ABSTRACT
CDK9 is a protein in constant development in cancer therapy. Herein we present an overview of the enzyme as a target for cancer therapy. We provide data on its characteristics and mechanism of action. In recent years, CDK9 inhibitors that have been designed with molecular modeling have demonstrated good antitumoral activity in vitro. Clinical studies of the drugs flavopiridol, dinaciclib, seliciclib, SNS-032 and RGB-286638 used as CDK9 inhibitors are also reviewed, with their additional targets and their relative IC50 values. Unfortunately, treatment with these drugs remains unsuccessful and involves many adverse effects. We could conclude that there are many small molecules that bind to CDK9, but their lack of selectivity against other CDKs do not allow them to get to the clinical use. However, drug designers currently have the tools needed to improve the selectivity of CDK9 inhibitors and to make successful treatment available to patients.
Keywords: CDK9, molecular modeling, antitumor, anticancer, drug development, cyclin, kinase
Introduction
CDK9 was first isolated and designated PITALRE, for the characteristic Pro-Ile-Thr-Ala-Leu-Arg-Glu motif by us.1 Its chromosomal mapping and phosphorilation sites were studied before it was named CDK9.2-4 In HIV studies, PITALRE was identified as the catalytic subunit of the positive transcription elongation factor b (P-TEFb), a protein kinase that hyperphosphorylate the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase II in vitro.5-7 Peng et al. are the ones that described P-TEFb as a novel CDK/cyclin pair, calling their subunits CDK9 and Cyclin T for the first time.8 After that, Cyclin T1, T2a, and T2b were identified. Each binds CDK9 and possesses P-TEFb activity, although 80% of CDK9 binds Cyclin T1, 10% binds T2a, and 10% binds T2b.9 A year later, Cyclin K was also found to interact with CDK9 in vivo.10 Herein, we provide data of the characteristics and mechanism of action of CDK9. Molecular modeling, in vitro and clinical studies of the drugs used as CDK9 inhibitors are also reviewed. Currently, the scientific community requires targeted cancer drugs to get the successful treatment to patients and drug designers have the tools needed to improve the selectivity of CDK9 inhibitors.
Mechanism of action
CDK9 is not a typical Cdc2-like kinase. It does not act in cell-cycle regulation processes; rather, it acts in differentiation processes.11 It is the catalytic subunit of P-TEFb that, in association with Cyclin T, has the ability to phosphorilate the CTD substrate of RNA polymerase II and reach the RNA transcription elongation.1-12 Although there are other cyclin-dependent kinases that are capable of phosphorilating the CTD, the only one that activates gene expression in a catalyst manner is CDK9. Therefore, Cyclin T/CDK9 is a dedicated kinase functioning in transcription, with CTD being the major functional target of the complex in vivo.12
Although the mechanism underlying CDK9 is complex and not totally elucidated, it is schemed and explained in Figure 1. The CTD of the RNA polymerase II comprises tandem repeats of the 7 amino acid sequence YSPTSPS, domain that is essential for the polymerase function in vivo.13 The CTD should be hyperphosphorylated to regulate elongation.14 The number of phosphorilation sites exceeds 50, serine being the predominant one.15 There are 2 main phosphorylations carried out by cyclin-dependent kinases (CDKs): the one of Ser5 (YSPTSer5PS) by CDK716,17 and the one of Ser2 (YSer2PTSPS) by CDK9.18 Firstly, CDK7 phosphorilates Ser5, allowing for the activation of RNA-Pol II.18-20 Next, the Ser5 phosphorilated RNA-Pol II is able to stimulate transcription of the RNA, but not its elongation.20,21 The productive elongation comes with the phosphorylation of Ser2 by P-TEFb.18,22-26 Therefore, P-TEFb (CDK9/Cyclin T or CDK9/Cyclin K) is essential in order to generate mature mRNAs in cells.
Figure 1.
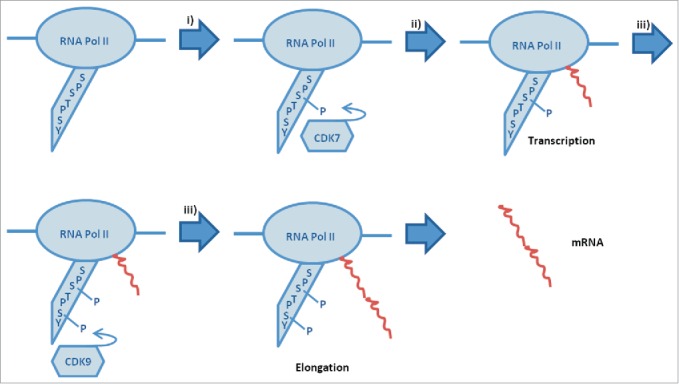
Scheme of RNA-Pol II sequence process: i) activation, ii) transcription and iii) elongation of RNA.
CDK9 Isoforms
There are 2 isoforms of the CDK9 protein: the major 42 kDa CDK9 isoform, and the minor 55 kDa isoform. The 42kDa isoform (CDK942) is the one originally identified as PITALRE.1 The second form of CDK9 (CDK955) is 13kDa larger than the protein originally identified,27 with a 117 residue terminal extension.28 Both isoforms were present in HeLa, NIH/3T3 human macrophages and mouse lung and liver tissues, but with different abundance.28,29 Although their phosphorylation patterns, studied with 144 peptide substrates, are identical, they possess different localization and expression patterns summarized in Table 1.28,29 These results suggest that the functions of the 2 isoforms should be distinguished, although there is not a concrete characterization of them in literature yet.
Table 1.
Differences in localization and expression patterns of CDK9 isoforms.28,29
| CDK942 | CDK955 | |
|---|---|---|
| Localization | Nucleoplasm | Nucleolus |
| Undifferentiated monocytes | High levels | Not detected |
| Macrophage differentiation | — | High levels |
| Primary lymphocytes | Level increased Promoter responsible to activate signals | Level decreased |
| Promoter in HeLa cells | Strong | — |
| Hepatocytes | Predominant form after cell cycle | Predominant form before cell cycle |
CDK955 is identical to CDK942, except for an additional 117 amino acid residues at the amino terminus.30 The promoter sequence has 2 transcription starts in CDK9. The segment that transcribed CDK942 mRNA is characterized by a GC-rich sequence,27,31 while CDK955 mRNA is transcribed by a TATA box, that is approximately 500 bp upstream of the mRNA transcription start-point.24,31 Liu et al. found that Ku70, a protein involved in DNA repair, specifically associates with the CDK955, but not with the 42kDa. These results again suggest that the functions of the 2 isoforms should be distinguished, and that CDK955 may play a role in the repair of DNA.
Molecular modeling in CDK9
Medicinal chemistry approaches in drug research and development have evolved alongside the progress observed in molecular modeling drug discovery. The amount of in silico studies significantly increased, stimulated by the detailed knowledge of CDK9 at the molecular level and by the advances in bioinformatics.
The computational study of the P-TEFb complex allows the identification of several CDK9 inhibitors. Currently, the most prominent method of blocking P-TEFb function is to directly inhibit the ATP-binding site of CDK9 (Fig. 2). Flavopiridol (1 [Table 2]) is an anticancer drug in phase II clinical trials with a broad specificity, as CDK inhibitor that binds the ATP site of CDK9.32-35 However, this strategy is not the most specific for drug discovery because the ATP binding pocket is reasonably conserved in the whole CDK family, with more than 12 CDKs involved. Moreover, the inhibitor has to compete with the molecules of ATP during binding, which are in high cellular concentrations.
Figure 2.
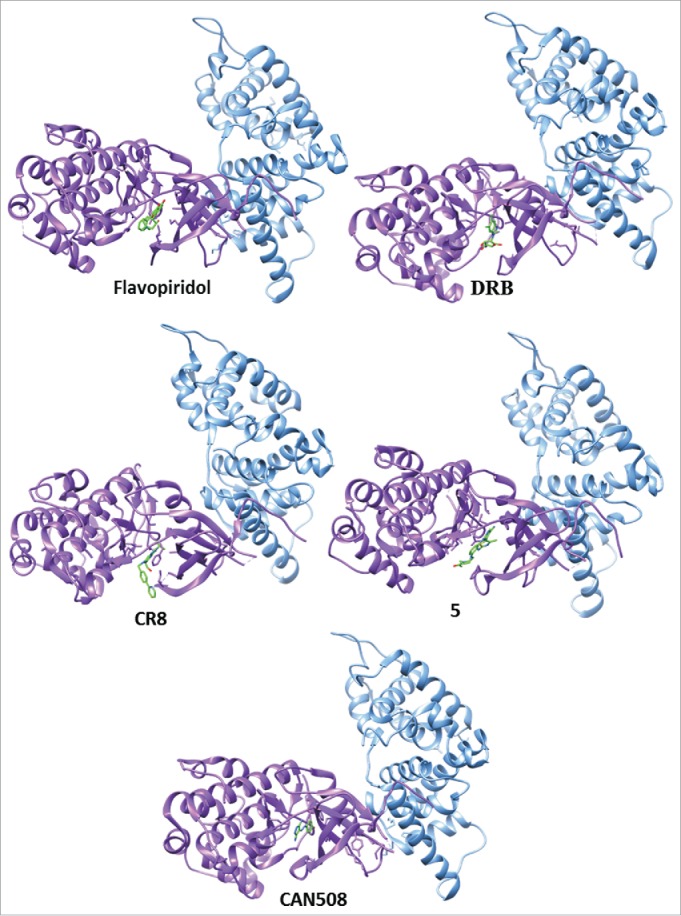
CDK9 inhibitors blocking the ATP-binding site (CDK9 is in purple and Cyclin T1 in blue).
Table 2.
CDK9 Inhibition K1 values by small molecules 1–10. 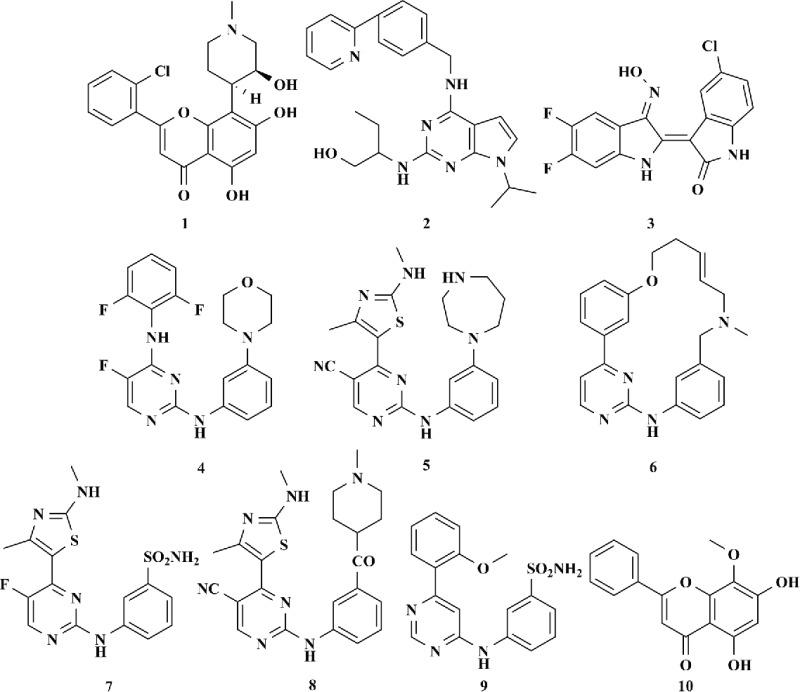
| Number | Name | Inhibition K1 (nM) |
|---|---|---|
| 1 | Flavopiridol39 | 4.59 |
| 2 | CR837,53 | 110 |
| 3 | Indirubin-3´-monoxime derivatives38 | 400 |
| 4 | 5-Fluoro-N2,N4-diphenylpyrimidine-2,4-diamines39 | 330 |
| 5 | 4-(thiazol-5-yl)-2-(phenylamino)pyrimidines40,50 | 7 |
| 6 | TG0248,49,54 | 3 |
| 7 | CDKI-7348 | 4 |
| 8 | 2,4,5-trisubstited pyrimidine derivatives49 | 14 |
| 9 | LCD00006751 | 44 |
| 10 | Wogonin52 | 190 |
Alternative approaches have been designed in order to increase the selectivity of CDK9 inhibitors (Fig. 2). One example is done by 5,6-dichlorobenzimidazone-1-β-D-ribofuranoside (DRB). It blocks the ATP binding site of CDK9 by halogen bond formation, inducing conformational changes in the glycine-rich loop of CDK9. This change of conformation contributes to a high affinity interaction.36 The pan-CDK inhibitor CR8 (2 [Table 2]) induces a downward movement of this loop in CDK9.37 The importance of halogen atoms in the molecular design of selective CDK9 inhibitors is reinforced by the discovery of the indirubin-3′-monoxime derivative (3) showed in Table 2.38 5-Fluoro-N2,N4-diphenylpyrimidine-2,4-diamines (4 [Table 2]) binds the ATP binding site of CDK9 with a different orientation from that of flavopiridol.39 However, the CDK9 inhibition by all these molecules (2–4) is lower than the one done by flavopiridol (1).
The study of the interactions of a series of substituted 4-(thiazol-5-yl)-2-(phenylamino)pyrimidines (5, Table 2) and of CAN508, a 4-arylazo-3,5-diamino-1H-pyrazole inhibitor, with the ATP binding site of CDK9 and CDK2 suggests that the ATP binding site of CDK9 is more malleable than that of CDK2 (Fig. 2), and can accommodate large and flexible compounds.40-42 These studies provide another approach for the selectivity of inhibitors toward CDK9 over CDK2.
Another example is the computational study of the CDK9/cyclin T1 protein-protein interaction done by Randjelovic et al.,43 where 2 peptide sequences were identified as potential inhibitors to bind the surface of CDK9. In this way, they directly interfere with the CDK9/Cyclin T1 complex formation. Furthermore, the small molecule 2-amino-8-hydroxyquinoline has been proposed as a potential inhibitor of the CDK9/Cyclin T1 interaction.44
F07 and F07#13 are 2 small molecules that despite being studied as anti-retroviral drugs, target the interface pocket of CDK9/Cyclin T1. The in silico analysis of Duyne et al. demonstrated a better binding by the drugs to the active form of CDK9.45
Small Molecules as CDK9 Inhibitors
Research in small molecules is fundamental for the discovery of a successful drug in targeted cancer treatment. Herein, we summarize some of the small molecules that have been designed as antitumor drugs with CDK9 inhibition (Table 2).
TG02 (6 [Table 2]) is one of the molecules with the best value of CDK9 inhibition K1 (3nM).46 However, it has not been designed as a CDK9 inhibitor specifically, as it also binds other kinases, such as Janus Kinase 2 and Fms-like tyrosine kinase-3.47 TG02 is a macrocycle that holds a phenylamino pyrimidine as CDKI-73 (7) and the 2,4,5-trisubstited pyrimidine derivative (8) showed in Table 2. These molecules have shown appreciable selectivity for CDK9 as CDK inhibitors, capable of activating caspase 3, reducing the level of Mcl-1 anti-apoptotic protein, and inducing cancer cell apoptosis in breast, colon and leukemia cancer cells.48,49
Other CDK inhibitors with the phenylamino pyrimidine in their structure that bind the ATP binding site and present CDK9 potency and selectivity are LDC000067 (9 [Table 2]) and the substituted 4-(thiazol-5-yl)-2-(phenylamino) pyrimidine (5 [Table 2). They have demonstrated potent anticancer activity against different cell lines, such as cervix, lung, breast and leukemia with down-regulation of Mcl-1.50,51 These small molecules holding a phenylamino pyrimidine could represent promising leads for the development of specific CDK9 inhibitors.
Wogonin, one of the active flavones from the natural herb Scutellaria balcalensis, should be highlighted as a CDK9 inhibitor (10 [Table 2]). It presents similarities with the structure of flavopiridol, and blocks the phosphorylation of the carboxy-terminal domain of RNA polymerase II at Ser2, resulting in apoptosis induction in leukemic T-cells in vitro.52
It should be considered that, although the search of the most specific CDK9 inhibitor is depicted, the activity of these inhibitors toward other kinases is not necessarily detrimental. The final judgement on the anticancer potential of a molecule should go through in vivo experimentation inescapably, where the overall therapeutic efficacy can be evaluated.
Clinical Trials of CDK9 Inhibitors
Randomized controlled trials are considered the most reliable methodology for acquiring adequate data to understand the benefits and risks of new drugs and how they are optimally utilized.55 Five CDK9 inhibitors that have been tested in clinical trials in the last years are reviewed in this paper, with additional data about the specific tumoral pathologies involved in each trial, their additional targets and their relative IC50 values listed in Table 3.
Table 3.
CDK9 Inhibitors in Clinical Trials.
| Clinical Trials |
||||
|---|---|---|---|---|
| Name | Additional Targets | Phase | Tumors* | IC50 (nM)** |
| Flavopiridol | CDK1, CDK2, CDK4, CDK6, CDK7, CDK9, GSK3β35,83 | II | AML56; PPC57; CLL58 | AML: 40084; Ov202: 10085; B-CLL: 10086 |
| I | RMM64; NHL65; CLL63,69; AML70; ALL70; ABLs70 | U266: 1087; RPMI-8226: 1087; JeKo-1: 7088; Molt-4: 10089; K562: 35089 | ||
| Dinaciclib | CDK1, CDK2, CDK5, CDK983,89 | II | ABC71; NSLC72; AML73; ALL73; | Breast: 890; Lung: 6–1490; Leukemia: 690; |
| I | CLL74; RMM76 | J558: 291 | ||
| Seliciclib | CDK1, CDK2, CDK5, CDK7, CDK9, CK1, GSK3A, DIRK1A,ERK183 | I | SAT78,79 | Mean: 740092 |
| SNS-032 | CDK1, CDK2, CDK4, CDK7, CDK983 | I | SAT80; CLL81; RMM81 | Leukemia:13993 |
| RGB-286638 | CDK1, CDK2, CDK4, CDK5, CDK6, CDK7, CDK982,83 | I | SAT82 | Myeloma: 10094 |
AML: Acute myelogenous leukemia; PPC: Primary peritoneal carcinoma; CLL: Chronic lymphocytic leukemia; RMM: Relapsed multiple myeloma; NHL: Non-Hodgkin's lymphoma; ALL: Acute lymphoblastic leukemia; ABLs: Acute byphenotypic leukemias; ABC: Advanced breast cancer; NSLC: Non-small cell lung cancer; SAT: Solid advanced tumors.
Half maximal inhibitory concentration of each drug against the cell lines indicated and expressed in nM.
Flavopiridol is the drug most often evaluated in clinical trials as a CDK9 inhibitor. A randomized phase II study of 2 schedules of flavopiridol given with cytosine arabinoside and mitoxantrone to patients with acute myelogenous leukemia (AML) garnered 58% complete response, although 8% of the patients left the study because of the adverse effects and 13% of them died.56 The complete response to the treatment in the other 2 phase II trials was approximately 2%. One of the studies observed combination with cisplatin in primary peritoneal carcinoma (PPC),57 while the other was only of flavopiridol in patients with leukemia.58 In this last study, all patients suffered adverse effects, with 87% high risk. It should be mentioned that 26% of the patients stopped the treatment with flavopiridol due to an adverse event.58
There is no complete response in 7 of the 12 flavopiridol clinical trials in Phase I studied.59-65 Many adverse effects and events were described in the trials, such as thrombocytopenia,59,60,63,64 embolism,60 neutropenia60,62-65 and fatigue.59,61,65 Therefore, more than the half of the Phase I studies did not satisfy the patients. In addition, there are only 3 studies with complete response under 10%,66-68 and one where 3 of the 9 patients had complete remission, but 8 presented anemia.69 The study where the percentage is higher is the one which observed the association to cytosine arabinoside and mitoxantrone mentioned before (40%), although 51% of the patients suffered tumor lysis syndrome.70
Dinaciclib is the other CDK9 inhibitor that has been featured in Phase I and II clinical trials throughout the last years. In three Phase II studies where it was involved, there was not a complete response to the treatment and 75–95% of the patients suffered adverse effects.71-73 Moreover, Phase I studies of the drug revealed several adverse effects and no complete response in any case.74-77
Yet another CDK9 inhibitor involved in Phase I studies is seliciclib. Although the clinical trials do not reveal many adverse effects, they also do not expose any complete response by the patients treated.78,79 A Phase I study enrolling SNS-032, a CDK 2, 7 and 9 inhibitor, was terminated during dose-escalation. 100% of the patients suffered clinical adverse effects.80 Other Phase I and pharmacologic study of the drug demonstrated that there is no response to antitumor activity in the patients, with 75% having adverse effects.81 RGB-286638, a novel multitargeted CDK inhibitor, including CDK9, revealed no complete response to the treatment and 23% adverse effects described by the patients in its first human trial.82
It should be mentioned that these aforementioned 5 inhibitors used in clinical trials (flavopiridol, dinaciclib, seliciclib, SNS-032, RGB-286638) are not selective to CDK9. They also inhibit other CDKs and other enzymes (Table 3). Therefore, unsuccessful treatment with these drugs involving many adverse effects could be due to its lack of selectivity.
Conclusion
CDK9 is a target in constant development in cancer therapy. CDK9 inhibitors have demonstrated good antitumoral activity in vitro. Although there are many small molecules that bind CDK9, the lack of selectivity against other CDKs and enzymes does not allow their clinical use. However, drug designers have the tools required to improve the selectivity of CDK9 inhibitors. Moreover, the scientific community requires targeted cancer drugs to offer patients successful treatment.
Disclosure of potential conflicts of interest
The authors confirm that the content in this article presents no conflict of interest.
Funding
This work was supported by the Fund for Sbarro Health Research Organization (SHRO) and for the Italian Association for Cancer Research (Associazione Italiana per la Ricerca sul Cancro, AIRC). The award of a postdoctoral grant from the Martín Escudero Foundation to Fátima Morales is gratefully acknowledged.
References
- [1].Graña X, De Luca A, Sang N, Fu Y, Claudio PP, Rosenblatt J, Morgan DO, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc Natl Acad Sci USA 1994; 91(9):3834-38; http://dx.doi.org/ 10.1073/pnas.91.9.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bullrich F, MacLachlan TK, Sang N, Druck T, Veronese ML, Allen SA, Chiorazzi N, Koff A, Heubner K, Croce CM, et al.. Chromosomal Mapping of Members of the cdc2 Family of Protein Kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk Inhibitor, p27Kip, to Regions Involved in Human Cancer. Cancer Res 1995; 55:1199-1205; PMID:7882308 [PubMed] [Google Scholar]
- [3].Garriga J, Segura E, Mayol X, Grubmeyer C, Graña X. Phosphorylation site specificity of the CDC2-related kinase PITALRE. Biochem J 1996; 320(3):983-9; PMID:9003389; http://dx.doi.org/ 10.1042/bj3200983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Luca A, Esposito V, Baldi A, Claudio PP, Fu Y, Caputi M, Pisano MM, Baldi F, Giordano A. CDC2-related kinase PITALRE phosphorylates pRb exclusively on serine and is widely expressed in human tissues. J Cell Physiol 1997; 172(2):265-73; PMID:9258347; http://dx.doi.org/ 10.1002/(SICI)1097-4652(199708)172:2%3c265::AID-JCP13%3e3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- [5].Yang X, Gold MO, Tang DN, Lewis DE, Aguilar-Cordova E, Rice AP, Herrmann CH. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA 1997; 94(23):12331-36; PMID:9356449; http://dx.doi.org/ 10.1073/pnas.94.23.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhu Y, Pe'ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews MB, Price DH. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev 1997; 11(20):2622-32; PMID:9334325; http://dx.doi.org/ 10.1101/gad.11.20.2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Falco G, Giordano A. CDK9: from basal transcription to cancer and AIDS. Cancer Biol Ther 2002; 1(4):342-7; PMID:12432243; http://dx.doi.org/ 10.4161/cbt.1.4.6113 [DOI] [PubMed] [Google Scholar]
- [8].Peng J, Marshall NF, Price DH. Identification of a cyclin subunit required for the function of Drosophila P-TEFb. J Biol Chem 1998; 273(22):13855-60; PMID:9593731; http://dx.doi.org/ 10.1074/jbc.273.22.13855 [DOI] [PubMed] [Google Scholar]
- [9].Peng J, Zhu Y, Milton JT, Price DH. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev 1998; 12(5):755-62; PMID:9499409; http://dx.doi.org/ 10.1101/gad.12.5.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fu TJ, Peng J, Lee G, Price DH, Flores O. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J Biol Chem 1999; 274(49):34527-30; PMID:10574912; http://dx.doi.org/ 10.1074/jbc.274.49.34527 [DOI] [PubMed] [Google Scholar]
- [11].De Falco G, Giordano A. CDK9 (PITALRE): a multifunctional cdc2-related kinase. J Cell Physiol 1998; 177(4):501-6; PMID:10092203; http://dx.doi.org/ 10.1002/(SICI)1097-4652(199812)177:4%3c501::AID-JCP1%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- [12].Napolitano G, Majello B, Licciardo P, Giordano A, Lania L. Transcriptional activity of positive transcription elongation factor b kinase in vivo requires the C-terminal domain of RNA polymerase II. Gene 2000; 254(1–2):139-45; PMID:10974544; http://dx.doi.org/ 10.1016/S0378-1119(00)00278-X [DOI] [PubMed] [Google Scholar]
- [13].Corden JL. Tails of RNA polymerase II. Trends Biochem Sci 1990; 15(10):383-7; PMID:2251729; http://dx.doi.org/ 10.1016/0968-0004(90)90236-5 [DOI] [PubMed] [Google Scholar]
- [14].Hirose Y, Manley JL. RNA polymerase II and the integration of nuclear events. Genes Dev 2000; 14(12):1415-29; PMID:10859161 [PubMed] [Google Scholar]
- [15].Dahmus ME. Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J Biol Chem 1996; 271(32):19009-12; PMID:8759772; http://dx.doi.org/ 10.1074/jbc.271.32.19009 [DOI] [PubMed] [Google Scholar]
- [16].Trigon S, Serizawa H, Conaway JW, Conaway RC, Jackson SP, Morange M. Characterization of the residues phosphorylated in vitro by different C-terminal domain kinases. J Biol Chem 1998; 273(12):6769-75; PMID:9506978; http://dx.doi.org/ 10.1074/jbc.273.12.6769 [DOI] [PubMed] [Google Scholar]
- [17].Spilianakis C, Kretsovali A, Agalioti T, Makatounakis T, Thanos D, Papamatheakis J. CIITA regulates transcription onset via Ser5-phosphorylation of RNA Pol II. EMBO J 2003; 22(19):5125-36; PMID:14517250; http://dx.doi.org/ 10.1093/emboj/cdg496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Medlin J, Scurry A, Taylor A, Zhang F, Peterlin BM, Murphy S. P-TEFb is not an essential elongation factor for the intronless human U2 snRNA and histone H2b genes. EMBO J 2005; 24(23):4154-65; PMID:16308568; http://dx.doi.org/ 10.1038/sj.emboj.7600876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ho CK, Shuman S. Distinct roles for CTD Ser-2 and Ser-5 phosphorylation in the recruitment and allosteric activation of mammalian mRNA capping enzyme. Mol Cell 1999; 3(3):405-11; PMID:10198643; http://dx.doi.org/ 10.1016/S1097-2765(00)80468-2 [DOI] [PubMed] [Google Scholar]
- [20].Lolli G. Binding to DNA of the RNA-polymerase II C-terminal domain allows discrimination between Cdk7 and Cdk9 phosphorylation. Nucleic Acids Res 2009; 37(4):1260-68; PMID:19136461; http://dx.doi.org/ 10.1093/nar/gkn1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oelgeschläger T. Regulation of RNA polymerase II activity by CTD phosphorylation and cell cycle control. J Cell Physiol 2002; 190(2):160-9; http://dx.doi.org/ 10.1002/jcp.10058 [DOI] [PubMed] [Google Scholar]
- [22].Price DH. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol Cell Biol 2000; 20(8):2629-34; PMID:10733565; http://dx.doi.org/ 10.1128/MCB.20.8.2629-2634.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ping YH, Rana TM. Tat-associated kinase (P-TEFb): a component of transcription preinitiation and elongation complexes. J Biol Chem 1999; 274(11):7399-404; PMID:10066804; http://dx.doi.org/ 10.1074/jbc.274.11.7399 [DOI] [PubMed] [Google Scholar]
- [24].Dahlberg O, Shilkova O, Tang M, Holmqvist P, Mannervik M. P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Drosophila Embryo Development. PLoS Genet 2015; 11(2):e1004971; PMID:25679530; http://dx.doi.org/ 10.1371/journal.pgen.1004971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Laitem C, Zaborowska J, Isa NF, Kufs J, Dienstbier M, Murphy S. CDK9 inhibitors define elongation checkpoints at both ends of RNA polymerase II-transcribed genes. Nat Struct Mol Biol 2015; 22(5):396-403; PMID:25849141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jonkers I, Kwak H, Lis JT. Genome-wide dynamics of Pol II elongation and its interplay with promoter proximal pausing, chromatin, and exons. eLife 2014; 3:e02407; PMID:24843027; http://dx.doi.org/ 10.7554/eLife.02407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shore SM, Byers SA, Maury W, Price DH. Identification of a novel isoform of Cdk9. Gene 2003; 307:175-82; PMID:12706900; http://dx.doi.org/ 10.1016/S0378-1119(03)00466-9 [DOI] [PubMed] [Google Scholar]
- [28].Shore SM, Byers SA, Dent P, Price DH. Characterization of Cdk9(55) and differential regulation of two Cdk9 isoforms. Gene 2005; 350(1):51-8; PMID:15780980; http://dx.doi.org/ 10.1016/j.gene.2005.01.015 [DOI] [PubMed] [Google Scholar]
- [29].Liu H, Herrmann CH. Differential localization and expression of the Cdk9 42k and 55k isoforms. J Cell Physiol 2005; 203(1):251-60; PMID:15452830; http://dx.doi.org/ 10.1002/jcp.20224 [DOI] [PubMed] [Google Scholar]
- [30].Liu H, Herrmann CH, Chiang K, Sung TL, Moon SH, Donehower LA, Rice AP. 55K isoform of CDK9 associates with Ku70 and is involved in DNA repair. Biochem Biophys Res Commun 2010; 397(2):245-50; PMID:20493174; http://dx.doi.org/ 10.1016/j.bbrc.2010.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Romano G, Giordano A. Role of the cyclin-dependent kinase 9-related pathway in mammalian gene expression and human diseases. Cell Cycle 2008; 7(23):3664-8; PMID:19029809; http://dx.doi.org/ 10.4161/cc.7.23.7122 [DOI] [PubMed] [Google Scholar]
- [32].De Azevedo WF Jr, Canduri F, Da Silveira NJ. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem Biophys Res Commun 2002; 293(1):566-71; PMID:12054639; http://dx.doi.org/ 10.1016/S0006-291X(02)00266-8 [DOI] [PubMed] [Google Scholar]
- [33].Canduri F, Da Silveira NJF, Camera JC Jr, De Azevedo WF Jr. Structural bioinformatics study of cyclin-dependent kinases complexed with inhibitors. Eclet Quím 2003; 28(1):45-53; http://dx.doi.org/ 10.1590/S0100-46702003000100006 [DOI] [Google Scholar]
- [34].Baumli S, Lolli G, Lowe ED, Troiani S, Rusconi L, Bullock AN, Debreczeni JE, Knapp S, Johnson LN. The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation. EMBO J 2008; 27(13):1907-18; PMID:18566585; http://dx.doi.org/ 10.1038/emboj.2008.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Caracciolo V, Laurenti G, Romano G, Carnevale V, Cimini AM, Crozier-Fitzgerald C, Gentile Warschauer E, Russo G, Giordano A. Flavopiridol induces phosphorylation of AKT in a human glioblastoma cell line, in contrast to siRNA-mediated silencing of Cdk9: Implications for drug design and development. Cell Cycle 2012; 11(6):1202-16; PMID:22391209; http://dx.doi.org/ 10.4161/cc.11.6.19663 [DOI] [PubMed] [Google Scholar]
- [36].Baumli S, Endicott JA, Johnson LN. Halogen bonds form the basis for selective P-TEFb inhibition by DRB. Chem Biol 2010; 17(9):931-6; PMID:20851342; http://dx.doi.org/ 10.1016/j.chembiol.2010.07.012 [DOI] [PubMed] [Google Scholar]
- [37].Krystof V, Baumli S, Fürst R. Perspective of cyclin-dependent kinase 9 (CDK9) as a drug target. Curr Pharm Des 2012; 18(20):2883-90; PMID:22571657; http://dx.doi.org/ 10.2174/138161212800672750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yan L, Lai F, Chen X, Xiao Z. Discovery of novel indirubin-3′-monoxime derivatives as potent inhibitors against CDK2 and CDK9. Bioorg Med Chem Lett 2015; 25(11):2447-51; PMID:25908517; http://dx.doi.org/ 10.1016/j.bmcl.2015.03.066 [DOI] [PubMed] [Google Scholar]
- [39].Gao J, Fang C, Xiao Z, Huang L, Chen CH, Wang LT, Lee KH. Discovery of novel 5-fluoro-N 2,N 4-diphenylpyrimidine-2,4-diamines as potent inhibitors against CDK2 and CDK9. MedChemComm 2015; 6(3):444-54; PMID:25914804; http://dx.doi.org/ 10.1039/C4MD00412D [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hole AJ, Baumli S, Shao H, Shi S, Huang S, Pepper C, Fischer PM, Wang S, Endicott JA, Noble ME. Comparative structural and functional studies of 4-(thiazol-5-yl)-2-(phenylamino)pyrimidine-5-carbonitrile CDK9 inhibitors suggest the basis for isotype selectivity. J Med Chem 2013; 56(3):660-70; PMID:23252711; http://dx.doi.org/ 10.1021/jm301495v [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Krystof V, Cankar P, Frysová I, Slouka J, Kontopidis G, Dzubák P, Hajdúch M, Srovnal J, de Azevedo WF Jr, Orság M, et al.. Four-arylazo-3,5-diamino-1H-pyrazole CDK inhibitors: SAR study, crystal structure in complex with CDK2, selectivity, and cellular effects. J Med Chem 2006; 49(22):6500-9; PMID:17064068; http://dx.doi.org/ 10.1021/jm0605740 [DOI] [PubMed] [Google Scholar]
- [42].Kryštof V, Rárová L, Liebl J, Zahler S, Jorda R, Voller J, Cankař P. The selective P-TEFb inhibitor CAN508 targets angiogenesis. Eur J Med Chem 2011; 46(9):4289-94; http://dx.doi.org/ 10.1016/j.ejmech.2011.06.035 [DOI] [PubMed] [Google Scholar]
- [43].Randjelović J, Erić S, Savić V. Computational study and peptide inhibitors design for the CDK9 - cyclin T1 complex. J Mol Model 2013; 19(4):1711-25; PMID:23296566; http://dx.doi.org/ 10.1007/s00894-012-1735-2 [DOI] [PubMed] [Google Scholar]
- [44].Randjelović J, Erić S, Savić V. In silico design of small molecule inhibitors of CDK9/cyclin T1 interaction. J Mol Graph Model 2014; 50:100-12; PMID:24769691; http://dx.doi.org/ 10.1016/j.jmgm.2014.04.002 [DOI] [PubMed] [Google Scholar]
- [45].Van Duyne R, Guendel I, Jaworski E, Sampey G, Klase Z, Chen H, Zeng C, Kovalskyy D, El Kouni MH, Lepene B, et al.. Effect of mimetic CDK9 inhibitors on HIV-1-activated transcription. J Mol Biol 2013; 425(4):812-29; PMID:23247501; http://dx.doi.org/ 10.1016/j.jmb.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, Tan YC, Hu C, Jayaraman R, William AD, et al.. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia 2012; 26(2):236-43; PMID:21860433; http://dx.doi.org/ 10.1038/leu.2011.218 [DOI] [PubMed] [Google Scholar]
- [47].Poulsen A, William A, Blanchard S, Nagaraj H, Williams M, Wang H, Lee A, Sun E, Teo EL, Tan E, et al.. Structure-based design of nitrogen-linked macrocyclic kinase inhibitors leading to the clinical candidate SB1317/TG02, a potent inhibitor of cyclin dependant kinases (CDKs), Janus kinase 2 (JAK2), and Fms-like tyrosine kinase-3 (FLT3). J Mol Model 2013; 19(1):119-30; PMID:22820730; http://dx.doi.org/ 10.1007/s00894-012-1528-7 [DOI] [PubMed] [Google Scholar]
- [48].Walsby E, Pratt G, Shao H, Abbas AY, Fischer PM, Bradshaw TD, Brennan P, Fegan C, Wang S, Pepper C. A novel Cdk9 inhibitor preferentially targets tumor cells and synergizes with fludarabine. Oncotarget 2014; 5(2):375-85; PMID:24495868; http://dx.doi.org/ 10.18632/oncotarget.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shao H, Shi S, Foley DW, Lam F, Abbas AY, Liu X, Huang S, Jiang X, Baharin N, Fischer PM, et al.. Synthesis, structure-activity relationship and biological evaluation of 2,4,5-trisubstituted pyrimidine CDK inhibitors as potential anti-tumour agents. Eur J Med Chem 2013; 70:447-55; PMID:24185375; http://dx.doi.org/ 10.1016/j.ejmech.2013.08.052 [DOI] [PubMed] [Google Scholar]
- [50].Shao H, Shi S, Huang S, Hole AJ, Abbas AY, Baumli S, Liu X, Lam F, Foley DW, Fischer PM, et al.. Substituted 4-(thiazol-5-yl)-2-(phenylamino)pyrimidines are highly active CDK9 inhibitors: synthesis, X-ray crystal structures, structure-activity relationship, and anticancer activities. J Med Chem 2013; 56(3):640-59; PMID:23301767; http://dx.doi.org/ 10.1021/jm301475f [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Albert TK, Rigault C, Eickhoff J, Baumgart K, Antrecht C, Klebl B, Mittler G, Meisterernst M. Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor. Br J Pharmacol 2014; 171(1):55-68; PMID:24102143; http://dx.doi.org/ 10.1111/bph.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Polier G, Ding J, Konkimalla BV, Eick D, Ribeiro N, Köhler R, Giaisi M, Efferth T, Desaubry L, Krammer PH, et al.. Wogonin and related natural flavones are inhibitors of CDK9 that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis 2011; 2:e182; PMID:21776020; http://dx.doi.org/ 10.1038/cddis.2011.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bettayeb K, Oumata N, Echalier A, Ferandin Y, Endicott JA, Galons H, Meijer L. CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene 2008; 27(44):5797-807; PMID:18574471; http://dx.doi.org/ 10.1038/onc.2008.191 [DOI] [PubMed] [Google Scholar]
- [54].Álvarez-Fernández S, Ortiz-Ruiz MJ, Parrott T, Zaknoen S, Ocio EM, San Miguel J, Burrows FJ, Esparís-Ogando A, Pandiella A. Potent antimyeloma activity of a novel ERK5/CDK inhibitor. Clin Cancer Res 2013; 19(10):2677-87; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2118 [DOI] [PubMed] [Google Scholar]
- [55].Tenaerts P, Madre L, Archdeacon P, Califf RM. The Clinical Trials Transformation Initiative: innovation through collaboration. Nat Rev Drug Discov 2014; 13(11):797-8; PMID:25359366; http://dx.doi.org/ 10.1038/nrd4442 [DOI] [PubMed] [Google Scholar]
- [56].Karp JE, Garrett-Mayer E, Estey EH, Rudek MA, Smith BD, Greer JM, Drye DM, Mackey K, Dorcy KS, Gore SD, et al.. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica 2012; 97(11):1736-42; PMID:22733022; http://dx.doi.org/ 10.3324/haematol.2012.062539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bible KC, Peethambaram PP, Oberg AL, Maples W, Groteluschen DL, Boente M, Burton JK, Gomez Dahl LC, Tibodeau JD, Isham CR, et al.. Mayo Phase 2 Consortium (P2C), North Central Cancer Treatment Group (NCCTG). A phase 2 trial of flavopiridol (Alvocidib) and cisplatin in platin-resistant ovarian and primary peritoneal carcinoma: MC0261. Gynecol Oncol 2012; 127(1):55-62; PMID:22664059; http://dx.doi.org/ 10.1016/j.ygyno.2012.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lanasa MC, Andritsos L, Brown JR, Gabrilove J, Caligaris-Cappio F, Ghia P, Larson RA, Kipps TJ, Leblond V, Milligan DW, et al.. Final results of EFC6663: a multicenter, international, phase 2 study of alvocidib for patients with fludarabine-refractory chronic lymphocytic leukemia. Leuk Res 2015; 39(5):495-500; PMID:25804339; http://dx.doi.org/ 10.1016/j.leukres.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fekrazad HM, Verschraegen CF, Royce M, Smith HO, Chyi Lee F, Rabinowitz I. A phase I study of flavopiridol in combination with gemcitabine and irinotecan in patients with metastatic cancer. Am J Clin Oncol 2010; 33(4):393-7; PMID:19884803; http://dx.doi.org/ 10.1097/COC.0b013e3181b2043f [DOI] [PubMed] [Google Scholar]
- [60].Dickson MA, Rathkopf DE, Carvajal RD, Grant S, Roberts JD, Reid JM, Ames MM, McGovern RM, Lefkowitz RA, Gonen M, et al.. A phase I pharmacokinetic study of pulse-dose vorinostat with flavopiridol in solid tumors. Invest New Drugs 2011; 29(5):1004-12; PMID:20461440; http://dx.doi.org/ 10.1007/s10637-010-9447-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ramaswamy B, Phelps MA, Baiocchi R, Bekaii-Saab T, Ni W, Lai JP, Wolfson A, Lustberg ME, Wei L, Wilkins D, et al.. A dose-finding, pharmacokinetic and pharmacodynamic study of a novel schedule of flavopiridol in patients with advanced solid tumors. Invest New Drugs 2012; 30(2):629-38; PMID:20938713; http://dx.doi.org/ 10.1007/s10637-010-9563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Luke JJ, D'Adamo DR, Dickson MA, Keohan ML, Carvajal RD, Maki RG, de Stanchina E, Musi E, Singer S, Schwartz GK. The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: preclinical investigations and results of a phase I dose-escalation clinical trial. Clin Cancer Res 2012; 18(9):2638-47; PMID:22374332; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Maddocks K, Wei L, Rozewski D, Jiang Y, Zhao Y, Adusumilli M, Pierceall WE, Doykin C, Cardone MH, Jones JA, et al. Reduced occurrence of tumor flare with flavopiridol followed by combined flavopiridol and lenalidomide in patients with relapsed chronic lymphocytic leukemia (CLL). Am J Hematol 2015; 90(4):327-33; PMID:25639448; http://dx.doi.org/ 10.1002/ajh.23946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hofmeister CC, Poi M, Bowers MA, Zhao W, Phelps MA, Benson DM, Kraut EH, Farag S, Efebera YA, Sexton J, et al.. A phase I trial of flavopiridol in relapsed multiple myeloma. Cancer Chemother Pharmacol 2014; 73(2):249-57; PMID:24241210; http://dx.doi.org/ 10.1007/s00280-013-2347-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jones JA, Rupert AS, Poi M, Phelps MA, Andritsos L, Baiocchi R, Benson DM, Blum KA, Christian B, Flynn J, et al.. Flavopiridol can be safely administered using a pharmacologically derived schedule and demonstrates activity in relapsed and refractory non-Hodgkin's lymphoma. Am J Hematol 2014; 89(1):19-24; PMID:23959599; http://dx.doi.org/ 10.1002/ajh.23568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dickson MA, Shah MA, Rathkopf D, Tse A, Carvajal RD, Wu N, Lefkowitz RA, Gonen M, Cane LM, Dials HJ, et al.. A phase I clinical trial of FOLFIRI in combination with the pan-cyclin-dependent kinase (CDK) inhibitor flavopiridol. Cancer Chemother Pharmacol 2010; 66(6):1113-21; PMID:20953860; http://dx.doi.org/ 10.1007/s00280-010-1269-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Holkova B, Kmieciak M, Perkins EB, Bose P, Baz RC, Roodman GD, Stuart RK, Ramakrishnan V, Wan W, Peer CJ, et al.. Phase I trial of bortezomib (PS-341; NSC 681239) and “nonhybrid” (bolus) infusion schedule of alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory indolent B-cell neoplasms. Clin Cancer Res 2014; 20(22):5652-62; PMID:25248382; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bose P, Perkins EB, Honeycut C, Wellons MD, Stefan T, Jacobberger JW, Kontopodis E, Beumer JH, Egorin MJ, Imamura CK, et al.. Phase I trial of the combination of flavopiridol and imatinib mesylate in patients with Bcr-Abl+ hematological malignancies. Cancer Chemother Pharmacol 2012; 69(6):1657-67; PMID:22349810; http://dx.doi.org/ 10.1007/s00280-012-1839-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stephens DM, Ruppert AS, Maddocks K, Andritsos L, Baiocchi R, Jones J, Johnson AJ, Smith LL, Zhao Y, Ling Y, et al.. Cyclophosphamide, alvocidib (flavopiridol), and rituximab, a novel feasible chemoimmunotherapy regimen for patients with high-risk chronic lymphocytic leukemia. Leuk Res 2013; 37(10):1195-9; PMID:23867058; http://dx.doi.org/ 10.1016/j.leukres.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Karp JE, Smith BD, Resar LS, Greer JM, Blackford A, Zhao M, Moton-Nelson D, Alino K, Levis MJ, Gore SD, et al.. Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood 2011; 117(12):3302-10; PMID:21239698; http://dx.doi.org/ 10.1182/blood-2010-09-310862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, Statkevich P, Zhu Y, Yao SL, Small K, et al.. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer 2014; 14(3):169-76; PMID:24393852; http://dx.doi.org/ 10.1016/j.clbc.2013.10.016 [DOI] [PubMed] [Google Scholar]
- [72].Stephenson JJ, Nemunaitis J, Joy AA, Martin JC, Jou YM, Zhang D, Statkevich P, Yao SL, Zhu Y, Zhou H, et al.. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 2014; 83(2):219-23; PMID:24388167; http://dx.doi.org/ 10.1016/j.lungcan.2013.11.020 [DOI] [PubMed] [Google Scholar]
- [73].Gojo I, Sadowska M, Walker A, Feldman EJ, Iyer SP, Baer MR, Sausville EA, Lapidus RG, Zhang D, Zhu Y, et al.. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol 2013; 72(4):897-908; PMID:23949430; http://dx.doi.org/ 10.1007/s00280-013-2249-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Fabre C, Gobbi M, Ezzili C, Zoubir M, Sablin MP, Small K, Im E, Shinwari N, Zhang D, Zhou H, et al.. Clinical study of the novel cyclin-dependent kinase inhibitor dinaciclib in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol 2014; 74(5):1057-64; PMID:25217392; http://dx.doi.org/ 10.1007/s00280-014-2583-9 [DOI] [PubMed] [Google Scholar]
- [75].Zhang D, Mita M, Shapiro GI, Poon J, Small K, Tzontcheva A, Kantesaria B, Zhu Y, Bannerji R, Statkevich P. Effect of aprepitant on the pharmacokinetics of the cyclin-dependent kinase inhibitor dinaciclib in patients with advanced malignancies. Cancer Chemother Pharmacol 2012; 70(6):891-8; PMID:23053255; http://dx.doi.org/ 10.1007/s00280-012-1967-y [DOI] [PubMed] [Google Scholar]
- [76].Kumar SK, LaPlant B, Chng WJ, Zonder J, Callander N, Fonseca R, Fruth B, Roy V, Erlichman C, Stewart AK, Mayo Phase 2 Consortium. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015; 125(3):443-8; PMID:25395429; http://dx.doi.org/ 10.1182/blood-2014-05-573741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nemunaitis JJ, Small KA, Kirschmeier P, Zhang D, Zhu Y, Jou YM, Statkevich P, Yao SL, Bannerji R. A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med 2013; 11:259; PMID:24131779; http://dx.doi.org/ 10.1186/1479-5876-11-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Le Tourneau C, Faivre S, Laurence V, Delbaldo C, Vera K, Girre V, Chiao J, Armour S, Frame S, Green SR, et al.. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer 2010; 46(18):3243-50; PMID:20822897; http://dx.doi.org/ 10.1016/j.ejca.2010.08.001 [DOI] [PubMed] [Google Scholar]
- [79].Benson C, White J, De Bono J, O'Donnell A, Raynaud F, Cruickshank C, McGrath H, Walton M, Workman P, Kaye S, et al.. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer 2007; 96(1):29-37; PMID:17179992; http://dx.doi.org/ 10.1038/sj.bjc.6603509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Heath EI, Bible K, Martell RE, Adelman DC, Lorusso PM. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Invest New Drugs 2008; 26(1):59-65; PMID:17938863; http://dx.doi.org/ 10.1007/s10637-007-9090-3 [DOI] [PubMed] [Google Scholar]
- [81].Tong WG, Chen R, Plunkett W, Siegel D, Sinha R, Harvey RD, Badros AZ, Popplewell L, Coutre S, Fox JA, et al.. Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. J Clin Oncol 2010; 28(18):3015-22; PMID:20479412; http://dx.doi.org/ 10.1200/JCO.2009.26.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Van der Biessen DA, Burger H, de Bruijn P, Lamers CH, Naus N, Loferer H, Wiemer EA, Mathijssen RH, de Jonge MJ. Phase I study of RGB-286638, a novel, multitargeted cyclin-dependent kinase inhibitor in patients with solid tumors. Clin Cancer Res 2014; 20(18): 4776-83; PMID:25024258; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0325 [DOI] [PubMed] [Google Scholar]
- [83].Romano G. Deregulations in the cyclin-dependent kinase-9-related pathway in cancer: implications for drug discovery and development. ISRN Oncol 2013; 305371; PMID:23840966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lew QJ, Tan CH, Gurumurthy M, Chu KL, Cheong N, Lane DP, Chao SH. NPMc+ AML cell line shows differential protein expression and lower sensitivity to DNA-damaging and p53-inducing anticancer compounds. Cell Cycle 2011; 10(12):1978-87; PMID:21558800; http://dx.doi.org/ 10.4161/cc.10.12.15859 [DOI] [PubMed] [Google Scholar]
- [85].Bible KC, Boerner SA, Kirkland K, Anderl KL, Bartelt D Jr, Svingen PA, Kottke TJ, Lee YK, Eckdahl S, Stalboerger PG, et al.. Characterization of an ovarian carcinoma cell line resistant to cisplatin and flavopiridol. Clin Cancer Res 2000; 6(2):661-70; PMID:10690552 [PubMed] [Google Scholar]
- [86].Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000; 96(2):393-7; PMID:10887097 [PubMed] [Google Scholar]
- [87].Semenov I, Akyuz C, Roginskaya V, Chauhan D, Corey SJ. Growth inhibition and apoptosis of myeloma cells by the CDK inhibitor flavopiridol. Leuk Res 2002; 26(3):271-80; PMID:11792416; http://dx.doi.org/ 10.1016/S0145-2126(01)00103-5 [DOI] [PubMed] [Google Scholar]
- [88].Venkataraman G, Maududi T, Ozpuyan F, Bahar HI, Izban KF, Qin JZ, Alkan S. Induction of apoptosis and down regulation of cell cycle proteins in mantle cell lymphoma by flavopiridol treatment. Leuk Res 2006; 30(11):1377-84; PMID:16624404; http://dx.doi.org/ 10.1016/j.leukres.2006.03.004 [DOI] [PubMed] [Google Scholar]
- [89].Jackman KM, Frye CB, Hunger SP. Flavopiridol displays preclinical activity in acute lymphoblastic leukemia. Pediatr Blood Cancer 2008; 50(4):772-8; PMID:18000861; http://dx.doi.org/ 10.1002/pbc.21386 [DOI] [PubMed] [Google Scholar]
- [90].Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, Seghezzi W, Paruch K, Dwyer MP, Doll R, et al.. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther 2010; 9(8):2344-53; PMID:20663931; http://dx.doi.org/ 10.1158/1535-7163.MCT-10-0324 [DOI] [PubMed] [Google Scholar]
- [91].Nguyen TK, Grant S. Dinaciclib (SCH727965) inhibits the unfolded protein response through a CDK1- and 5-dependent mechanism. Mol Cancer Ther 2014; 13(3):662-74; PMID:24362465; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wilson SC, Atrash B, Barlow C, Eccles S, Fischer PM, Hayes A, Kelland L, Jackson W, Jarman M, Mirza A, et al.. Design, synthesis and biological evaluation of 6-pyridylmethylaminopurines as CDK inhibitors. Bioorg Med Chem 2011; 19(22):6949-65; PMID:21982796; http://dx.doi.org/ 10.1016/j.bmc.2011.08.051 [DOI] [PubMed] [Google Scholar]
- [93].Walsby E, Lazenby M, Pepper C, Burnett AK. The cyclin-dependent kinase inhibitor SNS-032 has single agent activity in AML cells and is highly synergistic with cytarabine. Leukemia 2011; 25(3):411-9; PMID:21212792; http://dx.doi.org/ 10.1038/leu.2010.290 [DOI] [PubMed] [Google Scholar]
- [94].Cirstea D, Hideshima T, Santo L, Eda H, Mishima Y, Nemani N, Hu Y, Mimura N, Cottini F, Gorgun G, et al.. Small-molecule multi-targeted kinase inhibitor RGB-286638 triggers P53-dependent and -independent anti-multiple myeloma activity through inhibition of transcriptional CDKs. Leukemia 2013; 27(12):2366-75; PMID:23807770; http://dx.doi.org/ 10.1038/leu.2013.194 [DOI] [PMC free article] [PubMed] [Google Scholar]


