Abstract
Background
Despite substantial progress in the delivery of HIV prevention programs, some communities continue to experience high rates of HIV infection. We report on temporal trends in HIV prevalence in pregnant women in a community in rural KwaZulu-Natal (KZN) in South Africa.
Methods
Annual, anonymous cross-sectional HIV sero-prevalence surveys were conducted between 2001 and 2013 amongst first visit prenatal clinic attendees. The time periods 2001 to 2003 were defined as pre-antiretroviral therapy (ART), 2004 to 2008 as early ART and 2009 to 2013 as contemporary ART roll-out to correspond with the substantial scale-up of ART program.
Results
Overall, HIV prevalence rose from 35.3% [95% confidence interval (CI) 32.3–38.3] pre-ART (2001–2003) to 39.0% (CI: 36.8–41.1) in the early ART (2004–2008) to 39.3% (CI: 37.2–41.4) in the contemporary ART (2009–2013) roll-out periods. In teenage women (<20 years), HIV prevalence declined from 22.5% (CI: 17.5–27.5) to 20.7% (CI: 17.5–23.8) and to 17.2% (CI: 14.3–20.2) over the similar ART roll-out periods (p=0.046). Prevalence increased significantly in women 30 years and older (p<0.001) over the same time period largely due to survival following ART scale up. Teenage girls with male partners 20–24 and ≥25 years had a 1.7-fold (CI: 1.3–2.4; p=0.001) and 3-fold (CI: 2.1–4.3; p<0.001) higher HIV prevalence respectively.
Conclusion
Notwithstanding the encouraging decline in teenagers, the ongoing high HIV prevalence in pregnant women in this rural community, despite prevention and treatment programs, is deeply concerning. Targeted interventions for teenagers, especially for those in age-disparate relationships, are needed to impact this HIV epidemic trajectory.
Keywords: HIV, prevalence, young pregnant women, rural South Africa
Introduction
In 2012, the Joint United Nations Program on HIV/AIDS (UNAIDS) estimated that approximately 6.1 million (95% CI, 5.8–6.4 million) people in South Africa were living with human immunodeficiency virus (HIV), having the highest burden of infection in the world despite being home to <1% of the global population.1 The epidemic in South Africa which also has the highest number of AIDS cases globally has been described as “hyper-endemic, generalized and mature”.2 Preventing further infections in such settings requires an in depth understanding of the epidemic and to design customized locally responsive interventions accordingly.3–5
Since 2002, the two dose nevirapine regimen to prevent mother to child transmission (PMTCT) of HIV has been available to all pregnant HIV seropositive women who choose to have an HIV test and accept the PMTCT intervention. Parallel to these services, through the district hospitals, the South African Ministry of Health initiated antiretroviral therapy (ART) provision to HIV positive adults meeting the ART eligibility criteria of CD4 cell count of <200/μl. By March 2005, at least one service point for AIDS related care and treatment was established in each of the country’s 53 districts. By 2008 about 588 000 people had initiated ART;6 and contemporary ART roll-out continued to improve, with almost two million adults and children were receiving ART in 2011, representing just over 50% of those eligible for treatment under World Health Organization (WHO) guidelines of CD4 cell count of <350/μl.7, 8
In 2004, the Centre for the AIDS Programme of research in South Africa (CAPRISA) through its research facilities in rural Vulindlela, uMgungundlovu district, KwaZulu-Natal (KZN) initiated the free provision of ART to patients with AIDS who met the eligibility criteria defined by the Department of Health9. Over time the decentralization of services and nurse initiated management of ART through primary health care (PHC) clinics facilitated the roll-out, scale up and the retention of patients in ART programmes. In parallel, the burden of managing uncomplicated cases at referral hospitals also reduced. To enhance the rapid scale-up of ART, mobile teams help to initiate patients at hard to reach areas such as farms and for those having difficulty leaving their work premises to attend clinics. Community care givers play an important role and actively assist with tracing patients who default treatment and have been successful in reintroducing them back to the health facilities. Through this comprehensive and incremental approach, the highest HIV burden district of uMgungundlovu made remarkable progress on ART provision and the number of patients on ART increased from 61 230 in 201110 to 111 691 by 201411.
To monitor temporal trends in the evolving epidemic, the South African government has undertaken annual, anonymous, HIV prevalence surveys among pregnant women utilizing public sector prenatal clinics.12 These and other data13 have highlighted differences within and between South African provinces, with the province of KZN having the highest prevalence of HIV.14 As epidemics mature with increasing coverage of ART, prevalence of HIV infection is a less reliable marker of the evolving epidemic since survival improves and prevalence is expected to increase and masking of new infections. However, measuring HIV prevalence in young, pregnant women, <24 years of age provides a reliable indirect measurement of new infections as they are more likely to have recently become sexually active and therefore infection is more likely to be recent. Since 2001, we have conducted complementary annual HIV prevalence surveys in prenatal clinics in rural KZN in one of the three highest burden HIV health districts12 where extensive HIV prevention and treatments programs are being implemented. The purpose of this study was to assess the trends in HIV prevalence in pregnant women following the introduction and scale-up of ART and selected risk factors associated with HIV transmission in this setting.
Methods
Study setting
Surveys were undertaken in Vulindlela, a rural community located about 150 km west of Durban in the province of KZN, home to approximately 150 000 residents and characterized by high levels of poverty and unemployment.
Health care in Vulindlela is accessed from seven nurse-run PHC clinics that deliver at no cost an essential package of health services that includes prenatal care, family planning, childhood immunization, minor ailment services and management of common chronic conditions. HIV testing services are available at these clinics with pre-and post-test counseling.
Study procedures
The South African Department of Health’s National Antenatal Sentinel HIV and Syphilis Prevalence Surveys are conducted annually amongst pregnant women and blood samples are tested using a single enzyme-linked immunosorbent assay (ELISA) (Abbott Axsym System for HIV-1/HIV-2; Abbott Laboratories, Chicago, IL, USA) [10].
Coinciding with these surveys, we conducted cross sectional surveys from 1 October to 30 November of each year from 2001 to 2013. Consecutive pregnant women who presented for their first prenatal care visit at one of the seven primary health care clinics, regardless of age were eligible to participate.
All women were provided with study information on anonymized HIV testing for surveillance. As part of routine prenatal care blood samples are collected for hemoglobin and syphilis testing and women were requested to provide an additional sample for HIV testing. During each survey following verbal informed consent, trained nurses administered a questionnaire to obtain minimal key demographic and behavioral data. This included the age of the woman, her current partner’s age, if this was her first pregnancy, and dates of prior pregnancies, knowledge of HIV status and exposure to antiretroviral treatment (ART) were recorded on a standardized case report form labelled with a unique participant identification number. Peripheral blood was collected in prelabelled ethylenediaminetetraacetic acid (EDTA) and plain tubes. Samples were transported to the central laboratory in Durban for HIV testing after removal of all identifiers. With the aid of a unique participant identification number, HIV test results were linked to demographic and behavioral data; from 2004 to current partner’s age and prior pregnancies and from 2009 to exposure to ART. The survey protocol was in compliance with the World Health Organization’s (WHO) and South African Department of Health’s guidelines for the conduct of anonymized HIV surveys12, 15–17 and was reviewed and approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (Reference number E179/04).
Laboratory testing procedures
In 2001 and 2002, HIV testing was performed using an enzyme linked immunosorbent assay (ELISA) (Abbott AxSYM system for HIV-1/HIV-2, Abbott Park, Illinois, USA). In 2003, specimens were tested using a rapid assay (Abbott Determine, Abbott Park, Illinios, USA) with all positives confirmed using a second rapid assay (Capillus, Trinity Biotech, Bray, Wicklow, Ireland). From 2004 to 2013, specimens were tested by ELISA (Enzygnost, Dade Behring, Mannheim, Germany). Any specimen indicating an indeterminate result was further tested with the Abbott Determine rapid assay. These tests all perform similarly with sensitivities and specificities in excess of 98%.18
Data analysis
Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC, USA). All statistical tests are two sided and confidence intervals (CI) are at 95% level. Based on consistency in the age structure of the cohort over time, pooled summary data for women are reported for the time periods 2001 to 2003 (as pre-ART roll-out), 2004 to 2008 (early ART roll-out) and 2009 to 2013 (contemporary ART roll-out) to correspond with the delivery of the ART program in KZN7.
The overall HIV prevalence and 95% CI were calculated and reported for age stratified groups. The Cochran-Armitage χ2 test was used to test for linear trend in HIV prevalence over time. Log-binomial regression adjusted for previous pregnancy and clinic was used to determine whether age of current male sexual partner was associated with HIV infection in women. We included the clinic in the model to adjust for clustering because the clinics were of different sizes and HIV prevalence varied across clinics. We assumed that women who had been pregnant before were at high risk of HIV, and that previous pregnancy could confound age and HIV status. This analysis was stratified by the women’s age groups <20, 20–24 and ≥25 years.
Results
Demographic characteristics
Between 2001 and 2013, a total of 5075 pregnant women attending prenatal clinics in the rural Vulindlela sub district of KwaZulu-Natal were included in annual HIV prevalence surveys, with a mean of 390 (range 225–552) women surveyed per year.
The age distribution of surveyed women attending prenatal clinics showed that the proportion of women in the <20 year age group was 33.7% in the 2001–2003 period, 32.9% in the 2004–2008 period, and 30.6% in the 2009–2013 period and was the highest compared to other age groups. The proportion of women aged 20–24 years increased from 28.1%% in the 2001–2003 period to 30.3% in the period 2004–2008 and was 29.3% in the 2009–2013 period. However, the proportion of women in the age groups 25–29, 30–34 and ≥35 years was consistently lower across all time periods (Supplementary Table 1).
Temporal trends in HIV prevalence
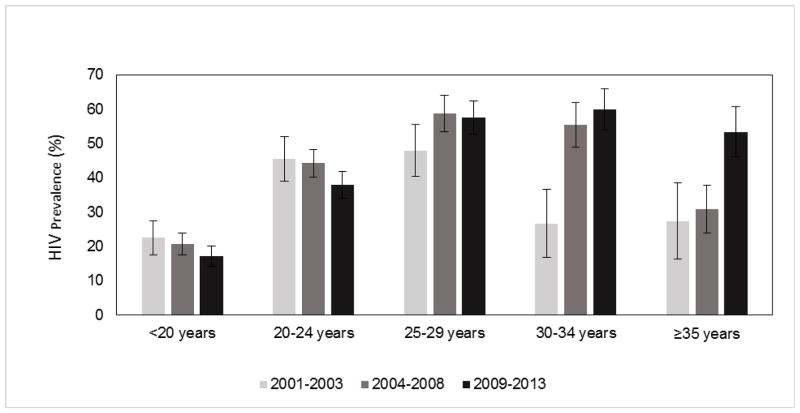
Table 1 shows the overall and age specific HIV prevalence. The overall HIV prevalence increased from 35.3% [95% confidence interval (CI) 32.3–38.3] in the period 2001–2003 to 39.0% (CI 36.8–41.1) in the period 2004–2008 to 39.3% (CI 37.2–41.4) in the 2009–2013 period (p=0.058). Age-stratified analysis showed that HIV prevalence in teenagers <20 years was 22.5% (CI 17.5–27.5) in the 2001–2003 period and decreased to 20.7% (CI 17.5–23.8) in the period 2004–2008 to 17.2% (CI 14.3–20.2) in the 2009–2013 period (p=0.046). Similarly, in women in the 20–24 year age group the prevalence was 45.5% (CI 38.9–52.0) in the period 2001–2003 and declined to 44.2% (CI 40.2–48.2) in the period 2004–2008 to 37.9% (CI 34.0–41.8) in the period 2009–2013 (p=0.018). In women aged 25–29 years, HIV prevalence remained relatively constant and was 47.9% (CI 40.3–55.5), 58.8% (CI 53.5–64.2) and 57.6% (CI 52.7–62.4); (p=0.085) over the same time period. Amongst women 30–34 years HIV prevalence increased from 26.7% (CI 16.7–36.7) to 55.5% (CI 49.0–62.0) to 59.9% (CI 53.9–66.0) (p<0.001) and in women 35 years and older HIV prevalence increased from 27.4% (CI 16.3–38.5) to 30.8% (CI 23.8–37.7) to 53.4% (CI 46.0–60.8); (p<0.001) over the same time periods. Figure 1 shows the decline in HIV prevalence in the <20 and 20 to 24 year age group, with prevalence increasing in the 25–29, 30–34 and ≥35 year age groups.
Table 1.
Temporal trends in HIV prevalence by age in pregnant women attending prenatal clinics in Vulindlela, rural KwaZulu-Natal by survey periods 2001–2003, 2004–2008 and 2009–2013.
| Age group | 2001–2003 | 2004–2008 | 2009–2013 | p-value† | |||
|---|---|---|---|---|---|---|---|
| n/N | % (95%CI) | n/N | % (95%CI) | n/N | % (95%CI) | ||
| <20 | 60/267 | 22.5 (17.5–27.5) | 133/644 | 20.7 (17.5–23.8) | 109/633 | 17.2 (14.3–20.2) | 0.046 |
| 20–24 | 101/222 | 45.5 (38.9–52.0) | 262/593 | 44.2 (40.2–48.2) | 230/607 | 37.9 (34.0–41.8) | 0.018 |
| 25–29 | 79/165 | 47.9 (40.3–55.5) | 190/323 | 58.8 (53.5–64.2) | 232/403 | 57.6 (52.7–62.4) | 0.085 |
| 30–34 | 20/75 | 26.7 (16.7–36.7) | 126/227 | 55.5 (49.0–62.0) | 151/252 | 59.9 (53.9–66.0) | <0.001 |
| ≥35 | 17/62 | 27.4 (16.3–38.5) | 52/169 | 30.8 (23.8–37.7) | 94/176 | 53.4 (46.0–60.8) | <0.001 |
|
| |||||||
| Total* | 345/977 | 35.3 (32.3–38.3) | 778/1996 | 39.0 (36.8–41.1) | 826/2102 | 39.3 (37.2–41.4) | 0.058 |
includes women with missing age
Cochran-Armitage χ2 test for linear trend
Figure 1.
Trends in HIV prevalence in pregnant women by survey period, 2001–2003, 2004–2008 and 2009–2013.
The trends in HIV prevalence in young women are presented in two-year age groups in Table 2. Given that HIV infection in women <24 years could be regarded as a reliable measure of incident infections19, thus has substantial bearing on assessing epidemic control progress in terms of reducing new infections19. In all survey periods, a substantial increase in HIV prevalence was observed as women transitioned from their late teens to early twenties (Table 2). Substantial reductions in HIV prevalence from 22.2% (CI 12.6–31.8) to 10.6% (CI 6.0–15.2) to 7.7% (CI 3.7–11.8); (p=0.003) were observed in the ≤16 year olds, but rapid increase in HIV prevalence from age 17 years and over were common to all study periods, with little variation over time. In this setting by age 24 years, 54.4% (CI 43.4–65.4), 51.1% (CI 43.9–58.2) and 46.3% (CI 39.6–52.9); (p=0.176) of young pregnant women were HIV positive in the periods 2001–2003, 2004–2008 and 2009–2013, respectively.
Table 2.
Temporal trends in HIV prevalence in ≤24 year old pregnant women attending prenatal clinics in Vulindlela, rural KwaZulu-Natal by survey periods 2001–2003, 2004–2008 and 2009–2013.
| Age group | 2001–2003 | 2004–2008 | 2009–2013 | p-value† | |||
|---|---|---|---|---|---|---|---|
| n/N | % (95%CI) | n/N | % (95%CI) | n/N | % (95%CI) | ||
| </=16 | 16/72 | 22.2 (12.6–31.8) | 18/170 | 10.6 (6.0–15.2) | 13/168 | 7.7 (3.7–11.8) | 0.003 |
| 17–18 | 34/144 | 23.6 (16.7–30.5) | 69/324 | 21.3 (16.8–25.8) | 68/336 | 20.2 (15.9–24.5) | 0.423 |
| 19–20 | 34/108 | 31.5 (22.7–40.2) | 99/300 | 33.0 (27.7–38.3) | 77/282 | 27.3 (22.1–32.5) | 0.245 |
| 21–22 | 34/86 | 39.5 (29.2–49.9) | 113/255 | 44.3 (38.2–50.4) | 82/240 | 34.2 (28.2–40.2) | 0.124 |
| 23–24 | 43/79 | 54.4 (43.4–65.4) | 96/188 | 51.1 (43.9–58.2) | 99/214 | 46.3 (39.6–52.9) | 0.176 |
|
| |||||||
| Total | 161/489 | 32.9 (28.8–37.1) | 395/1237 | 31.9 (29.3–34.5) | 339/1240 | 27.3 (24.9–29.8) | 0.007 |
Cochran-Armitage χ2 test for linear trend
Temporal trends in sexual partner age disparity and HIV prevalence
Data on age disparity in the sexual relationships engaged in by surveyed pregnant women were collected from 2003, and was available for 4160 women (82.0%). Overall, between 2003 and 2013, HIV prevalence was higher in women who reported currently having older sexual partners compared to those with partners either the same age or younger than themselves. Table 3 shows the association between current sexual partner’s age and HIV infection stratified by woman’s age. Amongst teenagers <20 years, the adjusted relative risk (ARR) for HIV infection with current sexual partner in the age group 20–24 years was 1.7 (CI 1.3–2.4); (p=0.001) and increased to 3.0 (CI 2.1–4.3); (p<0.001) if the current sexual partner was ≥25 years. For young women aged 20–24 years whose current sexual partner was <20 years, the ARR was 0.3 (CI 0.1–1.2); (p=0.097) but increased significantly to 1.3 (CI 1.2–1.6); (p<0.001) with a partner ≥25 years. In contrast for women ≥25 years the age of the current sexual partner was not associated with HIV infection, ARR=1.0 (CI 0.7–1.5); (p=0.869).
Table 3.
Multiple log binomial regression examining the association of current partners’ age and HIV infection among pregnant women attending antenatal clinics in rural KwaZulu-Natal, 2003–2013. Unadjusted and adjusted relative risk (RR), 95% confidence intervals (CI) for prevalent HIV infection
| Age group | Partner’s age group | HIV prevalence % (95%CI) | Unadjusted | Adjusted* | ||
|---|---|---|---|---|---|---|
| RR (95%CI) | p-value | ARR (95%CI) | p-value | |||
| <20 | <20 | 10.8 (7.8–13.7) | Reference | |||
| 20–24 | 20.4 (17.5–23.3) | 1.9 (1.4–2.6) | <0.001 | 1.7 (1.3–2.4) | 0.001 | |
| ≥25 | 32.9 (25.3–40.4) | 3.1 (2.1–4.4) | <0.001 | 3.0 (2.1–4.3) | <0.001 | |
|
|
||||||
| 20–24 | <20 | 10.0 (0.0 –23.1) | 0.3 (0.1–1.1) | 0.062 | 0.3 (0.1–1.2) | 0.097 |
| 20–24 | 35.1 (31.0–39.2) | Reference | ||||
| ≥25 | 46.6 (42.9–50.3) | 1.3 (1.2–1.5) | <0.001 | 1.3 (1.2–1.6) | <0.001 | |
|
|
||||||
| ≥25 | <20 | 0 | NE | |||
| 20–24 | 57.7 (38.7–76.7) | 1.1 (0.8–1.5) | 0.714 | 1.0 (0.7–1.4) | 0.869 | |
| ≥25 | 54.2 (51.8–56.7) | Reference | ||||
Adjusted for clinic and previous pregnancy
NE: Non estimable
Discussion
Our surveys highlight the consistently high burden of HIV infection borne by young pregnant women in this rural community, which may be explained at least in part by young women engaging in high-risk sexual intercourse. HIV prevalence has remained unprecedentedly high: in excess of 40% in women aged 20–24 years, and in excess of 50% in women aged 25–34 years. The overall high prevalence in this age group is deeply concerning despite the prevention and treatment programs and additional targeted interventions especially for those in age-disparate relationships, are needed to impact this HIV epidemic.
Whilst the roll-out of ART in 2004 in this community appears to have had a substantial impact on survival, age-specific HIV prevalence provides a clear explanation for lack of progress in reducing the disease burden despite the presence of numerous prevention interventions. Although there has been a significant decline in HIV prevalence in the ≤16 year age group, indeed, we report exceptionally high and relatively unchanged HIV prevalence in young women (17.2% in women <20 years in the 2009–2013 study period), which suggest that reductions in new infections in this population have been minimal, given that in generalized epidemic settings such as South Africa HIV prevalence in 15–24 year olds is considered a reliable measurement to approximate trends in incident infections.19, 20 Assuming that HIV transmission is highest during early and acute HIV infection, the burden of potential incident infections in these teenagers and young women is deeply concerning and has serious implications for fuelling the HIV epidemic in this community,21–24 though the slight declines in HIV prevalence in the teenage groups are promising against the existing sheer high burden of infection. Moreover, significantly altering epidemic trajectories in similar settings will require prevention interventions targeted at teenagers and young women, and by understanding the risk factors associated with HIV acquisition in this key population.
The association reported here between high HIV prevalence and increased age of sexual partner for women ≤24 years, that increases in strength in women <20 years of age is consistent with other reports.13, 25–29 There is an urgent need to understand the causality of this association and how it is evolving with time, as well as the reasons why teenagers and young women in this community engage sexually with older men. To what extent partnerships leading to sexual intercourse are impacted by the need for financial support in young women, illness and death in women over 25 years and other complex cultural factors is undetermined26.
Furthermore, monitoring the impact of ART provision and the survival of women over 25 years of age continues to be critical: the high burden of HIV observed in this age group reflects the cumulative effect of HIV acquisition, underscores the extent of the care burden, and may help inform future prevention and treatment efforts. Although HIV prevalence in South Africa varies widely by province and the generalizability of results is limited, our surveys starkly demonstrate the unprecedented scale of the HIV epidemic at a local level. The surveys also provide some bearing on the implications of the epidemic for the community. For example, the low proportion of pregnancies in pre-ART roll-out period (2001–2003) in women over 30 years reported here may be attributable to declines in fertility rate as a result of advancing HIV disease and high mortality rates in this group. 30–34
In this setting, HIV acquisition is almost synonymous with sexual debut, which is estimated at around age 16 years.27, 35 Further, the burden of teenage pregnancies in the community, which represent an average of 30% of the surveyed pregnancies sampled per year from 2001 to 2013, is concerning given associations with poor economic and educational outcomes.27, 36 Although several, largely government-initiated and school-based behavioural interventions and educational outreach programs have aimed to target the teenage female population, these data demonstrate their limited success in terms of reducing pregnancy and HIV infection rates in teenagers. Despite several behavioral interventions currently implemented to reduce the sexual transmission of HIV, including programs aimed at delaying sexual debut, preventing intergenerational sex, medical male circumcision, promoting condom use within the structured ABC guidelines (abstinence, being faithful to one’s partner, and condom use) and even with earlier access to ART, which has been shown to prevent HIV transmission in randomized clinical trials37 and observational cohorts38, HIV incidence and prevalence remains persistently high in teenage girls39–41. It is clear that these teenage girls are a key group for HIV prevention and understanding of HIV transmission dynamics in this population is a major gap in the knowledge of HIV epidemiology. The severity of the epidemic in this setting creates an imperative for the inclusion of teenagers <18 years in biomedical trials42 with both HIV incidence and other sexual and reproductive health endpoints.
There are several limitations to our study. Our survey population comprising pregnant women seeking prenatal care limits generalizability beyond this group as HIV prevalence is likely to be very different amongst similar age, non-pregnant women in this community.43 Furthermore pregnant women attending the clinic for the first time for a current pregnancy are provided with comprehensive health care and we included all women in the study, however, it is possible that some women might have been missed and therefore the prevalence could potentially be over-estimated. The subgroup analysis on the association between current sexual partner’s age and HIV infection stratified by woman’s age, although limited by the small sample size across each year, demonstrated that this effect is more distinct in teenagers <20 years of age. However, as women get older it is not clear whether boys and younger men are at risk of infection from older women. More detailed phylogenetic analysis of HIV-1 sequences and phylodynamic studies are needed to better understand the precise bi-directionality of infection to sexual partners across all age groups. As surveillance spanned over several years, the outcome measurement of HIV sero-positivity could potentially be misclassified due to the use of different laboratory methods and tests, even though these tests have a sensitivity and specificity in excess of 98.2% and have been used extensively in national surveillance programmes13, 44, 45. Nevertheless it is possible that these tests may yield non-specific reactions due to causes other than HIV, in low or high HIV prevalence settings and therefore the risk of misclassification. Whilst we aimed to collect data on ART use from 2009, we obtained a low response rate which could be due to HIV-related stigma and discriminations which remains pervasive in this community.46–49 We therefore relied on the district ART data10, 11
To conclude, notwithstanding limitations inherent in cross-sectional surveys and with behavioural data collection specifically of women’s recall bias of their partner’s age, these data demonstrate an unequivocal and unambiguously high pregnancy rate and HIV prevalence in this rural community and highlight partner age as an important risk factor in HIV acquisition among young women. Given the eight to ten year latency between HIV infection and AIDS related morbidity and mortality50, the continued scale-up of ART services at primary care clinics could be key to averting a further AIDS catastrophe in this setting. To what extent survival of these communities, already with limited economic and productive capacity, is compromised is hard to predict. Inclusion of young women in our quest for new biomedical and behavioral modalities to reduce HIV risk remains an urgent imperative.
Supplementary Material
Acknowledgments
We wish to thank all the women who participated and the clinic staff that assisted in these surveys. A special thanks to the Provincial Department of Health for their support.
Source of Funding
This work has been supported through the Centre for the AIDS Programme of Research in South Africa (CAPRISA), which was established as part of the Comprehensive International Program of Research on AIDS (CIPRA) and is supported by the National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH), US Department of Health and Human Services (DHHS) (grant no. 1U19 AI51794) and The US President’s Emergency Plan for AIDS Relief (PEPFAR) (grant # 5U2GPS001350). JAF, ABMK, NYZ and GM were supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant #D43TW00231). ABMK was also supported in part by the SA Medical Research Council.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic, 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr/UNAIDS_Global_Report__en.pdf.
- 2.Abdool Karim Q, Hassanally L. HIV Epidemic Types and Customized Prevention Responses. FOCUS, A Guide to AIDS Research and Counseling. 2008 Fall;23(4):1–4. [PubMed] [Google Scholar]
- 3.Chopra M, Lawn JE, Sanders D, et al. Achieving the health Millennium Development Goals for South Africa: challenges and priorities. Lancet. 2009;374(9694):1023–31. doi: 10.1016/S0140-6736(09)61122-3. [DOI] [PubMed] [Google Scholar]
- 4.Wilson D, Halperin DT. “Know your epidemic, know your response”: a useful approach, if we get it right. Lancet. 2008;372(9637):423–6. doi: 10.1016/S0140-6736(08)60883-1. [DOI] [PubMed] [Google Scholar]
- 5.Collins C, Coates TJ, Curran J. Moving beyond the alphabet soup of HIV prevention. AIDS. 2008;22(Suppl 2):S5–8. doi: 10.1097/01.aids.0000327431.82795.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adam MA, Johnson LF. Estimation of adult antiretroviral treatment coverage in South Africa. S Afr Med J. 2009;99(9):661–7. [PubMed] [Google Scholar]
- 7.Johnson LF. Access to Antiretroviral Treatment in South Africa, 2004–2011. Southern African Journal of HIV Medicine. 2012;13(1):22–7. doi: 10.4102/sajhivmed.v18i1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.South African National Department of Health. Department of Health Annual Report 2012/13. RP: 237/2012. http://africacheck.org/wp-content/uploads/2014/02/131016dohrreport.pdf.
- 9.Naranbhai V, Abdool Karim Q, Naidoo K, Yende-Zuma N, Abdool Karim SS. Sustainability of task-shifting for antiretroviral treatment. Lancet. 2012;380(9857):1907–8. doi: 10.1016/S0140-6736(12)62110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KwaZulu Natal Province. HIV/AIDS Quarterly Report, uMgungundlovu district. 2012 http://www.google.co.za/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=2&ved=0CCIQFjAB&url=http%3A%2F%2Fwww.umdm.gov.za%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_download%26gid%3D751%26Itemid%3D382&ei=emKuVIz9AYLmaumCgpAM&usg=AFQjCNEZ-XYUbrpR7YhCo9f2lzJYEF7M9w.
- 11.uMgungundlovu District Municipality. uMgungundlovu District Presentation Quarter 2 2014/2015. 2014 Nov 19; http://www.kznonline.gov.za/hivaids/councils/Provincial-Councils-on-AIDS/2014/uMgungundlovu%20Q2%202014%202015.pdf.
- 12.South African Department of Health. The 2012 National Antenatal Sentinel HIV and Herpes Simplex type-2 prevalence Survey, South Africa. 2014 http://www.health.gov.za/docs/reports/2013/report4.pdf.
- 13.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. [Google Scholar]
- 14.Coetzer M, Cilliers T, Papathanasopoulos M, et al. Longitudinal analysis of HIV type 1 subtype C envelope sequences from South Africa. AIDS Res Hum Retroviruses. 2007;23(2):316–21. doi: 10.1089/aid.2006.0207. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS/WHO. Working group on Global HIV/AIDS and STI Surveillance. 2000. Guidelines for Second Generation HIV Surveillance: The next decade. WHO/CDC/EDC/20005 UNAIDS/0003E. [Google Scholar]
- 16.South African Department of Health. The South African National Voluntary Counselling and Testing (VCT) HIV Prevention and Care Strategy. Pretoria. 2003 [Google Scholar]
- 17.South African National Department of Health. National HIV Counselling and Testing Policy Guidelines. 2010 http://www.uj.ac.za/EN/CorporateServices/ioha/Documentation/Documents/hct_policy_guidelines%202010.pdf.
- 18.Sickinger E, Stieler M, Kaufman B, et al. Multicenter evaluation of a new, automated enzyme-linked immunoassay for detection of human immunodeficiency virus-specific antibodies and antigen. J Clin Microbiol. 2004;42(1):21–9. doi: 10.1128/JCM.42.1.21-29.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghys PD, Kufa E, George MV. Measuring trends in prevalence and incidence of HIV infection in countries with generalised epidemics. Sex Transm Infect. 2006;82(Suppl 1):i52–6. doi: 10.1136/sti.2005.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UNAIDS/WHO. Report on the global HIV/AIDS epidemic 2008. 2008. “UNAIDS/0825E / JC1510E”. [Google Scholar]
- 21.Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22(9):1055–61. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- 22.Laga M, Schwartlander B, Pisani E, Sow PS, Carael M. To stem HIV in Africa, prevent transmission to young women. AIDS. 2001;15(7):931–4. doi: 10.1097/00002030-200105040-00014. [DOI] [PubMed] [Google Scholar]
- 23.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 24.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 25.Gregson S, Nyamukapa CA, Garnett GP, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet. 2002;359(9321):1896–903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 26.Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22(Suppl 4):S17–25. doi: 10.1097/01.aids.0000341774.86500.53. [DOI] [PubMed] [Google Scholar]
- 27.Pettifor AE, Rees HV, Kleinschmidt I, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19(14):1525–34. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 28.Harling G, Newell ML, Tanser F, Kawachi I, Subramanian SV, Barnighausen T. Do age-disparate relationships drive HIV incidence in young women? Evidence from a population cohort in rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2014;66(4):443–51. doi: 10.1097/QAI.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly RJ, Gray RH, Sewankambo NK, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr. 2003;32(4):446–51. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- 30.Mashego M, Johnson D, Frohlich J, Carrara H, Karim QA. High AIDS-related mortality among young women in rural KwaZulu-Natal. S Afr Med J. 2007;97(8):587–92. [PubMed] [Google Scholar]
- 31.Glynn JR, Buve A, Carael M, et al. Decreased fertility among HIV-1-infected women attending antenatal clinics in three African cities. J Acquir Immune Defic Syndr. 2000;25(4):345–52. doi: 10.1097/00042560-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Gray RH, Wawer MJ, Serwadda D, et al. Population-based study of fertility in women with HIV-1 infection in Uganda. Lancet. 1998;351(9096):98–103. doi: 10.1016/S0140-6736(97)09381-1. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JJ, Ronsmans C, Ezeh A, Gregson S. The population impact of HIV on fertility in sub-Saharan Africa. AIDS. 2004;18(Suppl 2):S35–43. doi: 10.1097/00002030-200406002-00005. [DOI] [PubMed] [Google Scholar]
- 34.Zaba B, Gregson S. Measuring the impact of HIV on fertility in Africa. AIDS. 1998;12(Suppl 1):S41–50. [PubMed] [Google Scholar]
- 35.Shisana O, Rehle T, Simbayi L, et al. South African National HIV Prevalence, HIV Incidence, Behaviour and Communication Survey. 2005. [Google Scholar]
- 36.Jukes M, Simmons S, Bundy D. Education and vulnerability: the role of schools in protecting young women and girls from HIV in southern Africa. AIDS. 2008;22(Suppl 4):S41–56. doi: 10.1097/01.aids.0000341776.71253.04. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdool Karim Q, Kharsany AB, Frohlich JA, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2011;40(4):922–30. doi: 10.1093/ije/dyq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdool Karim Q, Kharsany AB, Leask K, et al. Prevalence of HIV, HSV-2 and pregnancy among high school students in rural KwaZulu-Natal, South Africa: a bio-behavioural cross-sectional survey. Sex Transm Infect. 2014;90(8):620–6. doi: 10.1136/sextrans-2014-051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kharsany AB, Mlotshwa M, Frohlich JA, et al. HIV prevalence among high school learners - opportunities for schools-based HIV testing programmes and sexual reproductive health services. BMC public health. 2012;12:231. doi: 10.1186/1471-2458-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdool Karim Q, Kharsany AB, Frohlich JA, et al. HIV incidence in young girls in KwaZulu-Natal, South Africa--public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav. 2012;16(7):1870–6. doi: 10.1007/s10461-012-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mmbaga EJ, Leyna GH, Mnyika KS, Klepp KI. Comparison of HIV-1 prevalence and risk factors between pregnant, non-pregnant, all women and the general population in Tanzania: implications for second-generation surveillance. International journal of STD & AIDS. 2009;20(7):483–8. doi: 10.1258/ijsa.2008.008400. [DOI] [PubMed] [Google Scholar]
- 44.Shisana O, Simbayi L. Nelson Mandela/HSRC study of HIV/AIDS: South African national HIV prevalence, behavioural risks and mass media household survey. Cape Town: HSRC Press; 2002. [Google Scholar]
- 45.National Department of Health. The 2012 National Antenatal Sentinel HIV and Herpes Simplex type-2 prevalence Survey. South Africa: 2014. [Google Scholar]
- 46.Young SD, Hlavka Z, Modiba P, et al. HIV-related stigma, social norms, and HIV testing in Soweto and Vulindlela, South Africa: National Institutes of Mental Health Project Accept (HPTN 043) J Acquir Immune Defic Syndr. 2010;55(5):620–4. doi: 10.1097/QAI.0b013e3181fc6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peltzer K, Ramlagan S. Perceived stigma among patients receiving antiretroviral therapy: a prospective study in KwaZulu-Natal, South Africa. AIDS Care. 2011;23(1):60–8. doi: 10.1080/09540121.2010.498864. [DOI] [PubMed] [Google Scholar]
- 48.Abdool Karim SS. Stigma impedes AIDS prevention. Nature. 2011;474(7349):29–31. doi: 10.1038/474029a. [DOI] [PubMed] [Google Scholar]
- 49.Karim QA, Meyer-Weitz A, Mboyi L, et al. The influence of AIDS stigma and discrimination and social cohesion on HIV testing and willingness to disclose HIV in rural KwaZulu-Natal, South Africa. Global Public Health. 2008;3(4):351–65. [Google Scholar]
- 50.Munoz A, Sabin CA, Phillips AN. The incubation period of AIDS. AIDS. 1997;11(Suppl A):S69–76. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.