cells to sense their environment and detect antigens, but can also supply nutrients to both cancer cells and some unicellular organisms. However, little is known about the fate of the membrane components internalized via macropinosomes. This study explains how cells avoid the bulk digestion of their surface proteins. We identify several core components of this pathway and show that they are required for early recycling from macropinosomes. We demonstrate that this pathway is crucial for cells undergoing continuous macropinocytosis to maintain surface protein levels and is therefore physiologically important for such cells to sustain normal functions.
Keywords: phagocytosis, macropinocytosis, WASH, Dictyostelium, trafficking
Abstract
Macropinocytosis is an ancient mechanism that allows cells to harvest nutrients from extracellular media, which also allows immune cells to sample antigens from their surroundings. During macropinosome formation, bulk plasma membrane is internalized with all its integral proteins. It is vital for cells to salvage these proteins before degradation, but the mechanisms for sorting them are not known. Here we describe the evolutionarily conserved recruitment of the WASH (WASP and SCAR homolog) complex to both macropinosomes and phagosomes within a minute of internalization. Using Dictyostelium, we demonstrate that WASH drives protein sorting and recycling from macropinosomes and is thus essential to maintain surface receptor levels and sustain phagocytosis. WASH functionally interacts with the retromer complex at both early and late phases of macropinosome maturation, but mediates recycling via retromer-dependent and -independent pathways. WASH mutants consequently have decreased membrane levels of integrins and other surface proteins. This study reveals an important pathway enabling cells to sustain macropinocytosis without bulk degradation of plasma membrane components.
Macropinocytosis is the process by which cells extend protrusions and engulf large volumes of extracellular fluid. This process most likely originally evolved in unicellular protists such as the amoeba Dictyostelium discoideum as a means to obtain nutrients from the environment (1–3). Although this process is not necessary in most cells in multicellular organisms, macropinocytosis can still be induced in many mammalian cell lines (4–6) and is constitutively up-regulated in Ras-transformed cancer cells where it performs its original role in capturing nutrients (7).
Macropinocytosis is also physiologically important for many immune cells. Immature dendritic cells and macrophages both continuously take up their surrounding fluid by macropinocytosis to sample their environment and capture antigens for presentation (8). Unfortunately this process can provide a way for pathogens to gain entry into host cells and is thus exploited by a number of infectious agents such as viruses, bacteria, and even prions (9–13).
Despite their broad physiological relevance, relatively little is known about how macropinosomes are processed and mature within the cell. Macropinosomes differ from classical microendocytic pathways such as clathrin-mediated endocytosis (CME) in a number of important respects. Most notably, macropinosomes are much larger in size, and are often defined as aqueous endocytic vesicles larger than 200 nm in diameter, compared with ∼100 nm for clathrin-driven vesicles (14). Macropinosomes therefore have a much smaller surface-to-volume ratio, so, whereas they are highly efficient at fluid-phase uptake (15, 16), microendocytosis is relatively more important for membrane internalization (17).
Clathrin-coated pits are selective about which membrane proteins they endocytose. However, macropinosomes lack similar cargo-sorting coats. Although some specific proteins can be excluded from forming macropinosomes, macropinocytosis is largely considered to result in bulk internalization of the plasma membrane and all its constituent proteins (14, 18, 19). In axenic Dictyostelium cells, whereas microendocytosis can completely internalize the plasma membrane in ∼15 min (17, 20), we calculate that macropinocytosis alone turns over the entire cell surface, including membrane proteins, in ∼100 min (Materials and Methods). As these proteins will be degraded in bulk if not rapidly recovered, this represents a major challenge for the cell. However, whereas the rapid recycling of surface proteins from macropinosomes has been demonstrated in both primary mouse macrophages and Dictyostelium (20, 21), little is known of the mechanism.
Recent years have seen considerable advances in our understanding of how membrane proteins are sorted and recycled from endosomes. In particular, significant progress has been made in defining the role of actin in the segregation and extraction of specific proteins (22). Many studies describe a role for the WASH (WASP and SCAR homolog) complex in driving endosomal actin polymerization (23–30). WASH has been implicated in the endosome-to-surface trafficking of a number of cargos, including the transferrin receptor (TfnR), α5β1 integrins, and β2-adrenoreceptor as well as in targeting the epidermal growth factor receptor (EGFR) to the lysosome (23, 25, 28, 29, 31). The WASH complex also directly interacts with the retromer sorting complex and is thus required for the specific retrieval of retromer cargos from several endocytic compartments (23, 32).
In this study, we describe a role for the WASH complex in the retrieval of surface proteins from macropinosomes. Dictyostelium cells can use both macropinocytosis of liquid medium and phagocytosis of bacteria to grow. As a genetically tractable professional phagocyte, Dictyostelium has been well used for studies on phagosome maturation, and the pathways involved are highly conserved with those in mammalian macrophages (33–35). Much less is known about macropinosome maturation, but the large and frequent macropinosomes formed by laboratory strains of Dictyostelium make it ideal for investigations (2). Here we show that without WASH, cells are unable to prevent the degradation of the plasma membrane proteins captured by macropinocytosis. We further show that without this pathway, surface receptor levels are depleted and phagocytosis is perturbed. This demonstrates both the physiological importance of WASH-mediated recycling, as well as providing a mechanistic understanding of how surface proteins from macropinosomes and phagosomes are recovered.
Results
WASH Is Recruited to Early Macropinosomes and Phagosomes.
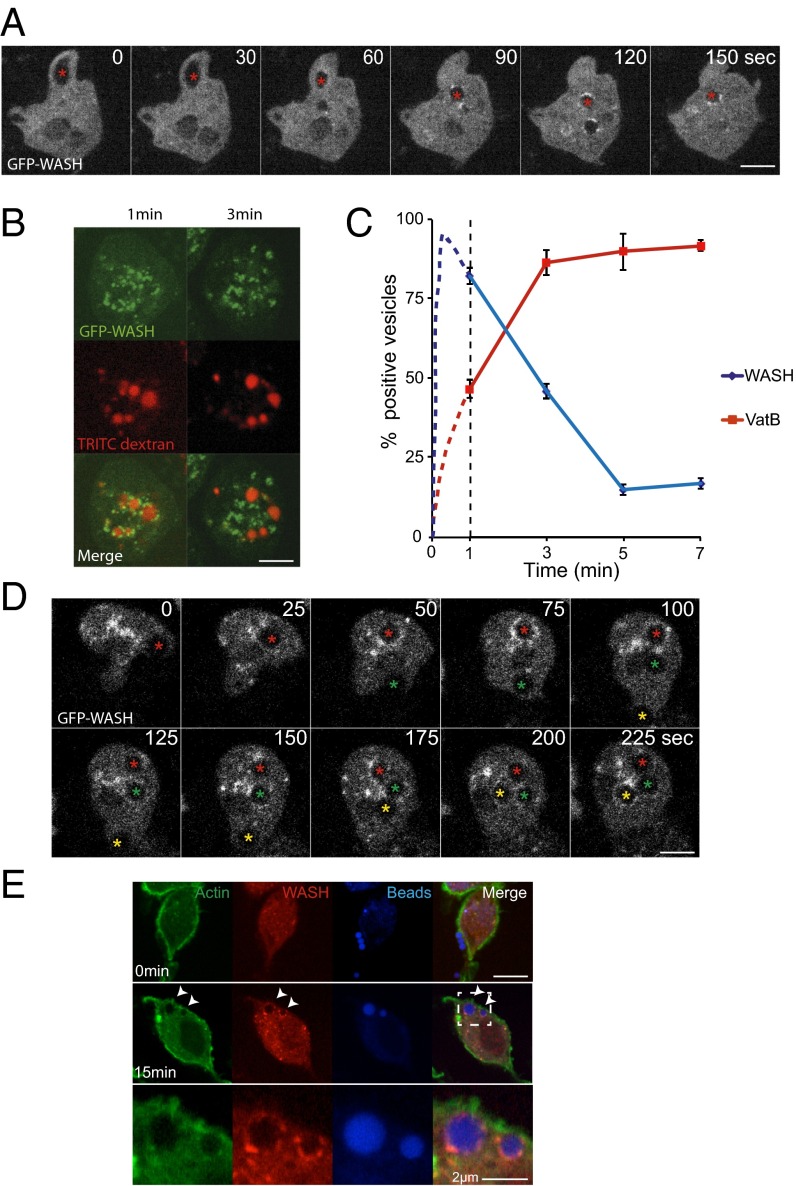
To determine if the WASH complex plays a role during the early stages of macropinosome maturation, we observed vegetative Dictyostelium cells expressing GFP–WASH by time-lapse microscopy. Whereas forming macropinocytic cups were devoid of GFP–WASH, we observed strong and extremely rapid recruitment immediately after closure; WASH is recruited within 1 min of the macropinosome sealing (Fig. 1A and Movie S1). Consistent with previous observations of both Dictyostelium postlysosomes and mammalian endosomes (23, 24, 27), GFP–WASH was restricted to multiple discrete subdomains on the macropinosome surface. This observation indicates a previously unrecognized role for WASH in early macropinosome maturation.
Fig. 1.
WASH is transiently recruited to early macropinosomes and phagosomes. (A) Time-lapse microscopy of Dictyostelium cells expressing GFP–WASH. Red star indicates a macropinosome as it forms and is subsequently internalized (Movie S1 shows full sequence). (B) GFP–WASH-expressing cells were given a 2-min pulse of TRITC–dextran before washing and imaging. GFP–WASH decorates all of the vesicles at the earliest time point (1 min) but is absent 2 min later. (C) Quantification of the cells treated as in B as well as the equivalent cells expressing GFP–VatB. Data for the first minute (before imaging was possible) is inferred from time-lapse imaging of cells in the absence of dextran as in A and represented as the dotted lines (n = 4, mean ± SD). (D) Time lapse of Dictyostelium cells phagocytosing 2 μm latex beads. This cell engulfs three individual beads, indicated by the asterisks (Movie S2 shows full sequence). (E) J774 murine macrophages were exposed to blue fluorescent latex beads and fixed and stained at the times indicated. (Scale bar, 5 μm in all images unless otherwise indicated.)
To define the temporal dynamics of WASH recruitment to macropinosomes, we exposed cells to a 2-min pulse of fluorescent dextran. This exposure allowed us to follow the synchronous maturation of multiple macropinosomes over time. Whereas macropinosomes were strongly decorated with GFP–WASH immediately after the pulse, recruitment was lost within 4 min (Fig. 1 B and C).
As phagocytosis shares many common elements with the macropinocytic pathway, we asked whether WASH is similarly recruited to phagosomes.
Upon phagocytosis of latex beads, GFP–WASH was recruited with identical dynamics to macropinosomes (Fig. 1D and Movie S2). To test whether phagosomal WASH recruitment was conserved in mammals, we looked in the mouse macrophage-like cell line J774.2. Cells were fixed and stained for endogenous WASH at several time points after addition of fluorescent beads (Fig. 1E). We again observed strong recruitment of WASH immediately after phagosome closure, indicating that this step is conserved between Dictyostelium and mammals.
Early WASH Recruitment Is Independent of V-ATPase Trafficking.
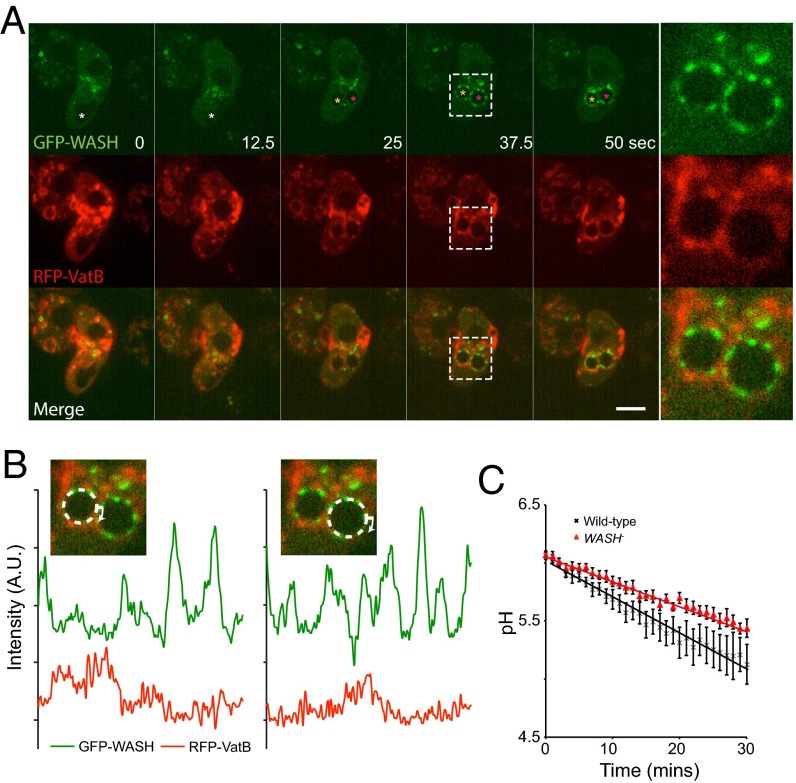
Previously, we showed that a late phase of WASH activity drives the sorting and removal of the V-ATPase during Dictyostelium lysosome to postlysosome transition (27). This finding occurred via direct interaction between the V-ATPase and WASH-generated actin, and arrival of WASH coincides with V-ATPase removal. We therefore asked whether WASH also regulates V-ATPase trafficking during early macropinosome maturation. Surprisingly, early macropinosomes acquired WASH and the V-ATPase simultaneously, with WASH accumulating slightly faster (Fig. 1C). Coexpression of GFP–WASH with RFP fused to the V-ATPase subunit VatM demonstrated that, whereas both were present in dynamic patches on the same vesicles, no spatial correlation between patches could be observed (Fig. 2 A and B and Movie S3).
Fig. 2.
Early WASH recruitment does not colocalize with the V-ATPase. (A) Time lapse of Dictyostelium cells coexpressing GFP–WASH and RFP–VatB. The forming macropinosome is marked by a white asterisk, which subsequently splits into two internal vesicles (yellow and pink asterisks). (Far Right) Enlargement of the box marked at 37.5 s (Movie S3 shows full sequence). (Scale bar, 5 μm.) (B) Quantification of the fluorescence intensity around the circumference of the two vesicles shown at 37.5 s. (C) The rate of phagosome acidification measured using pH-sensitive beads (n = 7, mean ± SEM).
If WASH-mediated V-ATPase recycling were constitutive, WASH activity on early macropinosomes and phagosomes would inhibit acidification. However, no increase in the rate of acidification was detected upon WASH disruption (Fig. 2C). Indeed, phagosomal acidification was actually slightly decreased, most likely due to decreased V-ATPase supply, as so much is sequestered in later compartments such as postlysosomes (27, 30). Thus, the early phase of WASH activity does not cause V-ATPase recycling, and WASH must act on different cargos at different stages during vesicle maturation.
WASH Drives Retromer Recycling from Both Early Macropinosomes and Postlysosomes.
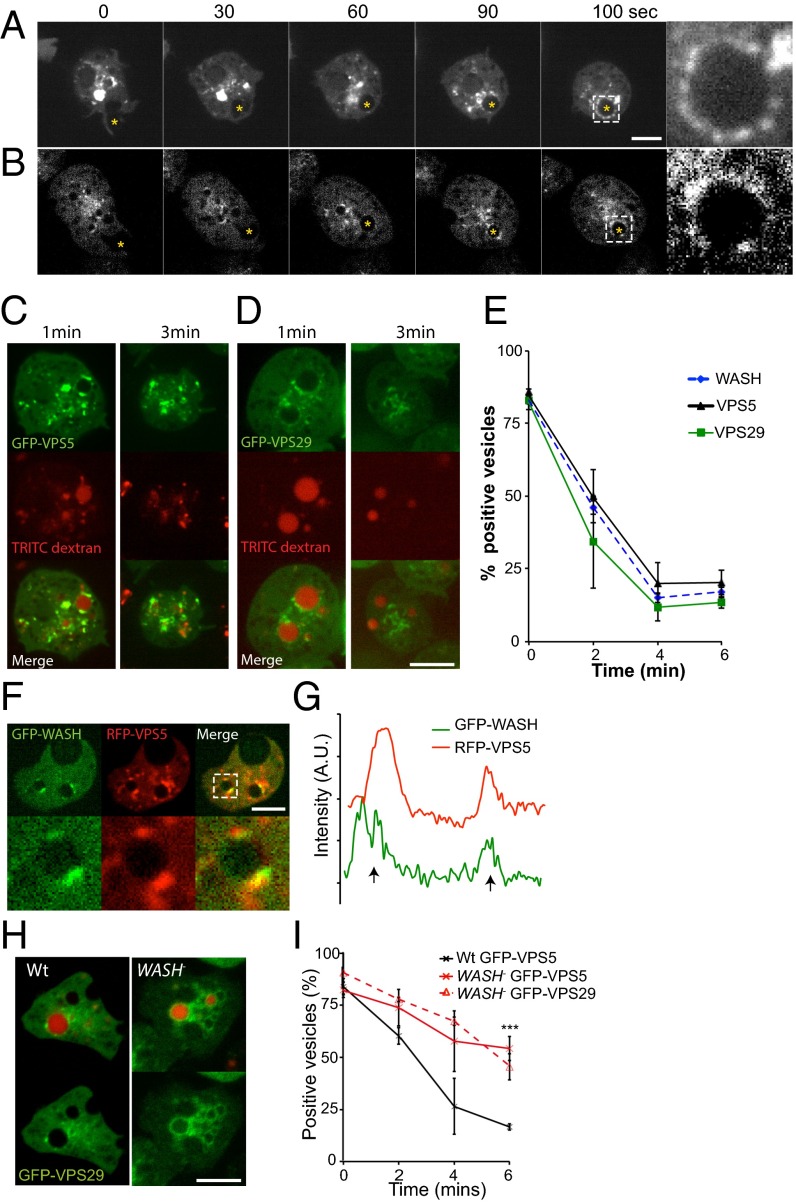
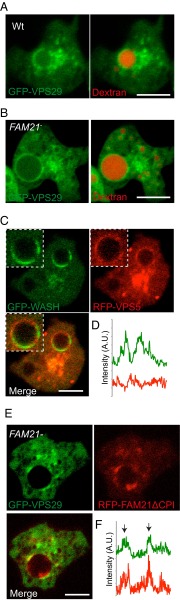
If WASH is not regulating V-ATPase trafficking, what are its functional roles in early macropinosomes? In mammalian cells, WASH mediates sorting from endosomes via direct interaction between the FAM21 subunit of the WASH complex and the VPS35 subunit of the retromer sorting complex (24, 32, 36). The retromer complex drives extraction of specific proteins at several trafficking steps by sequence-specific binding (37–39). The core retromer component Snx1 is also recruited to macropinosomes in mammalian cells (40, 41). We therefore investigated whether Dictyostelium WASH regulates the retromer during early macropinosome maturation. As the retromer consists of two subcomplexes, we expressed GFP fusions of both the sorting subcomplex member VPS29, and the membrane-associated subcomplex member VPS5. Both subunits localized to patches on macropinosomes immediately after internalization, with identical dynamics to WASH (Fig. 3 A–E and Movies S4 and S5). Coexpression of GFP–WASH and RFP–VPS5 confirmed that both proteins were restricted to the same patches on the macropinosome surface (Fig. 3 F and G).
Fig. 3.
WASH is required for retromer recycling. Time-lapse microscopy of wild-type cells expressing (A) GFP–VPS5 and (B) GFP–VPS29. Nascent macropinosomes are marked by yellow asterisks (Movies S4 and S5 show full sequences). (C) Images of GFP–VPS5- and (D) GFP–VPS29-expressing cells after exposure to a 2-min pulse of TRITC–dextran. (E) Quantification of retromer association (n = 4, mean ± SD). (F) Coexpression of GFP–WASH and RFP–VPS5. (Bottom) Enlargement of the boxed area and the fluorescence around this vesicle shown in G. (H) Localization of GFP–VPS29 in wild-type and WASH-null cells exposed to a 2-min pulse of TRITC–dextran. (I) Quantification of retromer recruitment to macropinosomes in WASH-null cells following a TRITC–dextran pulse (n = 3, ***P < 0.001, t test). Scale bars, 5 μm.) All error bars represent SD.
Although WASH was not required for retromer recruitment, disruption of WASH caused both GFP–VPS29 and GFP–VPS5 to localize uniformly on macropinosomes rather than to discrete patches (Fig. 3H). Both retromer subunits were also retained on macropinosomes much longer in WASH-null cells, demonstrating that WASH is important for retromer retrieval (Fig. 3I).
WASH and the retromer therefore functionally interact during early macropinosome maturation. Mechanistically, these data provide clear evidence that WASH activity sequesters the retromer into discrete membrane subdomains, driving recycling.
Multiple Mechanisms of WASH Recruitment and Sorting.
We next investigated the mechanism of WASH recruitment. The interaction between the VPS35 subunit of the retromer and multiple repeated motifs in the FAM21 tail is both necessary and sufficient to recruit the WASH complex to mammalian endosomes (32, 42). In contrast, WASH still localizes to postlysosomes in Dictyostelium FAM21 mutants (43), leading to speculation of whether the FAM21–retromer interaction is conserved (44).
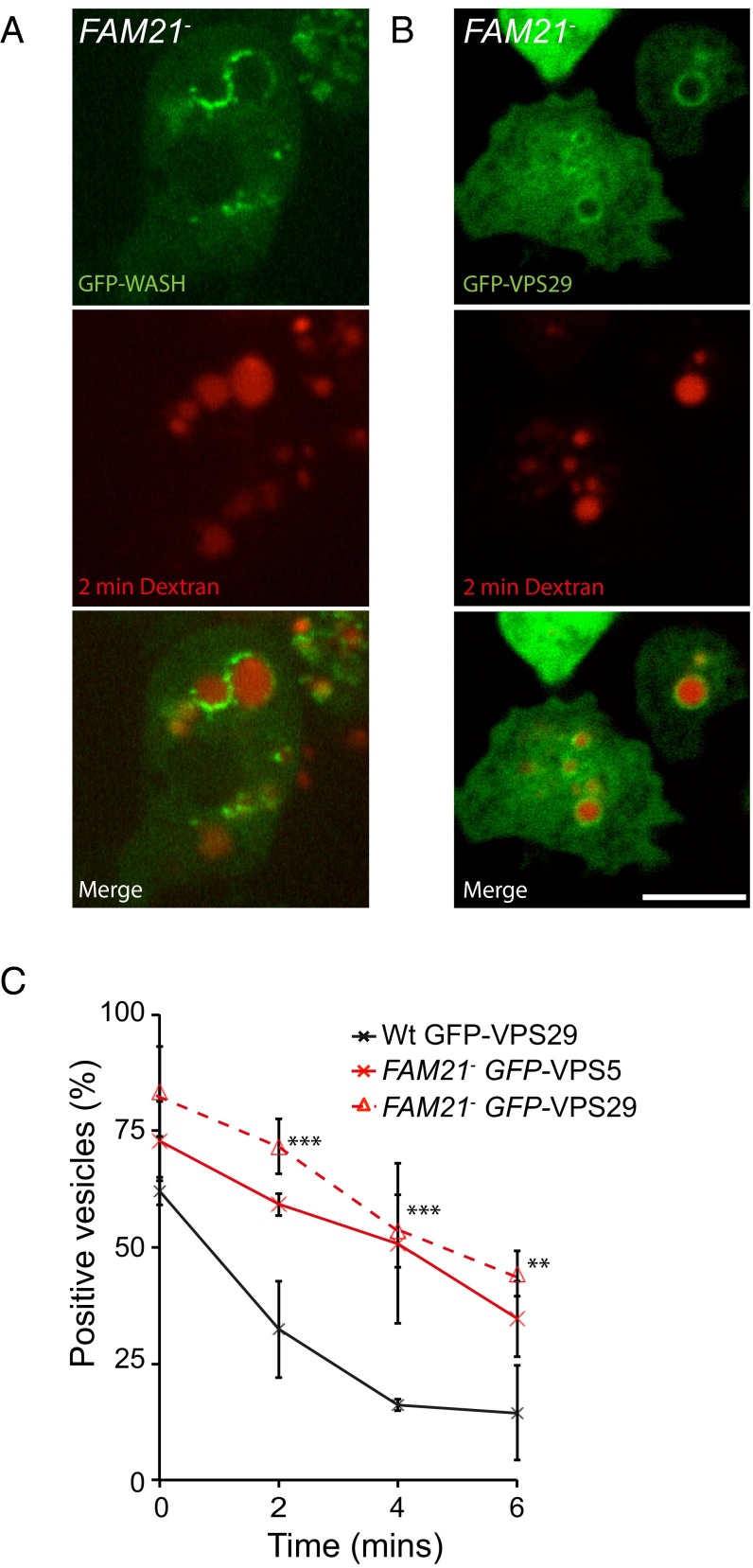
Although we have demonstrated a functional interaction between WASH and the retromer, we found that WASH was still recruited to early macropinosomes in the absence of FAM21 (Fig. 4A). Loss of FAM21 still blocked both retromer sequestration and retrieval (Fig. 4 B and C), demonstrating that, whereas FAM21 is essential to link WASH to the retromer, other interactions are sufficient for both WASH and retromer recruitment.
Fig. 4.
The FAM21–retromer interaction is not essential for early WASH recruitment. Images of FAM21-null cells expressing (A) GFP–WASH and (B) GFP–VPS29 immediately following a 2-min pulse of TRITC–dextran. (C) Quantitation of retromer subunit localization to TRITC–dextran-containing vesicles following a 2-min pulse in both wild-type and FAM21-null cells (n = 4, mean ± SD ***P < 0.005, **P < 0.05). (Scale bars, 5 μm.)
We also examined whether retromer interacts with WASH during the postlysosomal phase of WASH activity (27). Late endocytic compartments were specifically labeled by loading cells with TRITC–dextran before a 45-min chase with nonfluorescent medium. Patches of GFP–VPS29 were again detected on wild-type postlysosomes.
The enlarged postlysosomes that form in Dictyostelium FAM21 mutants provide a convenient means to investigate the FAM21–retromer interaction in vivo. Surprisingly, the retromer patches on postlysosomes became uniform when FAM21 was disrupted (Fig. 5 A and B). This finding was confirmed by coexpression of GFP–WASH and RFP–VPS5 in FAM21-null cells, again demonstrating that there was no enrichment of retromer in the patches of GFP–WASH that still form on FAM21-null postlysosomes (Fig. 5 C and D) (43). The interaction between retromer and WASH is maintained in both early and late phases.
Fig. 5.
Retromer is also present on postlysosomes and is recruited to WASH patches via FAM21. (A) Wild-type and (B) FAM21-null cells expressing GFP–VPS29 were exposed to TRITC–dextran for 2 h before washing out for 45 min before imaging. (C) FAM21-null cells coexpressing GFP–WASH and RFP–VPS5 grown in the presence of 5% dextran to enlarge the postlysosomal compartment. (D) Fluorescence intensity along the circumference of the boxed vesicle in C. (E) FAM21-null cells coexpressing GFP–VPS29 and RFP–FAM21ΔCPI. Fluorescence intensity around the enlarged vesicle is quantified in F. (Scale bars, 5 μm.)
To confirm the role of the FAM21 tail in binding the retromer, we reexpressed FAM21 containing its C-terminal repeats, but lacking the Capping Protein interaction site (FAM21ΔCPI) in FAM21-null cells. As Capping Protein binding is required for retrieval of the WASH complex itself, the truncated protein should rescue retromer binding but not WASH recycling (43). Consistently, coexpression of RFP–FAM21ΔCPI with GFP–VPS29 rescued sequestration of retromer to the same patches as the WASH complex (Fig. 5 E and F).
The WASH complex therefore functionally interacts with the retromer at both early and late stages of macropinosome maturation. This interaction is mediated via the well-known conserved interaction with FAM21, but is not essential for WASH recruitment. As we have previously shown (41), there must be other parallel mechanisms for WASH recruitment to vesicles. Importantly, whereas retromer requires interaction with FAM21 to be recycled, V-ATPase removal strictly relies on its direct interaction with actin and is still removed in FAM21-null cells (27, 43). Therefore, WASH is both recruited by, and mediates recycling by multiple independent mechanisms.
WASH Drives Recycling of Surface Proteins from Early Macropinosomes.
Phagosome and macropinosome membranes are remodeled in a complex and highly regulated manner. However, the mechanisms underpinning protein sorting before degradation are poorly understood. WASH has been shown to drive the recycling of several plasma membrane proteins from sorting endosomes back to the cell surface (28, 29, 45). We therefore asked whether the early phase of WASH and retromer activity provides a way to recover plasma membrane components from nascent macropinosomes before degradation.
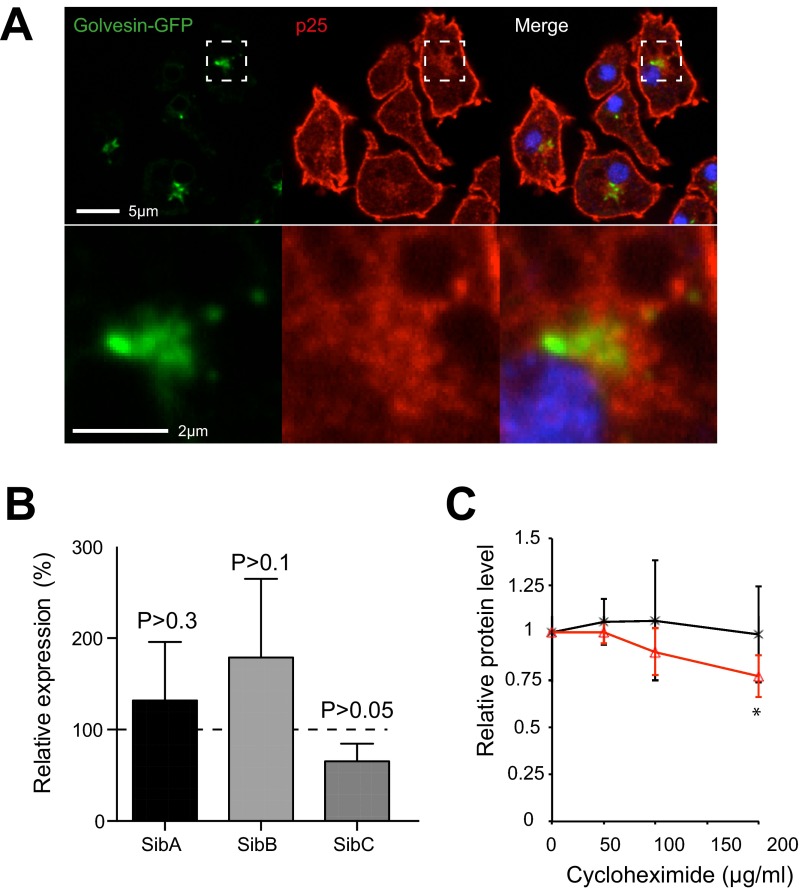
The rapid depletion of surface proteins from macropinosomes has previously been described in Dictyostelium (46, 47). Both the plasma membrane and a perinuclear recycling pool are marked by the protein p25, which is stripped from macropinosomes within the first minutes of internalization—at the same time that WASH and retromer are active. This recycling pool is in the same region, but does not overlap with the Golgi marker golvesin–GFP (Fig. S1A) (48). Interestingly, the p25 recycling compartment is also accessible to proteins lacking any cytosolic sorting signals, indicating nonspecific bulk recycling (46).
Fig. S1.
(A) p25 localizes to the perinuclear, and peri-Golgi region of the cell. Ax2 cells expressing the Golgi marker golvesin–GFP were fixed and stained with anti-p25 antibody. (B) Expression of surface proteins in WASH-null cells relative to Ax2, measured by qPCR. Values are mean ± SD (n = 4), no differences are statistically significant (P > 0.05, one-sample t test). (C) SibA levels are more sensitive to inhibition of protein synthesis by cyclohexamide treatment in WASH mutants. Cells were treated with the indicated concentrations of cycloheximide for 1 h, and endogenous SibA was measured by Western blot. Samples were normalized to the mitochondrial protein MCCC1 loading control; values plotted are the mean ± SD of four independent experiments (*P < 0.05 paired t test).
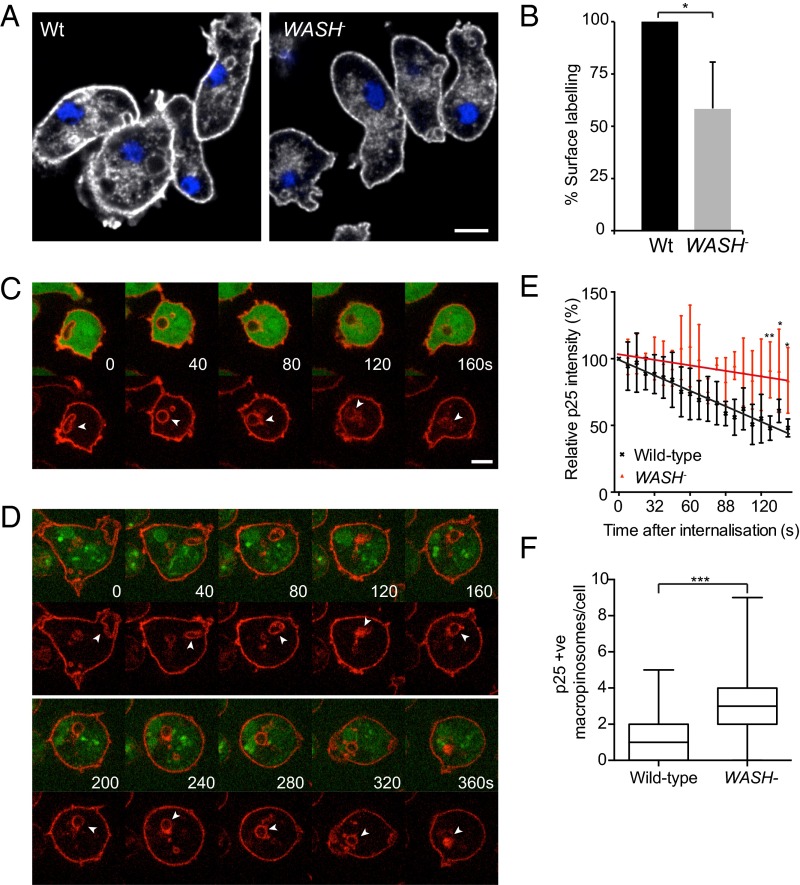
Failure to rapidly retrieve plasma membrane components from macropinosomes would lead to their degradation. Consistent with this hypothesis, immunostaining of WASH-null cells for endogenous p25 showed a significant depletion from both the surface and recycling pools (Fig. 6A). The surface levels of p25 were also measured by flow cytometry, indicating an almost 50% reduction in protein levels when WASH is disrupted (Fig. 6B).
Fig. 6.
WASH drives p25 recycling from macropinosomes. (A) Immunostaining of p25 in wild-type and WASH-null cells. (B) Intact cells were stained and surface levels of p25 measured by flow cytometry (n = 4, mean ± SD, *P < 0.05). (C and D) Live imaging of (C) wild-type and (D) WASH-null cells stained with fluorescently labeled anti-p25 antibody on ice before macropinocytosis was reinitiated at room temperature. Cells are expressing cytosolic GFP to identify intracellular vesicles, in which GFP is excluded; autofluorescent vesicles are also visible in D. Surface-labeled p25 is internalized and recycled from wild-type cells but not WASH nulls. (E) Quantitation of the fluorescence intensity of macropinosomes postinternalization. Values plotted are the mean ± SD of at least six independent movies, normalized to initial intensity (*P < 0.05, **P < 0.01). (F) Quantitation of p25-positive macropinosomes in wild-type and WASH-null cells. Cells were fixed and stained for endogenous p25, before z stacks were captured on a spinning disk microscope. Macropinosomes were defined as vesicles >0.5 μm in diameter and identified from z stacks. n > 200 cells over three independent experiments. ***P < 0.001 (Mann–Whitney test).
To further investigate p25 recycling, and to monitor macropinosomes specifically, we stained the surface of live cells with fluorescently labeled anti-p25 antibody at 4° C. Upon warming, macropinocytosis was reinitiated, and we were able to follow the internalization and retrieval of p25 by fluorescence microscopy (Fig. 6 C–E). In wild-type cells, the majority of p25 was removed within 2 min, but retrieval was much slower in WASH-null cells, which still retained ∼90% of the fluorescent signal at this time point (Fig. 6E and Movies S6 and S7). The rate of macropinocytosis is unaffected in WASH-null cells (27). Therefore, if p25 recycling is inhibited, WASH-null cells should contain more p25-positive macropinosomes. Consistently, we found almost twice as many p25-positive macropinosomes in fixed WASH mutants, despite the lower surface levels of the protein (Fig. 6F).
Dictyostelium Integrins Are Recycled via WASH.
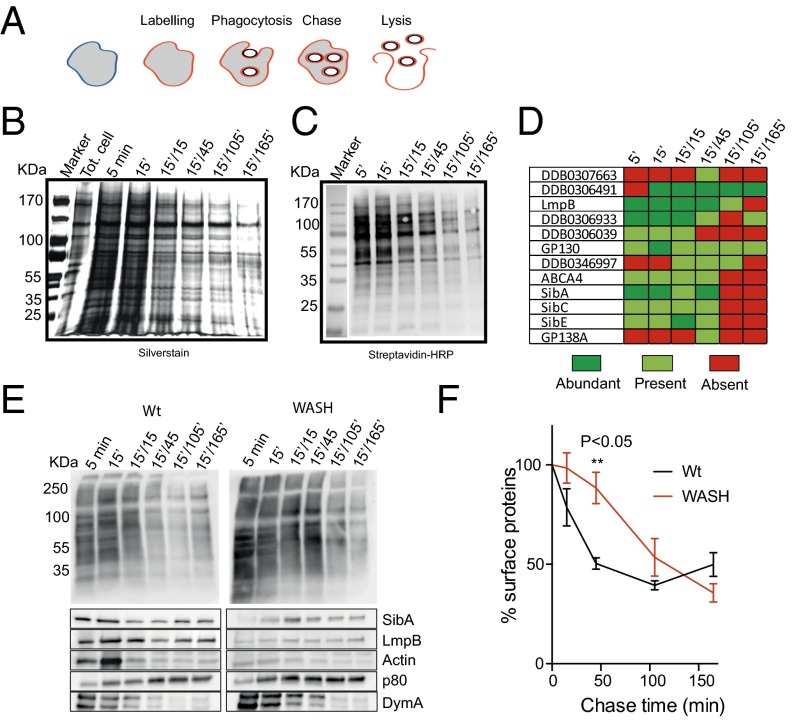
Although there is evidence that surface proteins may be excluded or enriched in phagosomal and macropinosome membranes, the specificity of protein internalization is unclear (49–51). To assess this lack of specificity, we labeled surface proteins by biotinylation and looked for their presence in purified early phagosomes. This experiment indicated that a large number of surface proteins were present in early phagosomes and decreased in abundance over time (Fig. 7).
Fig. 7.
Internalization of surface proteins by phagocytosis. (A) Outline of experimental procedure. The cell surface was labeled by biotinylation, before cells were allowed to phagocytose latex beads for 5 or 15 min. Beads were then washed out and phagosomes isolated at different time points, and surface-originated biotinylated proteins purified. These were analyzed by both (B) silver staining and (C) streptavidin Western blot. (D) Transmembrane proteins identified from each sample by mass spectrometry. Relative abundances were semiquantitatively assessed by number of peptides identified (n = 2). (E) Comparison of phagosome maturation in wild-type and WASH-null cells. Surface labeling and phagosome purification was done in parallel and probed with either streptavidin (Top) or specific antibodies (Bottom). (F) Quantification of biotinylated proteins on purified phagosomes, by densitometry of the entire lanes in blots such as in E (n = 2). All times indicated are in the format:pulse time (min)/chase time (min).
Several surface proteins internalized in phagosomes were then identified by mass spectrometry (Fig. 7D). Prominent among these are members of the Dictyostelium Sib family (similar to Integrin-β) that act as both adhesion and phagocytic receptors, as well as the lysosomal membrane glycoprotein LmpB (52). These data were confirmed by Western blot, indicating that endogenous SibA and LmpB are present on newly formed phagosomes, but are rapidly removed (Fig. 7E). Integrins are also internalized by macropinocytosis in mammalian cells (53) and α5β1 integrins have been reported to be recycled from mammalian endosomes by WASH (28, 29). We therefore asked whether WASH was also important to recycle integrins from macropinosomes.
To confirm a role for WASH in early phagosome recycling, we repeated the surface labeling and phagosome purification in WASH-null cells. In the mutants, retrieval of biotinylated surface proteins was significantly delayed, although it still occurred at later time points (Fig. 7 E and F). When we looked specifically at SibA and LmpB, however, no recycling from early phagosomes was observed, and there was also a complete loss of the transient accumulation of actin seen in wild-type cells. As controls, we also examined dynamin and p80. These well-characterized markers of maturation (54) were unaffected by loss of WASH, indicating a specific defect in surface protein recycling.
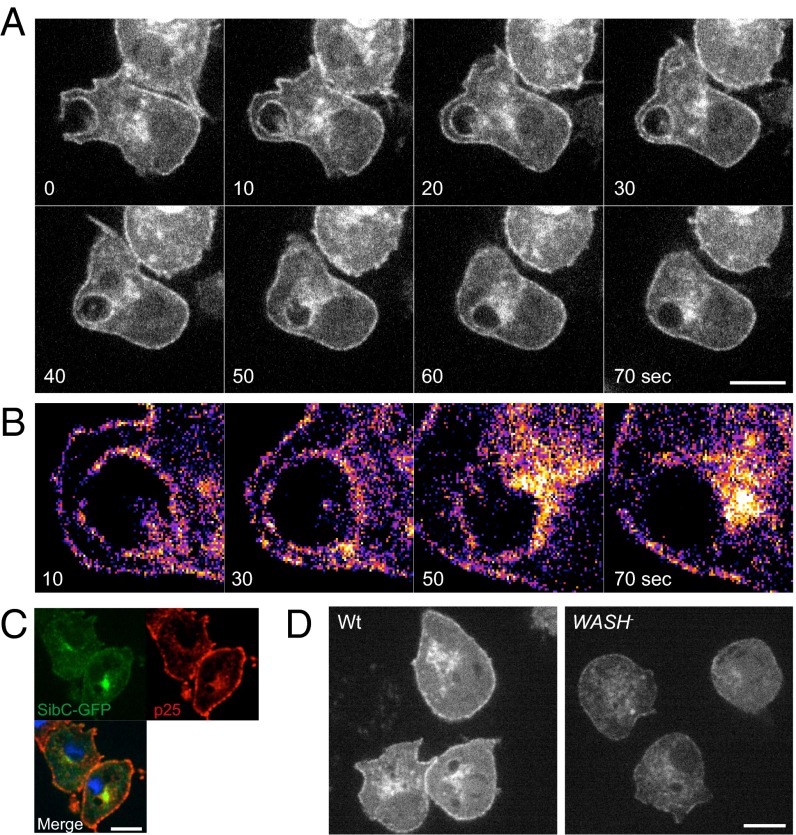
To observe integrin dynamics in live cells, we expressed GFP fused to the C terminus of SibC (SibC–GFP). In wild-type cells, SibC–GFP localized to both the plasma membrane and a perinuclear compartment. Importantly, SibC–GFP was neither excluded nor enriched on macropinocytic cups and is therefore efficiently internalized by this route (Fig. 8 A and B). In the first minute after internalization, SibC–GFP was rapidly removed from the macropinosome surface, appearing to be sequestered into a diffuse cluster of juxtanuclear vesicles (Fig. 8B and Movie S8). This finding is comparable to the recycling of antibody-labeled p25 (Fig. 6C) and both SibC–GFP and p25 colocalize at the juxtanuclear compartment, indicating that both proteins are recycled via the same route (Fig. 8C).
Fig. 8.
WASH is required to maintain integrin levels. (A) SibC–GFP is internalized by macropinosomes and rapidly recycled in wild-type cells. (B) Enlargement of the macropinosome in A, false-colored to clarify SibC–GFP intensity and localization following macropinsome internalization. (C) SibC–GFP-expressing wild-type cells fixed and stained for p25. (D) Localization of SibC–GFP in wild-type and WASH-null cells. (Scale bars, 5 μm.)
Consistent with the corecycling of SibC and p25, when we expressed SibC–GFP in WASH-null cells, the fusion protein was again severely depleted from both plasma membrane and juxtanuclear pools (Fig. 8D). In fact, the SibC–GFP depletion was so severe that we were unable to obtain enough signal for time-lapse fluorescence microscopy in WASH-null cells.
We also tested whether the observed decrease in protein levels was due to increased degradation, or decreased synthesis. To assess transcriptional regulation, we measured mRNA levels of SibA, B, and C by quantitative PCR (qPCR) but found no significant changes in expression (Fig. S1B). Increased rates of degradation were further confirmed by inhibiting protein synthesis with cycloheximide. Whereas higher drug concentrations or longer time periods could not be used as they blocked macropinocytosis, we consistently found that endogenous SibA levels decreased faster in WASH-null cells than wild-type controls, indicating increased degradation (Fig. S1C).
WASH therefore drives an early phase of recycling of surface proteins from macropinosomes and is required to prevent degradation and maintain plasma membrane levels of both p25 and Dictyostelium integrin homologs.
WASH Is Required for Efficient Phagocytosis.
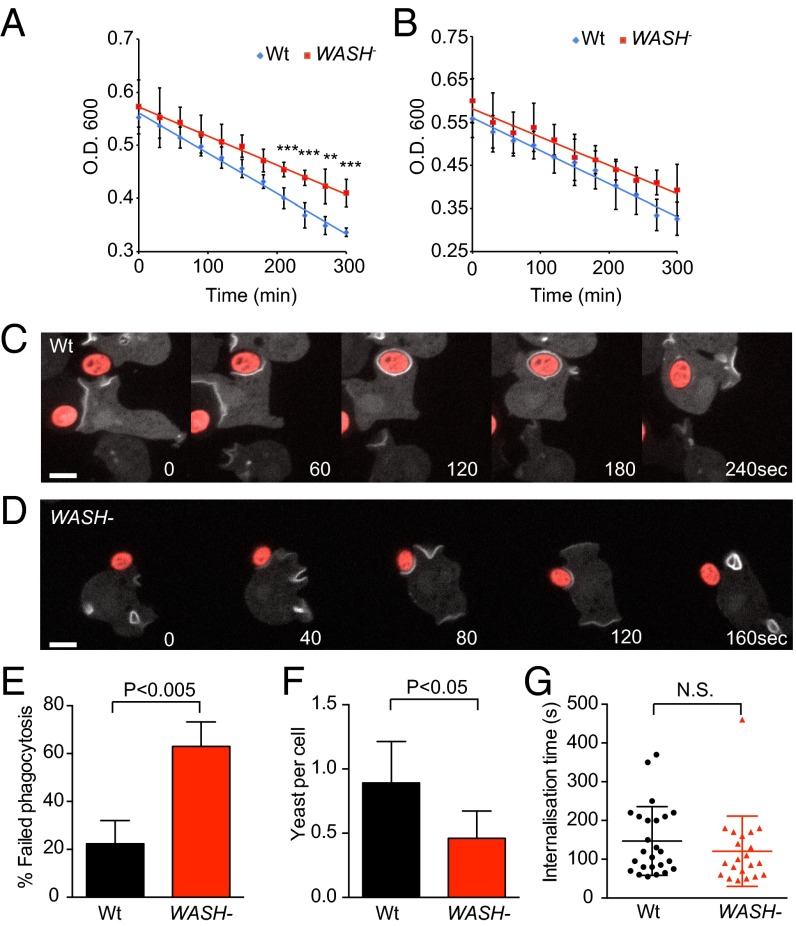
Reduction in the surface levels of proteins such as SibC should affect the ability of cells to bind bacteria (52). Previously, we showed that WASH-null cells grow poorly on bacteria, despite normal axenic growth (27, 30). As phagocytosis, but not macropinocytosis, is dependent on plasma membrane receptors, we measured the rate of bacterial uptake in WASH-null cells. Compared with wild-type, phagocytosis of Escherichia coli DH5α was decreased by 30% in WASH-null cells (Fig. 9A). This defect was less pronounced with Klebsiella aerogenes (Fig. 9B), consistent with the milder growth defect previously reported (30).
Fig. 9.
WASH is required for efficient phagocytosis. Phagocytosis of (A) E. coli DH5α and (B) K. aerogenes by wild-type and WASH-null Dictyostelium. Phagocytosis was measured by the decrease in turbidity of a bacterial suspension over time (n = 4, mean ± SD ***P < 0.001, **P < 0.01). (C) Wild-type and (D) WASH-null cells engulfing TRITC-labeled yeast. Cells are expressing the PI(3,4,5,)P3 reporter GFP–PHcrac to show phagosome formation and internalization, indicated by loss of the reporter. C is representative of normal, successful phagocytosis, whereas D is representative of a failed phagocytosis event frequently observed in WASH mutants. The frequency of phagocytic failure is quantified in E (mean ± SD of three independent experiments; >35 phagocytic events in total for each cell line). (F) The number of yeast engulfed in 30 min was significantly reduced (mean ± SD of four independent experiments, P < 0.05 paired t test); however, (G) the average time for successful phagocytic events was unaltered (n > 20 for each cell line).
To analyze phagocytosis in more detail, we also investigated the ability of WASH-null cells to engulf fluorescently labeled yeast. To clearly define phagocytic cup formation in live cells, we expressed the phosphatidyl [3,4,5] trisphosphate (PIP3) reporter GFP–PHcrac, which is strongly recruited to phagocytic and macropinocytic cups as they form (Fig. 9 C and D) (27, 55). Strikingly, we found that although WASH-null cells made frequent attempts to engulf yeast, over 60% of attempts failed, with the yeast detatching and escaping from the phagocytic cup (Fig. 9 D and E and Movie S9). In contrast, only 20% of phagocytic attempts failed in wild-type cells (Fig. 9C and Movie S10). Defective phagocytosis was further confirmed by quantifying the number of yeast engulfed in 30 min. We again found a significant reduction in WASH-null cells (Fig. 9F). In contrast, both cup morphology, and the speed at which successful phagocytic events progressed, were indistinguishable between WASH-null cells and wild-type (Fig. 9G). This finding indicates that there are no underlying defects in cup formation itself, consistent with the normal macropinocytosis in WASH mutants (27). These data demonstrate that WASH is required for efficient phagocytosis and are consistent with decreased ability to bind and retain prey within the forming phagocytic cup.
Discussion
Compared with microendocytic pathways such as CME, relatively little is known about how macropinosomes are remodeled postclosure. In this study, we show that both WASH and the retromer sorting complex are recruited to macropinosomes and phagosomes immediately after internalization. During this phase, we show that several plasma membrane proteins are retrieved and recycled and that WASH is essential to maintain the plasma membrane levels of these proteins. WASH therefore mediates an early recycling step, which prevents important surface proteins from being degraded. This finding is of particular importance during macropinocytosis, due to the largely nonspecific nature of membrane internalization, and without it, phagocytic cells are unable to hold onto their targets as they try to engulf them.
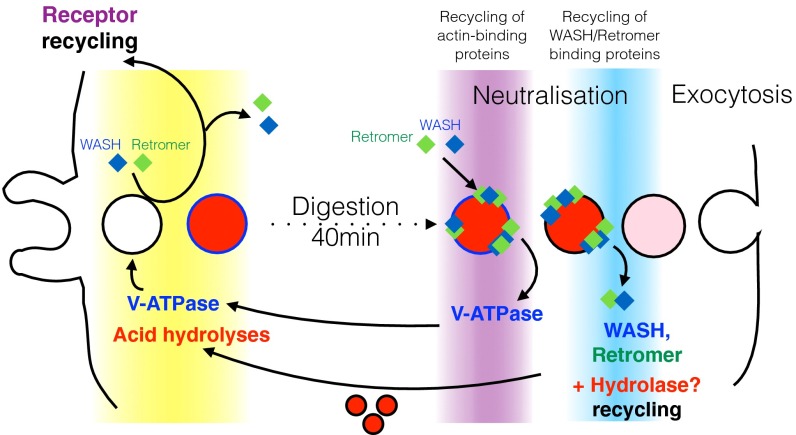
Importantly, we also show that WASH mediates recycling via multiple mechanisms (Fig. 10). Whereas retromer recycling is dependent on the interaction between VPS35 and FAM21 (24, 32, 36), the V-ATPase directly binds F-actin with submicromolar affinity (56, 57) and therefore only appears to require actin polymerization for sorting and removal. This is most clearly demonstrated during the postlysosomal transition of FAM21 mutant Dictyostelium cells. WASH normally removes both V-ATPase and retromer, but in FAM21-null cells (which retain the ability to polymerize actin on postlysosomes) (43), although V-ATPase is recycled, retromer is retained (43) (Fig. 4).
Fig. 10.
Multiple phases and functions of WASH in the Dictyostelium endocytic cycle. Model for the sequential activity of WASH and the multiple recycling mechanisms present, as macropinosomes and phagosomes transit through the cell.
WASH is also likely to mediate recycling independently of both the retromer and direct actin binding. By generating membrane subdomains, actin is thought to structurally stabilize the specialized tubules required for sequence-specific endosomal sorting (22, 31). In mammalian fibroblasts, loss of WASH also disrupts the nonsignal-mediated endosome-to-plasma membrane recycling of proteins such as the transferrin receptor (23). The presence of WASH and the retromer on early macropinosomes and phagosomes will therefore orchestrate complex sorting events by multiple mechanisms.
Interestingly, WASH does not appear to cause V-ATPase removal during its early phase of activity, when WASH and the V-ATPase arrive simultaneously (Fig. 2). Whether there is a specific mechanism to prevent this effect, or the transient recruitment of WASH is too brief to significantly inhibit acidification, is unclear. Nonetheless it seems unlikely that cells would use WASH to delay V-ATPase accumulation, as rapid acidification is crucial to efficiently kill potentially harmful bacteria.
We also provide evidence that, although the WASH complex interacts with the retromer via FAM21, other interactions are sufficient to recruit WASH when interaction with the retromer is lost. As the WASH complex and the retromer interact and colocalize at both early macropinosomes and postlysosomes, this evidence is perhaps surprising. However, the systematic disruption of WASH complex subunits previously indicated that there must be multiple signals to recruit the intact complex to membranes (43). Whereas the FAM21–VPS35 interaction appears to be the most important factor in the mammalian studies to date, additional unknown interactions are clearly sufficient in Dictyostelium. Whether or not these additional interactions occur, but are insufficient to drive recruitment in mammalian cells, remains to be determined, as does the possibility of tissue-specific pathways.
The presence of the retromer on Dictyostelium postlysosomes also begs the question of what its function might be. Dictyostelium cells differ from most mammalian cells in that they must continuously expel indigestible material that they take up from their environment. As Dictyostelium postlysosomes constitutively exocytose their contents, we speculate that the late phase of WASH and retromer activity provides a mechanism to retrieve hydrolases before they are lost by exocytosis. Indeed, the sequential delivery and retrieval of hydrolytic enzymes during phagosome maturation is well characterized (33) and a late hydrolase retrieval step was previously proposed by others (33, 58), who showed that hydrolase retention is disrupted by dominant negative Rab7—a now well-characterized regulator of the retromer (59).
One of the best characterized roles for the retromer is to facilitate acid hydrolase trafficking by driving the retrograde trafficking of the cation-independent mannose-6-phosphate receptor (CI-M6PR) from lysosomes back to the trans-Golgi network (TGN) (37). In the pH neutral Golgi, the CI-M6PR binds mannose-6-phosphate–tagged hydrolases to extract and deliver them to the lysosome. The acidic pH of the lysosome then causes the hydrolases to dissociate from the receptor, whereupon the retromer mediates CI-M6PR retrieval and return to the TGN (39, 60, 61). As the Dictyostelium postlysosomal transition is precipitated by neutralization, we hypothesize that the hydrolases will reassociate with their M6PR for retromer-mediated extraction before exocytosis. Consistent with this hypothesis, we previously found that disruption of either WASH or FAM21 leads to accumulation of hydrolases in postlysosomes (30). Importantly, the retention of hydrolases in postlysosomes, leading to decreased delivery to nascent phagosomes, will also reduce phagocyte function. Therefore, the inability of WASH-null cells to grow on a diverse range of bacteria is likely a composite phenotype of both reduced phagocytosis and reduced killing and digestion (30).
These studies describe an expanded view of WASH cell biology, whereby multiple phases of activity and sorting mechanisms operate throughout macropinosome and phagosome maturation (Fig. 10). Although Dictyostelium postlysosomes have no obvious parallel in most mammalian cells, both macropinocytosis and phagocytosis are highly conserved across evolution, and we show that the early phase of WASH activity is also present in mouse macrophages.
We have shown that WASH plays a critical role in recycling plasma membrane components from early macropinosomes and phagosomes. WASH is thus essential to maintain surface levels of phagocytic receptors. Whereas the specificity of WASH and/or retromer-mediated recycling is not currently clear, in epithelial cells, α5β1 integrins are recycled independently of the retromer (62). The Dictyostelium Sibs and the macrophage integrin-like phagocytic receptors are therefore likely to be sorted in a similar manner. Other phagocytic receptors, such as the Caenorhabditis elegans apoptotic corpse receptor CED-1, are recycled from phagosomes in a retromer-dependent manner (63). Therefore, both retromer-dependent and -independent sorting are likely to occur simultaneously. Although we are only beginning to understand the mechanisms regulating macropinosome maturation, there is growing evidence of the importance of macropinocytosis in numerous physiological contexts (64). The transient recruitment of WASH and the retromer is one of the first steps in macropinosome processing, but only future studies will reveal the complete path of these vesicles.
Materials and Methods
Estimation of Macropinosome Membrane Turnover.
It has previously been shown that axenic Dictyostelium strains form approximately two macropinosomes per minute, at an average diameter of 1.6 μm (16). However, in our laboratory strain of Ax2, macropinosomes are slightly smaller, averaging ∼1 μm in diameter. This size will internalize 6.3 μm2 of membrane every minute. The surface area of Dictyostelium has been estimated as 700 μm2 (65) and will therefore be completely turned over in ∼100 min.
Cell Strains and Culture.
Dictyostelium cells, routinely subcultured in HL5 medium (Formedium), adhered to plastic dishes at 22 °C. All mutants were generated in the Ax2 genetic background, with the exception of the previously described FAM21 nulls, which were in Ax4 (43). Appropriate wild-type controls were used in each case. Cells were transformed by electroporation and selected in either 20 μg/mL hygromycin (Invitrogen) or 10 μg/mL G418 (Sigma) as required. J774.2 mouse macrophages were obtained from ATCC and grown in Dulbecco’s modified Eagle’s medium supplemented with 10% (vol/vol) FCS.
Molecular Biology.
The GFP–WASH, GFP–WASH/RFP–VatB, and RFP–FAM21ΔCPI expression constructs were previously described (27, 43). Full-length coding sequences for VPS5, VPS29, and SibC (66) were amplified by PCR from a cDNA library generated from vegetative cells and sequenced. VPS5 and VPS29 were subcloned into pDM448 (67) to give the GFP-fusion expression vectors pJSK613 and -614, respectively. The SibC coding sequence was subcloned into the C-terminal GFP-fusion vector pDM1045 (a kind gift from Douwe Veltman, Medical Research Council Laboratory of Molecular Biology, Cambridge, UK). VPS5 was also cloned into the RFP-fusion shuttle vector pDM602, before subcloning as a NgoMIV fragment into the GFP–WASH expression vector to generate the GFP–WASH/RFP–VPS5 expression plasmid pLP151.
Microscopy and Image Analysis.
All images were captured using a Perkin-Elmer Ultraview VoX spinning disk confocal microscope running on an Olympus IX81 body with a UplanSApo 100× oil immersion objective (N.A. 1.4). Cells were illuminated with 405, 488, and 594 laser lines and images were captured on a Hammamatsu C9100-50 EM-CCD camera, using Volocity software (Perkin-Elmer). Images were quantified using ImageJ software (https://imagej.nih.gov/ij/), and circular linescans were generated using the Oval Profile plugin with a line thickness of 2 pixels.
Before live-cell imaging, cells were grown in SIH medium (Formedium) overnight to reduce autofluorescence. Dictyostelium pulse–chase experiments were performed by exposing cells in glass-bottomed dishes (Iwaki) to 1 mg/mL TRITC–dextran (Invitrogen) for 2 min. The dextran was then removed by five changes of medium before imaging. Random fields of view were captured every 2 min and the proportion of cells decorated with GFP was scored manually. At least four fields of view were captured for each time point and the experiments were repeated three times. J774.2 cells were seeded on coverslips before addition of 2 μm blue fluorescent beads (Sigma). Cells were kept on ice for 10 min before warming to 37 °C. Coverslips were removed at time intervals and fixed in 4% paraformaldehyde for 10 min before processing for immunofluorescence. Cells were stained with anti-WASH antibody HPA002689 (Atlas antibodies) and Alexa-488 phalloidin.
The anti-p25 monoclonal antibody H72 was a kind gift from Pierre Cosson, University of Geneva, Geneva (47), and was fluorescently labeled using Zenon mouse IgG1 Alexa-594 labeling kit (Life Technology). Live cells were stained in labeled antibody diluted 1:10 in SIH medium at 4 °C for 10 min, before washing and imaging at room temperature.
Measuring Phagosomal pH.
Phagosomal pH measurements were done as previously described (68). Briefly, cells seeded in a 96-well plate were fed beads labeled with both FITC and Alexa-594. The fluorescence of each fluorophore was then measured in a plate reader over time, and pH was determined using a standard curve. The values plotted are the mean and SD of at least four independent experiments.
Flow Cytometry.
To measure surface protein levels, log-phase Dictyostelium cells were washed once in KK2 buffer (0.1 M potassium phosphate pH 6.1) before resuspension and fixation in 4% paraformaldehyde for 10 min. Cells were then washed thrice in PBS, before blocking for 30 min in 2% BSA on a rotator for 30 min before antibody staining at room temperature for 30 min. Cells were washed by pelleting thrice in PBS before staining with Alexa-488–conjugated secondary antibody and analyzed by a flow cytometer.
Membrane Labeling and Phagosome Isolation.
Plasma membrane proteins were biotinylated as described previously (69). Briefly, 6 × 108 cells (108 cells per time point) were biotinylated with 2 mg of NHS-LC-biotin (Pierce Biotechnology) in Sorensen-sorbitol buffer (SSB) (15 mM KH2PO4, 2 mM Na2HPO4, 120 mM sorbitol) pH 8.0 at 4 °C for 10 min. Cells were washed thrice in SSB and unreacted cross-linker quenched with 100 mM glycine. Biotinylated cells were then resuspended in fresh HL5c media containing 0.8 μm latex beads (Sigma) at 180:1 for the indicated pulse–chase times. Phagosomes were isolated by flotation on sucrose gradients after cell lysis with a ball homogenizer (69) before lysis in RIPA buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS). Biotinylated proteins were then purified from lysates using streptavidin-coated beads.
For mass spectrometry analysis, biotinylated proteins were recovered from streptavidin-coated beads by 5-min incubation at 95 °C in loading buffer and analyzed by SDS/PAGE. Each lane was cut into 12 pieces and analyzed by mass spectrometry. Results represent data from two independent experiments. Only transmembrane proteins detected in the two experiments are shown. Relative abundance was assessed semiquantitatively by the number of detected peptides.
For Western blotting, proteins were separated by SDS/PAGE, before transfer and probing with the following previously published antibodies: rabbit polyclonal antibody (pAB) to GST-SibA (52), rabbit pAb to LmpB (70), mouse monoclonal antibody (mAb) to p80 (47), anti-actin mAB 224–236-1 (gift from G. Gerisch, Max Planck Institute, Martinsfried, Germany), and rabbit pAb against DymA (71).
Phagocytosis Assays.
Phagocytosis of bacteria was measured by following the decrease in turbidity of a bacterial suspension over time as they were engulfed by amoebas (72). Dictyostelium cells were grown on bacterial (K. aerogenes) clearing plates before the assay, before harvesting, washing free of bacteria, and adding 2 × 107 cells to a bacterial suspension with an OD600 of ∼0.6. The Dictyostelium/bacterial mixture was shaken at 180 rpm and OD600 measured at time intervals.
Heat-killed Saccharomyces cerevisiae were labeled with TRITC as previously described (73). Dictyostelium cells transformed with the GFP–PHCRAC expression plasmid pDM631 (74) were seeded in glass-bottomed dishes in SIH medium for 2 h before addition of yeast at fivefold excess. For time-lapse microscopy, cells were overlaid with a thin ∼1.5-mm thick sheet of 1% SIH agarose before imaging on a spinning disk microscope as above. Phagocytosis attempts were indicated by the strong accumulation of GFP–PHCRAC around the yeast. To quantify engulfed yeast, Dictyostelium and labeled yeast were again incubated at 5:1 ratio. After 30 min, the fluorescence of extracellular yeast was quenched with 20 μg/mL trypan blue, and brightfield and fluorescence images were captured on an inverted, widefield microscope. Over 100 cells were scored for each sample.
qPCR.
qPCR was performed exactly as previously described (75). Expression levels were normalized to Ig7 as a loading control and calculated relative to wild-type controls. Primers used were: SibA GGAAAGCAACACATTTCCGT/TCTCCAAGGTCCAAATCACC; SibB ATCCATTTGGCCCATTACAA/AGATCCGATGTGGGTGTTTC; SibC TATTCACCTGTCCCGGATTC/ATTTCCGGCAAGAACAAATG; and Ig7 TCCAAGAGGAAGAGGAGAACTGC/TGGGGAGGTCGTTACACCATTC.
Supplementary Material
Acknowledgments
We thank Ralph Graf for helpful suggestions. J.S.K. is supported by a Royal Society university research fellowship and Cancer Research UK provided an Institute Group award (to R.H.I.). The T.S. laboratory is supported by multiple grants from the Swiss National Science Foundation and T.S. is a member of iGE3 (www.ige3.unige.ch). S.A.J. is funded by the Medical Research Council, Department for International Development Career Development Award Fellowship MR/J009156/1, a Krebs Institute fellowship, Medical Research Foundation Grant R/140419, and Wellcome Trust Strategic Award 097377/Z/11/Z.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524532113/-/DCSupplemental.
References
- 1.Amyere M, et al. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291(6–7):487–494. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 2.Bloomfield G, et al. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife. 2015;4:4. doi: 10.7554/eLife.04940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yutin N, Wolf MY, Wolf YI, Koonin EV. The origins of phagocytosis and eukaryogenesis. Biol Direct. 2009;4:9. doi: 10.1186/1745-6150-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109(6 Pt 1):2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki N, Hatae T, Yamada T, Hirohashi S. Actinin-4 is preferentially involved in circular ruffling and macropinocytosis in mouse macrophages: Analysis by fluorescence ratio imaging. J Cell Sci. 2000;113(Pt 18):3329–3340. doi: 10.1242/jcs.113.18.3329. [DOI] [PubMed] [Google Scholar]
- 6.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol. 1993;61(1):44–53. [PubMed] [Google Scholar]
- 7.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Roche PA. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front Physiol. 2015;6:1. doi: 10.3389/fphys.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Pérez BE, Hernández-González JC, García-Nieto S, Luna-Herrera J. Internalization of a non-pathogenic mycobacteria by macropinocytosis in human alveolar epithelial A549 cells. Microb Pathog. 2008;45(1):1–6. doi: 10.1016/j.micpath.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Magzoub M, et al. N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochem Biophys Res Commun. 2006;348(2):379–385. doi: 10.1016/j.bbrc.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 11.Meier O, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158(6):1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanbo A, et al. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010;6(9):e1001121. doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watarai M, Makino S, Fujii Y, Okamoto K, Shirahata T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 2002;4(6):341–355. doi: 10.1046/j.1462-5822.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- 14.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10(4):364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 15.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5(11):424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 16.Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110(Pt 2):105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- 17.Aguado-Velasco C, Bretscher MS. Circulation of the plasma membrane in Dictyostelium. Mol Biol Cell. 1999;10(12):4419–4427. doi: 10.1091/mbc.10.12.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102(Pt 4):867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM, Brodie SE, Cohn ZA. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhaus EM, Soldati T. A myosin I is involved in membrane recycling from early endosomes. J Cell Biol. 2000;150(5):1013–1026. doi: 10.1083/jcb.150.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121(5):1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puthenveedu MA, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derivery E, et al. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17(5):712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17(5):699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duleh SN, Welch MD. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton (Hoboken) 2010;67(3):193–206. doi: 10.1002/cm.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia D, et al. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci USA. 2010;107(23):10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnell M, et al. Actin polymerization driven by WASH causes V-ATPase retrieval and vesicle neutralization before exocytosis. J Cell Biol. 2011;193(5):831–839. doi: 10.1083/jcb.201009119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zech T, et al. The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J Cell Sci. 2011;124(Pt 22):3753–3759. doi: 10.1242/jcs.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duleh SN, Welch MD. Regulation of integrin trafficking, cell adhesion, and cell migration by WASH and the Arp2/3 complex. Cytoskeleton (Hoboken) 2012;69(12):1047–1058. doi: 10.1002/cm.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King JS, et al. WASH is required for lysosomal recycling and efficient autophagic and phagocytic digestion. Mol Biol Cell. 2013;24(17):2714–2726. doi: 10.1091/mbc.E13-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temkin P, et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13(6):715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia D, Gomez TS, Billadeau DD, Rosen MK. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol Biol Cell. 2012;23(12):2352–2361. doi: 10.1091/mbc.E11-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotthardt D, et al. High-resolution dissection of phagosome maturation reveals distinct membrane trafficking phases. Mol Biol Cell. 2002;13(10):3508–3520. doi: 10.1091/mbc.E02-04-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuhaus EM, Soldati T. Molecular mechanisms of membrane trafficking. What do we learn from Dictyostelium discoideum? Protist. 1999;150(3):235–243. doi: 10.1016/S1434-4610(99)70026-X. [DOI] [PubMed] [Google Scholar]
- 35.Boulais J, et al. Molecular characterization of the evolution of phagosomes. Mol Syst Biol. 2010;6:423. doi: 10.1038/msb.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helfer E, et al. Endosomal recruitment of the WASH complex: Active sequences and mutations impairing interaction with the retromer. Biol Cell. 2013;105(5):191–207. doi: 10.1111/boc.201200038. [DOI] [PubMed] [Google Scholar]
- 37.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165(1):123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burd C, Cullen PJ. Retromer: A master conductor of endosome sorting. Cold Spring Harb Perspect Biol. 2014;6(2):a016774. doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165(1):111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JT, et al. The SNX-PX-BAR family in macropinocytosis: The regulation of macropinosome formation by SNX-PX-BAR proteins. PLoS One. 2010;5(10):e13763. doi: 10.1371/journal.pone.0013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim JP, Teasdale RD, Gleeson PA. SNX5 is essential for efficient macropinocytosis and antigen processing in primary macrophages. Biol Open. 2012;1(9):904–914. doi: 10.1242/bio.20122204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harbour ME, Breusegem SY, Seaman MN. Recruitment of the endosomal WASH complex is mediated by the extended ‘tail’ of Fam21 binding to the retromer protein Vps35. Biochem J. 2012;442(1):209–220. doi: 10.1042/BJ20111761. [DOI] [PubMed] [Google Scholar]
- 43.Park L, et al. Cyclical action of the WASH complex: FAM21 and capping protein drive WASH recycling, not initial recruitment. Dev Cell. 2013;24(2):169–181. doi: 10.1016/j.devcel.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Seaman MN, Gautreau A, Billadeau DD. Retromer-mediated endosomal protein sorting: all WASHed up! Trends Cell Biol. 2013;23(11):522–528. doi: 10.1016/j.tcb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piotrowski JT, Gomez TS, Schoon RA, Mangalam AK, Billadeau DD. WASH knockout T cells demonstrate defective receptor trafficking, proliferation, and effector function. Mol Cell Biol. 2013;33(5):958–973. doi: 10.1128/MCB.01288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charette SJ, Mercanti V, Letourneur F, Bennett N, Cosson P. A role for adaptor protein-3 complex in the organization of the endocytic pathway in Dictyostelium. Traffic. 2006;7(11):1528–1538. doi: 10.1111/j.1600-0854.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 47.Ravanel K, et al. Membrane sorting in the endocytic and phagocytic pathway of Dictyostelium discoideum. Eur J Cell Biol. 2001;80(12):754–764. doi: 10.1078/0171-9335-00215. [DOI] [PubMed] [Google Scholar]
- 48.Schneider N, et al. Golvesin-GFP fusions as distinct markers for Golgi and post-Golgi vesicles in Dictyostelium cells. Biol Cell. 2000;92(7):495–511. doi: 10.1016/s0248-4900(00)01102-3. [DOI] [PubMed] [Google Scholar]
- 49.Lefkir Y, et al. Involvement of the AP-1 adaptor complex in early steps of phagocytosis and macropinocytosis. Mol Biol Cell. 2004;15(2):861–869. doi: 10.1091/mbc.E03-06-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WL, Mason D, Schreiber AD, Grinstein S. Quantitative analysis of membrane remodeling at the phagocytic cup. Mol Biol Cell. 2007;18(8):2883–2892. doi: 10.1091/mbc.E06-05-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercanti V, et al. Selective membrane exclusion in phagocytic and macropinocytic cups. J Cell Sci. 2006;119(Pt 19):4079–4087. doi: 10.1242/jcs.03190. [DOI] [PubMed] [Google Scholar]
- 52.Cornillon S, et al. An adhesion molecule in free-living Dictyostelium amoebae with integrin beta features. EMBO Rep. 2006;7(6):617–621. doi: 10.1038/sj.embor.7400701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu Z, Noss EH, Hsu VW, Brenner MB. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J Cell Biol. 2011;193(1):61–70. doi: 10.1083/jcb.201007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gopaldass N, et al. Dynamin A, Myosin IB and Abp1 couple phagosome maturation to F-actin binding. Traffic. 2012;13(1):120–130. doi: 10.1111/j.1600-0854.2011.01296.x. [DOI] [PubMed] [Google Scholar]
- 55.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95(1):81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 56.Holliday LS, et al. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem. 2000;275(41):32331–32337. doi: 10.1074/jbc.M004795200. [DOI] [PubMed] [Google Scholar]
- 57.Vitavska O, Wieczorek H, Merzendorfer H. A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton. J Biol Chem. 2003;278(20):18499–18505. doi: 10.1074/jbc.M212844200. [DOI] [PubMed] [Google Scholar]
- 58.Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997;8(7):1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rojas R, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183(3):513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper AA, Stevens TH. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133(3):529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seaman MN. The retromer complex: endosomal protein recycling and beyond. J Cell Sci. 2012;125(Pt 20):4693–4702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol. 2012;197(2):219–230. doi: 10.1083/jcb.201111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen D, et al. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327(5970):1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- 64.Lim JP, Gleeson PA. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 65.Traynor D, Kay RR. Possible roles of the endocytic cycle in cell motility. J Cell Sci. 2007;120(Pt 14):2318–2327. doi: 10.1242/jcs.007732. [DOI] [PubMed] [Google Scholar]
- 66.Fey P, Dodson RJ, Basu S, Chisholm RL. One stop shop for everything Dictyostelium: DictyBase and the Dicty Stock Center in 2012. Methods Mol Biol. 2013;983:59–92. doi: 10.1007/978-1-62703-302-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veltman DM, Akar G, Bosgraaf L, Van Haastert PJ. A new set of small, extrachromosomal expression vectors for Dictyostelium discoideum. Plasmid. 2009;61(2):110–118. doi: 10.1016/j.plasmid.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Sattler N, Monroy R, Soldati T. Quantitative analysis of phagocytosis and phagosome maturation. Methods Mol Biol. 2013;983:383–402. doi: 10.1007/978-1-62703-302-2_21. [DOI] [PubMed] [Google Scholar]
- 69.Dieckmann R, Gopaldass N, Escalera C, Soldati T. Monitoring time-dependent maturation changes in purified phagosomes from Dictyostelium discoideum. Methods Mol Biol. 2008;445:327–337. doi: 10.1007/978-1-59745-157-4_21. [DOI] [PubMed] [Google Scholar]
- 70.Janssen KP, Rost R, Eichinger L, Schleicher M. Characterization of CD36/LIMPII homologues in Dictyostelium discoideum. J Biol Chem. 2001;276(42):38899–38910. doi: 10.1074/jbc.M103384200. [DOI] [PubMed] [Google Scholar]
- 71.Wienke DC, Knetsch ML, Neuhaus EM, Reedy MC, Manstein DJ. Disruption of a dynamin homologue affects endocytosis, organelle morphology, and cytokinesis in Dictyostelium discoideum. Mol Biol Cell. 1999;10(1):225–243. doi: 10.1091/mbc.10.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.King J, Insall RH. Parasexual genetics of Dictyostelium gene disruptions: Identification of a ras pathway using diploids. BMC Genet. 2003;4:12. doi: 10.1186/1471-2156-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rivero F, Maniak M. Quantitative and microscopic methods for studying the endocytic pathway. Methods Mol Biol. 2006;346:423–438. doi: 10.1385/1-59745-144-4:423. [DOI] [PubMed] [Google Scholar]
- 74.Veltman DM, Lemieux MG, Knecht DA, Insall RH. PIP₃-dependent macropinocytosis is incompatible with chemotaxis. J Cell Biol. 2014;204(4):497–505. doi: 10.1083/jcb.201309081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.King J, et al. Genetic control of lithium sensitivity and regulation of inositol biosynthetic genes. PLoS One. 2010;5(6):e11151. doi: 10.1371/journal.pone.0011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.