Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) results in almost half of all deaths caused by antibiotic resistant organisms. Current evidence suggests that MRSA infections are associated with antibiotic use. This study examined state-level data to determine whether outpatient antibiotic use was associated with hospital-acquired MRSA (HA-MRSA) infections. The 2013 Centers for Disease Control and Prevention (CDC) Healthcare-Associated Infections Progress Report was used to obtain HA-MRSA infection rates. Data on the number of antibiotic prescriptions with activity towards methicillin-sensitive Staphylococcus aureus (MSSA) at the state level were obtained from the 2013 Medicare Provider Utilization and Payment Data: Part D Prescriber Public Use File. Pearson's correlation coefficient was used to analyze the relationship between the number of antibiotic prescriptions and HA-MRSA infection rates. The average number of HA-MRSA infections was 0.026 per 1000 persons with the highest rates concentrated in Southeastern and Northeastern states. The average number of outpatient prescriptions per capita was 0.74 with the highest rates in Southeastern states. A significant correlation (ρ = 0.64, P <.001) between infections and prescriptions was observed, even after adjusting for non-reporting hospitals. This association provides evidence of the importance of appropriate antibiotic prescribing. Prescriber and heat map data may be useful for targeting antimicrobial stewardship programs in an effort to manage appropriate antibiotic use to help stop antibiotic resistance.
Keywords: Antibiotic resistance, Antimicrobial stewardship, Medicare, Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive Staphylococcus aureus
Introduction
Infectious disease is the second leading cause of death worldwide and the third leading cause of death in developed countries.1 Over the past 70 years, the use of antibiotics and other antimicrobial agents has greatly reduced morbidity and mortality from infectious diseases.2 However, as these drugs have been some of the most commonly prescribed medications over a long period of time, some bacteria have found ways to develop resistance towards antibiotics that are designed to kill them, rendering the drugs less effective.2 The economic cost of antibiotic resistance in the United States is estimated to be as high as $55 billion per year for both direct healthcare costs and additional costs to society associated with lost productivity.3–5 In the United States, more than 2 million people each year have an infection that is resistant to an antibiotic, leading to 23,000 deaths.4 Methicillin-resistant Staphylococcus aureus (MRSA) alone results in almost half of all deaths caused by antibiotic resistant organisms.4 Antibiotic resistance is a global problem putting current and future populations at substantial risk of injury, loss, and death and has been declared a substantial threat to public health and national security by the Infectious Diseases Society of America and Institute of Medicine.2,3
Antibiotic use is the single most important factor leading to antibiotic resistance.4 The problem of antibiotic resistance could be reduced if antibiotics were prescribed more appropriately.4 Up to 50% of antibiotics are not needed or are not optimally prescribed.4,6 There are trillions of microorganisms in the human body made up of both beneficial and potentially harmful bacteria.4,7 Among these microorganisms, antibiotic resistant bacteria may be present but in small enough numbers that infection does not develop.4 An example is nasal colonization or carriage of MRSA.8 Beneficial bacteria play a role in keeping harmful bacteria numbers low.4 When antibiotics are given, harmful as well as beneficial bacteria that protect the body from infection are killed.4 The few antibiotic resistant bacteria that are present are then allowed to multiply and infect the body causing an antibiotic resistant infection.4 This infection can ultimately spread to healthcare and community settings.4,9,10
Staphylococcus aureus (S. aureus) infections are among the most common and problematic.11 Penicillin resistance among S. aureus was detected within a decade after its introduction worldwide in the 1940s.2 In response, new antibiotics were developed but within a decade, MRSA was identified.2 This pattern of resistance continues to occur at an increasing rate with each new antibiotic developed.2 Recently, economic concerns have driven pharmaceutical companies away from new antibiotic approvals and developments, leaving few options left to treat antibiotic resistant S. aureus.1,2 Adding to the problem is S. aureus's ability to colonize and form biofilms on biomaterials in hospitals.10 Certain strains of MRSA form biofilms contributing to the spread of hospital-acquired (HA-MRSA), which is generally defined as a MRSA infection detected by a positive culture taken more than 48 hours after hospital admission and treated in the inpatient setting.10,12,13
This continual trend toward increased antibiotic resistance highlights the urgency of ensuring that inappropriate antibiotic use be decreased. A possible solution to antibiotic resistance is antimicrobial stewardship.14,15 Antimicrobial stewardship refers to a coordinated effort designed to improve the appropriate use of antimicrobials by promoting the selection of the optimal antimicrobial drug regimen, dose, duration of therapy, and route of administration. These efforts can ultimately reduce the cost of healthcare infections and can reduce the potential for developing antimicrobial resistant organisms.16 While prior studies have examined inappropriate inpatient antibiotic use, to our knowledge, the relationship between the use of outpatient antibiotics to treat methicillin-sensitive Staphylococcus aureus (MSSA) and the incidence of HA-MRSA has not been studied.
The goal of the study was to examine the association between Medicare Part D antibiotic prescriptions with bactericidal or bacteriostatic activity towards MSSA and HA-MRSA bacteremia infection rates (incidence of infections that were reported by healthcare facilities) by state. Our hypothesis was that states with heavy antibiotic use would also have high rates of HA-MRSA infections.
Methods
The study involved comparing per capita antibiotic prescriptions for Medicare beneficiaries to the number of HA-MRSA infections reported by hospitals at the state level. HA-MRSA infection data were obtained from the 2013 Centers for Disease Control and Prevention (CDC) Healthcare-Associated Infections (HAI) Progress Report which included the number of laboratory-confirmed HA-MRSA infections and the number of hospitals reporting for each state.17 Because all states had non-reporting hospitals, infection rates for each state were adjusted to estimate infection rates as if each state had 100% hospital reporting by dividing the infection rate by the percent of hospitals reporting.
Inclusion criteria for the numerator for antibiotic use were being enrolled in a Medicare Part D prescription drug plan and being prescribed systemic antibiotics with bactericidal or bacteriostatic activity towards MSSA according to the 2014 Sanford Guide to Antimicrobial Therapy.18
The 2013 Medicare Provider Utilization and Payment Data: Part D Prescriber Public Use File (PUF) identified providers using their National Provider Identifier (NPI) and the specific prescriptions that were dispensed on their behalf, listed by brand and generic name.19 For each prescriber and drug, the dataset includes the total number of prescriptions that were dispensed (original and refills), days supply, the total drug cost, and the provider's address.
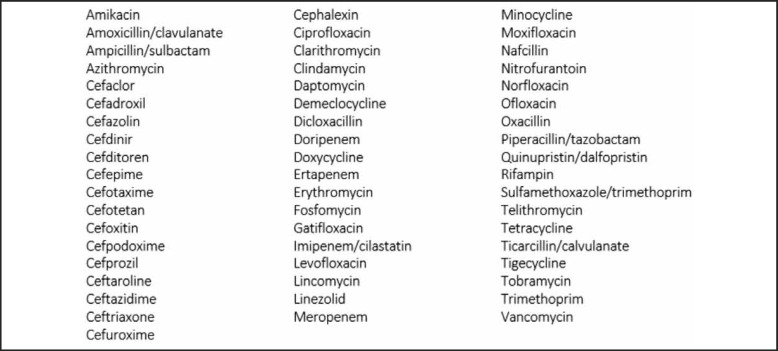
Claims data for all providers who prescribed antibiotic medications with MSSA coverage were downloaded and summarized at the state level. The antibiotics included are listed in Figure 1. The metric of interest was antibiotic prescriptions per 1000 persons aged 65 and over per state, which was obtained by dividing number of prescriptions by the state's population aged 65 and over. We examined the correlation between the number of prescriptions per 1000 persons aged 65 and over, and the number of HA-MRSA infections per 1000 persons in the general population at the state level in the United States (US) in 2013 using the Pearson's correlation coefficient. Scatterplots were used to detect outliers visually and the correlation coefficient was re-calculated after removing any outliers. Statistical analyses were conducted in Stata v.11 (College Station, TX). Following the guidance of the University of Hawai‘i Office of Research Compliance, we did not seek Institutional Review Board approval, as all of the data were de-identified and publicly available.
Figure 1.
Antibiotics and Activity Against MSSA by Generic Name.
Antibiotics included in this study by generic name with systemic bactericidal or bacteriostatic activity towards Methicillin-sensitive Staphylococcus aureus according to The Sanford Guide to Antimicrobial Therapy 2014.
Results
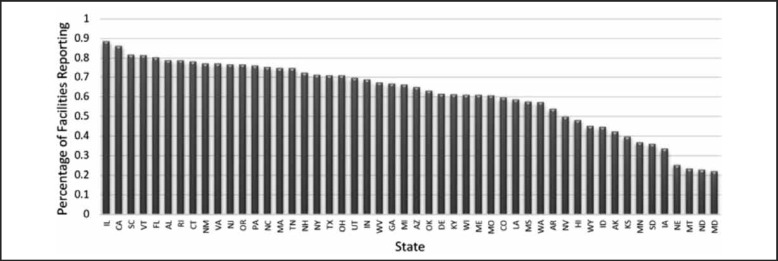
In 2013, the average percentage of hospitals reporting HA-MRSA in the US was 61.5%. Reporting by individual states varied from a high of 88.4% of hospitals reporting in Illinois to a low of 22.0% of hospitals reporting in Maryland (Figure 2).
Figure 2.
Percentage of Hospitals Reporting HA-MRSA Bacteremia Infections by State.
The 2013 percentage of each State's hospitals reporting the number of HA-MRSA infections in order from highest (left) to lowest (right). The average percentage in the United States of hospitals reporting these infections was 61.5%.
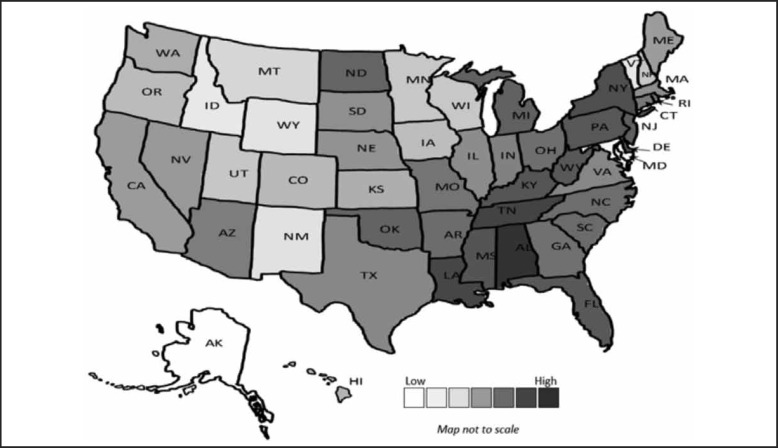
The US average of reported number of HA-MRSA infections per 1000 persons in the general population was 0.026 in 2013. The highest number of infections per 1000 persons were in Alabama (0.06) and the lowest in Alaska (0.003). Figure 3 displays a heat map of the infection rates by state. The states with the highest infection rates were primarily in Southeastern and Northeastern regions.
Figure 3.
HA-MRSA Bacteremia Infections per 1000 Persons.
The 2013 United States heat map of the number of HA-MRSA infections per 1000 persons with the lowest numbers in white and highest in dark gray.
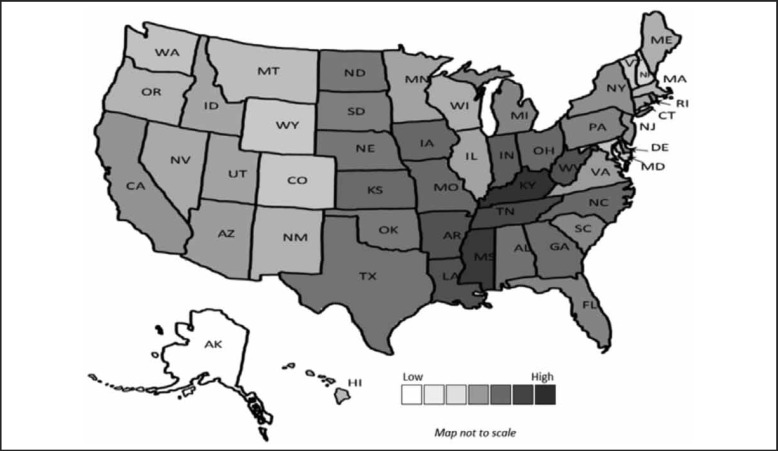
In 2013, over 33 million Medicare Part D MSSA covering antibiotics were prescribed. The average number of Medicare Part D MSSA covering antibiotic prescriptions per 1000 persons in those aged 65 and over in the US in 2013 was 0.74. The highest prescribing state was Kentucky (1.25) and the lowest prescribing state was Alaska (0.22). A heat map for the number of prescriptions in each state shows the highest prescribing states primarily in the Southeastern region (Figure 4).
Figure 4.
MSSA Antibiotic Prescriptions per 1000 Persons.
The 2013 United States heat map of the number of Medicare Part D MSSA antibiotic prescriptions per 1000 persons with the lowest numbers in white and highest in dark gray.
Less than half (48%) of facilities in Hawai‘i reported HA-MRSA infections. The HA-MRSA infection rate was 0.014 and the adjusted rate was 0.03, resulting in a state ranking of 15th and 16th, respectively, out of 50 in terms of lowest unadjusted and adjusted infection rate respectively. The Medicare Part D MSSA antibiotic prescribing rate was 0.5, resulting in a state ranking of 9th out of 50 in terms of lowest prescribing rate.
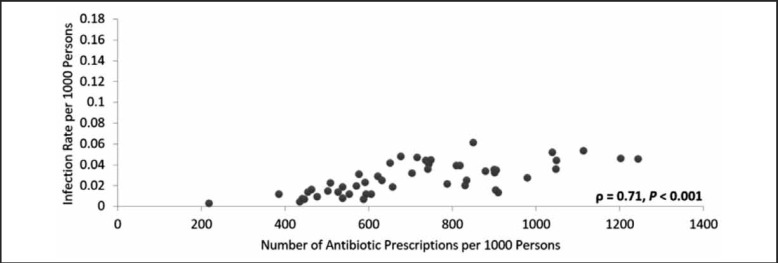
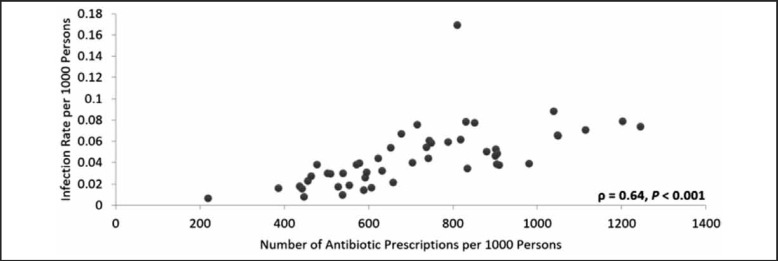
There was a positive, statistically significant correlation between the rate of HA-MRSA infections and the MSSA antibiotic prescription rate for each state (ρ = 0.72, P < .001, Figure 5). Because a varying proportion of hospitals reported infections in each state, a secondary analysis was performed using an infection rate adjusted for hospital non-reporting (Figure 6). The adjusted analysis resulted in a positive and statistically significant correlation (ϱ = 0.64, P < .001) that was less positive than the original correlation and also resulted in an outlier (North Dakota). The HA-MRSA rate for North Dakota increased from 0.038 to 0.169 after adjustment for hospital non-reporting. When the outlier was removed, this correlation increased considerably and became greater than that for the unadjusted results (ϱ = 0.78, P < .001).
Figure 5.
HA-MRSA Infection Rate Related to Number of Antibiotic Prescriptions by State, 2013.
The 2013 relationship between the HA-MRSA infection rate and the number of Medicare Part D MSSA covering antibiotic prescriptions in each state. Each state's value is represented as a single point on the figure. The relationship was examined using the Pearson's correlation coefficient which resulted in ρ = 0.71 with P <.001.
Figure 6.
HA-MRSA Infection Rate Related to Number of Antibiotic Prescriptions by State, 2013, Adjusted for Hospital Non-reporting.
The 2013 relationship between the HA-MRSA infection rate and the number of Medicare Part D MSSA covering antibiotic prescriptions in each state with adjusted values. The infection rates were recalculated after adjusting for non-reporting hospitals. The adjusted relationship was examined using the Pearson's correlation coefficient which resulted in ρ = 0.64 with P <.001.
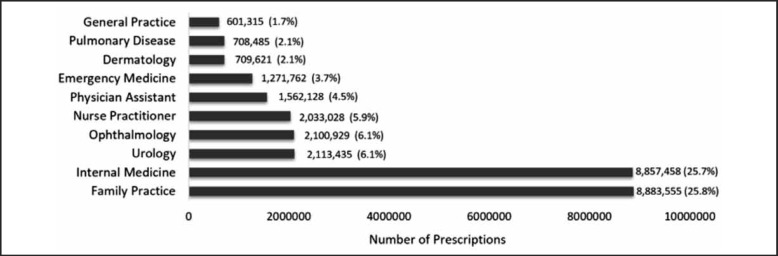
Eighty-four percent of all MSSA covering antibiotics (or 28.8 million) were prescribed by physicians and other providers in 10 specialty areas (Figure 7). Family practice and internal medicine were the top two prescribers, together prescribing more than half of all MSSA covering antibiotics. Family practice prescribed 25.8% and internal medicine prescribed 25.7%. The third highest prescribing specialty was urology that prescribed 6.1% of the total, followed by ophthalmology (6.1%), nurse practitioner (5.9%), physician assistant (4.5%), emergency medicine (3.7%), dermatology (2.1%), pulmonary disease (2.1%), and general practice (1.7%).
Figure 7.
Number of MSSA Antibiotic Prescriptions for Top 10 Specialties.
The 2013 top 10 specialties who prescribed Medicare Part D MSSA covering antibiotics in order from lowest (top) to highest (bottom) including the number of prescriptions and percentage of antibiotics prescribed out of the total number of prescribed MSSA antibiotics.
Discussion
In this study comparing HA-MRSA infection rates to Medicare Part D antibiotic prescriptions at the state level, we found a significant correlation between the presence of HA-MRSA and the number of outpatient antibiotic prescriptions with activity towards MSSA.
Both unadjusted HA-MRSA infection rates and those adjusted for hospital non-reporting were significantly correlated with MSSA antibiotic prescription rates. However, after adjusting for non-reporting hospitals, the correlation was weaker. The adjustment for non-reporting hospitals created an outlier value for North Dakota. North Dakota had a relatively high unadjusted infection rate and a very small percentage of hospitals reporting. One limitation of our adjustment is that we corrected for the number of hospitals reporting without regard to hospital size. It may be that in North Dakota, a large hospital reported their data and many small ones with a small number of cases did not report. In this case, we would have over-adjusted for non-reporting.
Previous research has shown an association between prior antibiotic use and MRSA colonization and infection at the population- and individual-level with an increased risk of morbidity and mortality.2,8,20,25 An association between primary care outpatient antibiotic prescribing and community-acquired MRSA (CA-MRSA) has also been shown.20,26 State level antibiotic prescriptions and MRSA wound infections have been studied.27 Research has been done on the decrease of MSSA susceptibility over the past decade, and hospital-acquired MRSA resistance rates have been compared with MSSA resistance rates.28,30 The spread of certain MRSA strains have been tracked across the United States.11 There have been studies showing an association between antibiotic use and HA-MRSA; however, these studies focused on a few antibiotics prescribed mainly from an inpatient setting.9,31,33 This study adds to the existing literature by examining the relation between outpatient antibiotic use and HA-MRSA infections.
Although antimicrobial stewardship programs are beneficial for reducing antimicrobial resistance, they are difficult to initiate. It takes coordination, economic resources, and in many cases, it adds to healthcare professionals' time and workload. Initiating these programs may not be feasible, especially at the national level. This study provides a possible way to help focus the initiation of antimicrobial stewardship programs in specific states or in specific healthcare specialties. States with high antibiotic prescribing rates or high infection rates may benefit from these programs more than states with low prescribing or low infection rates. More benefits could also be seen in specialties with higher antibiotic prescribing rates over those with lower prescribing rates.
In Hawai‘i, efforts have been made to initiate antimicrobial stewardship programs throughout the state, especially at the health system level. Hawai‘i ranked in the top third in terms of lowest infection rates in the US and in the top fifth in terms of the lowest prescribing rates. If the study's hypothesis is correct and higher prescribing rates means more antibiotic resistant infections, then since Hawai‘i has both relatively low prescribing rates and infection rates, Hawai‘i seems to be contributing to the development of HA-MRSA to a lesser extent than most other states. This relatively low contribution combined with efforts to initiate antimicrobial stewardship programs makes Hawai‘i appear to be a relatively safe state in terms of developing HA-MRSA.
There are several limitations to our study. First, only one year of data (2013) on antibiotic prescriptions was included, and data was only available for Medicare Part D subscribers. Hence, antibiotic use was only representative of disabled and/or elderly with Medicare Part D coverage, whereas the infection rates were representative of the entire state population. Although it would have been ideal to include antibiotic use for the general US population, we were limited by the availability of publicly available data. On the other hand, our analysis includes all patients with Medicare Part D coverage across the US, which provides a broad sampling of patients at risk for HA-MRSA. Second, we only had data on outpatient (not inpatient) antibiotic use. Third, our HA-MRSA data was hospital reported and not all hospitals submitted data. While we tried to adjust for non-reporting, we may have some measurement error in HA-MRSA rates. Moreover, we do not have information on HA-MRSA infections in people who did not go to the hospital. Also, we did not correct for spacial proximity so the significance of our correlations may be exaggerated. Finally, data were aggregated at the state level, not at the individual level, so we do not know whether the patients actually taking the antibiotics were the ones with HA-MRSA infections.
Conclusion
In summary, this study shows a significant correlation between outpatient Medicare Part D antibiotics with MSSA coverage and hospital-onset MRSA infections in the US. Although cause and effect cannot be established, this study supports the theory that the indiscriminate use of antibiotics is “pushing” MSSA towards developing into its drug-resistant counterpart, MRSA. This study, along with results from previous studies, suggests that antibiotic use in any setting may lead to MRSA infections in healthcare settings. This study also highlights how publicly available data aggregated at the prescriber level and heat map data may be able to help focus antimicrobial stewardship programs to specific specialties or states in order to reduce inappropriate antibiotic use. Antibiotic resistance is a dangerous problem. Our study helps to justify the need for immediate national action to avoid a post-antibiotic world.
Conflict of Interest
None of the authors identify any conflict of interest.
Disclosure Statement
Drs. Davis, Seto, and Taira were partially supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Numbers P20MD000173, U54MD007584, G12MD007601, and P20GM103466. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Morell EA, Balkin DM. Methicillin-resistant staphylococcus aureus: a pervasive pathogen highlights the need for new antimicrobial development. Yale J Biol Med. 2010;83(4):223–233. [PMC free article] [PubMed] [Google Scholar]
- 2.Ventola CL. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm Therapeut. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Littman J, Viens AM. The ethical significance of antimicrobial resistance. Public Health Ethics. 2015;8(3):209–224. doi: 10.1093/phe/phv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Office of Infectious Disease, author. Antibiotic resistance threats in the United States, 2013. 2013. Apr, [November 16, 2015]. Available at: http://www.cdc.gov/drugresistance/threat-report-2013.
- 5.Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- 6.Gupta K, Maclntyre A, Vanasse G, Dembry LM. Trends in prescribing β-lactam antibiotics for treatment of community-associated methicillin-resistant staphylococcus aureus infections. J Clin Microbiol. 2007;45(12):3930–3934. doi: 10.1128/JCM.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH Human Microbiome Project defines normal bacterial makeup of the body. 2012. Jun 13, [November 16, 2015]. National Institutes of Health Web site. http://www.nih.gov/news-events/news-releases/nih-human-microbiome-project-defines-normal-bacterial-makeup-body.
- 8.Costelloe C, Lovering A, Montgomery A, Lewis D, McNutty C, Hay AD. Effect of antibiotic prescribing in primary care on methicillin-resistant staphylococcus aureus carriage in community-resident adults: a controlled observational study. Int J Antimicrob Agents. 2012;39(2):135–141. doi: 10.1016/j.ijantimicag.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Struelens MJ. The epidemiology of antimicrobial resistance in hospital acquired infections: problems and possible solutions. BMJ. 1998;317(7159):652–654. doi: 10.1136/bmj.317.7159.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya S, Bir R, Majumdar T. Evaluation of multidrug resistant staphylococcus aureus and their association with biofilm production in a tertiary care hospital, Tripura, Northeast India. J Clin Diagn Res. 2015;9(9):DC01–DC04. doi: 10.7860/JCDR/2015/13965.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrel M, Perencevich EN, David MZ. USA 300 methicillin-resistant staphylococcus aureus, United States, 2000–2013. Emerg Infect Dis. 2015;21(11):1973–1980. doi: 10.3201/eid2111.150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson KB, Searle K, Stoddard GJ, Samore MH. Staphylococcus aureus and Vancomycin-resistant Enterococci in Rural Communities, Western United States. Emerg Infect Dis. 2005;11(6):895–903. doi: 10.3201/eid1106.050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Bayer A, Cosgrove SE, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children. Clin Infect Dis. 2011;52:1–38. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 14.Carreno JJ, Kenney RM, Bloome M, et al. Evaluation of pharmacy generalists performing antimicrobial stewardship services. Am J Health-Syst Pharm. 2015;72:1298–1303. doi: 10.2146/ajhp140619. [DOI] [PubMed] [Google Scholar]
- 15.Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc. 2011;86(11):1113–1123. doi: 10.4065/mcp.2011.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Promoting Antimicrobial Stewardship in Human Medicine, author. Infectious Diseases Society of America Web Site. 2012. [February 20, 2016]. http://www.antibioticsnow.org/Stewardship_Policy/
- 17.Healthcare-associated Infections (HAIs), author Centers for Disease Control and Prevention Web Site. 2015. Jan, [November 16, 2015]. http://www.cdc.gov/hai/progress-report/ Updated March 23, 2015.
- 18.Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Black D, Freedman DO, Pavia AT, Schwartz BS. The Sanford Guide to Antimicrobial Therapy 2014. 44th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014. [Google Scholar]
- 19.Centers for Medicare and Medicaid, author. Medicare Fee-For Service Provider Utilization & Payment Data Part D Prescriber Public Use File: A Methodological Overview. 2015. Apr, [June 15, 2015]. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Downloads/Prescriber_Methods.pdf.
- 20.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 21.Dancer SJ. The problem with cephalopsorins. J Antimicrob Chemother. 2001;48(4):463–478. doi: 10.1093/jac/48.4.463. [DOI] [PubMed] [Google Scholar]
- 22.Kardas-Sloma L, Boelle PY, Opatowski L, Brun-Buisson C, Guillemot D, Temime L. Impact of antibiotic exposure patterns on selection of community-associated methicillin-resistant staphylococcus aureus in hospital settings. Antimicrob Agents Chemother. 2011;55(10):4888–4895. doi: 10.1128/AAC.01626-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tacconelli E, Angelis GD, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008;61(1):26–38. doi: 10.1093/jac/dkm416. [DOI] [PubMed] [Google Scholar]
- 24.Schneider-Lindner V, Delaney JA, Dial S, Dascal A, Suissa S. Antimicrobial drugs and community-acquired methicillin-resistant staphylococcus aureus, United Kingdom. Emerg Infect Dis. 2007;13(7):994–1000. doi: 10.3201/eid1307.061561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba PS, Taneja J, Mishra B. Methicillin and Vancomycin resistant S. aureus. in hospitalized patients. J Glob Infect Dis. 2010;2(3):275–283. doi: 10.4103/0974-777X.68535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–1591. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer ML, Perencevich EN, Eber MR, et al. Optimizing antimicrobial prescribing: Are clinicians following national trends in methicillin-resistant staphylococcus aureus (MRSA) infections rather than local data when treating MRSA wound infections. Antimicrob Resist Infect Control. 2013;2:28. doi: 10.1186/2047-2994-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barfod TS, Wibore EA, Brauner JV, Knudsen JD. Changes in antimicrobial susceptibility patterns of klebsiella pneumoniae, escherichia coli and staphylococcus aureus over the past decade. Dan Med J. 2015;62(10):A5145. [PubMed] [Google Scholar]
- 29.Pate AJ, Terribilini RG, Ghobadi F, et al. Antibiotics for methicillin-resistant staphylococcus aureus skin and soft tissue infections: the challenge of outpatient therapy. Am J Emerg Med. 2014;32(2):135–138. doi: 10.1016/j.ajem.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Yoa Z, Peng Y, Chen X, et al. Healthcare associated infections of methicillin-resistant staphylococcus aureus: a case-control-control study. PLoS One. 2015;10(10):e0140604. doi: 10.1371/journal.pone.0140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight GM, Budd EL, Whitney L, et al. Shift in dominant hospital-associated methicillin-resistant staphylococcus aureus (HA_MRSA) clones over time. J Antimicrob Chemother. 2012;67(10):2514–2522. doi: 10.1093/jac/dks245. [DOI] [PubMed] [Google Scholar]
- 32.Shorr AF. Epidemiology of staphylococcus resistance. Clin Infect Dis. 2007;45:S171–S176. doi: 10.1086/519473. [DOI] [PubMed] [Google Scholar]
- 33.Aldeyab MA, Kearney MP, McElnay JC, et al. A point prevalence survey of antibiotic prescriptions: benchmarking and patterns of use. Br J Clin Pharmacol. 2011;71(2):293–296. doi: 10.1111/j.1365-2125.2010.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]