Abstract / Preface
Bacteria use quorum sensing to orchestrate gene expression programmes that underlie collective behaviours. Quorum sensing relies on the production, release, detection and group-level response to extracellular signalling molecules, which are called autoinducers. Recent work has discovered new autoinducers in Gram-negative bacteria, shown how these molecules are recognized by cognate receptors, revealed new regulatory components that are embedded in canonical signalling circuits and identified novel regulatory network designs. In this Review we examine how, together, these features of quorum sensing signal–response systems combine to control collective behaviours in Gram-negative bacteria and we discuss the implications for host–microbial associations and antibacterial therapy.
Introduction
Quorum sensing is a cell-to-cell communication process that enables bacteria to collectively modify behaviour in response to changes in the cell density and species composition of the surrounding microbial community. Quorum sensing involves the production, release and group-wide detection of extracellular signalling molecules, which are called autoinducers. Autoinducers accumulate in the environment as bacterial population density increases. Bacteria monitor changes in the concentration of autoinducers to track changes in their cell numbers and to collectively alter global patterns of gene expression. Processes that are controlled by quorum sensing, such as bioluminescence, the secretion of virulence factors, the production of public goods and the formation of biofilms, are unproductive and costly when undertaken by a single bacterial cell, but become effective when undertaken by the group1.
Both Gram-positive and Gram-negative bacteria use quorum sensing. Gram-positive systems typically use secreted oligopeptides and two-component systems, which consist of membrane-bound sensor kinase receptors and cytoplasmic transcription factors that direct alterations in gene expression. The biological roles of quorum sensing in Gram-positive bacteria have been extensively reviewed elsewhere2–4. In this Review, we focus on quorum sensing in Gram-negative bacteria and highlight unusual signalling molecules, novel regulatory components and heterogeneity in quorum sensing responses.
Four common features are found in nearly all known Gram-negative quorum sensing systems5. First, the autoinducers in such systems are acyl-homoserine lactones (AHLs) or other molecules that are synthesized from S-adenosylmethionine (SAM), and they are able to diffuse freely through the bacterial membrane. Second, autoinducers are bound by specific receptors that reside either in the inner membrane or in the cytoplasm. Third, quorum sensing typically alters dozens to hundreds of genes that underpin various biological processes. Fourth, in a process called autoinduction, autoinducer-driven activation of quorum sensing stimulates the increased synthesis of the autoinducer, which establishes a feed-forward loop that is proposed to promote synchronous gene expression in the population.
Gram-negative bacteria often use several autoinducers, and new studies are revealing the molecular determinants that provide the receptors extraordinary specificity in distinguishing between closely related molecules. Quorum sensing information is often integrated by small RNAs (sRNAs)6 that control target gene expression and that also function in feedback loops. Quorum sensing network architectures promote signalling fidelity, temporal control and flexible input–output dynamics. Important questions regarding quorum sensing are: how do bacterial cells prioritize one autoinducer over another? How do network features enable optimal performance? And what are the requirements that enable quorum sensing systems to tune their input–output relations to changing stimuli?
Quorum sensing underpins collective behaviours that often involve expensive public goods7. Placing such assets under collective control avoids exploitation of those goods. Nonetheless, recent evidence suggests that stochastic processes are also relevant; for example, phenotypic heterogeneity that stems from pathways that are controlled by quorum sensing may enable bet-hedging and division of labour among constituent members of a bacterial population8. How individual heterogeneity can be embedded in processes that are synchronously executed at the population level is being intensively investigated9. Quorum sensing heterogeneity could also be crucial for neighbouring cells that are not close relatives — for example, in the microbiota of the host10. Autoinducers and other molecules that are produced by both prokaryotic and eukaryotic organisms could be used for one-way, two-way or multi-way communication. Appropriate interpretation of the information that is contained in such chemical blends at the individual and population levels could be crucial for the survival of individual cells and for protection of the host and its established microbiota from bacterial, fungal or viral invaders. Indeed, in eukaryotic hosts, autoinducers provide probiotic functions, alter the composition of the microbiota, affect the expression of virulence genes and encourage pathogens to disperse from biofilms11.
This Review focuses on new Gram-negative bacterial autoinducers, receptors, design principles that control regulatory network architectures and the coordinated responses that quorum sensing controls. We discuss newly discovered functions that are mediated by quorum sensing, highlight their relevance for collective bacterial behaviours, the possibility of heterogeneity in quorum sensing responses, and we emphasize roles in host–bacterial interactions.
Autoinducers, receptors, and specificity
Bacteria that live in heterogeneous populations presumably encounter complex mixtures of autoinducers that are produced by themselves, their clonal siblings, close relatives, and their non-kin neighbours, which could be fierce competitors7,12,13. Thus, bacteria face the challenge of extracting information from mixtures of related and unrelated molecules. This issue is compounded by the fact that bacteria often rely on producing and detecting several autoinducers. How bacteria correctly interpret blends of molecules that are produced by themselves and by other species in the vicinity, and how they elicit appropriate and coordinated changes in gene expression in response to these blends are important questions.
Autoinducers
In Gram-negative bacteria, AHLs are the most common class of autoinducers. They have a core N-acylated homoserine-lactone ring and a 4–18 carbon acyl chain that can contain modifications14 (FIG. 1a). Hundreds of bacterial species contain LuxI-type synthases that produce these AHLs15. The length of the acyl chain can affect stability, which may have consequences for signalling dynamics16.
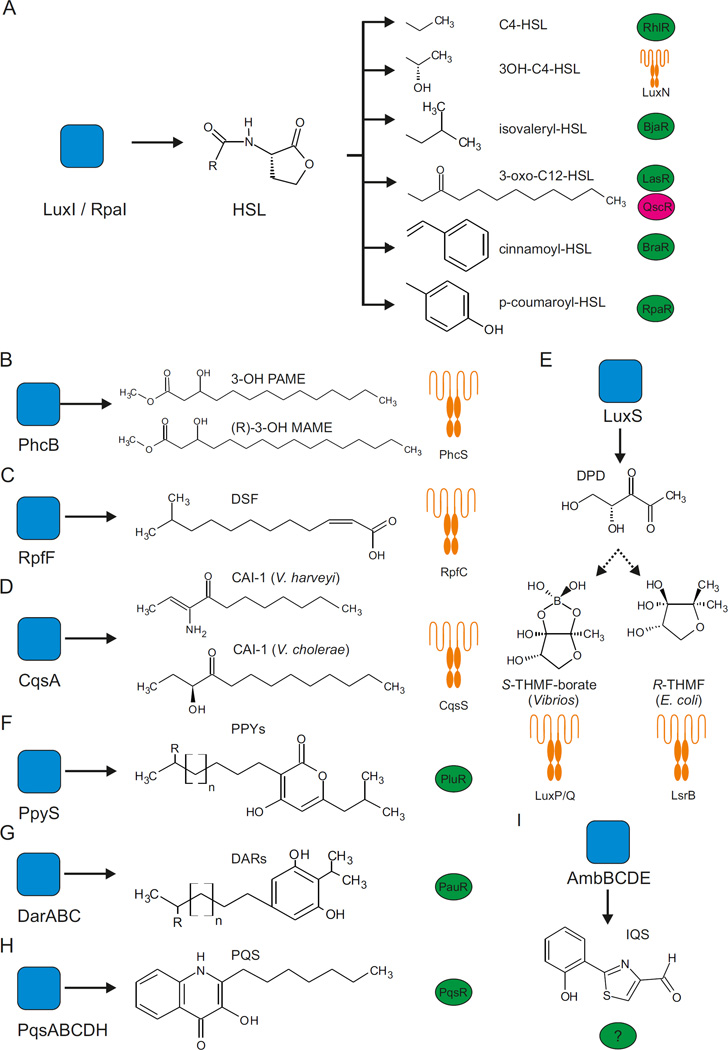
Figure 1. Quorum sensing synthases, autoinducers and receptors.
This figure shows the structures of various autoinducers together with their corresponding synthases (blue) and receptors (transcription factors are shown as green and pink ovals and transmembrane receptors are shown as orange schematics). a | Homoserine lactone (HSL) autoinducers that are produced by different Gram-negative bacteria. b | 3-hydroxypalmitic-acid-methyl-ester (3-OH PAME) and (R)-methyl-3-hydroxymyristate ((R)-3-OH MAME) are produced and detected by Ralstonia spp. c | Diffusible signal factor (DSF) is used for quorum sensing in Xanthomonas campestris. d | The CAI-1 autoinducer synthase (CqsA) and the CqsS receptor system produces and recognizes various cholera autoinducer 1 (CAI-1) molecules. The Vibrio harveyi and Vibrio cholerae CAI-1 molecules are shown. e | 4,5-dihydroxy-2,3-pentanedione (DPD) is synthesized by all LuxS enzymes and is thus the universal precursor to the widespread family of quorum sensing autoinducers that are collectively designated as autoinducer 2 (AI-2). In the presence of boron, AI-2 forms (2S,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran-borate (S-THMF-borate), the active autoinducer in Vibrio spp. In the absence of boron, AI-2 exists as (2R,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran (R-THMF), the active autoinducer in enteric bacteria. The LuxPQ and LsrB receptor schematics shown are meant to designate that autoinducer recognition occurs in the periplasm, not the cytoplasm. LuxP and LsrB are homologues of ribose binding proteins. LuxP functions in conjunction with the two-component sensor kinase protein LuxQ and LsrB functions together with a membrane-spanning ATP binding cassette (ABC) transporter complex. f | 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde (IQS) is produced by Pseudomonas aeruginosa. The IQS receptor is currently unknown. g | The 2-heptyl-3-hydroxy-4-quinolone (PQS) system is one of several quorum sensing systems in P. aeruginosa. h | Photorhabdus asymbiotica uses dialkylresorcinols (DARs) for cell–cell communication. i | PpyS of Photorhabdus luminescens produces several photopyrones, which are sensed by the PluR transcriptional regulator. E. coli, Escherichia coli; RpaI, 4-coumaroyl-homoserine lactone synthase.
LuxI enzymes produce AHLs by deriving the lactone moiety from SAM, and, in most cases, the particular acyl chain is obtained from intermediates of fatty acid biosynthesis. One remarkable exception is the plant-associated photosynthetic bacterium Rhodopseudomonas palustris in which the LuxI-type enzyme, 4-coumaroyl-homoserine lactone synthase (RpaI), produces p-coumaroyl-homoserine lactone (HSL), for which the acyl group comes from the host metabolite p-coumarate17 (FIG. 1a). Using a plant-derived compound enables R. palustris to connect its quorum sensing response to bacterial population density and the availability of plant consumables. Other plant-associated bacteria synthesize unusual HSL autoinducers. Bradyrhizobium japanicum and Aeromonas spp. produce isovaleryl-HSL18, whereas Bradyrhizobium BTAi produces cinnamoyl-HSL19 (FIG. 1a), but all of these species use bacterial substrates.
Two other plant-associated bacteria, Ralstonia solanacearum and Xanthomonas campestris, produce atypical autoinducers. Depending on the strain, the PhcB protein of R. solanacearum synthesizes one of two related autoinducers, 3-hydroxypalmitic-acid-methyl-ester (3-OH PAME)20 and (R)-methyl-3-hydroxymyristate ((R)-3-OH MAME; FIG. 1b)21. These autoinducers control virulence and the formation of biofilms22. X. campestris also uses cis-11-methyl-2-dodecenoic acid, which is known as diffusible signal factor (DSF; FIG. 1c), to modulate transitions between its planktonic and biofilm-associated lifestyles23. Structural homologues of DSF have been discovered recently, including in human pathogens, such as Pseudomonas aeruginosa and Burkholderia cenocepacia24. All DSF-type molecules are synthesized by RpfF proteins25 (FIG. 1c). Interestingly, one organism can generate several DSF signals, all of which are synthesized by a single RpfF protein26.
Many bacteria produce and detect several autoinducers. The bioluminescent marine bacterium Vibrio harveyi was the first bacterium that was discovered to use several autoinducers and it remains the model for understanding how bacteria process chemical blends. V. harveyi uses three autoinducers for intra-species, intra-genera and inter-species communication27 to regulate approximately 600 target genes28. V. harveyi produces a canonical AHL, 3OH-C4-HSL (HAI-1; FIG. 1a), using the LuxM synthase29,30. Surprisingly, LuxM is not a LuxI homologue, but it carries out analogous reactions using SAM and fatty-acid intermediates as substrates31. As far as is known, only V. harveyi and its closest relative Vibrio parahaemolyticus produce HAI-1, which suggests that this autoinducer is used for intra-species communication5.
V. harveyi also uses (Z)-3-aminoundec-2-en-4-one as an autoinducer (FIG. 1d). The related molecule, (S)-3-hydroxytriecan-4-one, was discovered first as an autoinducer in Vibrio cholerae32 (FIG. 1d). Collectively, these molecules are called cholera autoinducer 1 (CAI-1). In V. cholerae, the CAI-1 autoinducer synthase (CqsA) acts on SAM and decanoyl-CoA to produce amino-CAI-1, which is immediately converted, possibly spontaneously, into CAI-1. Both amino-CAI-1 and CAI-1 are biologically active; however, CAI-1 predominates in cell-free culture fluids33–35. Amino-CAI-1 is more stable than CAI-1 (REF. 33), which raises the possibility that CAI-1 promotes a rapid response to fluctuations of autoinducer. CqsA enzymes exist in all Vibrio spp. and they can produce various CAI-1 moieties that have different acyl chain lengths and modifications. Vibrio spp. respond to each other’s CAI-1s with different affinities than to their own CAI-1s, which suggests that CAI-1 is used for intra-Vibrio communication. Curiously, other than Vibrio spp., cqsA homologues exist in the distantly related bacteria Legionella pneumophila and Janthinobacterium sp. HH01 (REFS 36,37). In L. pneumophila, the corresponding autoinducer, 3-hydroxypentadecane-4-one (LAI-1), regulates DNA uptake and host cell interaction, which implicates LAI-1 in inter-kingdom communication38.
The final autoinducer in V. harveyi is autoinducer 2 (AI-2), which consists of a set of interconverting autoinducer molecules that are all derived from 4,5-dihydroxy-2,3-pentanedione (DPD; FIG. 1e)39. LuxS, the DPD synthase, is present in more than 500 bacterial species, making AI-2 the most common bacterial autoinducer identified to date40. DPD is highly reactive and it spontaneously cyclizes into various furanone moieties. Specific bacterial species detect different forms of DPD as their active AI-2 signals. For example, in V. harveyi, AI-2 contains boron41, whereas, in Escherichia coli and Salmonella spp., the AI-2 signal is a non-borated cyclized DPD moiety42 (FIG. 1e). As the different DPDs rapidly interconvert, AI-2 provides a means for inter-species communication13,43. Certain bacteria, such as P. aeruginosa, do not encode LuxS and thus do not make AI-2. Nonetheless, they can detect AI-2 produced by other bacterial species, and AI-2 alters their gene expression programmes44.
P. aeruginosa uses two canonical AHL autoinducers (FIG. 1a) as well as non-AHL autoinducers for quorum sensing. Specifically, cyclic dipeptides (2,5-diketopiperazines; DKPs) are generated by tRNA-dependent cyclodipeptide synthases45 and 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde (IQS) is produced by proteins that are encoded by the non-ribosomal peptide synthase gene cluster ambBCDE46 (FIG. 1f). In addition, a quinolone (2-heptyl-3-hydroxy-4-quinolone, known as PQS; FIG. 1g) is used as an autoinducer47. PQS is produced by proteins that are encoded by the pqsABCDH genes, and, together with the two AHLs, controls the formation of biofilms and the production of virulence factors48. Quinolones are widely known for their antibiotic and anticancer activities49, which demonstrates the multi-functionality of particular autoinducers.
Autoinducer multi-functionality has also been reported for Photorhabdus species. Photorhabdus asymbiotica is an insect and human pathogen that produces dialkylresorcinols50 (DARs; FIG. 1h), whereas Photorhabdus luminescens, in which virulence is limited to nematodes, synthesizes photopyrone autoinducers51 (PPYs; FIG. 1i). In addition to quorum sensing, PPYs function as insect toxins, whereas DARs have antibiotic activity50. Eight different PPYs (PPYA, PPYB, PPYC, PPYD, PPYE, PPYF, PPYG and PPYH) are produced by the PpyS synthase and the DarB ketosynthase produces 2,5-dialkylcyclohexane-1,3-diones (CHDs) from fatty-acid-derived precursors, which can be further oxidized into DARs by the DarA aromatase52.
Receptors and specificity
Commonly, Gram-negative bacteria use LuxR-type receptors, which are cytoplasmic transcription factors that detect AHLs produced by partner LuxI-type synthases. LuxR proteins contain two functional domains: an amino-terminal ligand-binding domain and a carboxy-terminal DNA-binding domain53. In the absence of the cognate autoinducer, most LuxR-type receptors fail to fold and are degraded. By contrast, LuxR proteins that are bound to an autoinducer are stable, dimerize and bind to DNA54,55. LuxR–autoinducer complexes associate with short DNA sequences termed ‘lux boxes’ upstream of target genes56– 58. Interestingly, EsaR, the LuxR-type protein from Pantoea stewartii, functions as a transcriptional repressor in the absence of its cognate autoinducer and releases DNA following autoinducer binding59,60.
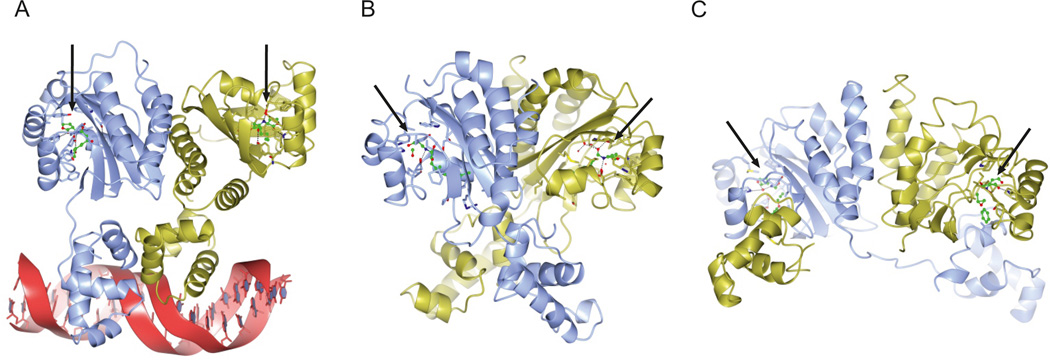
Structures of four full-length LuxR-type receptors have been solved: TraR (FIG. 2a) from Agrobacterium tumefaciens55,61 and Rhizobium sp. NGR234 (REF. 62), QscR (FIG. 2b) from P. aeruginosa63 and CviR (FIG. 2c) from Chromobacterium violaceum64. Structures of the ligand-binding domains of LasR65 from P. aeruginosa and SdiA66 from E. coli have also been solved. In all cases, LuxR-type receptors form homodimers. The N-terminal regions of LuxR-type receptors resemble GAF and PAS domains, which are well-known mediators of signal transduction processes67. The C-terminal regions have DNA-binding helix–turn–helix domains, which are characteristic of many bacterial transcription factors68. Polar residues in the N termini, including three highly conserved tryptophan residues, contact the HSL moiety of the autoinducer, which defines the binding orientation. Residues that provide hydrophobic and van der Waals interactions to acyl chain moieties are less conserved. The acyl chains can occupy the binding pocket in different configurations: short AHLs are extended and point toward the solvent, whereas long chains are bent and face the interior of the pocket69. Seemingly, LuxR proteins use a combination of amino-acid variation and flexibility in the binding pocket to achieve AHL binding specificity.
Figure 2. Structures of LuxR-type quorum sensing receptors.
This figure shows the crystal structures of four LuxR-type receptors. a | TraR from Agrobacterium tumefaciens bound to autoinducer and DNA (Protein Data Bank (PDB) entry 1L3L). b | QscR from Pseudomonas aeruginosa bound to autoinducer (PDB entry 3SZT). c | CviR from Chromobacterium violaceum bound to an inhibitor called chlorolactone (PDB entry 3QP5). The arrows denote the positions of the ligands. The structures of the ligand-binding domains of all three proteins are similar; however, whereas TraR (panel a) adopts an asymmetric dimer, QscR (panel b) and CviR (panel c) form nearly symmetric cross-subunit architectures. The locations and conformations that are adopted by the DNA-binding domains differ substantially, enabling (panels a and b) or preventing (panel c) DNA binding and transcriptional activation of target genes.
Approximately 76% of annotated LuxR proteins belong to the so-called LuxR-solo class of transcription factors70, that is, they have no accompanying LuxI synthases. This suggests that many more autoinducers could exist that are produced by non-LuxI synthases or that are supplied by other bacteria and modulate the activity of these receptors. QscR from P. aeruginosa is probably the best-characterized LuxR-solo receptor. QscR has relaxed ligand-binding specificity compared with the two non-solo LuxR receptors, LasR and RhlR. Indeed, QscR activates target gene expression at nanomolar concentrations of C8-HSL, C10-HSL, 3-oxo-C10-HSL, C12-HSL, 3-oxo-C12-HSL and C14-HSL71 (FIG. 1a). Thus, QscR may be used by P. aeruginosa to detect autoinducers that are produced by cohabitating species, such as Burkholderia cepacia72.
The second major class of Gram-negative quorum sensing receptors are the two-component membrane-bound histidine kinases that signal to cytoplasmic transcription factors through phosphorylation. The best-studied examples come from V. harveyi and V. cholerae5. HAI-1, CAI-1 and AI-2 are detected by LuxN73, CqsS32 and LuxQ74, respectively (FIG. 1a,d,e). LuxN is specific to V. harveyi, whereas the other two receptors (CqsS and LuxQ) are conserved in V. cholerae. The detection of AI-2 also requires the periplasmic protein LuxP75. LuxN and CqsS are predicted to contain nine and six transmembrane spanning helices, respectively, which prevent the use of structure-based methods to define autoinducer detection and specificity determinants. Rather, receptor mutagenesis coupled with permutation of the AHL and CAI-1 ligands revealed the LuxN and CqsS binding pockets and uncovered the ‘gatekeeper’ amino acids that are crucial for distinguishing between autoinducers12,76. Both receptors show strict specificity for their cognate ligands. Indeed, LuxN is not activated by any AHL variant and longer AHLs function as potent antagonists12, which suggests that V. harveyi detects non-cognate autoinducers that are produced by competitors and, in response, turns off quorum sensing to avoid the exploitation of its public goods. With respect to CqsS, CAI-1 derivatives that have altered acyl chains fail to activate CqsS, whereas enlargement of the head group converts the autoinducer into an antagonist76.
The crystal structures of LuxP in complex with the periplasmic domain of LuxQ were determined74,75. In the absence of AI-2, the two LuxPQ complexes form a symmetric heterotetramer, which, following AI-2 binding, undergoes a substantial conformational change. Protomer rotation in the periplasmic region breaks the symmetry of the LuxPQ–LuxPQ tetramer, which prevents the phosphorylation of the cytoplasmic domains (see below). In LuxP from V. harveyi, two positively charged arginine residues that are located in the binding pocket stabilize the boron-complexed, negatively charged AI-2 moiety and facilitate hydrogen bonding of the ligand to five additional amino acids. Interestingly, AI-2 binding also promotes clustering of LuxPQ–LuxPQ tetramers, which can influence AI-2 sensitivity and response dynamics75.
Several proteobacteria have an alternative AI-2 detection system. The lsrACDBFGE (lsr stands for LuxS regulated) operon encodes an ATP-binding cassette transporter (ABC transporter) that internalizes AI-2. The operon also encodes enzymes that are responsible for the degradation of AI-2 (REF.40). The operon is regulated by the LsrR repressor, which binds to a processed AI-2 product in the cytoplasm. In this case, LsrB is the equivalent of LuxP and is located in the periplasm (FIG. 1e). LsrB binds to a boron-free cyclized AI-2 moiety. Three crystal structures of LsrB complexed with AI-2 show that, despite sequence and structural variation, six highly conserved amino acids drive the interaction of LsrB with AI-2 (REF. 77). Given the low percentage (~11%) of sequence identity between LuxP-like and LsrB-like receptors, it is possible that additional, yet undiscovered, AI-2 receptors exist.
Quorum sensing network architectures
To accurately execute quorum sensing behaviours, bacteria must detect, interpret and integrate extracellular chemical information and convert that information into changes in gene expression. How bacteria achieve these feats is especially interesting when several autoinducers are used and in mixed species consortia78. Moreover, the information can be corrupted by internal noise (such as fluctuations in transcript or protein numbers), external changes (temperature, pH, osmolarity, and so on), or if competing bacteria contribute or consume autoinducers, and all of these features require compensation. Systems biology approaches have uncovered common network design principles that occur in quorum sensing systems that are able to overcome these issues79. Below, to illustrate these principles, we discuss the two most common canonical network architectures using Pseudomonas spp. and Vibrio spp. as examples.
Pseudomonas spp. quorum sensing
Pseudomonas spp., specifically P. aeruginosa, use a dense network of quorum sensing receptors and regulators (FIG. 3). The major P. aeruginosa receptors are LuxR-type receptors that, following autoinducer binding in the cytoplasm, function as DNA-binding transcriptional activators80. There are currently four well-known quorum sensing pathways in P. aeruginosa: two LuxR and LuxI-type systems called LasR and LasI and RhlR and RhlI, the PqsR-controlled quinolone system and the IQS system that functions under phosphate-limiting conditions46.
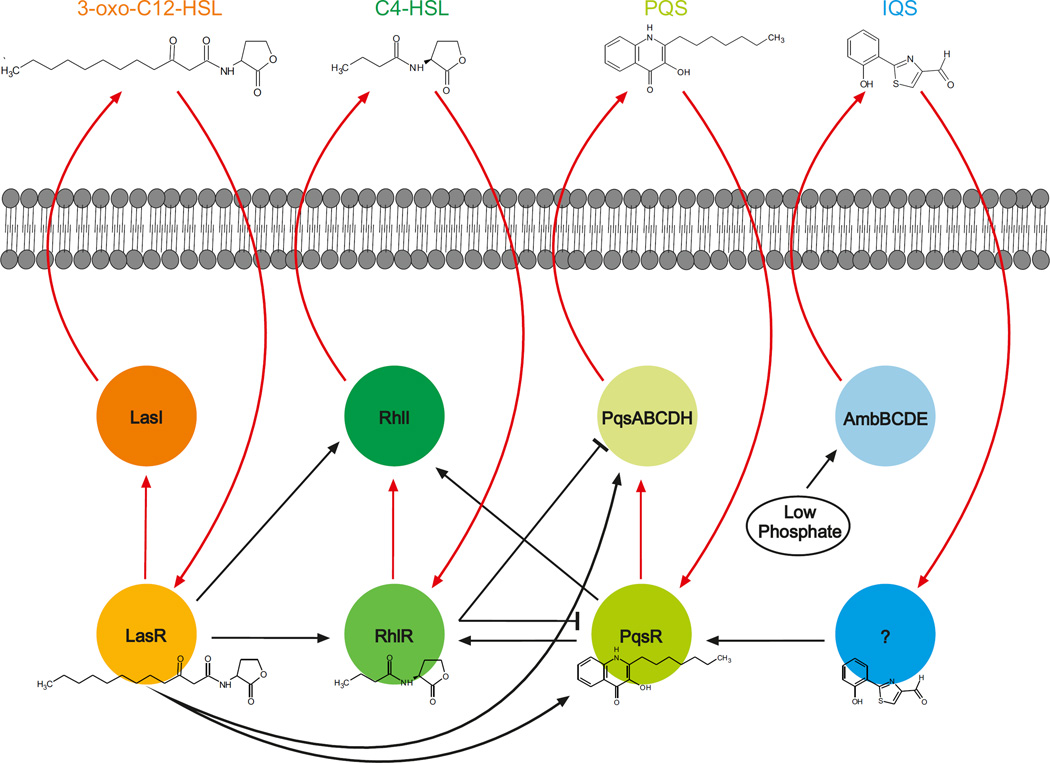
Figure 3. Quorum sensing circuits in Pseudomonas aeruginosa.
The four autoinducer synthases, LasI, RhlI, PqsABCDH and AmbBCDE, produce the autoinducers, 3-oxo-C12-homoserine lactone (HSL), C4-HSL, 2-heptyl-3-hydroxy-4-quinolone (PQS) and 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde (IQS), respectively. 3-oxo-C12-HSL, C4-HSL and PQS, are recognized by cytoplasmic transcription factors. The receptor for IQS is currently unknown. The production of the IQS signal is induced under phosphate starvation. The individual circuits are highly interconnected and involve autoinduction (red arrows).
The systems are organized in a hierarchy with LasR at the top of the cascade (FIG. 3). LasR, in complex with 3-oxo-C12-HSL (FIG. 1a), activates a large regulon of downstream genes that includes the lasI synthase gene, which leads to autoinduction81. The LasR–autoinducer complex also activates the expression of rhlR and rhlI, which encode the second quorum sensing pathway48, and the pqsR and pqsABCDH genes, which encode the PQS system82. RhlR operates similarly to LasR, and when bound to C4-HSL (FIG. 1a), activates its own regulon that includes rhlI and thereby establishes the second autoinduction feed-forward loop83,84. The PqsR–PQS complex feeds back to activate rhlRI85, which connects the three signalling modules. In addition, RhlR inhibits the expression of pqsR and pqsABCD, and this loop is suggested to ensure the correct ratio of 3-oxo-C12-HSL to C4-HSL, which, in turn, dictates the activation of PQS86. A recent survey of regulators that affect quorum sensing in P. aeruginosa listed 13 transcription factors of which 10 repressed and 3 activated Rhl-directed and/or Las-directed functions48. This high degree of interconnectivity highlights how several intracellular and extracellular cues are integrated to modulate the quorum sensing output. Presumably, fine-tuning the response through several layers of regulation enables robust cell–cell communication under diverse conditions.
Interestingly, RhlR is a key quorum sensing component in P. aeruginosa that controls the expression of virulence genes80. As rhlI can be activated by either LasR or PqsR, together with RhlR bound to C4-HSL, at least one other autoinducer is required for pathogenicity. In wild-type P. aeruginosa, the additional required autoinducer is usually supplied by the Las system. However, isolates of P. aeruginosa from patients with cystic fibrosis frequently have mutations in lasR87. In this case, the phosphate starvation protein PhoB can override the necessity for LasR through the activation of IQS production. In turn, IQS activates the expression of the pqs genes (FIG. 3), which produces the additional required autoinducer through activation of rhl expression46. This alternative by-pass mechanism makes virulence gene expression in P. aeruginosa immune to mutations in LasR, which could be particularly relevant during chronic infection48.
Vibrio spp. quorum sensing
V. harveyi and V. cholerae provide the second example of a canonical quorum sensing circuit; in this example, the system relies on membrane-bound receptors. Although the advantages and disadvantages of cytoplasmic DNA-binding transcription factors versus membrane-bound receptors are not fully understood, one issue is clear: both types of system must avoid responding to endogenously produced autoinducers before achieving ‘a quorum’. Rapid degradation of LuxR-type proteins in the absence of autoinducer prevents the premature activation of quorum sensing in Pseudomonas-type systems88, whereas localization of the receptors to the membrane in Vibrio-type systems decouples the cytosolic production of autoinducers from detection in the periplasm5.
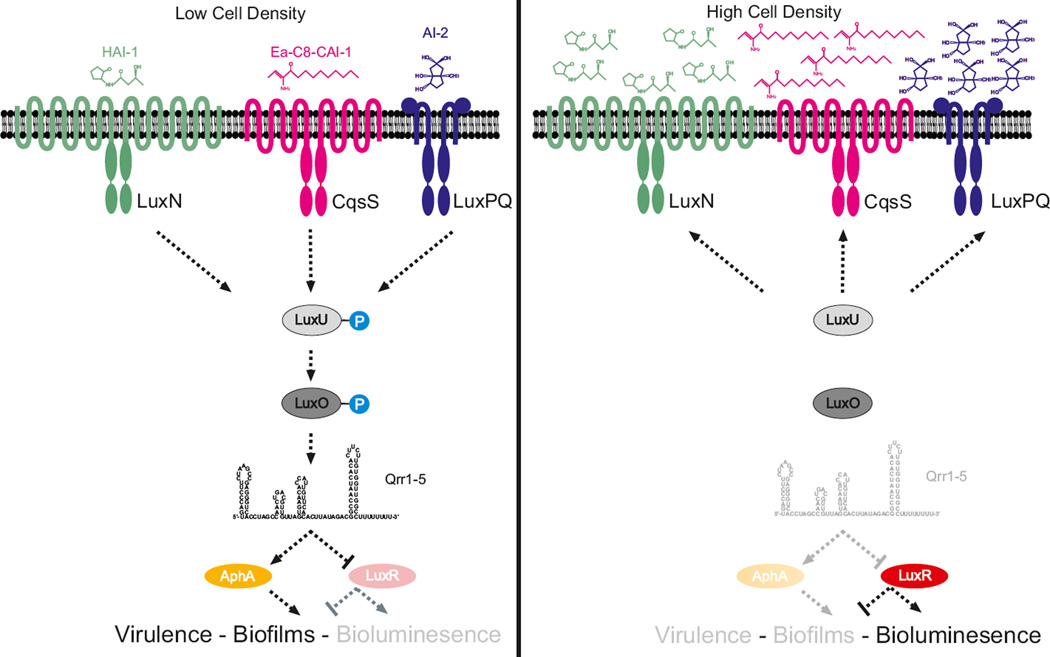
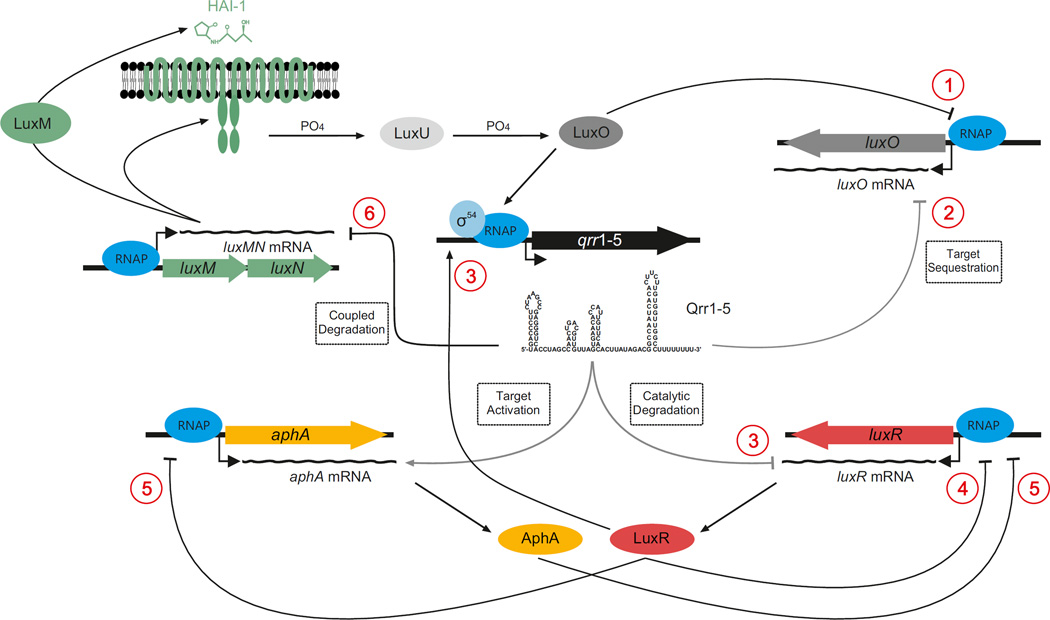
V. harveyi and V. cholerae use CqsS and LuxPQ as quorum sensing receptors, which interact with CAI-1 and AI-2, respectively. In addition, V. harveyi uses a third HAI-1 binding receptor, LuxN. In both species, the signalling relays are arranged in parallel (FIG. 4). In the absence of autoinducers, LuxN, LuxPQ and CqsS are kinases that autophosphorylate and shuttle phosphate to LuxU, which passes the phosphate to the response regulator LuxO89. Phosphorylated LuxO functions together with σ54 (REF. 90) to activate the transcription of genes that encode four (V. cholerae) or five (V. harveyi) homologous sRNAs, known as the quorum regulatory sRNAs (Qrr sRNAs)91. The Qrr sRNAs are Hfq-dependent sRNAs that regulate gene expression by base-pairing with target mRNAs and altering translation6,92. The Qrr sRNAs activate or repress the translation of 20 mRNAs93. Most importantly, they activate translation of the mRNA that encodes the low cell density master regulator, AphA, and they repress translation of the mRNAs that encode the high cell density master regulators, LuxR in V. harveyi and HapR in V. cholerae91 (FIG. 4).
Figure 4. Quorum sensing circuits in Vibrio harveyi.
Left panel: Signal transduction at low cell densities. During this stage, autoinducer levels are low and the LuxN, LuxPQ and CqsS receptors act as kinases. LuxO is phosphorylated and the quorum regulatory small RNAs (Qrr sRNAs) Qrr1, Qrr2, Qrr3, Qrr4 and Qrr5 (Qrr1–5) are transcribed. The Qrr sRNAs repress luxR and activate aphA. AphA controls genes that are involved in individual behaviours and activates genes that are required for virulence and the formation of biofilms (in Vibrio cholerae). Right panel: Signal transduction at high cell densities. During this stage, autoinducer levels are high and the LuxN, LuxPQ and CqsS receptors function as phosphatases. LuxO is dephosphorylated, the Qrr1–5 sRNAs are not transcribed; therefore, AphA is not produced, whereas LuxR is produced. LuxR controls genes that are required for group behaviours, including genes that are responsible for bioluminescence (in Vibrio harveyi). AI-2, autoinducer 2; Ea-C8-CAI-1, (Z)-3-aminoundec-2-en-4-one; HAI-1, 3OH-C4-homoserine lactone.
At high cell density, autoinducer binding inhibits autophosphorylation, which enables the phosphatase activities of the receptors to dominate94. Dephosphorylated LuxO is inactive, which terminates expression of the qrr genes. In the absence of the Qrr sRNAs, luxR or hapR is dere-pressed and aphA is not activated. Under this condition, LuxR or HapR is produced and it activates genes that underpin collective quorum sensing behaviours (FIG. 4).
In addition, the Qrr sRNAs repress luxMN, which encode an autoinducer synthase and receptor pair95 (FIG. 5), and repress translation of luxO96. The Qrr sRNAs repress luxR or hapR through catalytic degradation of the mRNA, repress luxMN by coupled degradation of the mRNA, repress the translation of luxO by sequestering luxO mRNA, and they activate aphA by revealing the ribosome binding site97. Although catalytic degradation of the luxR or hapR mRNA by the Qrr sRNAs does not alter the Qrr pool, coupled degradation and sequestration remove Qrr sRNAs from the system97. These regulatory mechanisms are crucial for the maintenance of appropriate Qrr pools and overall quorum sensing dynamics (FIG. 5).
Figure 5. Feedback loops control Vibrio harveyi quorum sensing dynamics.
Six different feedback loops are embedded in the Vibrio harveyi quorum sensing circuit. a | LuxO autorepresses its own transcription. b | The quorum regulatory small RNAs (Qrr sRNAs) inhibit luxO translation by mRNA target sequestration. c | LuxR activates qrr transcription. The Qrr sRNAs, in turn, inhibit the production of LuxR by catalytic degradation of the luxR mRNA. d | LuxR represses its own transcription. e | AphA and LuxR reciprocally repress each other’s transcription. f | Base pairing of the Qrr sRNAs with the luxMN mRNA facilitates degradation of the RNA duplex (coupled degradation). The arrows denote activation. Inhibitory arrows denote repression. Grey arrows indicate post-transcriptional regulation. All of these feedback loops except the Qrr-to-luxMN loop also exist in Vibrio cholerae. In V. cholerae, LuxR is known as HapR. HAI-1, 3OH-C4-homoserine lactone; RNAP, RNA polymerase.
The probability that a particular receptor is in the kinase or phosphatase state is dictated by the difference in free energy between the two configurations73. This molecular architecture is analogous to chemotaxis receptors in E. coli and suggests the general relevance of two-state, free-energy models for bacterial sensor kinases98.
Importantly, because all quorum sensing receptors in Vibrio spp. have both kinase and phosphatase activity, and transfer phosphate to and from the same phosphorelay protein, LuxU, quorum sensing can never be fully turned on or fully turned off unless all the autoinducers are present or absent, respectively.
Quorum sensing dynamics in Vibrio spp. are further modulated by the above-mentioned feedback loops as well as other regulatory feedbacks that tune the information that flows through the network. There are six known feedback loops (FIG. 5): First, LuxO autorepresses its own transcription99. Second, the Qrr sRNAs sequester the luxO mRNA, which represses luxO translation96,97. Both of these loops limit the production of LuxO at low cell densities, which sets the lower limit below which the Qrr sRNAs, and thus quorum sensing, cannot be further repressed97,100. Third, LuxR or HapR activates the expression of the qrr genes101, and the Qrr sRNAs feedback to destabilize the luxR or hapR mRNA91. This double loop makes LuxR-driven or HapR-driven quorum sensing transitions faster102. Fourth, LuxR or HapR represses its own transcription, which avoids the runaway production of LuxR or HapR at high cell densities, which places a limit on the possible quorum sensing output103,104. Fifth, AphA and LuxR or HapR reciprocally repress one another’s transcription, which ensures maximal production of AphA at low cell density and maximal production of LuxR or HapR at high cell density103. Sixth, at low cell density, the Qrr sRNAs facilitate the degradation of luxMN mRNA, which decreases the synthesis and detection of HAI-1. This loop de-emphasizes the HAI-1 signal at low cell density and enhances HAI-1 sensitivity at high cell density95. Presumably, at low cell density, numbers of non-kin cells are crucial to track but at high cell density, monitoring and cooperating with kin cells are key. Indeed, theoretical work suggests that in mixed-species communities, the broad signal AI-2 is more informative during the early stages of biofilm formation, whereas species-specific autoinducers dominate in single-species communities or during later stages of biofilm formation105,106. Together, all of these feedback loops guarantee optimal dynamics, fidelity and smooth transitions between quorum sensing states.
Quorum sensing functions
Traditionally, quorum sensing was defined as cell–cell communication among bacteria that results in changes in transcription factor activity, and thus, changes in gene expression. Quorum sensing-directed behaviours were defined as those that require all of the bacteria in the population to act in unison to make the behaviours successful107,108. New research broadens these definitions by showing inter-kingdom communication109, responses by intracellular small-molecule chemical signals110, and heterogeneity in gene expression that is controlled by quorum sensing8.
Quorum sensing has long been known to control the production of virulence factors and the formation of biofilms3. Similarly, biofilms and virulence are known to rely on intracellular second-messenger signalling molecules, including cyclic dimeric guanosine monophosphate (c-di-GMP) and cyclic adenosine monophosphate (cAMP)111. This overlap is exemplified in B. cenocepacia: the DSF-family autoinducer cis-2-dodecenoic acid (BDSF) binds to RpfR, which is a protein that contains GGDEF and EAL domains. The binding of BDSF to RpfR causes a decrease in the intracellular concentration of c-di-GMP, which affects swarming motility, the formation of biofilms and virulence112. There are other examples of quorum sensing connections to c-di-GMP and cAMP in Vibrio spp., pseudomonads and other Gram-negative pathogens113. Linking quorum sensing to nucleotide-based second messengers enables the conversion of complex information encoded in autoinducer blends into a single, general intracellular signalling molecule.
Quorum sensing behaviours are often studied in isolation, that is, in well-mixed, shaken cultures and/or in the absence of cooperating or competing microorganisms. However, non-uniform growth conditions and/or mixed communities influence the functions of quorum sensing114. For example, fluid flow, especially in complex geometries, influences the temporal and regional activation of quorum sensing-controlled biofilm formation genes among individual members of V. cholerae communities, which leads to complex patterns of colonization115,116. In the oral cavity, which is non-uniform and subject to flow, AI-2-based communication is required for the formation of multispecies biofilms and the development of dental plaque117. In other biofilm communities, quorum sensing promotes competition, at least among non-kin. For example, in V. cholerae, quorum sensing activates type VI secretion, causing lysis of neighbouring non-kin cells118–120, which promotes the scavenging of DNA from lysed cells and horizontal gene transfer121.
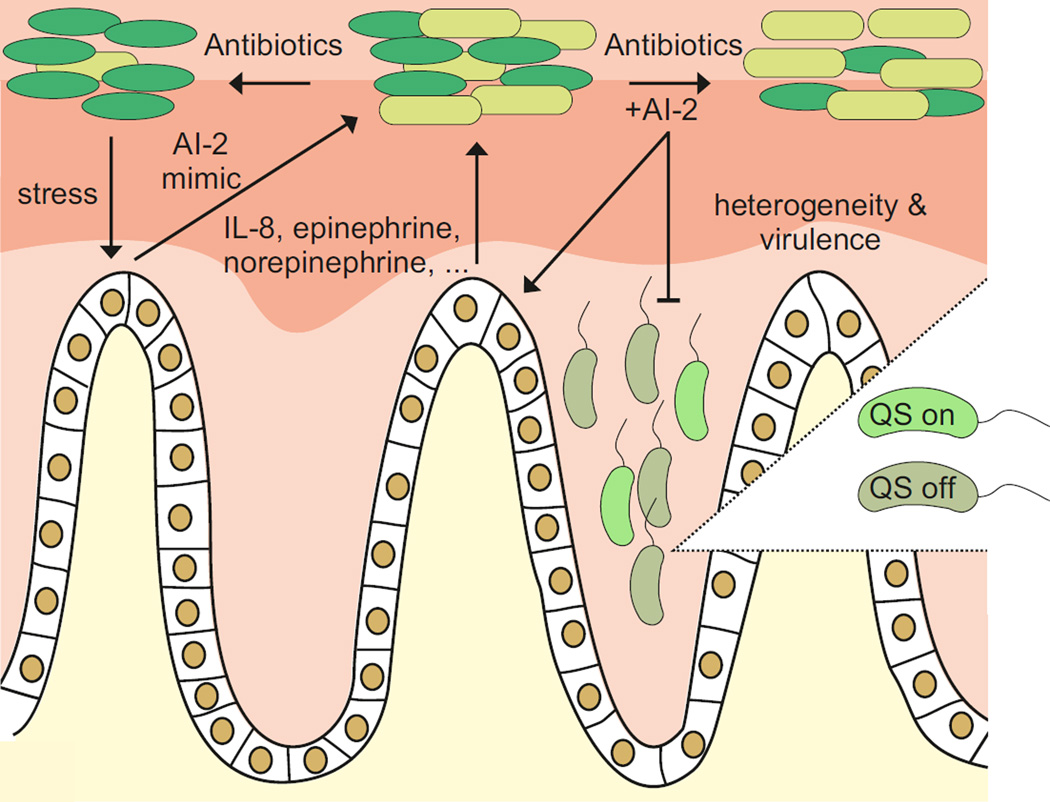
In the gut, AI-2 signalling was recently reported to promote the expansion of Firmicutes over that of Bacteroidetes after antibiotic treatment, which suggests that quorum sensing at least partially shapes the composition of the microbiota122 (FIG. 6). Interestingly, a much greater proportion of species in the Firmicutes than in the Bacteroidetes encode functional AI-2 signalling systems. Furthermore, AI-2 produced by the gut commensal bacterium Blautia obeum (formerly known as Ruminococcus obeum) restricts the virulence of V. cholerae, which is relevant during recovery from cholera123. Interestingly, the V. cholerae receptor that is relevant for AI-2 sensing under these conditions is the LuxR-solo transcription factor, VqmA, rather than LuxPQ124,125.
Figure 6. AI-2-mediated quorum sensing in the mammalian gut.
Gut microorganisms communicate using autoinducer 2 (AI-2). Treatment with antibiotics can alter the composition of the microbiota, which can be ameliorated by modulating levels of AI-2. Eukaryotic cells produce cytokines, such as interleukin-8 (IL-8), in response to AI-2. Hormones (adrenaline and noradrenaline) and AI-2 mimics are produced by the host and can be detected by bacteria. Quorum sensing can alter phenotypic heterogeneity among isogenic members of a bacterial population, which affects virulence-related traits, such as biofilm formation.
Modulation of the gut microbiome and/or its activities can also result from inter-kingdom autoinducer signalling109. For example, exposure of mammalian epithelial cells to AI-2 induces the production of the inflammatory cytokine interleukin-8 (REF. 126). AI-2 produced by P. aeruginosa causes apoptosis in some mammalian cell types127. Conversely, enteric bacteria detect the hormones adrenaline and noradrenaline produced by the host using the sensor kinases QseC and QseE, respectively128. Most recently, mammalian epithelial cells were found to release an AI-2 mimic in response to bacteria or to the disruption of tight junctions. The AI-2 mimic is detected by the bacterial AI-2 receptor, LuxP or LsrB, and it activates quorum sensing-driven gene expression in the bacteria129 (FIG. 6). Exploiting AI-2 as a general communication signal, as opposed to other species-specific autoinducers, could be a strategy that enables the host to maximally communicate with and drive global changes in gene expression in mixed populations such as those that exist in the gut. Similarly, diverse plants and algae produce autoinducer mimics that influence quorum sensing-controlled behaviours in their bacterial colonizers, although, in many cases, the significance of these interactions is unclear130.
Finally, not all quorum sensing-pathways promote the synchronization of gene expression among all group members (FIG. 6). This phenotypic heterogeneity is considered an important bet-hedging strategy131. Quorum sensing-driven heterogeneity has been extensively studied in V. harveyi and can be attributed to the phosphorylation status of LuxO (FIG. 4), which has consequences for the formation of biofilms132,133. Nonconformist cells have also been reported in other systems; however, in most cases, the molecular mechanisms that underlie heterogeneity are not defined8. Recent work in Pseudomonas putida suggests that the production of autoinducers can be heterogonous in immature biofilms and that autoinducers can trigger self-induction of quorum sensing functions in individual cells9, which indicates that the biological function of a quorum sensing signal can vary depending on the growth conditions.
Conclusions
Chemical communication among bacteria through quorum sensing is a central feature of bacterial life that enables bacteria to take a census of the population and discern who their neighbours are, whether they are kin or non-kin, and/or friend or foe. Quorum sensing enables bacteria to orchestrate collective behaviours. In this Review, we have summarized how quorum sensing systems function using a similar set of operating principles, which are embedded in the physical and chemical properties of the autoinducers, the corresponding receptors and their downstream regulators. Quorum sensing is crucial for many bacterial processes, and not surprisingly, synthetic modulators of quorum sensing are being actively pursued to alter bacterial behaviour on demand (BOX 1). It is possible that the principles that underlie bacterial quorum sensing networks are also crucial for collective behaviours in higher organisms. For example, social insects, such as honeybees and ants, use quorum sensing to determine nesting sites134,135. Another tantalizing example is that animal hair follicles can only regenerate in concert with nearby follicles, and this collective process follows a quorum sensing-like logic136. This and other new research raise the exciting, but now plausible possibility that quorum sensing is not restricted to microorganisms, but rather, is a general mechanism that functions throughout the tree of life.
Text Box 1: Synthetic Quorum-Sensing Modulators.
Disabling bacterial quorum sensing with small molecules has been proposed as a strategy to prevent the formation of biofilms and pathogenicity. The quorum sensing circuits of Pseudomonas aeruginosa and Vibrio cholerae contain several possible targets.
In P. aeruginosa, LasR antagonists have been identified that are derivatives of the native acyl-homoserine lactone (AHL)137–140. Structurally unrelated compounds have also been developed and some LasR inhibitors are also RhlR inhibitors141. For example, meta-bromo-thiolactone (mBTL) partially represses LasR and RhlR, and blocks the production of virulence factors and the formation of biofilms140. Some small molecules can be antagonists of one receptor (for example, RhlR) and agonists of another receptor (for example, LasR)142, which highlights the inherent complexity in successfully developing anti-quorum-sensing approaches. LasR and RhlR can have opposing regulatory roles for some targets (for example, LasR represses and RhlR activates certain targets and vice versa) and other regulatory pathways can have a role142,143. PqsR inhibitors have been synthesized based on the natural 2-heptyl-3-hydroxy-4-quinolone (PQS) ligand135,136 and have recently been demonstrated to function as anti-virulence agents144–146. Of note, blocking the function of PqsR with a small molecule can also interfere with the formation of persister cells147.
Blocking autoinducer synthases is another option; for example, the biosynthesis of PQS depends on anthranilate as an intermediate, and its production can be inhibited by the anthranilate analogue, methyl anthranilate148. A screen for inhibitors of the LasI and RhlI synthases identified salicylic acid, tannic acid and trans-cinnamaldehyde. Follow-up mechanistic analyses showed that tannic acid and trans-cinnamaldehyde inhibit RhlI149.
In V. cholerae, quorum sensing represses the production of virulence factors and promotes biofilm dispersal. Thus, molecules that prematurely activate quorum sensing are being pursued for therapeutic development. The addition of synthetic cholera autoinducer-1 (CAI-1) represses the production of cholera toxin and the toxin co-regulated pilus27,32. Several small-molecule CqsS agonists have been identified that are specific for Vibrio spp.150,151. An alternative possibility is the inhibition of LuxO, which activates quorum sensing. A high-throughput screen led to the identification of a set of 6-thio-5-azauracil derivatives, such as AzaU, that are potent inhibitors of LuxO ATPase activity152. AzaU has broad-spectrum activity against pathogenic Vibrio spp. However, AzaU is specific for LuxO proteins and does not antagonize other NtrC homologues and thus, AzaU does not affect growth. Finally, a synthetic inhibitor of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidase (MTAN; also known as Pfs), an enzyme that is involved in the synthesis of CAI-1 and autoinducer 2 (AI-2)35,39, blocks quorum sensing in V. cholerae153. Although MTAN inhibitors block the production of autoinducers and the formation of biofilms, deletion of the gene that encodes MTAN does not prevent biofilm development, which indicates that MTAN inhibitors could operate by a pleiotropic mechanism154.
Few clinical trials that involve these molecules have been conducted to date. One concern is that the inhibition of quorum sensing could increase the prevalence of virulent genotypes155. Identification of the most effective, resistance-proof and reliable quorum sensing-modulators is a challenging task. Nonetheless, the promise of this innovative strategy for antimicrobial treatment in times of emerging multidrug-resistant bacterial pathogens has led to substantial interest and activity.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute, NIH Grant 5R01GM065859, and National Science Foundation Grant MCB-0948112 (to B.L.B.). K.P. was supported by DFG Grant PA2820/1.
Glossary
- Two-component systems
A large group of signal-transduction circuits that typically consist of a membrane-bound histidine sensor kinase that detects a specific environmental stimulus and a cognate response regulator that mediates the cellular response, primarily through transcriptional regulation of target genes.
- Small RNAs (sRNAs)
Bacterial small RNAs (sRNAs) are a heterogeneous group of post-transcriptional regulators that often act together with the chaperone Hfq.
- Public goods
Common-pool resources that are frequently present in biological and social systems. Public goods are available to all members of the community, irrespective if a member contributed to their production or not. Therefore, public goods are prone to exploitation by non-producers.
- Feed-forward loop
A common regulatory network motif in biological pathways. The feed-forward loop is composed of two input factors (usually transcriptional regulators), one of which regulates the other, such that both jointly regulate a downstream target genes.
- Bet-hedging
A survival strategy that reduces the temporal variance in fitness at the expense of a reduced arithmetic mean fitness.
- GAF and PAS domains
Domains that are often conserved in signaling proteins in which they function as ligand binding domains.
- van der Waals interactions
Weak attractive or repulsive forces between molecules or atomic groups that donot result from covalent bonds or electrostatic interactions between ions or ionic groups.
- ATP-binding casstte transporter (ABC transporter)
A member of a large superfamily of small molecule transport systems that are present in all phyla.
- σ54
An alternative sigma factor in bacteria that is encoded by the rpoN gene, which was originally identified as a regulator of genes that are involved in nitrogen metabolism.
- Hfq
A globally acting RNA-binding protein that facilitates base pairing of bacterial small RNAs with their target mRNAs.
- Cyclic dimeric guanosine monophosphate (c-di-GMP)
A second messenger molecule used in signal transduction in a wide variety of bacteria.
- Cyclic adenosine monophosphate (cAMP)
A second messenger molecule important in many biological processes in organisms, ranging from bacteria to humans.
- GGDEF domain and EAL domains
Protein domains that are ubiquitous in bacteria and function to synthesize and degrade the intracellular signalling molecule cyclic dimeric guanosine monophosphate (c-di-GMP), respectively.
- Type VI secretion
Systems that are used by Gram-negative bacteria to inject effector proteins and virulence factors from across the interior of one bacterial cell into another cell called the prey.
- Horizontal gene transfer
The exchange of genetic information between organisms in a manner other than by traditional reproduction. Horizontal gene transfer is key for acquisition of antibiotic resistance in bacteria and horizontal gene transfer also has an important role in evolution and generation of diversity.
- Persister cells
Isogenic members of a bacterial population that have entered a non-growing or extremely slow-growing physiological state, which makes them tolerant to a wide range of antimicrobials.
References
- 1.Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77:73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 5.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papenfort K, Vogel J. Regulatory RNA in bacterial pathogens. Cell Host Microbe. 2010;8:116–127. doi: 10.1016/j.chom.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol. 2013;67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 8.Grote J, Krysciak D, Streit WR. Phenotypic Heterogeneity, a Phenomenon That May Explain Why Quorum Sensing Does Not Always Result in Truly Homogenous Cell Behavior. Appl Environ Microbiol. 2015;81:5280–5289. doi: 10.1128/AEM.00900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carcamo-Oyarce G, Lumjiaktase P, Kummerli R, Eberl L. Quorum sensing triggers the stochastic escape of individual cells from Pseudomonas putida biofilms. Nat Commun. 2015;6:5945. doi: 10.1038/ncomms6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt SL, Pena-Diaz J, Finlay BB. Chemical communication in the gut: Effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe. 2015;34:106–115. doi: 10.1016/j.anaerobe.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Gill EE, Franco OL, Hancock RE. Antibiotic adjuvants: diverse strategies for controlling drug-resistant pathogens. Chem Biol Drug Des. 2015;85:56–78. doi: 10.1111/cbdd.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke X, Miller LC, Bassler BL. Determinants governing ligand specificity of the Vibrio harveyi LuxN quorum-sensing receptor. Mol Microbiol. 2015;95:127–142. doi: 10.1111/mmi.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 15.Case RJ, Labbate M, Kjelleberg S. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J. 2008;2:345–349. doi: 10.1038/ismej.2008.13. [DOI] [PubMed] [Google Scholar]
- 16.von Bodman SB, Willey JM, Diggle SP. Cell-cell communication in bacteria: united we stand. J Bacteriol. 2008;190:4377–4391. doi: 10.1128/JB.00486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer AL, et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–599. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 18.Lindemann A, et al. Isovaleryl-homoserine lactone, an unusual branched-chain quorum-sensing signal from the soybean symbiont Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 2011;108:16765–16770. doi: 10.1073/pnas.1114125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlgren NA, Harwood CS, Schaefer AL, Giraud E, Greenberg EP. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proc Natl Acad Sci U S A. 2011;108:7183–7188. doi: 10.1073/pnas.1103821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flavier AB, Clough SJ, Schell MA, Denny TP. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol. 1997;26:251–259. doi: 10.1046/j.1365-2958.1997.5661945.x. [DOI] [PubMed] [Google Scholar]
- 21.Kai K, et al. Methyl 3-Hydroxymyristate, a Diffusible Signal Mediating phc Quorum Sensing in Ralstonia solanacearum. Chembiochem. 2015;16:2309–2318. doi: 10.1002/cbic.201500456. [DOI] [PubMed] [Google Scholar]
- 22.Genin S, Denny TP. Pathogenomics of the Ralstonia solanacearum species complex. Annu Rev Phytopathol. 2012;50:67–89. doi: 10.1146/annurev-phyto-081211-173000. [DOI] [PubMed] [Google Scholar]
- 23.Tao F, Swarup S, Zhang LH. Quorum sensing modulation of a putative glycosyltransferase gene cluster essential for Xanthomonas campestris biofilm formation. Environ Microbiol. 2010;12:3159–3170. doi: 10.1111/j.1462-2920.2010.02288.x. [DOI] [PubMed] [Google Scholar]
- 24.Ryan RP, An SQ, Allan JH, McCarthy Y, Dow JM. The DSF Family of Cell-Cell Signals: An Expanding Class of Bacterial Virulence Regulators. PLoS Pathog. 2015;11:e1004986. doi: 10.1371/journal.ppat.1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, et al. The Multiple DSF-family QS Signals are Synthesized from Carbohydrate and Branched-chain Amino Acids via the FAS Elongation Cycle. Sci Rep. 2015;5:13294. doi: 10.1038/srep13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Y, Wu J, Eberl L, Zhang LH. Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl Environ Microbiol. 2010;76:4675–4683. doi: 10.1128/AEM.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 28.van Kessel JC, Rutherford ST, Shao Y, Utria AF, Bassler BL. Individual and combined roles of the master regulators AphA and LuxR in control of the Vibrio harveyi quorum-sensing regulon. J Bacteriol. 2013;195:436–443. doi: 10.1128/JB.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 30.Cao JG, Meighen EA. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- 31.Hanzelka BL, et al. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J Bacteriol. 1999;181:5766–5770. doi: 10.1128/jb.181.18.5766-5770.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins DA, et al. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–886. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- 33.Kelly RC, et al. The Vibrio cholerae quorum-sensing autoinducer CAI-1: analysis of the biosynthetic enzyme CqsA. Nat Chem Biol. 2009;5:891–895. doi: 10.1038/nchembio.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng WL, et al. Signal production and detection specificity in Vibrio CqsA/CqsS quorum-sensing systems. Mol Microbiol. 2011;79:1407–1417. doi: 10.1111/j.1365-2958.2011.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Y, Perez LJ, Ng WL, Semmelhack MF, Bassler BL. Mechanism of Vibrio cholerae autoinducer-1 biosynthesis. ACS Chem Biol. 2011;6:356–365. doi: 10.1021/cb1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spirig T, et al. The Legionella autoinducer synthase LqsA produces an alpha-hydroxyketone signaling molecule. J Biol Chem. 2008;283:18113–18123. doi: 10.1074/jbc.M801929200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hornung C, et al. The Janthinobacterium sp. HH01 genome encodes a homologue of the V. cholerae CqsA and L. pneumophila LqsA autoinducer synthases. PLoS One. 2013;8:e55045. doi: 10.1371/journal.pone.0055045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon S, et al. Inter-kingdom Signaling by the Legionella Quorum Sensing Molecule LAI-1 Modulates Cell Migration through an IQGAP1-Cdc42-ARHGEF9-Dependent Pathway. PLoS Pathog. 2015;11:e1005307. doi: 10.1371/journal.ppat.1005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 40.Pereira CS, Thompson JA, Xavier KB. AI-2-mediated signalling in bacteria. FEMS Microbiol Rev. 2013;37:156–181. doi: 10.1111/j.1574-6976.2012.00345.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 42.Miller ST, et al. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Surette MG, Miller MB, Bassler BL. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A. 1999;96:1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan K, Dammel C, Stein J, Rabin H, Surette MG. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol. 2003;50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 45.Campbell J, Lin Q, Geske GD, Blackwell HE. New and unexpected insights into the modulation of LuxR-type quorum sensing by cyclic dipeptides. ACS Chem Biol. 2009;4:1051–1059. doi: 10.1021/cb900165y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, et al. A cell-cell communication signal integrates quorum sensing and stress response. Nat Chem Biol. 2013;9:339–343. doi: 10.1038/nchembio.1225. [DOI] [PubMed] [Google Scholar]
- 47.Pesci EC, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heeb S, et al. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev. 2011;35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brameyer S, Kresovic D, Bode HB, Heermann R. Dialkylresorcinols as bacterial signaling molecules. Proc Natl Acad Sci U S A. 2015;112:572–577. doi: 10.1073/pnas.1417685112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brachmann AO, et al. Pyrones as bacterial signaling molecules. Nat Chem Biol. 2013;9:573–578. doi: 10.1038/nchembio.1295. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs SW, et al. Formation of 1,3-cyclohexanediones and resorcinols catalyzed by a widely occurring ketosynthase. Angew Chem Int Ed Engl. 2013;52:4108–4112. doi: 10.1002/anie.201210116. [DOI] [PubMed] [Google Scholar]
- 53.Smith D, et al. Variations on a theme: diverse N-acyl homoserine lactone-mediated quorum sensing mechanisms in gram-negative bacteria. Sci Prog. 2006;89:167–211. doi: 10.3184/003685006783238335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swem LR, et al. A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell. 2009;35:143–153. doi: 10.1016/j.molcel.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang RG, et al. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 56.Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 57.Engebrecht J, Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984;81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens AM, Dolan KM, Greenberg EP. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc Natl Acad Sci U S A. 1994;91:12619–12623. doi: 10.1073/pnas.91.26.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schu DJ, Scruggs JM, Geissinger JS, Michel KG, Stevens AM. Acyl-homoserine lactone recognition and response hindering the quorum-sensing regulator EsaR. PLoS One. 2014;9:e107687. doi: 10.1371/journal.pone.0107687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Bodman SB, Majerczak DR, Coplin DL. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc Natl Acad Sci U S A. 1998;95:7687–7692. doi: 10.1073/pnas.95.13.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vannini A, et al. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 2002;21:4393–4401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G, Jeffrey PD, Fuqua C, Shi Y, Chen L. Structural basis for antiactivation in bacterial quorum sensing. Proc Natl Acad Sci U S A. 2007;104:16474–16479. doi: 10.1073/pnas.0704843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lintz MJ, Oinuma K, Wysoczynski CL, Greenberg EP, Churchill ME. Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor. Proc Natl Acad Sci U S A. 2011;108:15763–15768. doi: 10.1073/pnas.1112398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, et al. A strategy for antagonizing quorum sensing. Mol Cell. 2011;42:199–209. doi: 10.1016/j.molcel.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bottomley MJ, Muraglia E, Bazzo R, Carfi A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem. 2007;282:13592–13600. doi: 10.1074/jbc.M700556200. [DOI] [PubMed] [Google Scholar]
- 66.Yao Y, et al. Structure of the Escherichia coli quorum sensing protein SdiA: activation of the folding switch by acyl homoserine lactones. J Mol Biol. 2006;355:262–273. doi: 10.1016/j.jmb.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 67.Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 68.Galperin MY. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol. 2006;188:4169–4182. doi: 10.1128/JB.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Nair SK. Quorum sensing: how bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. 2012;21:1403–1417. doi: 10.1002/pro.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hudaiberdiev S, et al. Census of solo LuxR genes in prokaryotic genomes. Front Cell Infect Microbiol. 2015;5:20. doi: 10.3389/fcimb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JH, Lequette Y, Greenberg EP. Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Mol Microbiol. 2006;59:602–609. doi: 10.1111/j.1365-2958.2005.04960.x. [DOI] [PubMed] [Google Scholar]
- 72.Riedel K, et al. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology. 2001;147:3249–3262. doi: 10.1099/00221287-147-12-3249. [DOI] [PubMed] [Google Scholar]
- 73.Swem LR, Swem DL, Wingreen NS, Bassler BL. Deducing receptor signaling parameters from in vivo analysis: LuxN/AI-1 quorum sensing in Vibrio harveyi . Cell. 2008;134:461–473. doi: 10.1016/j.cell.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 75.Neiditch MB, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng WL, et al. Probing bacterial transmembrane histidine kinase receptor-ligand interactions with natural and synthetic molecules. Proc Natl Acad Sci U S A. 2010;107:5575–5580. doi: 10.1073/pnas.1001392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pereira CS, de Regt AK, Brito PH, Miller ST, Xavier KB. Identification of functional LsrB-like autoinducer-2 receptors. J Bacteriol. 2009;191:6975–6987. doi: 10.1128/JB.00976-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elias S, Banin E. Multi-species biofilms: living with friendly neighbors. FEMS Microbiol Rev. 2012;36:990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 79.Goryachev AB. Design principles of the bacterial quorum sensing gene networks. Wiley Interdiscip Rev Syst Biol Med. 2009;1:45–60. doi: 10.1002/wsbm.27. [DOI] [PubMed] [Google Scholar]
- 80.Jimenez PN, et al. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seed PC, Passador L, Iglewski BH. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deziel E, et al. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci U S A. 2004;101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winson MK, et al. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ventre I, et al. Dimerization of the quorum sensing regulator RhlR: development of a method using EGFP fluorescence anisotropy. Mol Microbiol. 2003;48:187–198. doi: 10.1046/j.1365-2958.2003.03422.x. [DOI] [PubMed] [Google Scholar]
- 85.McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–2708. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao H, et al. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc Natl Acad Sci U S A. 2001;98:14613–14618. doi: 10.1073/pnas.251465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffman LR, et al. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros. 2009;8:66–70. doi: 10.1016/j.jcf.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci U S A. 2001;98:1507–1512. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi . Mol. Microbiol. 1999;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 90.Lilley BN, Bassler BL. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol Microbiol. 2000;36:940–954. doi: 10.1046/j.1365-2958.2000.01913.x. [DOI] [PubMed] [Google Scholar]
- 91.Lenz DH, et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae . Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao Y, Feng L, Rutherford ST, Papenfort K, Bassler BL. Functional determinants of the quorum-sensing non-coding RNAs and their roles in target regulation. EMBO J. 2013;32:2158–2171. doi: 10.1038/emboj.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei Y, Ng WL, Cong J, Bassler BL. Ligand and antagonist driven regulation of the Vibrio cholerae quorum-sensing receptor CqsS. Mol Microbiol. 2012;83:1095–1108. doi: 10.1111/j.1365-2958.2012.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Teng SW, et al. Active regulation of receptor ratios controls integration of quorum-sensing signals in Vibrio harveyi. Mol Syst Biol. 2011;7:491. doi: 10.1038/msb.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tu KC, Long T, Svenningsen SL, Wingreen NS, Bassler BL. Negative feedback loops involving small regulatory RNAs precisely control the Vibrio harveyi quorum-sensing response. Mol Cell. 2010;37:567–579. doi: 10.1016/j.molcel.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng L, et al. A qrr noncoding RNA deploys four different regulatory mechanisms to optimize quorum-sensing dynamics. Cell. 2015;160:228–240. doi: 10.1016/j.cell.2014.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sourjik V, Wingreen NS. Responding to chemical gradients: bacterial chemotaxis. Curr Opin Cell Biol. 2012;24:262–268. doi: 10.1016/j.ceb.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Svenningsen SL, Tu KC, Bassler BL. Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. EMBO J. 2009;28:429–439. doi: 10.1038/emboj.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hunter GA, Vasquez FG, Keener JP. A mathematical model and quantitative comparison of the small RNA circuit in the Vibrio harveyi and Vibrio cholerae quorum sensing systems. Phys Biol. 2013;10:046007. doi: 10.1088/1478-3975/10/4/046007. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Tu KC, Ong NP, Bassler BL, Wingreen NS. Protein-level fluctuation correlation at the microcolony level and its application to the Vibrio harveyi quorum-sensing circuit. Biophys J. 2011;100:3045–3053. doi: 10.1016/j.bpj.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Svenningsen SL, Waters CM, Bassler BL. A negative feedback loop involving small RNAs accelerates Vibrio cholerae’s transition out of quorum-sensing mode. Genes Dev. 2008;22:226–238. doi: 10.1101/gad.1629908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rutherford ST, van Kessel JC, Shao Y, Bassler BL. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 2011;25:397–408. doi: 10.1101/gad.2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin W, Kovacikova G, Skorupski K. Requirements for Vibrio cholerae HapR binding and transcriptional repression at the hapR promoter are distinct from those at the aphA promoter. J Bacteriol. 2005;187:3013–3019. doi: 10.1128/JB.187.9.3013-3019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Long T, et al. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 2009;7:e68. doi: 10.1371/journal.pbio.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nadell CD, Xavier JB, Levin SA, Foster KR. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 2008;6:e14. doi: 10.1371/journal.pbio.0060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bassler BL. Small talk: cell-to-cell communication in bacteria. Cell. 2002;109:421–424. doi: 10.1016/s0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 109.Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol. 2009;12:192–198. doi: 10.1016/j.mib.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srivastava D, Waters CM. A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP. J Bacteriol. 2012;194:4485–4493. doi: 10.1128/JB.00379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pesavento C, Hengge R. Bacterial nucleotide-based second messengers. Curr Opin Microbiol. 2009;12:170–176. doi: 10.1016/j.mib.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Deng Y, et al. Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover. Proc Natl Acad Sci U S A. 2012;109:15479–15484. doi: 10.1073/pnas.1205037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kalia D, et al. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev. 2013;42:305–341. doi: 10.1039/c2cs35206k. [DOI] [PubMed] [Google Scholar]
- 114.Persat A, et al. The mechanical world of bacteria. Cell. 2015;161:988–997. doi: 10.1016/j.cell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. Solutions to the public goods dilemma in bacterial biofilms. Curr Biol. 2014;24:50–55. doi: 10.1016/j.cub.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim MK, Ingremeau F, Zhao A, Bassler BL, Stone HA. Local and global consequences of flow on bacterial quorum sensing. Nature Microbiology. 2016;1:15005. doi: 10.1038/nmicrobiol.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rickard AH, Campagna SR, Kolenbrander PE. Autoinducer-2 is produced in saliva-fed flow conditions relevant to natural oral biofilms. J Appl Microbiol. 2008;105:2096–2103. doi: 10.1111/j.1365-2672.2008.03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shao Y, Bassler BL. Quorum regulatory small RNAs repress type VI secretion in Vibrio cholerae. Mol Microbiol. 2014;92:921–930. doi: 10.1111/mmi.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng J, Shin OS, Cameron DE, Mekalanos JJ. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc Natl Acad Sci U S A. 2010;107:21128–21133. doi: 10.1073/pnas.1014998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A. 2010;107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borgeaud S, Metzger LC, Scrignari T, Blokesch M. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015;347:63–67. doi: 10.1126/science.1260064. [DOI] [PubMed] [Google Scholar]
- 122.Thompson JA, Oliveira RA, Djukovic A, Ubeda C, Xavier KB. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015;10:1861–1871. doi: 10.1016/j.celrep.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 123.Hsiao A, et al. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature. 2014 doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Z, Hsiao A, Joelsson A, Zhu J. The transcriptional regulator VqmA increases expression of the quorum-sensing activator HapR in Vibrio cholerae. J Bacteriol. 2006;188:2446–2453. doi: 10.1128/JB.188.7.2446-2453.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Papenfort K, Forstner KU, Cong JP, Sharma CM, Bassler BL. Differential RNA-seq of Vibrio cholerae identifies the VqmR small RNA as a regulator of biofilm formation. Proc Natl Acad Sci U S A. 2015;112:E766–E775. doi: 10.1073/pnas.1500203112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zargar A, et al. Bacterial secretions of nonpathogenic Escherichia coli elicit inflammatory pathways: a closer investigation of interkingdom signaling. MBio. 2015;6:e00025. doi: 10.1128/mBio.00025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shiner EK, et al. Pseudomonas aeruginosa autoinducer modulates host cell responses through calcium signalling. Cell Microbiol. 2006;8:1601–1610. doi: 10.1111/j.1462-5822.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 128.Karavolos MH, Winzer K, Williams P, Khan CM. Pathogen espionage: multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol Microbiol. 2013;87:455–465. doi: 10.1111/mmi.12110. [DOI] [PubMed] [Google Scholar]
- 129.Ismail AS, Valastyan JS, Bassler BL. A Host-Produced Autoinducer-2 Mimic Activates Bacterial Quorum Sensing. Cell Host Microbe. 2016 doi: 10.1016/j.chom.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Helman Y, Chernin L. Silencing the mob: disrupting quorum sensing as a means to fight plant disease. Mol Plant Pathol. 2015;16:316–329. doi: 10.1111/mpp.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508. doi: 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- 132.Plener L, et al. The phosphorylation flow of the Vibrio harveyi quorum-sensing cascade determines levels of phenotypic heterogeneity in the population. J Bacteriol. 2015;197:1747–1756. doi: 10.1128/JB.02544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anetzberger C, Pirch T, Jung K. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi. Mol Microbiol. 2009;73:267–277. doi: 10.1111/j.1365-2958.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- 134.Franks NR, et al. Not everything that counts can be counted: ants use multiple metrics for a single nest trait. Proc Biol Sci. 2006;273:165–169. doi: 10.1098/rspb.2005.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seeley TD, Visscher PK. Group decision making in nest-site selection by honey bees. Apidologie. 2004;35:101–116. [Google Scholar]
- 136.Chen CC, et al. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell. 2015;161:277–290. doi: 10.1016/j.cell.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McInnis CE, Blackwell HE. Design, synthesis, and biological evaluation of abiotic, non-lactone modulators of LuxR-type quorum sensing. Bioorg Med Chem. 2011;19:4812–4819. doi: 10.1016/j.bmc.2011.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Geske GD, Mattmann ME, Blackwell HE. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators. Bioorg Med Chem Lett. 2008;18:5978–5981. doi: 10.1016/j.bmcl.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stacy DM, et al. Synthesis and biological evaluation of triazole-containing N-acyl homoserine lactones as quorum sensing modulators. Org Biomol Chem. 2013;11:938–954. doi: 10.1039/c2ob27155a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Loughlin CT, et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ishida T, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl Environ Microbiol. 2007;73:3183–3188. doi: 10.1128/AEM.02233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Welsh MA, Eibergen NR, Moore JD, Blackwell HE. Small molecule disruption of quorum sensing cross-regulation in pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J Am Chem Soc. 2015;137:1510–1519. doi: 10.1021/ja5110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Welsh MA, Blackwell HE. Chemical Genetics Reveals Environment-Specific Roles for Quorum Sensing Circuits in Pseudomonas aeruginosa. Cell Chem Biol. 2016 doi: 10.1016/j.chembiol.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ilangovan A, et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR) PLoS Pathog. 2013;9:e1003508. doi: 10.1371/journal.ppat.1003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu C, et al. Discovery of antagonists of PqsR, a key player in 2-alkyl-4-quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem Biol. 2012;19:381–390. doi: 10.1016/j.chembiol.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 146.Lu C, Maurer CK, Kirsch B, Steinbach A, Hartmann RW. Overcoming the unexpected functional inversion of a PqsR antagonist in Pseudomonas aeruginosa: an in vivo potent antivirulence agent targeting pqs quorum sensing. Angew Chem Int Ed Engl. 2014;53:1109–1112. doi: 10.1002/anie.201307547. [DOI] [PubMed] [Google Scholar]
- 147.Starkey M, et al. Identification of anti-virulence compounds that disrupt quorum-sensing regulated acute and persistent pathogenicity. PLoS Pathog. 2014;10:e1004321. doi: 10.1371/journal.ppat.1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Calfee MW, Coleman JP, Pesci EC. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2001;98:11633–11637. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang CY, et al. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci Rep. 2014;4:7245. doi: 10.1038/srep07245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bolitho ME, et al. Small molecule probes of the receptor binding site in the Vibrio cholerae CAI-1 quorum sensing circuit. Bioorg Med Chem. 2011;19:6906–6918. doi: 10.1016/j.bmc.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Faloon P, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Discovery of Two, Structurally Distinct Agonists of Vibrio cholerae Quorum Sensing Acting via the CqsS Membrane Receptor. [PubMed] [Google Scholar]
- 152.Ng WL, Perez L, Cong J, Semmelhack MF, Bassler BL. Broad spectrum pro-quorum-sensing molecules as inhibitors of virulence in vibrios. PLoS Pathogens. 2012;8:e1002767. doi: 10.1371/journal.ppat.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gutierrez JA, et al. Transition state analogs of 5’-methylthioadenosine nucleosidase disrupt quorum sensing. Nat Chem Biol. 2009;5:251–257. doi: 10.1038/nchembio.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Silva AJ, Parker WB, Allan PW, Ayala JC, Benitez JA. Role of methylthioadenosine/S-adenosylhomocysteine nucleosidase in Vibrio cholerae cellular communication and biofilm development. Biochem Biophys Res Commun. 2015;461:65–69. doi: 10.1016/j.bbrc.2015.03.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kohler T, Perron GG, Buckling A, van Delden C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000883. doi: 10.1371/journal.ppat.1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]