Significance
In all organisms, morphological and functional diversity is the product of cell type-specific genetic programs. Asymmetric cell division in Caulobacter yields daughter cells that differ functionally due to the differential read-out of their genomes. Here, we report the discovery of GapR, a conserved DNA-binding protein required for cell cycle progression. We show that GapR only associates with DNA sequences of high adenine and thymine (AT) content, colocating with cell cycle master regulators that control genes mediating swarmer cell development. GapR protein distributes asymmetrically, accumulating on the compacted chromosome of the daughter swarmer cell compartment prior to division. We argue that Caulobacter has co-opted a protein that associates with AT-rich DNA to provide spatial control during an asymmetric cell division.
Keywords: Caulobacter, nucleoid-associated protein, asymmetry, AT-rich, cell cycle
Abstract
Faithful cell cycle progression in the dimorphic bacterium Caulobacter crescentus requires spatiotemporal regulation of gene expression and cell pole differentiation. We discovered an essential DNA-associated protein, GapR, that is required for Caulobacter growth and asymmetric division. GapR interacts with adenine and thymine (AT)-rich chromosomal loci, associates with the promoter regions of cell cycle-regulated genes, and shares hundreds of recognition sites in common with known master regulators of cell cycle-dependent gene expression. GapR target loci are especially enriched in binding sites for the transcription factors GcrA and CtrA and overlap with nearly all of the binding sites for MucR1, a regulator that controls the establishment of swarmer cell fate. Despite constitutive synthesis, GapR accumulates preferentially in the swarmer compartment of the predivisional cell. Homologs of GapR, which are ubiquitous among the α-proteobacteria and are encoded on multiple bacteriophage genomes, also accumulate in the predivisional cell swarmer compartment when expressed in Caulobacter. The Escherichia coli nucleoid-associated protein H-NS, like GapR, selectively associates with AT-rich DNA, yet it does not localize preferentially to the swarmer compartment when expressed exogenously in Caulobacter, suggesting that recognition of AT-rich DNA is not sufficient for the asymmetric accumulation of GapR. Further, GapR does not silence the expression of H-NS target genes when expressed in E. coli, suggesting that GapR and H-NS have distinct functions. We propose that Caulobacter has co-opted a nucleoid-associated protein with high AT recognition to serve as a mediator of cell cycle progression.
The organization of chromosomal DNA in prokaryotes, which lack structural equivalents of histones, is attributed to a diverse family of nucleoid-associated proteins (NAPs) (1). Although a growing body of evidence points to a physical chromosome that is ordered into regular, structured domains in bacteria (2–6), very little is known about the establishment and dynamics of these domains or the mechanism(s) by which NAPs integrate chromosome topology and gene regulation.
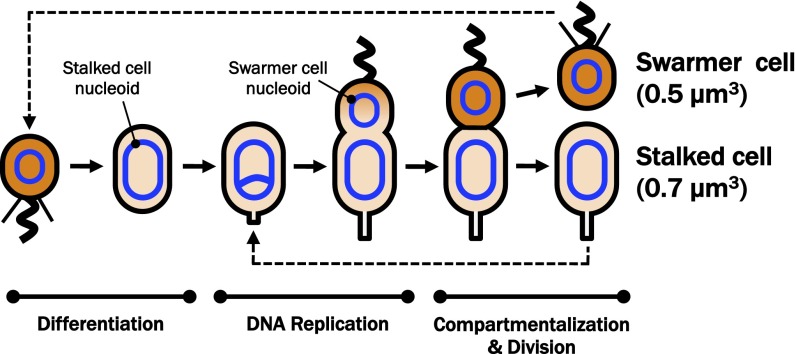
The topological problem of gene expression is compounded by the existence of cell type-specific genetic programs that underlie differentiation and specialization. The bacterium Caulobacter crescentus uses cell type-specific genetic programs to regulate growth, differentiation, and cell cycle progression (7). During each round of the cell cycle, Caulobacter divides asymmetrically to yield a stalked cell, which immediately initiates a new round of DNA replication and cell cycle progression, and a motile swarmer cell, which does not reinitiate the cell cycle until it differentiates into a stalked cell (Fig. 1). Each morphotype expresses a unique complement of genes that encode cell type-specific functions (e.g., motility, chemotaxis, encapsulation, or chromosome replication), the regulation of which can be attributed, at least in part, to an ensemble of interdependent, spatiotemporally restricted global transcriptional regulators that are organized into a highly robust control circuit (7–15). However, the control of more than 40% of cell cycle-regulated promoters cannot be accounted for by these master regulators (14), implying the existence of additional regulatory proteins that influence development through interaction(s) with the Caulobacter genome. Such factors could include NAPs, which can directly or indirectly modulate gene regulation (1). NAPs with broad roles in the regulation of chromosome dynamics and condensation (e.g., HU, IHF, and SMC) have been identified in Caulobacter (4, 16–18).
Fig. 1.
The C. crescentus cell cycle. Caulobacter exists as one of two independent morphotypes, the swarmer cell and stalked cell, which are differently sized and which have distinct polar appendages. The swarmer cell is unable to initiate chromosome replication and does not grow or divide. Following differentiation, the stalked cell initiates DNA replication and segregation of DNA. As the cell cycle progresses, an asymmetric predivisional cell arises that elaborates a flagellum at the nascent swarmer cell pole, which forms opposite the stalked pole. Completion of chromosome segregation is followed by the compartmentalization of a small swarmer and large stalked cell cytoplasmic space and ultimately by complete cytokinesis. Each asymmetric division event yields two cells with identical genomes but significantly different cytoplasmic volumes into which those genomes must be packaged.
We report the discovery of GapR, a NAP that globally interacts with the GC-rich Caulobacter genome at high adenine and thymine (AT) loci. We demonstrate through ChIP-seq analysis that GapR associates with promoters of cell cycle-controlled genes bound by master transcriptional regulators, including more than 90% of sites bound by MucR1 (12), a recently identified transcription factor that directs the establishment of swarmer cell identity. The depletion or overexpression of GapR (growth-associated A/T-binding protein involved in regulation) causes pleiotropic defects in cell growth and division. We provide evidence for the asymmetric accumulation of GapR in the swarmer compartment of predivisional cells despite constitutive GapR synthesis across the cell cycle, implying the presence of a posttranslational mechanism for asymmetric distribution of this essential DNA-associated protein. Conversely, the histone-like nucleoid structuring protein from Escherichia coli (H-NS), which also exhibits affinity for regions of high AT (19), does not localize preferentially to the swarmer compartment of predivisional Caulobacter cells. H-NS is also functionally distinct from GapR; whereas H-NS is dispensable and silences promoters of high AT content in E. coli, GapR associates with expressed genes and is essential for Caulobacter viability. The work presented here establishes a link between the recognition of chromosomal sites of high AT content and swarmer cell-specific functions through an essential NAP and suggests that GapR function may impact the establishment and/or maintenance of cell type-specific regulatory programs controlling Caulobacter cell cycle progression.
Results
Identification of Putative Essential DNA-Associated Proteins in Caulobacter.
Top-level control of the Caulobacter cell cycle is achieved through the coordinated activity of transcription factors that temporally restrict the expression of genes with cell type-specific functions (20, 21). To identify additional factors that have an essential role in cell cycle control, we mined the list of essential genes identified by global Tn-seq analysis of the Caulobacter genome (22) and characterized those proteins predicted to have DNA-binding activity. We used a combination of primary-sequence, secondary-structure, and domain-based annotation algorithms (SI Materials and Methods) to generate functional predictions for the encoded products. This analysis yielded a candidate list of putative essential DNA-binding proteins not previously identified in Caulobacter. Here, we describe the characterization of one of these candidates, CCNA_03428 (hereafter referred to as GapR), a small (89-residue) protein comprising a single domain of unknown function.
GapR has little sequence similarity to characterized proteins. The nearest characterized homologs are the DNA-binding transcriptional regulator DsbA from bacteriophage T4, the prokaryotic MerR-family transcriptional regulators, the nucleic-acid-binding arm of valyl-tRNA synthetase, and the coiled-coil domain of the eukaryotic intermediate filament protein vimentin (Table S1). Although the functions of these weak homologs imply DNA-binding activity, the function of GapR cannot be predicted from primary sequence alone given the paucity of high-confidence homologs of known function.
Table S1.
Pfam domain homologs of GapR
| Domain ID | Function | P value |
| PF10073 | DUF2312 (domain of unknown function) | 2 × 10−47 |
| PF11126 | Transcriptional regulator DsbA (from bacteriophage T4) | 1.2 × 10−4 |
| PF10458 | Nucleic acid-binding domain of valyl-tRNA synthetase | 1.5 × 10−4 |
| PF00038 | Vimentin (eukaryotic intermediate filament protein) | 4.3 × 10−4 |
| PF09278 | MerR-like transcriptional regulator | 5.3 × 10−4 |
| PF12325 | TATA modulatory factor 1 (binds eukaryotic TATA box) | 1 × 10−3 |
GapR Is Essential for Normal Growth and Cell Division in Caulobacter.
We attempted to delete the gapR gene from the chromosome to test the predicted essentiality of gapR and to determine the consequences of its dysregulation, but were unable to isolate a stable ∆gapR strain. This finding is consistent with the prediction that gapR is an essential gene in Caulobacter (22). To determine whether the inability to construct a ∆gapR strain is indeed due to the essentiality of GapR, we attempted to construct a gapR depletion strain using standard techniques but found that gapR expression from either the PxylX promoter or the PvanA promoter did not restore a WT phenotype to a ∆gapR strain, indicating that gapR transcription needs to be precisely controlled for optimal fitness (SI Text, Viable ∆gapR Mutants of Caulobacter NA1000 Cannot Be Isolated).
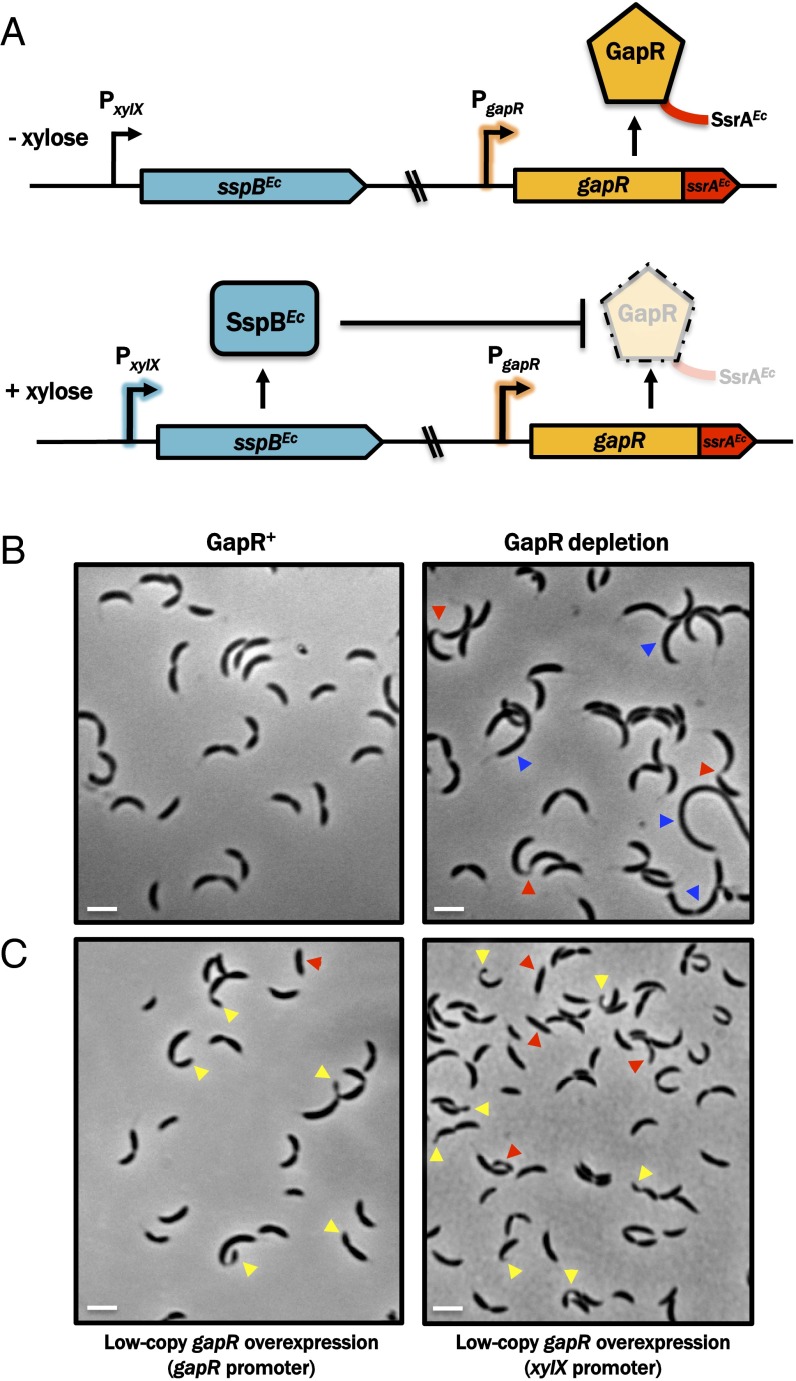
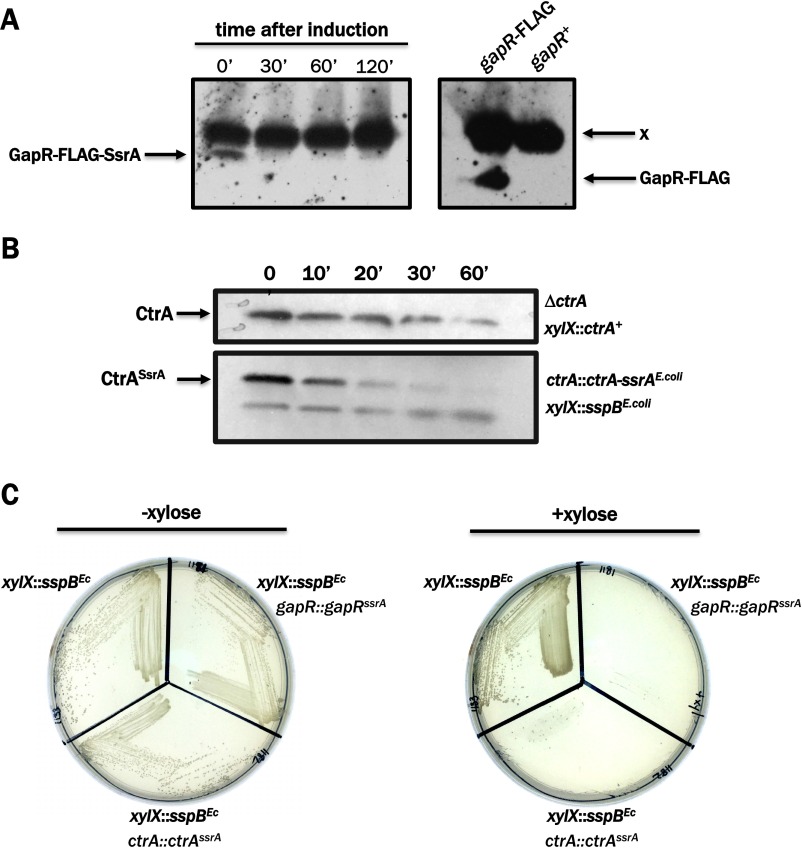
To circumvent this problem, we constructed a strain in which gapR is expressed at native levels but GapR protein abundance is dynamically controlled by inducible regulated proteolysis. This regulation was accomplished by exploiting the species-specific activity of the E. coli SspB adapter protein, which targets mutant derivatives of C-terminally ssrA-tagged proteins for degradation in a highly specific manner (23). By tagging the native gapR allele in frame with a sequence encoding an E. coli SspB-dependent ssrA tag (-AANDENYSENYADAS) and placing the sspB gene from E. coli under control of the xylose-inducible PxylX promoter, we generated a Caulobacter strain in which GapR is conditionally degraded by growing cells in the presence of xylose (Fig. 2A). This system enables the efficient clearance of GapR (Fig. S1A) and offers improved depletion kinetics compared with conventional methods involving inducible heterologous promoters (Fig. S1B) while preserving native transcriptional and translational regulation. The GapR “proteolytic depletion” strain grows in the absence of xylose (i.e., when expression of sspBEcoli is silenced), but does not grow on media containing xylose, suggesting that SspB-dependent proteolysis of GapR is sufficient to cause lethality (Fig. S1C). Because the expression of sspBEcoli in Caulobacter has no effect on viability (Fig. S1C), we conclude that gapR is an essential gene.
Fig. 2.
Depletion and overexpression of GapR cause morphological defects. (A) Schematic of the E. coli SsrA/SspB-based inducible proteolysis system co-opted for the specific degradation of GapR in Caulobacter, which is tagged with an SspB-dependent E. coli SsrA tag (SsrAEc) and expressed at the native genomic locus to preserve native levels and regulation. In the absence of xylose, the xylX promoter is inactive and sspB is not expressed. Addition of xylose leads to production of E. coli SspB (SspBEc), which promotes ClpXP-dependent proteolysis of SsrAEc-tagged GapR. (B) The GapR proteolytic depletion strain described in A was grown to midexponential phase in M2G and then propagated for an additional 2 h in the absence (permissive condition; Left) or presence (depletion condition; Right) of 0.3% (wt/vol) xylose. GapR-depleted cells exhibit incomplete separation (red arrowheads) and filamentation (blue arrowheads). (Scale bar, 1 μm.) (C) gapR was overexpressed in WT Caulobacter NA1000 on low-copy plasmids constitutively from the native promoter (Left) or for 4 h from the xylose-inducible xylX promoter [0.3% (wt/vol) xylose; Right]. GapR overexpression leads to morphological defects (red arrowheads) and aberrant division events (yellow arrowheads). (Scale bar, 1 μm.)
Fig. S1.
The E. coli SsrA/SspB system promotes efficient clearance of tagged proteins in Caulobacter. (A) A Caulobacter strain carrying xylose-inducible sspBEc and expressing SsrA-tagged GapR-FLAG was grown to midexponential phase in M2G and then grown in the presence of 0.3% xylose with samples collected for SDS/PAGE at 30, 60, and 120 min following the initiation of depletion. Western blotting was performed using α-FLAG polyclonal antisera. “x” indicates an endogenous protein that cross-reacts with the antisera used and represents a convenient loading control. (B) Kinetic comparison of a conventional gene depletion method with the SsrA/SspB proteolytic depletion method. A Caulobacter strain expressing the sole allele of ctrA from the xylX promoter (Top) was grown to midexponential phase in M2+xylose (permissive condition), washed and resuspended in M2G (depletion condition), and then incubated for 60 min. Additionally, a Caulobacter strain carrying xylose-inducible sspBEc and expressing SsrA-tagged CtrA from the native ctrA promoter (Bottom) was grown to midexponential phase in M2G (permissive condition) and then incubated for 60 min following the addition of 0.3% xylose (depletion condition). In both cases, samples were collected for SDS/PAGE at 10, 20, 30, and 60 min following the initiation of depletion, and Western blotting was performed using α-CtrA antisera. (C) Caulobacter strains carrying xylose-inducible sspBEc and expressing SsrA-tagged GapR, SsrA-tagged CtrA, or no tagged protein were grown in rich media (PYE) in the absence (Left) or presence (Right) of 0.3% xylose.
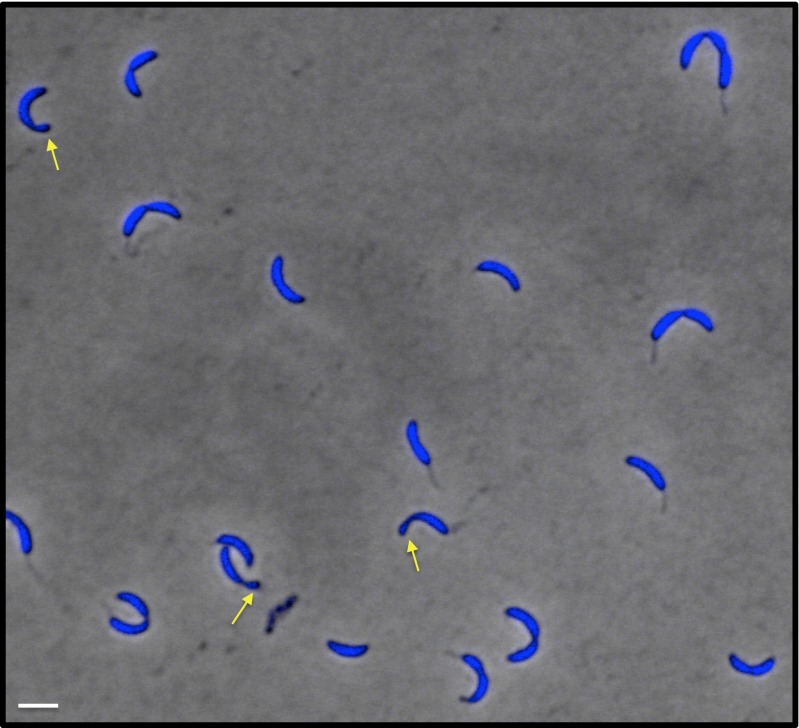
Using our proteolytic depletion strain, we determined the physiological consequences of induced GapR elimination. A culture of the GapR proteolytic depletion strain was grown to midexponential phase, incubated for 4 h in the presence or absence of xylose, and examined microscopically. Although cells grown in the absence of xylose (in which GapR was present) displayed normal shapes and sizes, cells grown in GapR-depleting conditions (i.e., + xylose) exhibited a variety of growth defects. Specifically, some cells became filamentous and formed conjoined vermiform minicell daughters that never separated from the mother (18% of 141 cells; Fig. 2B). Fluorescence microscopy of GapR-depleted cells stained with DAPI confirmed that these minicells contain DNA (Fig. S2). These phenotypic abnormalities indicate that GapR is critical for normal growth and cell division.
Fig. S2.
Polar minicells induced by GapR depletion contain DNA. The GapR proteolytic depletion strain was grown to midexponential phase in M2G, propagated for 4 h in the presence of 0.3% xylose, stained with DAPI, and imaged by phase-contrast and epifluorescence microscopy. Small minicells periodically observed at the new poles GapR-depleted cells exhibit incomplete separation (yellow arrows). (Scale bar, 1 μm.)
To determine whether gapR overexpression compromises Caulobacter growth, we expressed gapR from low-copy-number (pRMCS) or high-copy-number (pBMCS) replicating vectors under control of the native promoter (PgapR). gapR+ merodiploid strains carrying low-copy PgapR-gapR could be constructed, but these strains formed small, slow-growing colonies. Viable strains carrying high-copy pBMCS::PxylX-gapR or pBMCS::PgapR-gapR constructs could not be obtained, indicating that GapR causes lethality Caulobacter when constitutively expressed at high levels.
We examined cells in which gapR was overexpressed from a low-copy-number plasmid either from its native promoter or an inducible PxylX promoter (Fig. 2C). We found pleiotropic defects in Caulobacter cell cycle progression that included filamentation, morphological defects, and the formation of polar minicells (8% of 546 cells display polar minicells). These data demonstrate the requirement for tight regulation of GapR concentration in Caulobacter and suggest that GapR influences an array of critical cellular processes related to growth, division, and cell cycle progression.
GapR Globally Binds the Caulobacter Chromosome at AT-Rich Loci.
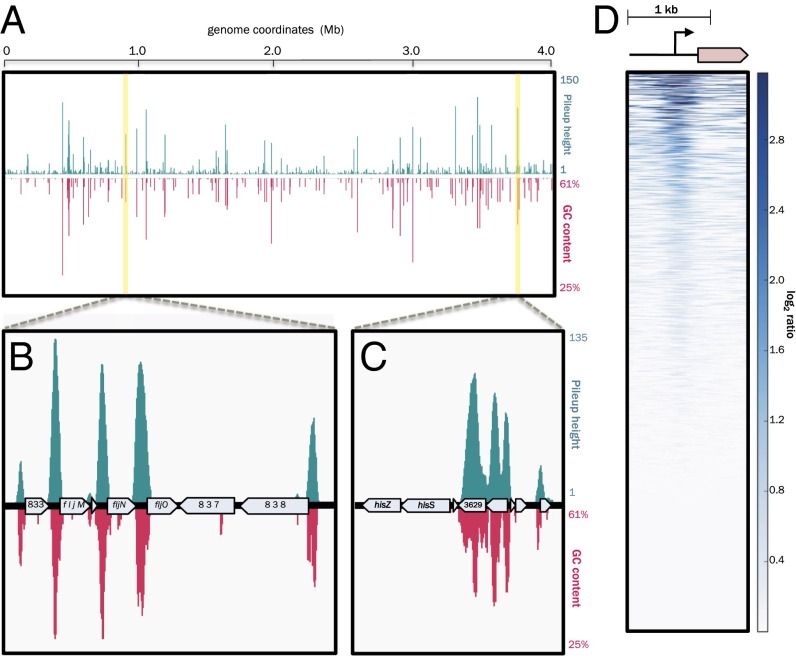
Given the putative DNA-binding activity implied by our bioinformatic analysis of GapR, we wished to determine whether GapR associates with DNA and to simultaneously identify all potential sites of GapR occupancy in vivo through chromatin immunoprecipitation coupled to deep sequencing (ChIP-seq). To ensure selective and sensitive immunoprecipitation of GapR, we generated strains in which gapR is replaced at the native locus with an allele encoding an N- or C-terminally FLAG-tagged variant (FLAG-GapR or GapR-FLAG, respectively). Each of the resulting strains grew normally, indicating that the addition of an epitope tag to either terminus of GapR does not compromise its function. These strains, along with a WT control strain expressing untagged GapR, were grown to midexponential phase, incubated briefly with a crosslinking agent, and subjected to chromatin immunoprecipitation (ChIP) using an IP-grade α-FLAG antibody. GapR-bound DNA was purified and subjected to deep sequencing. As a control, we performed ChIP-seq on a strain expressing FLAG-tagged CtrA, encoded at its native locus (Dataset S1), and compared our CtrA ChIP-seq profile with that obtained previously using custom antisera specific for CtrA (12), which showed good concordance. The ChIP-seq profiles of the FLAG-GapR and GapR-FLAG strains were strongly correlated, with somewhat higher signal observed in the FLAG-GapR sample. ChIP-seq profiling revealed broad GapR occupancy of the Caulobacter chromosome (Fig. 3A and Dataset S2), with 599 peaks above background (q-value threshold = 0.0001; SI Materials and Methods). Although the Caulobacter genome is 91.6% genic, we predominantly observed GapR ChIP-seq peak summits in intergenic regions of the chromosome, particularly among the most significant peaks: 76.5% of the top 200 GapR peak summits were located within intergenic regions, demonstrating a clear propensity for GapR to bind between, rather than within, genes.
Fig. 3.
GapR binds globally to AT-rich regions of the Caulobacter genome. (A) Genome-wide FLAG-GapR ChIP-seq profile (blue peaks) with read counts normalized to reads per kilobase per million (RPKM) plotted against local GC content (magenta peaks) as calculated using a 100-bp sliding window across the NA1000 genome. Regions highlighted in yellow are featured in greater detail to reveal intergenic (B) and intragenic (C) GapR ChIP-seq enrichment. Seventy-six percent of the top 200 GapR ChIP-seq peak summits lie within intergenic regions. (D) Enrichment of GapR peaks in promoter regions. Each row in the heatmap represents a Caulobacter ORF (stretched or compressed to 750 bp and oriented as shown in the gene cartoon above the plot such that the start codon occupies the same position in each row), as well as the 1,000 bp preceding the translation start site of each ORF (not stretched or compressed). Rows were subdivided into 10-bp bins, with each bin colored to reflect the degree of GapR ChIP-seq signal enrichment (bluer color = greater GapR enrichment). Finally, rows were sorted by maximum bin value (log2 of GapR ChIP-seq signal enrichment) from highest (Top) to lowest (Bottom). Heatmaps were generated using deepTools (SI Materials and Methods).
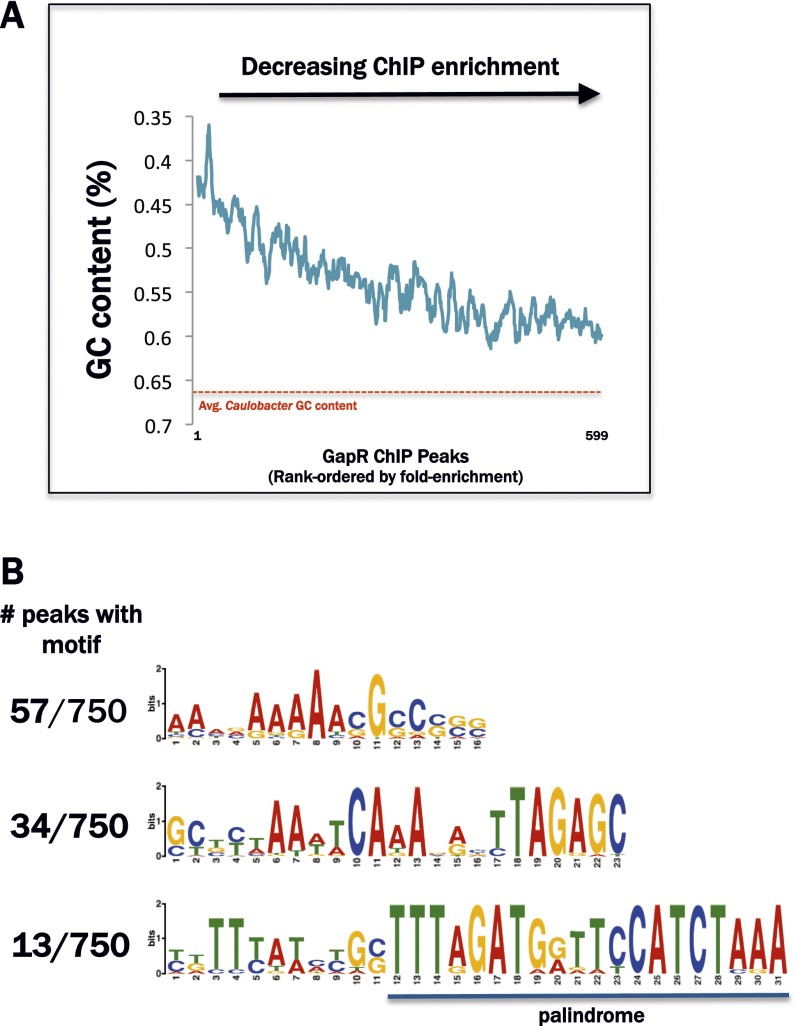
The Caulobacter genome exhibits a marked nucleotide bias with an overall GC content of ∼67% (24). However, intergenic regions of the otherwise GC-rich genome are punctuated frequently by local spikes in AT content that can reach 75% A/T. To determine whether GapR binding is correlated with a local bias in nucleotide content, we calculated the local GC content across the Caulobacter genome using sliding window analysis and measured the correlation between GapR occupancy and AT content. We indeed observed a striking positive correlation between GapR ChIP-seq signal strength and local AT content (Fig. 3 A–C and Fig. S3A), with an average GC content of only 45% among sequences comprising the top 100 GapR peaks.
Fig. S3.
GapR associates nonspecifically with AT-rich loci in the Caulobacter genome. (A) Line graph of GC content surrounding GapR ChIP-seq peak summits. All called GapR ChIP-seq peaks (q-value < 0.0001) were rank ordered by relative enrichment above background and sliding window averages in GC content (10-bp window size) of the 100-bp region centered on each corresponding peak summit were plotted. The red dashed line indicates the average GC content of the Caulobacter genome. (B) Position weight matrices (PWMs) of top hits from MEME-based motif analysis of GapR ChIP-seq peaks. For each PWM, a fraction is given that corresponds to the number of GapR peaks containing at least one occurrence of the associated motif.
To determine whether the AT-richness of GapR-associated sites reflects an AT-rich consensus motif or nonspecific affinity for high AT DNA, we mined the sequences under GapR peaks for a common motif that could represent a consensus sequence for GapR binding (25). We were unable to identify any sequence motif(s) common to more than 10% of the GapR-bound regions (Fig. S3B). This result, in light of the correlation between GapR ChIP-seq enrichment and local AT richness, suggests that the association of GapR with DNA is specified by local AT content rather than by an explicit consensus binding sequence. Indeed, DNA-associated proteins with generic affinity for AT-rich sequences have been described in various prokaryotic and eukaryotic organisms, including mammals (26–32).
GapR Associates with Promoters That Are Active During Normal Growth and Differentiation.
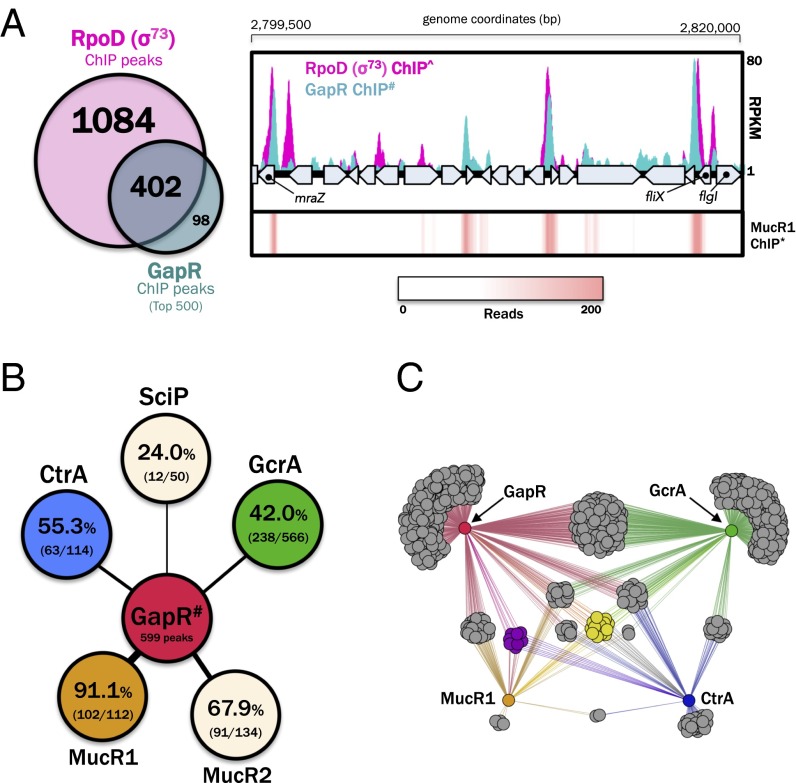
Given its association with intergenic loci, we next looked for the intersection of GapR-bound loci with annotated genomic features (i.e., genes and promoters). We found that GapR primarily associates with the 5′ regulatory regions of genes, as indicated by the clustering of GapR peaks within a 200-bp window preceding annotated Caulobacter ORFs (Fig. 3D). To determine whether GapR associates with promoters that are normally active during growth, we measured overlap between the GapR ChIP-seq profile with that of the housekeeping sigma factor in Caulobacter (σ73, i.e., RpoD) trapped on DNA in initiation complexes with target promoters as a consequence of rifampicin treatment (33). We observed a correlation between GapR and RpoD occupancy, with 80.4% of the top 500 GapR peaks overlapping an RpoD peak (Fig. 4A), indicating that GapR primarily associates with active promoters.
Fig. 4.
GapR binds at active promoters controlled by master regulators of cell cycle progression. (A) Overlap between the top 500 FLAG-GapR ChIP-seq peaks and all RpoD ChIP-seq peaks (Left), with a specific genomic region featured (Right) to indicate the presence of shared binding sites between RpoD, GapR, and MucR1 (RpoD ChIP data obtained from ref. 33; MucR1 ChIP-seq data from ref. 12). MucR1 ChIP-seq signal is presented as a heatmap of piled reads. (B) ChIP-seq peak overlap between GapR and master transcriptional regulators bound to promoters of cell cycle-regulated genes. Percentages in the colored circles represent the proportion of ChIP-seq peaks for the indicated master regulator (e.g., CtrA and SciP) that intersect a GapR ChIP-seq peak (median peak width = 400 bp), with each fraction expressing the number of peaks that intersect a GapR binding site out of the total number of peaks identified for that TF. The length and thickness of the lines connecting the shapes reflect the degree of overlap in occupancy between GapR and the master TFs shown. MucR1/2 and SciP ChIP-seq data and peak calls were obtained from ref. 12; GcrA ChIP-seq data were obtained from ref. 33 and peaks were called using the same workflow used for GapR/CtrA (see SI Materials and Methods for details). (C) Network diagram of intergenic regions (gray, purple, and yellow nodes) associated with different proteins (red, green, blue, and orange nodes). Connections (lines) indicate the presence of a ChIP-seq peak connecting an intergenic region to one or more of the proteins. Intergenic regions are clustered with others that share the same combination of connections to given proteins. Three-way intersection of the three proteins MucR1, CtrA, and GapR is represented by two clusters of intergenic regions: the purple cluster (no GcrA peak associated) and the yellow cluster (GcrA peak also associated). The total number of observed three-way intersections in intergenic regions is greater than expected (under the assumption that ChIP-seq peaks of these three proteins are independently distributed over the chromosome; 21 observed vs. 11.2 expected; SI Materials and Methods). Genes associated with all of the chromosomal GapR-CtrA-MucR1 overlapping sites are listed in Table S2.
GapR ChIP-seq peaks were observed near an abundance of genes whose expression is known to vary over the course of the cell cycle. For example, GapR ChIP-seq signal is enriched at promoters for genes that encode structural proteins and regulators of the swarmer-specific polar appendages (e.g., pilA, cpaA, flaFN, fljKLMNO, tipF), a swarmer-specific protease (perP), a cell type-specific encapsulation system (hvyA, pssZ, CCNA_00162-168, CCNA_00465-472) (11), components of the divisome (ftsZ), and the replisome (dnaA, dnaE), as well as cell cycle signaling factors (e.g., cckA, podJ, pleA) and master regulators of the cell cycle (ctrA, sciP, dnaA, gcrA). Because the expression of each of these genes varies as a function of the cell cycle, we used transcriptomic data obtained from synchronous Caulobacter populations throughout the cell cycle to determine whether GapR-bound promoters commonly exhibit cell cycle-dependent activity. Specifically, we identified the set of Caulobacter genes defined as cell cycle-regulated by two independent groups (14, 34) and determined how frequently the transcription start sites (TSSs) for these genes are bound by GapR (based on ChIP-seq data, from mixed population). We found that GapR peaks overlap nearly one-third of all cell cycle-regulated promoters (31.6%, Pearson’s χ2 test, P < 0.01; Dataset S3), although cell cycle-regulated promoters are not, in general, more AT-rich than noncell cycle-regulated promoters (SI Text, Cell Cycle-Regulated Promoters Are Not Especially AT-Rich). These findings further suggesting a physiological connection between GapR and the regulation of cell cycle progression.
Many cell cycle-regulated genes are subject to combinatorial control by two or more master regulators of cell cycle progression (14). To determine whether GapR binds promoters that are also occupied by known master regulators, we compared the GapR genome occupancy profile with that of transcriptional regulators known to modulate target gene expression in a cell cycle-dependent manner. We observed overlap between GapR occupancy and ChIP-seq peaks corresponding to each of the known master regulators and found statistically significant pairwise overlap in occupancy between GapR and each of the regulators CtrA, MucR1, MucR2, and GcrA (Pearson’s χ2 test, P < 2.2 × 10−16; SI Materials and Methods, Fig. 4 B and C, and Fig. S4A). The intersection between GapR and MucR1 ChIP-seq peaks is particularly noteworthy, as 91% of all MucR1 peaks overlap a GapR peak (102/112; Fig. 4B).
Fig. S4.
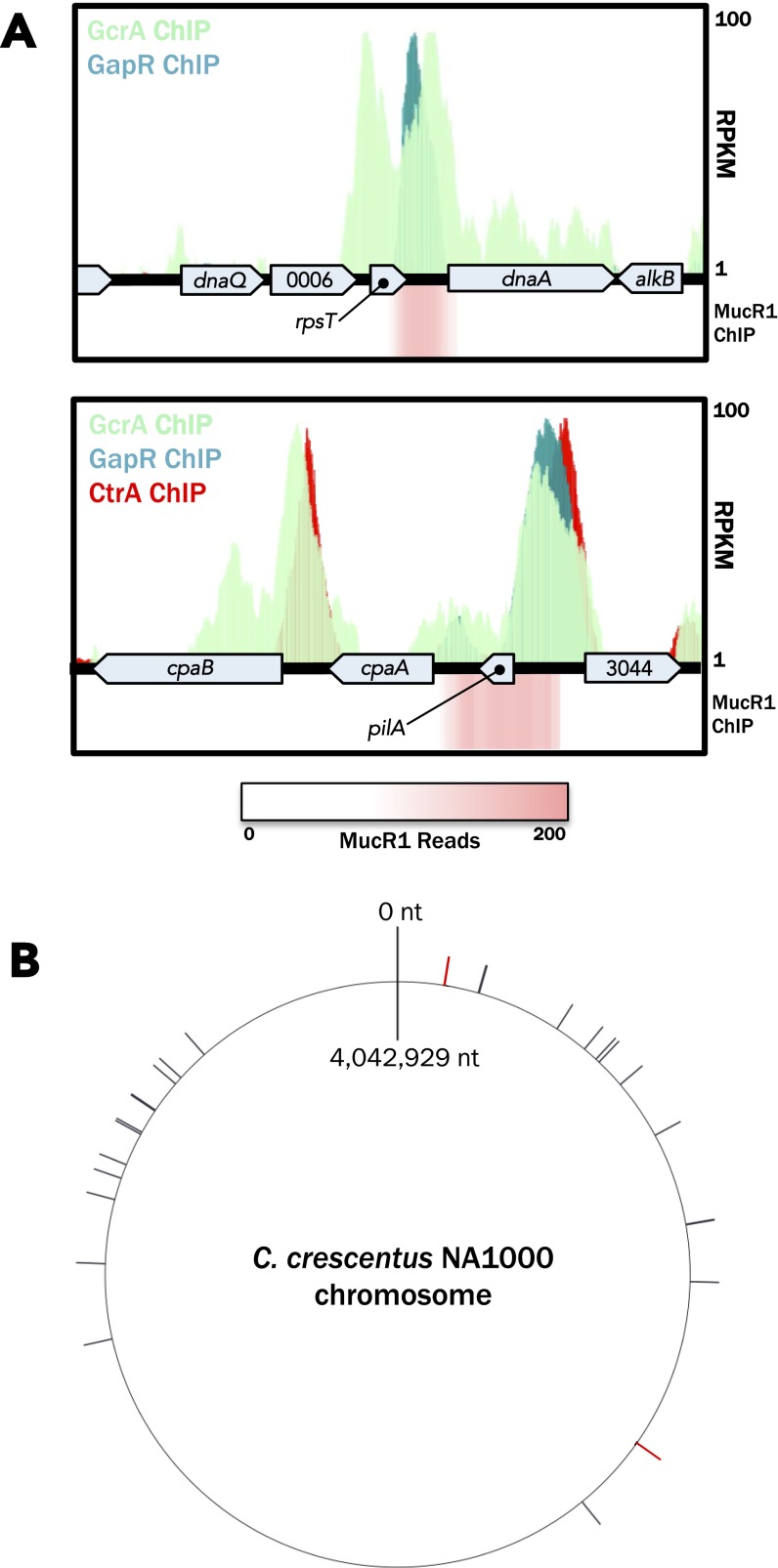
Overlap between the GapR ChIP-seq peaks and master regulator ChIP-seq peaks. (A) Specific genomic regions are shown to indicate the presence of shared binding sites between GapR, CtrA, and GcrA. MucR1 ChIP-seq signal is presented as a heatmap of piled reads. (B) A circular map of the Caulobacter NA1000 genome indicating the positions of all overlapping MucR1 and CtrA ChIP-seq peaks (tick marks). All but two of these sites (colored in red) also overlap with a GapR ChIP-seq peak. Nearly all of these sites lie in the origin-proximal half of the chromosome. The genes associated with these binding sites are largely swarmer specific in timing of expression or function, in agreement with the hypothesis that MucR1 represses swarmer-specific CtrA-regulated genes in S-phase (12) (Table S2).
CtrA and MucR1 together control the S → G1 transition by regulating the expression of many swarmer-specific genes (12). Although sites bound independently by either CtrA or MucR1 are distributed throughout the Caulobacter chromosome, those sites bound by both CtrA and MucR1 are almost entirely found in the origin-proximal half of the chromosome (binomial test, P < 0.01 for MucR1, P < 0.05 for CtrA; SI Materials and Methods). Further, GapR ChIP-seq peaks overlap a vast majority of these sites (24/26, 92.3%; Fig. S4B and Table S2). Overall, the overlap in occupancy of cell cycle-regulated promoters suggests that GapR is connected to the master regulatory circuit that drives cell cycle progression and cell type specification.
Table S2.
Caulobacter genes adjacent to sites associated with overlapping GapR, MucR1, and CtrA ChIP-seq peaks
 |
Minor Changes in Transcript Abundances Are Observed Following GapR Depletion or Overexpression.
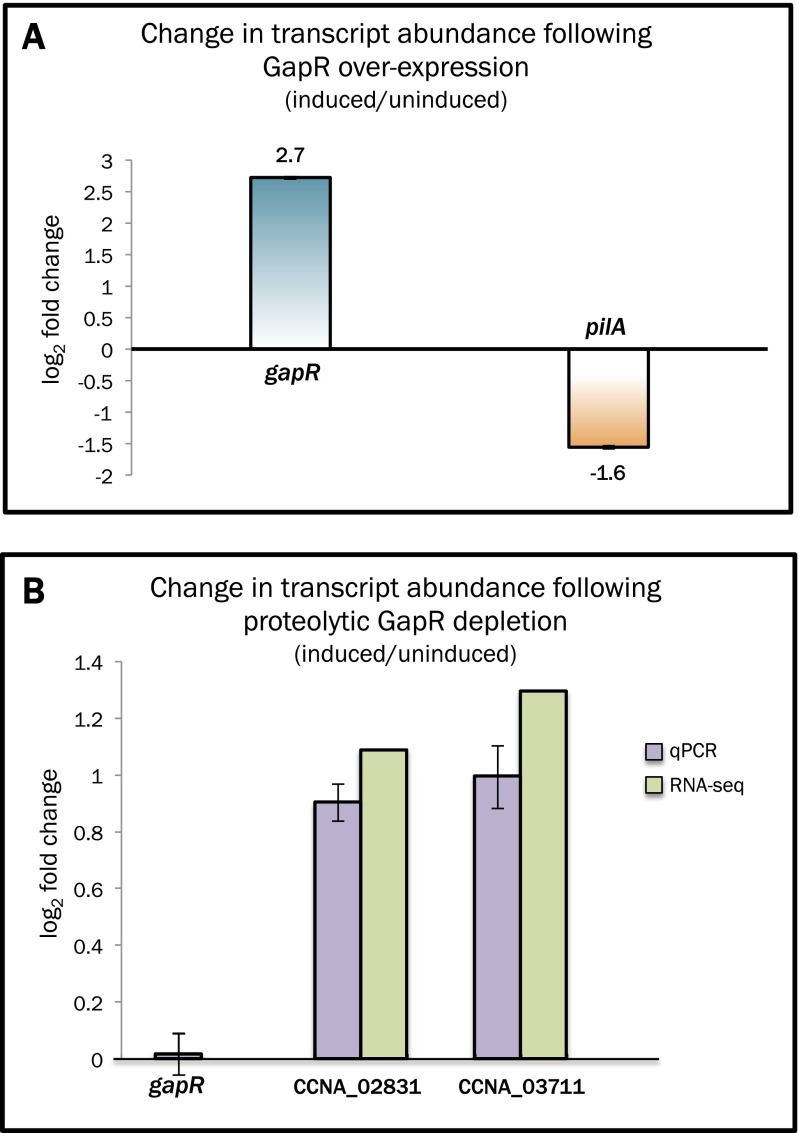
GapR exhibits widespread association with promoters and overlapping occupancy with known master transcriptional regulators; this is consistent with a role for this protein in regulating gene expression. To determine whether GapR is involved in transcriptional control, we selected individual genes whose promoters are occupied by GapR and quantitatively assessed transcript abundance following GapR overexpression or depletion. We found that overexpression (Fig. S5A) or depletion (Fig. S5B) of GapR leads to at least a twofold decrease or increase in expression, respectively, of candidate GapR target genes evaluated (pilA, CCNA_02831, CCNA_03711). To determine the global regulatory response to GapR perturbation, if any, we performed mRNA-seq following proteolytic clearance of GapR and conducted differential gene expression analysis compared with a GapR-replete strain to identify any GapR-dependent differences in transcription. In general, there are relatively few significant global changes in gene expression, with <1% of transcripts displaying differential expression following GapR depletion (Dataset S4). We identified 18 genes that that are differentially expressed on proteolytic depletion of GapR (Table S3); in 72% of these cases, the promoters of these genes overlap a GapR ChIP-seq peak. We also identified two genes that are differentially expressed up on mock depletion (in the gapR+ mock depletion strain), but whose abundance changes in opposite directions between mock and true GapR depletion (Table S4). Additionally, we found that the vast majority of genes associated with GapR ChIP-seq peaks do not exhibit a greater than twofold change in expression following GapR depletion, suggesting that transcriptional regulation is an indirect or secondary function of GapR.
Fig. S5.
GapR modulation influences expression of GapR-bound genes. (A) Bar graph showing the fold change (log2) in expression of the gapR and pilA from in a strain of Caulobacter where the PgapR promoter on a low-copy replicating plasmid promotes constitutive expression of gapR (pRMCS::gapR), as determined by qRT-PCR. (B) Bar graph showing the fold change (log2) in expression of the indicated GapR-bound genes following induction of GapR proteolysis (1 h) as determined by qRT-PCR and RNA-seq.
Table S3.
C. crescentus transcripts differentially expressed following GapR depletion
| Gene | Fold change (log2) | Gene product | GapR ChIP-seq peak in promoter? |
| vanB | 2.31 | Vanillate demethylase oxidoreductase subunit VanB | Yes |
| CCNA_01844 | 1.70 | DNA integration/recombination/inversion protein | Yes |
| CCNA_01605 | 1.44 | NADPH-dependent FMN reductase family protein | No |
| CCNA_03487 | 1.31 | Xonular occludens toxin, zot-like protein | Yes |
| CCNA_03711 | 1.30 | Ribosome-associated factor Y | Yes |
| CCNA_03364 | 1.27 | Conserved hypothetical protein | No |
| CCNA_01932 | 1.18 | 1-Acyl-sn-glycerol-3-phosphate acyltransferase | No |
| CCNA_03820 | 1.14 | Outer-membrane lipoproteins carrier protein | Yes |
| CCNA_02831 | 1.09 | Conserved hypothetical protein | Yes |
| ibpA | 1.07 | Inositol ABC transporter, periplasmic inositol-binding protein IbpA | Yes |
| CCNA_03175 | 1.06 | Conserved hypothetical protein | No |
| CCNA_03194 | 1.05 | Conserved inner membrane protein | Yes |
| CCNA_00788 | 1.04 | Transporter, major facilitator superfamily | Yes |
| CCNA_02158 | 1.03 | GT2 family glycosyltransferase | Yes |
| CCNA_02115 | 1.03 | Secreted pectate lyase-family protein | Yes |
| CCNA_02523 | −1.02 | Hypothetical protein | Yes |
| stpC | −1.17 | Stalk cross-band protein StpC | Yes |
| CCNA_02362 | −1.20 | Hypothetical protein | No |
Table S4.
Two C. crescentus transcripts that are both differentially expressed but change in different directions following GapR depletion vs. mock depletion
| Gene | Gene product | Fold change (log2) | GapR-associated (overlapping GapR ChIP-seq peak)? | ||
| GapR depletion | Negative control | Difference | |||
| CCNA_02543 | LSU ribosomal protein L33P | 0.7 | −1.0 | 1.7 | Yes |
| CCNA_01609 | Alkyl sulfatase | −0.8 | 2.2 | 3.0 | No |
GapR Forms Compact Clusters Enriched in the Swarmer Compartment of the Predivisional Cell.
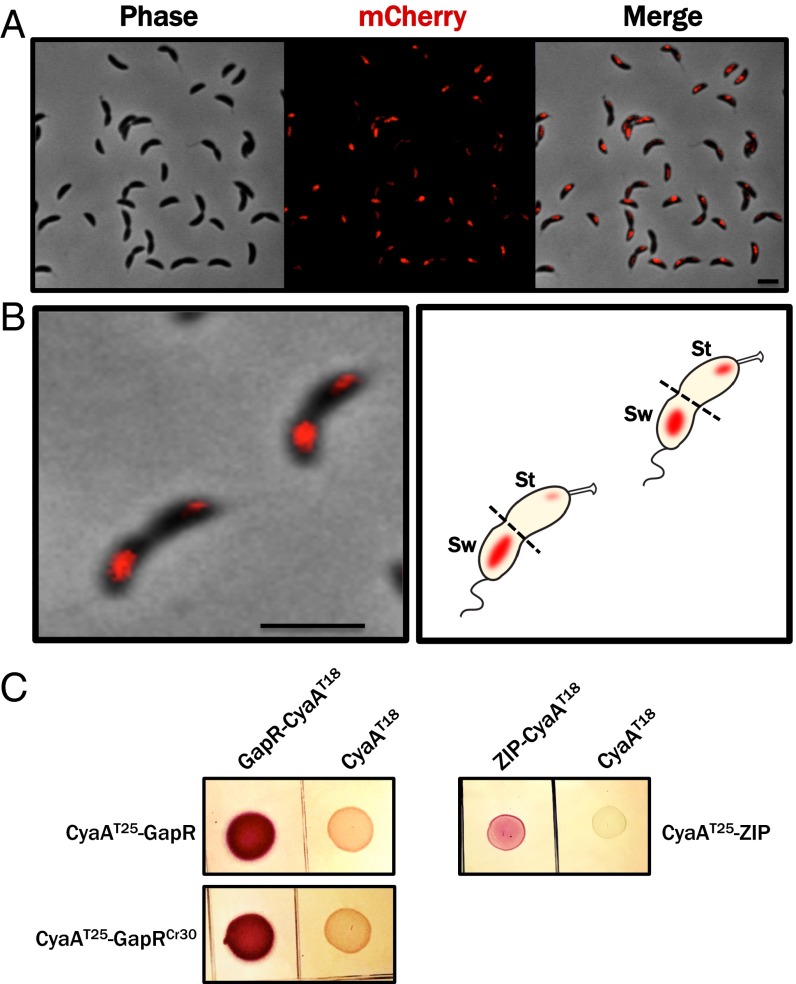
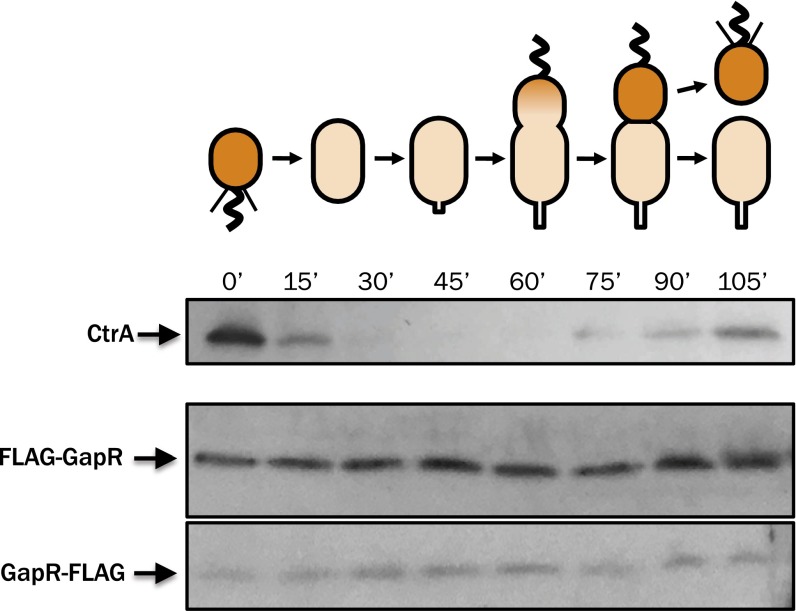
The physical association between GapR occupancy and cell cycle-activated genes is somewhat surprising in light of time-resolved translational profiling experiments that show little variation in GapR synthesis over the Caulobacter cell cycle (∼1.7-fold change) (35). To resolve this incongruity, we examined the subcellular distribution of GapR in vivo, reasoning that cell type-specific functions might be achieved through biased localization in lieu of “just-in-time” expression. We therefore generated fusions of GapR to the fluorescent protein mCherry and visualized the localization of the fusion protein in single cells (Fig. 5A). We observed in dividing Caulobacter cells a marked asymmetry in the intracellular distribution of mCherry-GapR, with the fluorescent signal found primarily in the swarmer compartment of at least half of the predivisional cells observed (Fig. 5B). This biased localization is not likely to be the result of swarmer compartment-specific expression of gapR, because GapR protein levels are roughly constant across the cell cycle in synchronous populations (Fig. S6).
Fig. 5.
GapR exhibits biased subcellular localization and self-associates in vivo. (A) WT Caulobacter NA1000 containing chromosomal mCherry-gapR under control of the PxylX promoter was grown to midexponential phase in M2G, grown an additional 2 h in the presence of 0.3% xylose, and then imaged by phase contrast and epifluorescence microscopy. (Scale bar, 1 μm.) (B) Biased swarmer compartment localization of mCherry-GapR in predivisional cells, presented as a fluorescence/phase contract overlay (Left) and as a diagrammatic projection (Right). (Scale bar, 1 μm.) (C) Bacterial two-hybrid assay indicating direct GapR-GapR and GapR-GapRCr30 interactions in vivo. Reconstitution of split adenylate cyclase (CyaA) activity, which implies a direct interaction between the domains fused to the T18 and T25 subunits of CyaA, is indicated by a Lac+ (red colony) phenotype on MacConkey agar. GapR or GapRCr30 was fused in-frame to the C terminus of CyaAT25 and coexpressed with GapR-CyaAT18 or, as a negative control, CyaAT18 alone (Right). As a positive control, the self-associating leucine zipper domain of yeast GCN4 (ZIP) was fused to the T18 and T25 fragments of CyaA (Left).
Fig. S6.
GapR expression is constitutive across the cell cycle. Synchronous cultures of Caulobacter NA1000 (Top) or derivative strains expressing FLAG-GapR or GapR-FLAG (Middle and Bottom, respectively) were allowed to proceed through the cell cycle in M2G. SDS/PAGE samples were harvested in 15-min increments, and Western blots were performed using α-CtrA or α-FLAG antisera.
Within single swarmer or stalked cells, we found that mCherry-GapR forms asymmetric foci in both cell types (Fig. 5A). In Caulobacter, and unlike in E. coli, the nucleoid fills the entire cytoplasmic space, as evidenced by the absence of DNA-free regions in cells stained with DNA-binding fluorescent dyes (16, 18); nevertheless, the fluorescent signal corresponding to GapR occupies only a fraction of the cytoplasm. The localized aggregation of GapR therefore suggests that GapR occupies regions of the Caulobacter chromosome that are particularly close to one another in 3D space.
GapR Self-Associates.
The formation of H-NS clusters in E. coli requires both DNA binding and homo-oligomerization (36, 37). The clustering of GapR we observed in Caulobacter suggests that this NAP also multimerizes. Indeed, we observed that GapR is capable of self-association using a bacterial two-hybrid assay that reports on bimolecular complementation of E. coli adenylate cyclase (CyaA), an enzyme whose activity can be reconstituted when the two essential functional domains are, respectively, fused to each of two cytoplasmic proteins that directly interact (Fig. 5C, Left) (38).
GapR was fused in frame to each functional domain of adenylate cyclase (CyaAT18 and CyaAT25), and complementary fusions were coexpressed in a strain of E. coli that cannot activate the cAMP-dependent reporter gene lacZ unless CyaAT18 and CyaAT25 are brought into close proximity through the direct interaction of their fusion partners (38). Coexpression of GapR-CyaAT18 with either GapR-CyaAT25 or CyaAT25-GapR resulted in a Lac+ phenotype (Fig. 5C, Right), indicating that GapR can bind other GapR molecules. Although this assay does not reveal the stoichiometry of GapR multimers, we can infer that GapR is minimally capable of forming dimers in vivo; our results do not exclude the possibility that GapR forms higher-order oligomers in Caulobacter. Because auto-association of GapR was detected in an orthologous host (E. coli), we additionally conclude that these GapR-GapR interactions are direct and do not require additional Caulobacter-derived factors.
GapR Activity Is Conserved Among α-Proteobacteria.
We identified 1,036 GapR homologs in sequenced genomes, 1,025 of which were found in the α-proteobacterial clade, indicating that GapR is essentially restricted to this lineage. Furthermore, GapR is nearly ubiquitous among the α-proteobacteria, with homologs identified in all but one of the free-living species in that clade. Indeed, GapR is a “signature” protein that is distinctive of the α-proteobacterial class (39–41). GapR homologs are also encoded within the genomes of several bacteriophages that infect α-proteobacterial hosts (including the Caulobacter-specific phage ΦCr30), suggesting that GapR is targeted or co-opted as a facet of the host-phage interaction.
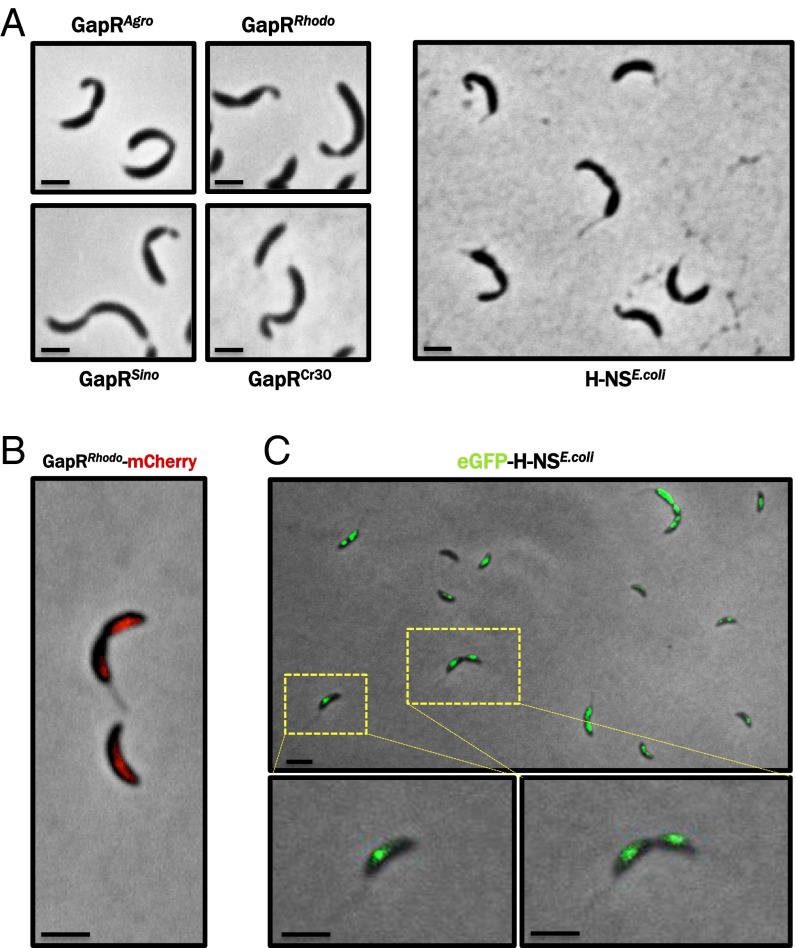
To test the possibility that homologs of Caulobacter GapR exhibit a conserved function, we cloned GapR homologs from several divergent α-proteobacterial species (Agrobacterium tumefaciens, Rhodobacter capsulatus, and Sinorhizobium meliloti), as well as from the caulophage ΦCr30, and induced the expression of each in a WT Caulobacter strain. We found that strains overexpressing gapR homologs from each of these organisms led to the same morphological and cell division defects caused by overexpression of the native gapR gene (Fig. 6A), suggesting a conserved function and providing evidence for an ancestral, common role for GapR proteins across the α-proteobacteria and, curiously, their phages. In addition, the results of a bacterial two-hybrid assay indicate that the ΦCr30 homolog of GapR (GapRCr30) interacts with GapR (Fig. 5C), suggesting that the phage-encoded homolog may directly bind Caulobacter GapR during the course of ΦCr30 infection.
Fig. 6.
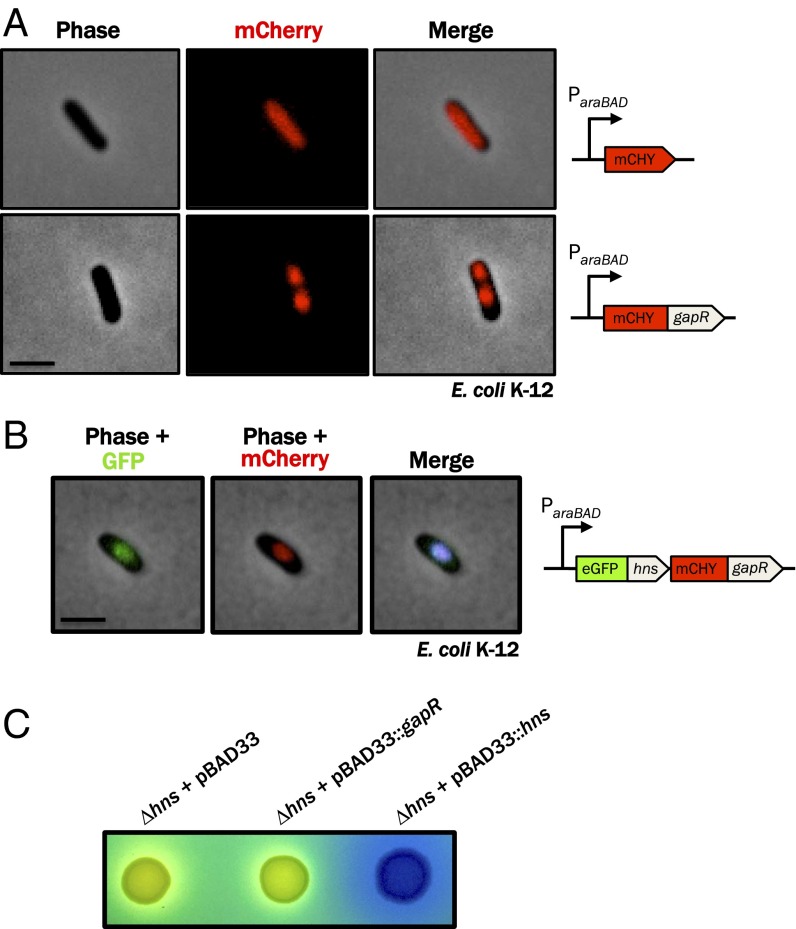
GapR-like activity and localization of GapR homologs and E. coli H-NS in Caulobacter. (A) GapR homologs from divergent α-proteobacteria and Caulobacter-specific bacteriophage ΦCr30 (Left) or E. coli hns (Right) were introduced into C. crescentus NA1000 on a low-copy replicating plasmid driven by the vanillate-inducible vanA promoter, grown for 4 h in M2G after addition of 0.5 mM vanillate and imaged by phase contrast microscopy. Agro, Agrobacterium tumefaciens; Rhodo, Rhodobacter capsulatus; Sino, Sinorhizobium meliloti; Cr30, Caulophage ΦCr30. (Scale bar, 1 μm.) (B) The GapR homolog from R. capsulatus (Rcc02587) was tagged with mCherry, expressed in NA1000 from the chromosomal PxylX promoter in the presence of 0.3% xylose and visualized by epifluorescence microscopy (shown overlaid with the phase contrast image), revealing an asymmetric distribution within predivisional cells. (Scale bar, 1 μm.) (C) eGFP-tagged H-NS from E. coli was expressed from the xylose promoter and its localization visualized as described above, showing a symmetric distribution within predivisional cells. (Scale bar, 1 μm.)
As the function and localization of a protein are often linked, we also visualized the localization that α-proteobacterial GapR homologs adopt in Caulobacter. We therefore fused the GapR homolog from Rhodobacter capsulatus (GapRRhodo) to mCherry, expressed this fusion in Caulobacter, and monitored the localization of the mCherry-GapRRhodo fusion protein in live cells. We found that GapRRhodo expressed in Caulobacter exhibited the same asymmetric distribution in predivisional cells as does Caulobacter GapR (Fig. 6B), providing additional evidence for a conserved, species-independent behavior of this novel family of NAPs.
In Caulobacter, precise protein localization is frequently achieved through protein-protein interactions that underlie hierarchical localization dependency networks. The fact that GapRRhodo exhibits a GapR-like localization pattern in Caulobacter is remarkable in light of the fact that these homologs are only 45% identical (Fig. S7), reducing the likelihood that specific protein-protein interactions involving GapRRhodo in R. capsulatus would be preserved in Caulobacter (42). Given the relatively low probability of conserved interactions between GapRRhodo and endogenous Caulobacter proteins, we asked whether clustering of GapR might instead be a consequence of generic binding to AT-rich regions of the genome, which have indeed been shown to colocalize in E. coli through association with the AT-associated NAP known as H-NS (37). If binding to AT-rich DNA is sufficient to establish the localization pattern observed for GapR, then an orthogonal AT-rich DNA-binding protein that bears no resemblance to any Caulobacter protein should nonetheless adopt a GapR-like localization pattern in Caulobacter. To test this, we expressed in Caulobacter a fluorescent protein fused to E. coli H-NS, a protein that lacks a homolog in Caulobacter and that is known to autonomously bind AT-rich DNA in vivo and in vitro. We observed that H-NS, like GapR, forms discrete subpolar clusters in Caulobacter, supporting the notion that GapR localizes through interactions with AT-rich loci on the Caulobacter chromosome. However, unlike GapR homologs, H-NS is apparently not asymmetrically distributed in Caulobacter, suggesting that swarmer-specific accumulation of GapR is a property specific to that protein family (Fig. 6C).
Fig. S7.
Sequence alignment of GapR homologs shown to induced GapR-like toxicity. GapR and its four homologs characterized in this report (GapRRhodo, GapRSino, GapRAgro, and GapRCr30; Fig. 6A) were aligned using ClustalW. Columns are marked when all homologs share the same residue (*), physicochemically similar residues (:), or residues with more generic common feature (.).
GapR Function Is Distinct from That of H-NS–Like Proteins.
GapR and H-NS each bind AT-rich DNA and form clusters in Caulobacter, suggesting that these divergent DNA-binding proteins may perform a common function in their respective hosts. We reasoned that if GapR and H-NS are functionally equivalent, then (i) H-NS should exhibit GapR-like activity in Caulobacter and (ii) GapR should exhibit H-NS–like activity in E. coli.
To test these predictions, we first overexpressed H-NS in Caulobacter to determine whether the phenotypes observed on GapR overexpression can also be induced by H-NS. When hns was expressed in Caulobacter from the vanillate-inducible PvanA promoter on a low-copy replicating plasmid, we observed the same defects in cell shape, size, and division that occur on gapR overexpression, including the polar minicells that are typical of gapR mutant strains (Fig. 6A). This finding suggests that the constitutive binding of AT-rich DNA may be sufficient to cause the defects associated with GapR overexpression.
DNA binding-deficient variants of H-NS fail to form localized clusters in live E. coli (37), indicating that the association of H-NS with AT-rich DNA underlies its localization. To test whether GapR exhibits H-NS–like activity in E. coli, we first asked whether GapR adopts an H-NS–like subcellular localization by expressing fluorescently labeled GapR in a ∆hns E. coli mutant and determining its localization. Although the expression of mCherry alone led to a homogeneous, diffuse fluorescent signal that occupied the entire cytoplasmic space, mCherry-GapR formed two discrete clusters per cell during exponential growth (Fig. 7A), a localization pattern that has been previously observed for H-NS using both diffraction-limited and superresolution fluorescence microscopy (37). On simultaneous coexpression of H-NS and GapR fused to eGFP and mCherry (respectively) in E. coli, we were able to observe a precise overlap in the subcellular distributions of the two fluorescent proteins (Fig. 7B), confirming a shared localization pattern and indicating a common nucleoid association profile for H-NS and GapR in E. coli. This observation further implies that the localization of GapR, at least in E. coli cells, is a consequence of recognizing AT-rich DNA (37).
Fig. 7.
GapR recapitulates the subcellular localization, but not the function, of H-NS in E. coli. (A) An E. coli ∆hns mutant expressing mCherry alone (Top) or mCherry-GapR (Bottom) under control of the arabinose-inducible araBAD promoter was grown for 2 h in rich media with induction (0.02% arabinose) and imaged by epifluorescence and phase contrast microscopy. (Scale bar, 1 μm.) (B) An E. coli ∆hns mutant containing a plasmid expressing both mCherry-GapR and eGFP-HNS from a single araBAD promoter was grown and imaged as in A. (C) E. coli ∆hns mutant strains transformed with pBAD33 containing gapR, pBAD33 containing hns (positive control), or empty vector (negative control) were spotted onto salicin agar (which reports on expression of the cryptic bgl operon) seeded with 0.02% arabinose. Yellow colony color, Bgl+ (no complementation); blue colony color, Bgl− (complementation). (Scale bar, 1 μm.)
H-NS is a global transcriptional silencer that represses the expression of ∼5% of E. coli genes. Accordingly, we next determined whether GapR can perform the silencing function of H-NS. One locus H-NS silences is the bgl operon, which encodes a functional phospho-β-glucosidase and a transport system for its substrate(s); ∆hns mutants exhibit a Bgl+ phenotype and can use β-glucosides as a sole carbon source (43). We expressed gapR in a ∆hns mutant of E. coli under control of the arabinose inducible ParaBAD promoter and monitored the expression of the bgl operon using a colorimetric assay that reports on the metabolism of salicin, a β-glucoside. Although the trans expression of hns+ in a ∆hns background restored the Bgl− phenotype, and although gapR can be efficiently expressed in E. coli (Fig. 7 A and B), expression of gapR did not result in repression of the bgl operon, because ∆hns strains expressing gapR retained the Bgl+ phenotype (Fig. 7C). Taken together, these findings show that, although GapR retains the AT-binding property of H-NS, it seems to lack the capacity to act as an autonomous transcriptional silencer. This observation distinguishes these two NAPs and suggests that although both GapR and H-NS associate with AT-rich regions of DNA, they are nonetheless functionally divergent.
Discussion
GapR Is an Essential DNA-Associated Proteins.
This report describes the discovery of GapR, a NAP that globally binds the Caulobacter chromosome at AT-rich loci and plays an essential role in the growth and division of the dimorphic bacterium Caulobacter crescentus. GapR is required for viability, but is also toxic when expressed at high levels, indicating that normal growth also requires robust regulation of GapR abundance. Strains depleted for or overexpressing gapR exhibit compromised growth, and at the single-cell level, defects in cell size, cell shape, and division are observed. Because there is a positive correlation between GapR ChIP-seq enrichment and average local AT content, and because overexpression of the exogenous AT-binding protein H-NS in Caulobacter reproduces the GapR overexpression phenotype, it is conceivable that GapR overproduction leads to ectopic association with nonnative binding sites (once all native sites are occupied), which directly or indirectly leads to the disruption of one or more developmental processes. Lethal overproduction of AT-binding NAPs has been previously observed, with overexpression of H-NS in E. coli leading to extreme condensation of the nucleoid and fatal inhibition of macromolecular synthesis (44). Although the mechanism of cell cycle disruption by excess GapR in Caulobacter remains to be determined, we can conclude that its activity and/or abundance must be regulated so as to mitigate the disruptive effects of unchecked GapR accumulation in the cell.
Caulobacter GapR is unusual among NAPs in that it is essential for viability; although H-NS family members can influence many processes through silencing of as much as 5% of the bacterial genome (31), they are often dispensable in the γ-proteobacterial species in which they are found (29, 45, 46). Notable exceptions include the ortholog of H-NS in Salmonella and the semiredundant pair of H-NS-like NAPs in Pseudomonas aeruginosa, MvaT/MvaU (32, 47). In both of these cases, the essential role of these NAPs is to silence the expression of horizontally acquired genes that are toxic when constitutively expressed. This phenomenon is unlikely to explain the essentiality of GapR, as most promoters bound by GapR are transcriptionally active (as evidenced by the presence of bound RpoD at >80% of these sites), and as we do not observe global de-repression of GapR-associated genes after GapR depletion (as is observed following deletion of hns in E. coli) (19). Our results therefore do not support an H-NS–like role for GapR in direct transcriptional silencing of target genes, suggesting that GapR represents a NAP family that is functionally distinct from H-NS–like proteins and whose divergent functions are critical for cell growth and division.
GapR Is a Signature α-Proteobacterial Protein.
GapR comprises a single domain of unknown function (DUF2312) that is ubiquitous among the α-proteobacteria, with gapR coding sequences found in nearly all members of this clade (40, 41); this includes endosymbionts with minimal genomes (e.g., Wolbachia). This conservation indicates a common, ancient ancestor for all orthologous GapR sequences in the α-proteobacteria. We have shown here that GapR homologs from various α-proteobacterial species can recapitulate the toxic consequences of gapR overproduction in Caulobacter, indicating conservation of function activity between GapR orthologs.
Not only is GapR conserved throughout the α-proteobacteria, but several bacteriophages with α-proteobacterial hosts, including the Caulobacter-specific lytic phage ΦCr30, also encode GapR homologs. It is noteworthy that ΦCr30 carries a homolog of Caulobacter GapR, a protein that accumulates in the swarmer compartment of predivisional cells, as this phage exhibits a specific tropism for the swarmer cell due to an inability to infect stalked cells (11). The identification of an AT-associated GapR-like protein in ΦCr30 is also particularly intriguing when considered in the context of genomic nucleotide bias: whereas the Caulobacter genome is 67.2% GC, the ΦCr30 genome is instead markedly GC poor (38.2% GC) (48). When we express the GapRCr30 homolog in Caulobacter, we observe growth and division defects. We also observe that GapRCr30 directly binds GapR in a bacterial two-hybrid experiment. These observations together are consistent with the notion that GapRCr30 is a viral “mimic” protein (49) that acts by inhibiting, antagonizing, displacing, or otherwise subverting GapR and hijacking the critical biological process(es) in which it is engaged. Recently, the divergent swarmer-specific Caulobacter phage ΦCbK and several of its sequenced relatives were shown to encode a homolog of the cell cycle transcriptional regulator GcrA (50, 51), indicating that phage-encoded mimics could act by modulating the Caulobacter cell cycle. We expect that investigation into the role of GapRCr30 in the phage infection cycle will inform our understanding of the essential function of GapR in Caulobacter and may additionally reveal novel virulence strategies used by bacteriophages.
The GapR homolog from a phylogenetically distinct α-proteobacterium (Rhodobacter capsulatus) adopts a GapR-like localization pattern, including not only subcellular clustering, but also asymmetric distribution to the swarmer compartment in the predivisional stage when expressed in Caulobacter. This observation highlights the functional conservation between these homologs despite their phylogenetic distance. E. coli H-NS, by contrast, does not exhibit this type of asymmetry, implying a GapR-specific mechanism that drives the swarmer-specific accumulation of this NAP. We infer that AT binding is the primary driver of GapR subcellular clustering, however, because the R. capsulatus GapR homolog and E. coli H-NS form foci like GapR in single Caulobacter cells. Although it is possible that GapR is passively distributed to the swarmer cell compartment in predivisional cells through an interaction with AT-rich DNA, the lack of swarmer compartment-specific enrichment of H-NS expressed in Caulobacter may instead indicate that additional mechanisms (e.g., compartment-specific proteolysis, active translocation, posttranslational modification, or upstream polarity factors) contribute to this effect.
GapR Associates Primarily with the Swarmer Cell Nucleoid.
Two separate lines of evidence support the possibility that the swarmer and stalked cell types of Caulobacter exhibit distinct nucleoid compaction or higher-order nucleoid structures. First, the nucleoids of swarmer and stalked cells exhibit markedly different sedimentation rates and contain distinct complements of associated protein (8, 52–54). Second, because the chromosome fills the cytoplasmic space in both daughter compartments (16), the swarmer nucleoid must be packaged into only ∼70% the volume afforded to that of the stalked cell nucleoid. It has been suggested that asymmetries in swarmer and stalked cell nucleoid structure are the consequence of one or more asymmetrically distributed NAPs that impart cell type-specific structure to the Caulobacter nucleoid (12). We are intrigued by the possibility that GapR, a protein that localizes to the swarmer nucleoid during cell division, represents a histone-like factor that contributes to the swarmer/stalked cell nucleoid asymmetry in Caulobacter.
GapR associates with hundreds of sites across the genome yet localizes to a discrete focus in vivo. This clustering suggests that distant genomic loci are colocalized in 3D space, as has been observed for H-NS–bound loci in E. coli (37). In addition to H-NS, multiple NAPs are known to promote long-range intrachromosomal interactions through DNA bending, looping, compaction, and the stabilization of discrete subdomains (4, 55–63). It may be that the self-association of GapR molecules links together noncontiguous loci that contain AT-rich sequences.
The findings presented here provide the foundation for the hypothesis that the accumulation of GapR in the swarmer cell compartment contributes to the control of swarmer cell fate. Conformation-capture experiments reveal a relationship between gene expression and chromosome structure in Caulobacter (4,6); if GapR plays a role in mediating nucleoid conformation of the swarmer, it could also establish or otherwise influence the program of gene expression that is specifically activated in the swarmer cell during the S → G1 transition.
GapR Is Linked to Spatiotemporal Regulation of Cell Cycle-Controlled Genes.
Although its synthesis is constitutive across the cell cycle, we have shown that GapR associates with cell cycle-regulated genes and has hundreds of binding sites in common with the known master cell cycle regulators, indicating a role for this essential NAP in cell cycle control. By comparing the ChIP-seq footprint of GapR against those of other master regulators, we discovered that GapR shares in vivo target loci with the master cell cycle regulators SciP, CtrA, GcrA, and MucR1/2. Although GapR ChIP-seq peaks overlap hundreds of intergenic regions in Caulobacter, we have shown that the extent of GapR overlap with binding sites of each of the regulators CtrA, GcrA, MucR1, and MucR2 is statistically significant (SI Materials and Methods). GapR overlaps most extensively with MucR1, covering more than 90% of its binding sites. MucR1/2 are homologous transcriptional regulators that are critical in establishing the swarmer cell developmental state during the S → G1 transition, presumably by inhibiting expression of G1-specific CtrA-regulated genes in the S phase. Products of these swarmer-activated genes include the pilus subunit protein PilA, the buoyancy switch factor and encapsulation inhibitor HvyA, and, importantly, the G1-specific CtrA inhibitor SciP (12).
In Caulobacter, the chromosomal positions of genes dictate not only their timing of replication/segregation but also their spatial placement in the Caulobacter cell (64). Two separate observations support the possibility that the spatial positioning of a gene locus is important for its proper expression. First, chromosomal rearrangements that have occurred in Caulobacter species have largely preserved the distances between rearranged genes and the origin, suggesting that the longitudinal subcellular positions of genes are also generally conserved (65). Second, we observe that promoters bound by CtrA and MucR1, whose timing of expression and downstream gene function are largely swarmer specific, are almost completely restricted to the origin-proximal half of the chromosome and therefore to the stalked and flagellar pole-proximal regions of the cell (the majority of these genes are listed in Table S2). Proteins like MucR1/2 and CtrA that control expression of these swarmer genes may interact with spatially restricted proteins, such as GapR, to carry out their tasks.
The overlap of GapR with nearly all MucR1-occupied CtrA sites raises the possibility that GapR may be directly involved in MucR1/2 function, i.e., modulating expression of CtrA-regulated genes and controlling the S → G1 switch. This connection between GapR and the activation of the swarmer cell program is even more striking in light of the subcellular distribution of GapR in predivisional cells, where the majority of GapR localizes asymmetrically to the swarmer cell compartment. Although MucR1/2 have been shown to repress CtrA-regulated genes, it remains unknown how such repression is restricted to S-phase (12). We hypothesize that the asymmetric distribution of GapR to the swarmer compartment provides spatial regulation to MucR1/2 activity. Because perturbation of GapR function leads only to minor changes in transcript abundances, we predict that this regulation is either indirect or that it fine-tunes essential events. We propose that GapR is a high AT-associated protein that has been co-opted to mediate the faithful asymmetric division of Caulobacter cells.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
Strains and plasmids used in this study are detailed in Tables S5 and S6. Plasmid construction, strain engineering, and growth conditions are described in SI Materials and Methods.
Table S5.
C. crescentus and E. coli strains used in this study
| Strain | Genotype and relevant features | Phenotype | Source |
| C. crescentus | |||
| NA1000 | Caulobacter crescentus CB15N | Synchronizable WT laboratory strain | (52) |
| DPR564 | NA1000 + xylX::gapR | KanR | This study |
| DPR1174 | NA1000 + vanA::gapR | KanR | This study |
| DPR1133 | NA1000 + xylX::EcsspB (b3228, E. coli) | SpecR XylR | This study |
| DPR1181 | NA1000 + xylX::sspB + gapR-EcssrAADAS (EcssrAADAS = AANDENYSENYADAS) | SpecR KanR XylS | This study |
| DPR1385 | NA1000 + xylX::sspB + gapR-M2-EcssrAADAS | SpecR KanR XylS | This study |
| DPR1182 | NA1000 + xylX::sspB + ctrA-EcssrAADAS | SpecR KanR XylS | This study |
| LS2523 | NA1000 + xylX::ctrA + ∆ctrA | Xylose-dependent, SpecR KanR | (88) |
| DPR630 | NA1000 + gapR::gapR-M2 | KanR | This study |
| DPR781 | NA1000 + gapR::ctrA-M2 | KanR | This study |
| DPR412 | NA1000 + gapR::M2-gapR | KanR | This study |
| DPR1136 | NA1000 + xylX::gapR-mCherry | KanR | This study |
| DPR1135 | NA1000 + xylX::mCherry-gapR | KanR | This study |
| DPR1238 | NA1000 + xylX::mCherry-gapRRhodo (rcc02587; Rhodobacter capsulatus) | KanR | This study |
| DPR1250 | NA1000 + xylX::eGFP-hns (b1237, E. coli) | KanR | This study |
| DPR1251 | NA1000 + pRMCS::PgapR-gapR | KanR | This study |
| DPR518 | NA1000 + pRXMCS::gapR | ChlorR | This study |
| DPR1166 | NA1000 + pRVMCS::gapR | KanR | This study |
| DPR1172 | NA1000 + pRVMCS::gapRAgro (Agau_L100364, Agrobacterium tumefaciens) | KanR | This study |
| DPR1173 | NA1000 + pRVMCS::gapRRhodo | KanR | This study |
| DPR1171 | NA1000 + pRVMCS::gapRSino (SMc04009; Sinorhizobium meliloti) | KanR | This study |
| DPR1170 | NA1000 + pRVMCS::gapRΦCr30 (OZ74_gp140; Caulobacter phage ΦCr30) | KanR | This study |
| DPR1169 | NA1000 + pRVMCS::hns | KanR | This study |
| E. coli | |||
| NEB Turbo | F' proA+B+ lacIq ∆lacZM15/fhuA2 ∆(lac-proAB) glnV galK16 galE15 R(zgb-210::Tn10)TetS endA1 thi-1 ∆(hsdS-mcrB)5 | Cloning strain; exhibits rapid growth | New England Biolabs |
| S17-1 | Ec294::[RP4-2(Tc::Mu)(Km::Tn7)] tra+ recA pro res | TpR, SmR, Transfers plasmids carrying oriT | Biomedal Lifescience |
| MC4100 | F- araD139 (argF-lac)U169 rpsL150 relA1 flb5301 deoC1 ptsF25 thi | WT laboratory strain | (67) |
| DPR1254 | MC4100 ∆hns::kan | KanR | This study |
| DPR1350 | MC4100 ∆hns::kan + pBAD33::hns | KanR ChlorR | This study |
| DPR1351 | MC4100 ∆hns::kan + pBAD33::gapR | KanR ChlorR | This study |
| DPR1357 | MC4100 ∆hns::kan + pBAD33::eGFP-hns | KanR ChlorR | This study |
| DPR1352 | MC4100 ∆hns::kan + pBAD33::mCherry-gapR | KanR ChlorR | This study |
| DPR1358 | MC4100 ∆hns::kan + pBAD33::mCherry-gapR_eGFP-hns | KanR ChlorR | This study |
| DPR1355 | MC4100 ∆hns::kan + pBAD33 | KanR ChlorR | This study |
| BTH101 | F-, cya-99, araD139, galE15, galK16, rpsL1, hsdR2, mcrA1, mcrB1 | Reporter strain for bacterial two-hybrid assay | Euromedex |
| DPR1382 | BTH101 + pUT18C + pKT25-ZIP | AmpR KanR | This study |
| DPR1366 | BTH101 + pUT18C-ZIP + pKT25-ZIP | AmpR KanR | This study |
| DPR1383 | BTH101 + pUT18C + pKT25::gapR | AmpR KanR | This study |
| DPR1361 | BTH101 + pUT18C::gapR + pKT25::gapR | AmpR KanR | This study |
| DPR1384 | BTH101 + pUT18C + pKT25::gapRΦCr30 | AmpR KanR | This study |
| DPR1362 | BTH101 + pUT18C::gapR + pKT25::gapRΦCr30 | AmpR KanR | This study |
Table S6.
Plasmids used in this study
| Plasmid | Description | Marker(s) | Source |
| pNPTS138 | For two-step recombination-mediated gene excision and replacement | nptI (KanR), sacB (SucS) | (67) |
| pMCS-1 | For chromosomal integration at any genomic locus of interest | aadA (SpecR) | (70) |
| pMCS-2 | For chromosomal integration at any genomic locus of interest | nptI | (70) |
| pXCHYC-1 | For generating chromsomal transcriptional fusions to the xylX promoter (PxylX) | aadA | (70) |
| pVCHYC-2 | For generating chromsomal transcriptional fusions to the vanA promoter (PvanA) | nptI | (70) |
| pXCHYC-2 | For generating chromsomal transcriptional fusions to the xylX promoter (PxylX) | nptI | (70) |
| pRXMCS-2 | Low-copy replicating vector with PxylX promoter for xylose-inducible overexpression | nptI | (70) |
| pRXMCS-6 | Low-copy replicating vector with PxylX promoter for xylose-inducible overexpression | cat (ChlorR) | (70) |
| pRVMCS-2 | Low-copy replicating vector with PvanA promoter for vanillate-inducible overexpression | nptI | (70) |
| pBXMCS-2 | High-copy replicating vector with PxylX promoter for xylose-inducible overexpression | nptI | (70) |
| pBAD33 | Replicating vector with ParaBAD promoter for arabinose-inducible expression | cat | (72) |
| pUT18C | For generating IPTG-inducible fusions of CyaAT18 to the N terminus of a gene of interest | bla (AmpR) | Euromedex |
| pKT25 | For generating IPTG-inducible fusions of CyaAT25 to the N terminus of a gene of interest | nptI | Euromedex |
| pUT18C-ZIP | Positive control plasmid for two-hybrid assay (gives Lac+ phenotype w/ pKT25-ZIP) | bla | Euromedex |
| pKT25-ZIP | Positive control plasmid for two-hybrid assay (gives Lac+ phenotype w/ pUT18C-ZIP) | nptI | Euromedex |
| pXCHYC-2::gapR | For single-step integration of gapR at the xylX locus | nptI | This study |
| pVCHYC-2::gapR | For single-step integration of gapR at the vanA locus | nptI | This study |
| pNPTS138::∆gapR::Ω | For deletion of gapR and replacement with the Ω cassette | nptI aadA | This study |
| pXCHYC-1::sspB | For single-step integration of E. coli sspB at the xylX locus | aadA | This study |
| pMCS-2::'gapR-EcssrAADAS | For single-step tagging of chromosomal gapR with the E. coli ssrAADAS tag | nptI | This study |
| pMCS-2::'ctrA-EcssrAADAS | For single-step tagging of chromosomal ctrA with the E. coli ssrAADAS tag | nptI | This study |
| pMCS-2::'gapR-M2-EcssrAADAS | For single-step tagging of chromosomal gapR with FLAG and E. coli ssrAADAS tags | nptI | This study |
| pMCS-2::'gapR-M2 | For single-step tagging of chromosomal gapR with a C-terminal FLAG tag | nptI | This study |
| pMCS-2::PgapR-M2-gapR' | For single-step tagging of chromosomal gapR with an N-terminal FLAG tag | nptI | This study |
| pMCS-2::'ctrA-M2 | For single-step tagging of chromosomal ctrA with a FLAG tag | nptI | This study |
| pXCHYC-2::gapR-mCherry | For single-step integration of gapR-mCherry at the xylX locus | nptI | This study |
| pXCHYC-2::mCherry-gapR | For single-step integration of mCherry-gapR at the xylX locus | nptI | This study |
| pXCHYC-2::mCherry-gapRRhodo | For single-step integration of mCherry-gapRRhodo at the xylX locus | nptI | This study |
| pXCHYC-2::eGFP-hns | For single-step integration of eGFP-hns at the xylX locus | nptI | This study |
| pRMCS-2::PgapR-gapR | For low-copy expression of gapR from its native promoter | nptI | This study |
| pBMCS-2::PgapR-gapR | For high-copy expression of gapR from its native promoter | nptI | This study |
| pRXMCS-6::gapR | For low-copy expression of gapR from the PxylX promoter | cat | This study |
| pBXMCS-2::gapR | For high-copy expression of gapR from the PxylX promoter | nptI | This study |
| pRVMCS-2::gapR | For low-copy expression of gapR from the PvanA promoter | nptI | This study |
| pRVMCS-2::gapRAgro | For low-copy expression of gapRAgro from the PvanA promoter | nptI | This study |
| pRVMCS-2::gapRRhodo | For low-copy expression of gapRRhodo from the PvanA promoter | nptI | This study |
| pRVMCS-2::gapRSino | For low-copy expression of gapRSino from the PvanA promoter | nptI | This study |
| pRVMCS-2::gapRΦCr30 | For low-copy expression of gapRΦCr30 from the PvanA promoter | nptI | This study |
| pRVMCS-2::hns | For low-copy expression of hns from the PvanA promoter | nptI | This study |
| pBAD33::hns | For arabinose-inducible expression of hns | cat | This study |
| pBAD33::gapR | For arabinose-inducible expression of gapR | cat | This study |
| pBAD33::mCherry-gapR | For arabinose-inducible expression of mCherry-gapR | cat | This study |
| pBAD33::eGFP-hns | For arabinose-inducible expression of eGFP-hns | cat | This study |
| pBAD33::mCherry-gapR_eGFP-hns | For arabinose-inducible expression of eGFP-hns and mCherry-gapR (synthetic operon) | cat | This study |
| pBAD33::mCherry | For arabinose-inducible expression of mCherry | cat | This study |
| pKT25::gapR | For IPTG-inducible expression of GapR fused to the C terminus of CyaAT25 | nptI | This study |
| pUT18C::gapR | For IPTG-inducible expression of GapR fused to the C terminus of CyaAT18 | bla | This study |
| pKT25::gapRΦCr30 | For IPTG-inducible expression of GapRΦCr30 fused to the C terminus of CyaAT25 | nptI | This study |
ChIP-Seq.
A full description of methods used for cell crosslinking and harvesting, chromatin immunoprecipitation, deep sequencing, and data processing is provided in SI Materials and Methods.
Subcellular Localization of Fluorescently Tagged Proteins.
C. crescentus strains were cultured to log phase in peptone yeast extract medium (PYE) containing appropriate antibiotic. Where necessary, gene expression was induced by adding 0.3% xylose for 120 min during growth at 30 °C before imaging by phase contrast and epifluorescence microscopy. E. coli strains were grown to A600 of about 0.2 and induced with 0.2% arabinose for 90 min during growth at 37 °C before imaging.
Western Blot Analysis.
Cultures were grown overnight in PYE and back-diluted 1:100 into fresh media. One-milliliter samples were collected from cultures grown in each condition at OD600 = 1. Harvested samples were normalized, pelleted (10,000 × g, 10 min), resuspended in SDS/PAGE sample buffer, lysed by incubating at 100 °C for 15 min, and subjected to electrophoresis through gradient (4–12%) SDS/PAGE gels. Rabbit polyclonal antisera that recognize the FLAG epitope (1:5,000 dilution; Sigma-Aldrich) or CtrA (1:10,000 dilution) were used for immunoblots. Protein bands were visualized using the Western Lightning ECL antibody detection kit (PerkinElmer) and Hyblot CL film (Denville Scientific).
SI Materials and Methods
Bacterial Strains and Growth Condition.
All Caulobacter crescentus strains used in this study derive from the laboratory strain CB15N (NA1000). Bacterial two-hybrid assays were performed in ∆cyaA lacZ reporter strains of E. coli K-12 (38); all other E. coli experiments were performed in the laboratory strain MC4100 (66). Genomic DNA from the α-proteobacterial strains Agrobacterium tumefaciens F2, Rhodobacter capsulatus PAS100, Sinorhizobium meliloti 1021, and the α-proteobacterial bacteriophage ΦCr30 was isolated by incubating bacterial colonies or cell-free ΦCr30 lysates at 100 °C in the presence of 10% (wt/vol) Chelex-100 resin (Bio-Rad).
For all experiments, Caulobacter strains were grown at 30 °C in a rich PYE or in minimal media supplemented with 0.2% glucose (M2G) or 0.3% xylose (M2X), and E. coli strains were grown at 30 °C (for bacterial two-hybrid experiments) or 37 °C (for all other experiments) in rich Luria-Bertani media (LB). The bgl complementation assay was performed by spotting appropriate strains on salicin agar containing 0.02% arabinose and 0.01% bromothymol blue (45). When appropriate, the growth medium was supplemented with 0.3% d-xylose, 0.2% l-arabinose, 500 µM vanillate, 5 µg/mL kanamycin, 125 µg/mL ampicillin, 25 µg/mL spectinomycin, and/or 5 µg/mL streptomycin.
For synchronization, Caulobacter cells were grown in 20 mL M2G with antibiotics to OD600 ∼0.3, harvested by pelleting at 6,000 × g, washed and resuspended in 1 mL cold M2 salts, and combined 1:1 with Percoll (Sigma-Aldrich). The cell suspension was centrifuged for 20 min at 10,000 × g at 4 °C. The swarmer band at the bottom of the Percoll gradient was isolated, washed three times in 1 mL ice-cold M2 salts, added to prewarmed M2G media, and grown with aeration at 30 °C.
For induced proteolysis experiments, cells were grown in M2G to midexponential phase, washed once in M2 media, and added to M2X to induce expression of sspBEcoli. Samples were harvested at indicated time points following sspBEcoli induction and analyzed by phase-contrast and fluorescence microscopy or subjected to SDS/PAGE electrophoresis and Western blotting.
Caulobacter Strain Construction.
All gene replacement constructs (e.g., gapR::FLAG-gapR or ∆gapR::Ω) were generated using derivatives of the suicide vector pNPTS138 that carries the kanamycin resistance determinant (nptI) and the levansucrase gene (sacB), a counterselectable marker that restricts growth in the presence of sucrose (67). Target constructs were cloned between two ∼500-bp sequences that flank the gene/site to be replaced or modified. These plasmids were transferred into Caulobacter NA1000 by conjugation through E. coli S17-1, with selection on PYE plates containing kanamycin (to select for cointegrates) and nalidixic acid (to counter select against the E. coli donor; NA1000 is intrinsically resistant to nalidixic acid) (68). pNPTS138 does not replicate in Caulobacter and can only be propagated through genome integration via homologous recombination. Kanamycin-resistant cointegrates were screened for sucrose sensitivity and the presence of integrated plasmid DNA was verified by PCR and sequencing using primers that flank the target locus.
Genome modifications were made with these pNPTS138 derivatives using a conventional two-step, recombination-based sucrose counter selection procedure. Colonies containing the integrated plasmid at the target locus were grown to saturation in rich media under nonselective conditions (no kanamycin added) and plated on PYE agar containing 3% (wt/vol) sucrose to select for plasmid excision (indicated by loss of the sacB marker). Sucrose-resistant, kanamycin-sensitive colonies were scored for the presence of the target construct at the genomic locus by diagnostic PCR.
Caulobacter transformation, conjugation, and generalized transduction were performed using standard methods as described elsewhere (69).
Plasmid Construction.
All non-pNPTS138 plasmid-based constructs generated in this study for use in Caulobacter were assembled using custom derivatives of the pMT collection vectors (70) that were modified within the multiple cloning site to enable type IIs restriction/ligation cloning (71) with custom adapters. High-copy (pB) and low-copy (pR) vectors from the pMT collection were used for gene overexpression, and integrating (pMCS) vectors were used for single-step generation of transcriptional fusions and C-terminal translational fusions.
Plasmid constructs generated for arabinose-dependent gene expression in E. coli were built using the pBAD system (72) or a dual plasmid system compatible with ∆cyaA strains (38, 73) used in bacterial two-hybrid experiments. ORFs were cloned into these plasmids by linearizing each parent vector with a restriction enzyme targeting the multiple cloning site and combining linearized vector with a PCR product comprising the ORF and flanking homology to the target vector through the Gibson Assembly reaction (74).
Sequences of oligonucleotides or plasmids used in this study are available on request.
ChIP-seq.
Saturated cultures of each ChIP strain (CtrA-FLAG, GapR-FLAG, FLAG-GapR, NA1000) were diluted in rich media (PYE) to OD600 = 0.01 and cultures were grown to midexponential phase (OD600 = 0.4–0.6) at 30 °C with aeration. Crosslinking was initiated through the addition of 1% formaldehyde directly to each culture followed by incubation at room temperature for 10 min (with aeration) before quenching with 2.5 M glycine and a series of three washes in cold 1× PBS. Cells were resuspended in TE (10 mM Tris, pH 8.0, 1 mM EDTA) and lysed using a high-efficiency lysozyme (ReadyLyse; Epicentre) according to supplier instructions in the presence of protease inhibitor mixture (Sigma). SDS was added to lysates to a final concentration of 0.1% and samples were sonicated (Covaris E220; Covaris) to generate DNA fragments with an average length of 300–700 bp. Lysates were cleared following sonication by centrifugation at 15,000 × g for 10 min at 4 °C to remove insoluble material; 1% Triton X-100 was added to each sample, and proteins that nonspecifically bind Protein A or the bead support used during immunoprecipitation were precleared by incubating the lysates with Dynabeads (Thermo Fisher) conjugated to Protein A. Five percent of each precleared lysate was reserved as input sample. FLAG-tagged proteins were immunoprecipated using α-M2 antibody (F7425, Sigma) coupled to magnetic beads blocked in BSA and glycogen. Beads were washed in low-salt buffer (50 mM Hepes, 1% Triton X-100, 150 mM NaCl), high-salt buffer (50 mM Hepes, 1% Triton X-100, 500 mM NaCl), and LiCl buffer (TE with 1% Triton X-100, 0.5% Nonidet P-40, and 150 mM LiCl), and the target protein was eluted in TE containing 1% SDS in the presence of competing 3x-FLAG peptide (Sigma). The eluates were adjusted to 300 mM NaCl and treated with RNase A and Proteinase K. Formaldehyde cross-links were reversed by overnight incubation at 65 °C with shaking, and pure ChIP DNA was obtained using the ChIP DNA Clean & Concentrator kit (Zymo Research).
ChIP DNA quality and fragment length distribution were measured on an Agilent 2100 Bioanalyzer at the Protein and Nucleid Acid (PAN) facility in the Stanford School of Medicine. Indexed, Illumina-compatible deep sequencing libraries were prepared and multiplexed using the Accel NGS 2S Plus DNA Library Kit (Swift Biosciences) according to the manufacturer’s instructions. Libraries were sequenced (paired-end, 125 cycles) on an Illumina MiSeq instrument at the Stanford PAN facility. Reads were processed and mapped to the NA1000 genome independently using software tools available on a public Galaxy instance (75, 76) and CLC Genomics Workbench (Qiagen). Datasets were visualized using the Integrative Genomics Viewer (77).
ChIP-seq Data Analysis.
Paired-end Illumina reads were mapped onto the Caulobacter NA1000 genome (CP001340.1) using Bowtie2 (78). Mapped reads were filtered, formatted, and normalized to reads per kilobase per million (RPKM) in Galaxy (75, 76, 79). FLAG-GapR, GapR-FLAG, and CtrA-FLAG ChIP-seq peaks were called using MACS2 (80) relative to ChIP DNA isolated from the control background strain NA1000, which was subjected to the complete ChIP-seq protocol but does not express any FLAG-tagged proteins. The q-value [false discovery rate (FDR)] cutoff for called peaks was 0.0001. Peaks were rank-ordered according to fold-enrichment (fold-enrichment values across all 788 called FLAG-GapR peaks range from 8-fold to 139-fold; the median enrichment is 22-fold). Raw reads from published ChIP-seq experiments were obtained for RpoD (33), GcrA (33), MucR1/2 (12), and SciP (12) from the European Nucleotide Archive and analyzed using the same workflow described above for GapR and CtrA. ChIP-seq peaks for MucR1, MucR2, and SciP were previously defined as 50-bp genomic intervals in which a significant enrichment in reads was observed (12). These 50-bp bins were extended by 100 bp in each direction to yield 250-bp intervals before overlap analysis. Intersection of ChIP-seq peaks (as shown in Fig. 4B) is defined as any amount of overlap between genomic intervals covered by GapR ChIP-seq peaks and ChIP-seq peaks for the indicated master regulators. Consensus sequences common to subsets of GapR-associated loci were identified by scanning peak sequences for conserved motifs using MEME (25, 81) and MEME-ChIP (82). Heatmap visualization (Fig. 3D) of enriched GapR ChIP-seq signal in intergenic/promoter regions was accomplished using deepTools (83). Each row of the heatmap represents a separate ORF and upstream region in Caulobacter. To generate the heat map, the ChIP-seq read counts near the translation start site of every ORF in the NA1000 genome was collected, internally normalized to total sample sequencing coverage, and binned into 10-bp bins. To create each heatmap row, the intensity of each bin in the row was calculated as the log2(ratio) of the corresponding normalized signal from the FLAG-GapR ChIP-seq data and the NA1000 (negative control) ChIP-seq data. Rows were sorted by maximum bin value, from highest (top) to lowest (bottom).
RNA Extraction and Reverse Transcription.
RNA was extracted using TRIzol from Life Technologies following manufacturer’s suggestions, using Zymo RNA Clean and Concentrator spin columns. RNA was DNase-digested after elution with Baseline Zero DNase (Epicentre). DNase-treated RNA was then either processed further for library prep or was reverse transcribed for qPCR using SuperScript III First-Strand Reverse Transcription kit (Thermo Fisher Scientific). For the RT step, random hexamers were used to prime the reaction, and RNaseH was used to hydrolyze template RNA.
RNA-seq Library Preparation and Sequencing.
Paired-end libraries for RNA-seq were prepared by first removing rRNA from the DNase-digested RNA (RNA Extraction and Reverse Transcription) using the Ribo-Zero Magnetic Kit (Epicentre) following manufacturer’s protocols. The remaining steps of the library prep was performed using ScriptSeq v2 (Epicentre) following manufacturer’s protocols. The cDNA libraries were sequenced on an Illumina NextSeq at the Stanford Functional Genomics Core Facility.
RNA-seq Data Analysis.
Sequences were aligned to the NA1000 genome (CP001340.1) using CLC workbench version 8.0. Unmapped reads were discarded. Raw counts were normalized using the DESeq method in R. Once normalized, the NOISeq package for R was used to simulate replicate data and to then calculate a probability that each gene was differentially expressed (pDE) between induced and uninduced depletion in each strain. Genes whose pDEs were equal to or above 0.9 and whose absolute log2 fold change in expression was greater than or equal to 1 qualified as differentially expressed within that strain. The genes in Table S3 are the transcripts detected as differentially expressed in the DPR1181 GapR depletion strain and whose pDE <0.9 in the DPR1133 control strain. Table S3 excludes three noncoding RNAs and two genes associated with transposable elements that would otherwise be listed (Dataset S4). Table S4 lists genes that were each determined to be differentially expressed after mock GapR depletion in the control strain DPR1133, but whose direction of change upon induction differs between the two strains DPR1133 and DPR1181. This analysis was also performed (using the relevant data) to identify the transcripts that were differentially expressed after proteolytic depletion of CtrA.
qPCR.
Extracted RNA was DNase digested and subject to RT (RNA Extraction and Reverse Transcription) before quantitative PCR (qPCR). qPCR was performed on an Applied Biosciences Fast7500 and using FastSYBR Master Mix from Thermo. The thermocycling conditions are as follows: after initial heating at 95 °C for 20 s, for 40 cycles: 95 °C for 3 s, 60 °C for 30 s. Primers for pilA (fwd: 5′-CTGGTTTAGGAGACCAAGTC-3′, rev: 5′-ATCAGGGCGACGATCAGG-3′, efficiency = 98%), gapR (fwd: 5′-CATGGAGCAGATCAAGGAAGTC-3′, rev: 5′-GGATACGGACCACCTTCTTGAG-3′, efficiency = 96%), CCNA_03711 (fwd: 5′-ATCGGGTCAGCAACTCATCA-3′, rev:5′-CCTTTTGTAGCGCCTGATGC-3′, efficiency = 92%), and CCNA_02831 (fwd: 5′-AGCGCAATTCAACCAGTTCC-3′, rev: 5′-GCCCAATTGTTTTCCGACCA-3′, efficiency = 96%) were used to measure expression levels of these four genes. Rho was used as an endogenous control for all experiments, using primers from ref. 84 with measured amplification efficiency = 97% (84).
Statistical Tests for Analysis of Overlapping Chromosomal Features.
GapR ChIP-seq peaks and overlap cycle-regulated TSSs.
A Pearson’s χ2 test with Yates’ continuity correction was performed to determine whether GapR ChIP-seq peaks are significantly enriched over cell cycle-regulated (CCR) TSSs in Caulobacter using TSSs from ref. 14. The χ2 test was performed by classifying TSSs as CCR or non-CCR [TSSs determined to be cell cycle-regulated by both Zhou et al. (14) and Fang et al. (34) qualified as CCR here; Dataset S3] and also as GapR-bound or not GapR-bound (by checking for overlap between ChIP-seq peak intervals and TSS coordinates). Overlap was called if the single base pair of a TSS overlapped any part of a GapR peak. The expected number of overlaps of GapR peaks and cell cycle-regulated TSSs (assuming independent distribution over the chromosome) was calculated as less than 83, whereas 103 such overlaps were observed (Pearson’s χ2 test, P < 0.01). In all, GapR peaks overlap 31.6% of cell cycle-regulated TSSs. P values were calculated using R.
GapR ChIP-seq peaks overlap transcription factor ChIP-seq peaks.
A Pearson’s χ2 test with Yates’ continuity correction was performed to determine whether GapR footprints overlapped significantly with those of other transcription factors based on ChIP-seq peaks. Specifically, intergenic regions in Caulobacter (CP001340.1) were tested for the presence of GapR ChIP-seq peaks, as well as for the presence of overlapping ChIP-seq peak of another gene product (CtrA, GcrA, MucR1, MucR2, SciP). Intergenic regions were classified as containing ChIP-seq peaks of both, only one, or neither gene product, and a contingency table was built from these overlap statistics. To contain both types of peaks, an intergenic region was required to contain two peaks that actually overlapped (for highest stringency). In this test, 1-bp overlap between any two features qualified as overlap. The details about the ChIP-seq data used are listed above (ChIP-seq Data Analysis) and P values were calculated in R. For SciP and GapR, P > 0.05, so GapR peaks are not reported as being significantly enriched for SciP peaks.
Three-way and four-way intersections in a network diagram (for Fig. 4C).
In Fig. 4C we display intergenic regions that are connected by their association with specific proteins. For any intergenic region, two or more proteins need not overlap one another directly to be connected; instead, they must simply overlap the same intergenic regions. This method of calling overlap leads to adequate representation of the intersections between ChIP-seq profiles of the different proteins; of the intergenic regions harboring ChIP-seq peaks for MucR1, CtrA, and GapR, for example, only 1 of these 21 intergenic regions contains ChIP-seq peaks for two transcription factors that do not directly overlap. Moreover, detection of direct overlap of two gene products in a given intergenic region by ChIP-seq may not be required for those two proteins to share control of nearby genes or to function together. In this test, 1-bp overlap between any two features qualified as overlap. We used Gephi 0.9.1 (85) to make the diagram. MucR2 is left out of the Network Diagram for simplicity and because MucR2 does not bind over intergenic regions containing ChIP-seq peaks for both GapR and CtrA without MucR1. Intergenic regions not associated with ChIP-seq peaks of any of these proteins are not included in the diagram. We then calculated the expected number of intergenic regions bound by all three regulators under the assumption that these three kinds of ChIP-seq peaks are independently distributed over the chromosome. This value was calculated by multiplying the expected number of intergenic regions containing CtrA and MucR1 by the frequency with which intergenic MucR1 peaks overlap GapR peaks, as well as by the frequency with which intergenic CtrA peaks overlap GapR peaks (same as the frequency for all CtrA peaks, 55.3%). The expected number of intergenic regions under these assumptions is 11.7.
Binomial test for asymmetrical distribution of MucR1-bound CtrA sites on the chromosome.
To confirm whether the distribution of MucR1-bound CtrA sites on the chromosome were significantly biased toward the origin-proximal half of the chromosome, an exact binomial test was performed. The number of trials was 26 (because there are a total of 26 sites on the chromosome where these two TFs overlap) and the number of successes was 22 (because 22 such sites lie in the origin-proximal half of the chromosome). Finally, the probability that a given protein might be found in the origin-proximal half of the chromosome was set equal to the fraction of that protein’s total ChIP-seq peaks found in that half of the chromosome. P values were calculated using R. For CtrA, P = 0.015, and, for MucR1, P = 0.0047. Even though GapR binds nearly all of these peaks, there is no bias in the localization of GapR peaks to one hemisphere of the chromosome over the other (only 52% of GapR ChIP-seq peaks are located in the origin-proximal half of the chromosome).
SI Text
Viable ∆gapR Mutants of Caulobacter NA1000 Cannot Be Isolated.
We sought to confirm the predicted essentiality of gapR by first attempting to isolate a stable ∆gapR insertion/deletion strain (∆gapA::Ω, where the Ω cassette confers dual resistance to streptomycin and spectinomycin) by conventional allelic replacement using the suicide vector pNPTS138 (67) containing the Ω cassette sandwiched between upstream and downstream sequences that flank the gapR ORF in the NA1000 genome. Although we were able to generate spectinomycin-resistant (SpecR) gapR+ ∆gapR::Ω intermediate cointegrates, all 100 isolates screened following counterselection on sucrose proved to be WT (gapR+) revertants (indicated by the fact that all sucrose-resistant isolates tested were also SpecS), suggesting that the gapR product is indeed essential for viability in Caulobacter.
Our first attempt to construct a gapR depletion strain used a conventional strategy in which the sole allele of gapR in the genome is controlled by the xylose-inducible PxylX promoter or the vanillate-inducible PvanA promoter at their respective genomic loci (xylX and vanA) (70). Although we were able to successfully replace gapR with the Ω cassette in xylX::gapR or vanA::gapR merodiploid strains in the presence of xylose or vanillate (respectively), indicating that ectopic gapR expression restores viability of a ∆gapR::Ω mutant, the resulting strains grew poorly in both rich and minimal media even in the presence of inducer, suggesting that constitutive gapR expression from the PxylX and PvanA promoters may be insufficient to maintain appropriate levels of GapR. The PvanA-gapR strain was particularly unstable and rapidly accumulated suppressor mutations, as evidenced by the appearance of fast-growing colonies that no longer require vanillate for growth.
Depletion of GapR Does Not Cause Global Changes in Transcript Abundances.
To determine whether GapR directly contributes to the activity of bound promoters, we used RNA-seq to quantify the relative abundance of all Caulobacter mRNAs in cells depleted for GapR (+0.3% xylose) compared with GapR+ cells (− xylose). As a positive control, we also performed RNA-seq on strains depleted for the master regulator CtrA, the regulon for which has been established through a variety of targeted and global transcriptomic experiments (86–88). We grew GapR or CtrA proteolytic depletion strains to midexponential phase in M2G and then backdiluted the growing cultures in M2 minimal media containing glucose (to maintain normal levels of GapR or CtrA) or xylose (to induce PxylX-dependent sspB expression and promote SspB-mediated proteolysis of GapR or CtrA). Following 1 h of parallel growth in permissive or depleting conditions, which is sufficient for the elimination of ssrA-tagged proteins using our depletion method (Fig. S1), we harvested cells from each condition, extracted total RNA, and generated libraries of rRNA-depleted, barcoded cDNA that was ultimately subjected to deep sequencing.
Comparative analysis of transcript levels in CtrA-depleted cultures (i.e., following growth in xylose) compared with CtrA-replete cultures revealed significant differences in transcript abundances for genes belonging to the known CtrA regulon, including genes involved in regulation of cell cycle progression (e.g., sciP, ccrM, rcdA, pleD, divK, perP, dgcB), motility and chemotaxis (e.g., che, mcpA, motA), and polar appendage biogenesis (cpa, hfa, hfs, flg, fli, flj, pilA). This finding confirms the validity of our targeted depletion approach, which reveals the transcriptional consequences of SspB-directed depletion of a tagged transcription factor.
Conversely, we detected few significant changes in mRNA abundance relative to the mock depletion control when GapR was depleted using this method; only 20 genes (Tables S2 and S3) display changes in expression that differ substantially between the experimental and control strains at 1 h after initiating the proteolysis of GapR.
These findings were verified using an independent method in which a panel of candidate GapR target genes we selected for qRT-PCR (Fig. S5B). As a template, total RNA was extracted, processed, and reverse transcribed (as described in SI Materials and Methods) from the GapR depletion strain following 60 min of GapR depletion (cells were pelleted as technical replicates from the very same cultures from which RNA was extracted for RNA-seq experiments). In addition, qPCR was performed to measure gene expression in a strain of Caulobacter that constitutively overexpresses gapR transcript from the native gapR promoter on a low-copy plasmid (Fig. S5A).
Unlike the transcription silencing, AT-binding NAP H-NS, whose depletion leads to the activation of hundreds of repressed genes in the E. coli genome (19, 89, 90), depletion of GapR seems not to induce a similarly broad effect in Caulobacter when depleted. On the other hand, the slight (approximately twofold) change in transcript abundance observed for several genes associated with GapR peaks on GapR overexpression or depletion indicates a possible, albeit minor, role for GapR in direct regulation of gene expression.
Although it remains possible that GapR influences gene expression on longer timescales, the transcript abundance of GapR-associated genes was generally unchanged after GapR was eliminated from the cell. The lack of an obvious effect on gene expression distinguishes this nucleoid-associated protein from conventional transcriptional repressors or activators like CtrA, whose target genes were significantly induced or silenced, suggesting that any effect GapR has on gene expression is likely to be indirect, or minimally, that GapR’s mechanism of action differs from the mechanism of action of canonical transcription factors.
Cell Cycle-Regulated Promoters Are Not Especially AT-Rich.
Because GapR associates with both regions of high AT content and with cell cycle-regulated genes, we determined the general relationship between AT content and cell cycle regulation of Caulobacter promoters. For each TSS in Caulobacter, the GC content of a 100-bp window containing the TSS at its center was calculated. TSSs were considered either CCR or non-CCR for this analysis only if they were classified the same way (as CCR or as non-CCR) in two independent studies performed by Zhou et al. (14) and Fang et al. (34) (Dataset S3). The median and interquartile ranges (IQRs) of AT content of these CCR and non-CCR promoters were very similar (40% median AT content, 8% IQR for CCR promoters vs. 39% median AT content, 7% IQR for non-CCR promoters). Therefore, the observation that GapR associates with nearly one-third of all cell cycle-regulated promoters is not a trivial consequence of the observation that it binds AT-rich DNA.
GapR ChIP-seq Peaks Are Primarily Intergenic.
Intergenic regions were determined using the Cauobacter genome to which ChIP-seq peaks were aligned (NA1000, CP001340.1) (35). The extent of overlap between GapR ChIP-seq peaks and intergenic regions in Caulobacter was explored beyond what is presented in the main text. Specifically, using liberal requirements for overlap (minimum 1-bp overlap between a peak and an intergenic region required), 99.3% of all GapR ChIP-seq peaks overlapped intergenic regions. Using more strict requirements for calling overlap, i.e., where at least 60% of the shorter element must covered by the other element to call overlap, 89.5% of all GapR ChIP-seq peaks overlapped intergenic regions.
GapR Overlaps MucR1-Bound CtrA Sites on the Chromosome.
GapR ChIP-seq peaks overlap a vast majority of the sites on the chromosome where CtrA overlaps with MucR1, according to the ChIP-seq data referenced in SI Materials and Methods (24/26, 92.3%, throughout both intergenic and genic regions). Further analysis of ChIP-seq data revealed that GapR associates with nearly all (21/23, 91.3%) of the intergenic regions bound by both MucR1 and CtrA peaks, exceeding the expected number (see network diagram in Fig. 4C; 11.7 intergenic regions expected vs. 21 observed, SI Materials and Methods). In contrast, despite some pairwise overlap between the ChIP-seq footprints of the three regulators MucR1, CtrA, and GcrA, no intergenic regions that harbor both MucR1 and CtrA are also bound by the master regulator GcrA (without also being bound by GapR; Fig. 4C).
Supplementary Material
Acknowledgments
We thank Emma Chory, Gerald Crabtree, Elizabeth Zuo, and Michael Eckart for outstanding technical assistance, and members of the L.S. laboratory for critical review of this manuscript. This work was supported by Ruth L. Kirchstein NIH F32 Postdoctoral Fellowship F32GM109650 (to D.P.R.), National Science Foundation (NSF) Graduate Research Fellowship DGE-114747 (to M.D.M.), Gordon & Betty Moore Foundation Life Science Research Foundation Postdoctoral Fellowship GBMF2550.03 (to K.L.), NSF Inspire Award CCF344284 (to D.L.D. and H.H.M.), and NIH Grants R01 GM32506 and R35 GM11807101 (to L.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: Sequence data have been deposited to the Sequence Read Archive (SRA) (accession nos. SRR4076697–SRR4076704).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612579113/-/DCSupplemental.
References
- 1.Dillon SC, Dorman CJ. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol. 2010;8(3):185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 2.Cagliero C, Grand RS, Jones MB, Jin DJ, O’Sullivan JM. Genome conformation capture reveals that the Escherichia coli chromosome is organized by replication and transcription. Nucleic Acids Res. 2013;41(12):6058–6071. doi: 10.1093/nar/gkt325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorman CJ. Genome architecture and global gene regulation in bacteria: Making progress towards a unified model? Nat Rev Microbiol. 2013;11(5):349–355. doi: 10.1038/nrmicro3007. [DOI] [PubMed] [Google Scholar]
- 4.Le TB, Imakaev MV, Mirny LA, Laub MT. High-resolution mapping of the spatial organization of a bacterial chromosome. Science. 2013;342(6159):731–734. doi: 10.1126/science.1242059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marbouty M, et al. Condensin- and replication-mediated bacterial chromosome folding and origin condensation revealed by Hi-C and super-resolution imaging. Mol Cell. 2015;59(4):588–602. doi: 10.1016/j.molcel.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Umbarger MA, et al. The three-dimensional architecture of a bacterial genome and its alteration by genetic perturbation. Mol Cell. 2011;44(2):252–264. doi: 10.1016/j.molcel.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick CL, Viollier PH. Decoding Caulobacter development. FEMS Microbiol Rev. 2012;36(1):193–205. doi: 10.1111/j.1574-6976.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo MF, Shapiro L, Gober J. Asymmetric expression of the gyrase B gene from the replication-competent chromosome in the Caulobacter crescentus predivisional cell. J Bacteriol. 1993;175(21):6970–6981. doi: 10.1128/jb.175.21.6970-6981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domian IJ, Quon KC, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90(3):415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 10.Holtzendorff J, et al. Oscillating global regulators control the genetic circuit driving a bacterial cell cycle. Science. 2004;304(5673):983–987. doi: 10.1126/science.1095191. [DOI] [PubMed] [Google Scholar]
- 11.Ardissone S, et al. Cell cycle constraints on capsulation and bacteriophage susceptibility. eLife. 2014;3:3. doi: 10.7554/eLife.03587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fumeaux C, et al. Cell cycle transition from S-phase to G1 in Caulobacter is mediated by ancestral virulence regulators. Nat Commun. 2014;5:4081. doi: 10.1038/ncomms5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gora KG, et al. A cell-type-specific protein-protein interaction modulates transcriptional activity of a master regulator in Caulobacter crescentus. Mol Cell. 2010;39(3):455–467. doi: 10.1016/j.molcel.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B, et al. The global regulatory architecture of transcription during the Caulobacter cell cycle. PLoS Genet. 2015;11(1):e1004831. doi: 10.1371/journal.pgen.1004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collier J. Regulation of chromosomal replication in Caulobacter crescentus. Plasmid. 2012;67(2):76–87. doi: 10.1016/j.plasmid.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Jensen RB, Shapiro L. The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc Natl Acad Sci USA. 1999;96(19):10661–10666. doi: 10.1073/pnas.96.19.10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SF, Thompson MA, Schwartz MA, Shapiro L, Moerner WE. Super-resolution imaging of the nucleoid-associated protein HU in Caulobacter crescentus. Biophys J. 2011;100(7):L31–L33. doi: 10.1016/j.bpj.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MA, Shapiro L. An SMC ATPase mutant disrupts chromosome segregation in Caulobacter. Mol Microbiol. 2011;82(6):1359–1374. doi: 10.1111/j.1365-2958.2011.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorman CJ. H-NS: A universal regulator for a dynamic genome. Nat Rev Microbiol. 2004;2(5):391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 20.Goley ED, Toro E, McAdams HH, Shapiro L. Dynamic chromosome organization and protein localization coordinate the regulatory circuitry that drives the bacterial cell cycle. Cold Spring Harb Symp Quant Biol. 2009;74:55–64. doi: 10.1101/sqb.2009.74.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams HH, Shapiro L. System-level design of bacterial cell cycle control. FEBS Lett. 2009;583(24):3984–3991. doi: 10.1016/j.febslet.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christen B, et al. The essential genome of a bacterium. Mol Syst Biol. 2011;7:528. doi: 10.1038/msb.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith KL, Grossman AD. Inducible protein degradation in Bacillus subtilis using heterologous peptide tags and adaptor proteins to target substrates to the protease ClpXP. Mol Microbiol. 2008;70(4):1012–1025. doi: 10.1111/j.1365-2958.2008.06467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nierman WC, et al. Complete genome sequence of Caulobacter crescentus. Proc Natl Acad Sci USA. 2001;98(7):4136–4141. doi: 10.1073/pnas.061029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey TL, et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server Issue):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui F, Zhurkin VB. Distinctive sequence patterns in metazoan and yeast nucleosomes: Implications for linker histone binding to AT-rich and methylated DNA. Nucleic Acids Res. 2009;37(9):2818–2829. doi: 10.1093/nar/gkp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon BR, et al. Structural basis for recognition of AT-rich DNA by unrelated xenogeneic silencing proteins. Proc Natl Acad Sci USA. 2011;108(26):10690–10695. doi: 10.1073/pnas.1102544108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timchenko T, Bailone A, Devoret R. Btcd, a mouse protein that binds to curved DNA, can substitute in Escherichia coli for H-NS, a bacterial nucleoid protein. EMBO J. 1996;15(15):3986–3992. [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon BR, Imperial R, Wang L, Navarre WW, Liu J. Lsr2 of Mycobacterium represents a novel class of H-NS-like proteins. J Bacteriol. 2008;190(21):7052–7059. doi: 10.1128/JB.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding P, et al. A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT. PLoS Pathog. 2015;11(6):e1004967. doi: 10.1371/journal.ppat.1004967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali SS, Xia B, Liu J, Navarre WW. Silencing of foreign DNA in bacteria. Curr Opin Microbiol. 2012;15(2):175–181. doi: 10.1016/j.mib.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Navarre WW, et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313(5784):236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 33.Haakonsen DL, Yuan AH, Laub MT. The bacterial cell cycle regulator GcrA is a σ70 cofactor that drives gene expression from a subset of methylated promoters. Genes Dev. 2015;29(21):2272–2286. doi: 10.1101/gad.270660.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang G, et al. Transcriptomic and phylogenetic analysis of a bacterial cell cycle reveals strong associations between gene co-expression and evolution. BMC Genomics. 2013;14:450. doi: 10.1186/1471-2164-14-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrader JM, et al. The coding and noncoding architecture of the Caulobacter crescentus genome. PLoS Genet. 2014;10(7):e1004463. doi: 10.1371/journal.pgen.1004463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueguchi C, Seto C, Suzuki T, Mizuno T. Clarification of the dimerization domain and its functional significance for the Escherichia coli nucleoid protein H-NS. J Mol Biol. 1997;274(2):145–151. doi: 10.1006/jmbi.1997.1381. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Li GW, Chen C, Xie XS, Zhuang X. Chromosome organization by a nucleoid-associated protein in live bacteria. Science. 2011;333(6048):1445–1449. doi: 10.1126/science.1204697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 1998;95(10):5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RS. Protein signatures distinctive of alpha proteobacteria and its subgroups and a model for alpha-proteobacterial evolution. Crit Rev Microbiol. 2005;31(2):101–135. doi: 10.1080/10408410590922393. [DOI] [PubMed] [Google Scholar]
- 40.Gupta RS, Mok A. Phylogenomics and signature proteins for the alpha proteobacteria and its main groups. BMC Microbiol. 2007;7:106. doi: 10.1186/1471-2180-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kainth P, Gupta RS. Signature proteins that are distinctive of alpha proteobacteria. BMC Genomics. 2005;6:94. doi: 10.1186/1471-2164-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis AC, Jones NS, Porter MA, Deane CM. What evidence is there for the homology of protein-protein interactions? PLOS Comput Biol. 2012;8(9):e1002645. doi: 10.1371/journal.pcbi.1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad I, Schaefler S. Regulation of the beta-glucoside system in Escherchia coli K-12. J Bacteriol. 1974;120(2):638–650. doi: 10.1128/jb.120.2.638-650.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spurio R, et al. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: Ultramicroscopic and molecular autopsy. Mol Gen Genet. 1992;231(2):201–211. doi: 10.1007/BF00279792. [DOI] [PubMed] [Google Scholar]
- 45.May G, Dersch P, Haardt M, Middendorf A, Bremer E. The osmZ (bglY) gene encodes the DNA-binding protein H-NS (H1a), a component of the Escherichia coli K12 nucleoid. Mol Gen Genet. 1990;224(1):81–90. doi: 10.1007/BF00259454. [DOI] [PubMed] [Google Scholar]
- 46.Dorman CJ. Virulence gene regulation in Shigella. Ecosal Plus. 2004;1(1) doi: 10.1128/ecosalplus.8.9.3. [DOI] [PubMed] [Google Scholar]
- 47.Castang S, Dove SL. Basis for the essentiality of H-NS family members in Pseudomonas aeruginosa. J Bacteriol. 2012;194(18):5101–5109. doi: 10.1128/JB.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ely B, Gibbs W, Diez S, Ash K. The Caulobacter crescentus transducing phage Cr30 is a unique member of the T4-like family of myophages. Curr Microbiol. 2015;70(6):854–858. doi: 10.1007/s00284-015-0799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elde NC, Malik HS. The evolutionary conundrum of pathogen mimicry. Nat Rev Microbiol. 2009;7(11):787–797. doi: 10.1038/nrmicro2222. [DOI] [PubMed] [Google Scholar]
- 50.Gill JJ, et al. The Caulobacter crescentus phage phiCbK: Genomics of a canonical phage. BMC Genomics. 2012;13:542. doi: 10.1186/1471-2164-13-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panis G, Lambert C, Viollier PH. Complete genome sequence of Caulobacter crescentus bacteriophage φCbK. J Virol. 2012;86(18):10234–10235. doi: 10.1128/JVI.01579-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132(1):294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evinger M, Agabian N. Caulobacter crescentus nucleoid: Analysis of sedimentation behavior and protein composition during the cell cycle. Proc Natl Acad Sci USA. 1979;76(1):175–178. doi: 10.1073/pnas.76.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward D, Newton A. Requirement of topoisomerase IV parC and parE genes for cell cycle progression and developmental regulation in Caulobacter crescentus. Mol Microbiol. 1997;26(5):897–910. doi: 10.1046/j.1365-2958.1997.6242005.x. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, Mallick B, Nagaraja V. Direct regulation of topoisomerase activity by a nucleoid-associated protein. Nucleic Acids Res. 2014;42(17):11156–11165. doi: 10.1093/nar/gku804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skoko D, et al. Mechanism of chromosome compaction and looping by the Escherichia coli nucleoid protein Fis. J Mol Biol. 2006;364(4):777–798. doi: 10.1016/j.jmb.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brackley CA, Taylor S, Papantonis A, Cook PR, Marenduzzo D. Nonspecific bridging-induced attraction drives clustering of DNA-binding proteins and genome organization. Proc Natl Acad Sci USA. 2013;110(38):E3605–E3611. doi: 10.1073/pnas.1302950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tupper AE, et al. The chromatin-associated protein H-NS alters DNA topology in vitro. EMBO J. 1994;13(1):258–268. doi: 10.1002/j.1460-2075.1994.tb06256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmerman SB. Shape and compaction of Escherichia coli nucleoids. J Struct Biol. 2006;156(2):255–261. doi: 10.1016/j.jsb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Summers EL, et al. The structure of the oligomerization domain of Lsr2 from Mycobacterium tuberculosis reveals a mechanism for chromosome organization and protection. PLoS One. 2012;7(6):e38542. doi: 10.1371/journal.pone.0038542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azam TA, Ishihama A. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J Biol Chem. 1999;274(46):33105–33113. doi: 10.1074/jbc.274.46.33105. [DOI] [PubMed] [Google Scholar]
- 62.Swiercz JP, Nanji T, Gloyd M, Guarné A, Elliot MA. A novel nucleoid-associated protein specific to the actinobacteria. Nucleic Acids Res. 2013;41(7):4171–4184. doi: 10.1093/nar/gkt095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleine Borgmann LA, Ries J, Ewers H, Ulbrich MH, Graumann PL. The bacterial SMC complex displays two distinct modes of interaction with the chromosome. Cell Reports. 2013;3(5):1483–1492. doi: 10.1016/j.celrep.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Viollier PH, Shapiro L. Spatial complexity of mechanisms controlling a bacterial cell cycle. Curr Opin Microbiol. 2004;7(6):572–578. doi: 10.1016/j.mib.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Ash K, et al. A comparison of the Caulobacter NA1000 and K31 genomes reveals extensive genome rearrangements and differences in metabolic potential. Open Biol. 2014;4(10):140128. doi: 10.1098/rsob.140128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casadaban MJ. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 67.Ried JL, Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57(2-3):239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 68.Kirkpatrick CL, Viollier PH. Synthetic interaction between the TipN polarity factor and an AcrAB-family efflux pump implicates cell polarity in bacterial drug resistance. Chem Biol. 2014;21(5):657–665. doi: 10.1016/j.chembiol.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 69.Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 1991;204:372–384. doi: 10.1016/0076-6879(91)04019-k. [DOI] [PubMed] [Google Scholar]
- 70.Thanbichler M, Iniesta AA, Shapiro L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 2007;35(20):e137. doi: 10.1093/nar/gkm818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engler C, Kandzia R, Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 2008;3(11):e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Battesti A, Bouveret E. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods. 2012;58(4):325–334. doi: 10.1016/j.ymeth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 74.Gibson DG. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011;498:349–361. doi: 10.1016/B978-0-12-385120-8.00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blankenberg D, et al. Galaxy: A web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol. 2010;19:19.10.1–19.10.21. doi: 10.1002/0471142727.mb1910s89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giardine B, et al. Galaxy: A platform for interactive large-scale genome analysis. Genome Res. 2005;15(10):1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robinson JT, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor J, Schenck I, Blankenberg D, Nekrutenko A. Using galaxy to perform large-scale interactive data analyses. Curr Protoc Bioinformatics. 2007;19:10.5.1–10.5.25. doi: 10.1002/0471250953.bi1005s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bailey TL, Johnson J, Grant CE, Noble WS. The MEME Suite. Nucleic Acids Res. 2015;43(W1):W39-49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Machanick P, Bailey TL. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics. 2011;27(12):1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T. deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42(Web Server Issue):W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazzon RR, Lang EA, Silva CA, Marques MV. Cold shock genes cspA and cspB from Caulobacter crescentus are posttranscriptionally regulated and important for cold adaptation. J Bacteriol. 2012;194(23):6507–6517. doi: 10.1128/JB.01422-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacomy M, Venturini T, Heymann S, Bastian M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One. 2014;9(6):e98679. doi: 10.1371/journal.pone.0098679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci USA. 2002;99(7):4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laub MT, McAdams HH, Feldblyum T, Fraser CM, Shapiro L. Global analysis of the genetic network controlling a bacterial cell cycle. Science. 2000;290(5499):2144–2148. doi: 10.1126/science.290.5499.2144. [DOI] [PubMed] [Google Scholar]
- 88.Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84(1):83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 89.Dorman CJ, Deighan P. Regulation of gene expression by histone-like proteins in bacteria. Curr Opin Genet Dev. 2003;13(2):179–184. doi: 10.1016/s0959-437x(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 90.Dorman CJ. Global regulators and environmental adaptation in Gram-negative pathogens. Clin Microbiol Infect. 2009;15(Suppl 1):47–50. doi: 10.1111/j.1469-0691.2008.02684.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.