Abstract
Thymic stromal lymphopoietin (TSLP) and IL-7 are cytokines that signal via the IL-7 receptor alpha (IL-7Rα) to exert both overlapping and unique functions during early stages of mouse B cell development. In human B lymphopoiesis the requirement for IL-7Rα signaling is controversial and the roles of IL-7 and TSLP are less clear. Here we evaluated human B cell production using novel in vitro and xenograft models of human B cell development that provide selective IL-7 and human TSLP (hTSLP) stimulation. We show that in vitro human B cell production is almost completely blocked in the absence of IL-7Rα stimulation and that either TSLP or IL-7 can provide a signal critical for the production and proliferation of human CD19+ PAX5+ pro-B cells. Analysis of primary human bone marrow (BM) stromal cells show that they express both IL-7 and TSLP providing an in vivo source of these cytokines. Using novel xenograft models we show that the in vivo production of human pro-B cells under the influence of mouse IL-7 is reduced by anti-IL-7 neutralizing antibodies, and this loss can be restored by hTSLP at physiological levels. These data establish the importance of IL-7Rα-mediated signals for normal human B cell production.
Keywords: TSLP, IL-7, human B lymphopoiesis, pro-B cell, pre-B cell
Introduction
IL-7Rα signaling is required for B lymphopoiesis in adult mice, although fetal B cell production is IL-7Rα-independent [1]. IL-7Rα is a component of two distinct cytokine receptor complexes with different secondary chains, one activated by IL-7 and the other by TSLP [2]. In adult mice where IL-7 is knocked out, there is a block in B cell precursor production that can be restored by TSLP, but only at the supra-physiological levels achieved in TSLP transgenic mice [3].
The role of IL-7Rα signals in human B cell development is controversial [4, 5]. Patients with defective IL-7R signaling exhibit severe combined immunodeficiency (SCID) characterized by a loss of T cells [6-9]. Although B cell function may be compromised in these patients, B cell numbers are normal [10], suggesting that human B lymphopoiesis differs from that in mice with respect to the requirement for IL-7Rα-mediated signals. However, these data are obtained during the first year of life consistent with fetal B cell production through an IL-7Rα-independent process similar to mouse. Early in vitro studies also suggested that human B cell production is IL-7-independent, however, these were often performed using fetal hematopoietic sources or culture models that produced mouse IL-7 which was later shown to activate human IL-7Rα [11]. Although fetal B cell production is not dependent on IL-7 or TSLP, both of these cytokines can stimulate in vitro proliferation of human fetal B cell precursors [12].
Our previous in vitro studies, performed using a human-only co-culture model, showed that human B cell production beyond the fetal period is increasingly dependent on a signal that can be provided by IL-7 [13]. The ability of TSLP to replace IL-7 in providing this signal is unknown and the importance of IL-7Rα stimulation for in vivo human B lymphopoiesis has not been demonstrated. Recently we developed a novel xenograft model and it showed that physiological levels of human TSLP (hTSLP) increase human B cell production, in vivo, in context of IL-7 [14]. Here we use novel in vitro and xenograft models, engineered to provide selective IL-7 and hTSLP stimulation, to evaluate the requirement for IL-7Rα signals in post-fetal human B cell production and the ability of hTSLP to induce these signals at physiological levels.
Results and Discussion
TSLP or IL-7 can provide a signal critical for the in vitro production of human B cell precursors
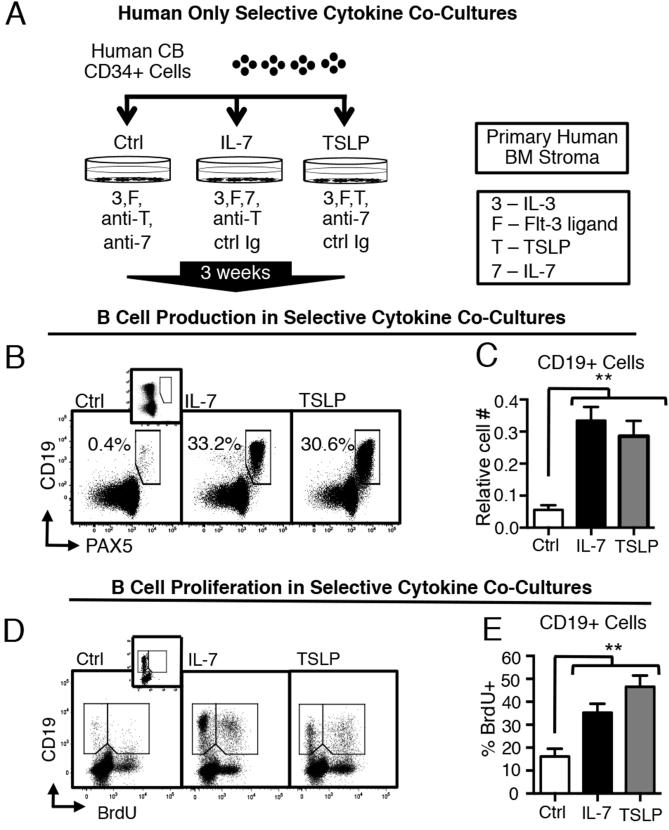
To investigate the role of IL-7Rα signals induced by TSLP and IL-7 in early stages of human B cell production, we developed a human-only co-culture model that supports the production of human pro-B cells and provides selective IL-7 or TSLP stimulation. Human umbilical cord blood (CB) CD34+ cells were cultured with primary human bone marrow (BM) stromal cells in media supplemented with human serum. Selective cytokine stimulation was achieved with combinations of exogenous human IL-7 or TSLP and with neutralizing antibodies to counter activity of endogenously produced IL-7 and TSLP (Fig 1A). CB CD34+ cells were grown under selective cytokine conditions for 3 weeks. Culture progeny were harvested and stained for flow cytometry to identify CD19+ PAX5+ B lineage cells. Control cultures that lacked TSLP or IL-7 produced small numbers of B lineage cells (Fig 1B). In contrast, TSLP and IL-7 cultures showed robust production of CD19+ PAX5+ B lineage cells (Fig 1B). Cultures with TSLP or IL-7 produced similar numbers of B cell precursors (Fig 1C).
Figure 1. TSLP can replace IL-7 in supporting the in vitro production and proliferation of human CD19+ PAX5+ B cell precursors.
(A) Human-only cultures were produced by plating primary human CB CD34+ cells on primary human BM stroma. Selective cytokine stimulation was achieved by supplementing cultures with exogenous IL-7 (5 ng/ml) or TSLP (10 ng/ml) or neutralizing antibodies to TSLP (anti-) or IL-7 (anti-7) at 1 ug/ml and 10 ng/ml respectively, as indicated. Cultures without a particular neutralizing antibody were supplemented, as a control, with non-specific isotype-matched antibodies (ctrl Ig). All culture conditions included IL-3 and Flt-3 ligand at 1 ng/ml. Cultures were maintained for 3 weeks, then harvested and stained for flow cytometry. (B) Dot plots of CD19 vs. PAX5 staining in each culture condition are shown (representative of n=3 independent experiments). PAX5 fluorescence minus one (FMO) control is displayed in inset. (C) Graphed are the relative numbers of CD19+ cells (mean± SEM) generated in vitro under indicated conditions at three weeks (n=14). Relative cell number is defined as the number of CD19+ cells/HSC generated in each condition divided by the sum of CD19+ cells/HSC in all conditions. (D) Representative dot plots of CD19 vs BrdU staining in indicated conditions. BrdU FMO control is displayed in the inset of the Ctrl dot plot. (E) Graphed is the percent BrdU+ cells (mean± SEM) in the total CD19+ population generated under indicated conditions at three weeks (n=8). Additional gating is shown in Fig. S1 in online supporting data. Statistical analysis was performed using one-tailed, paired t-test, *p<0.05, **p<0.001. Each comparison is versus the control condition. Error bars represent mean ± SEM.
To determine if TSLP-induced proliferation contributes to the increased production of human B cell precursors from CB CD34+ cells, we compared BrdU incorporation in cells generated under selective cytokine conditions (Fig 1D). Proliferation in TSLP cultures was significantly increased as compared to control cultures lacking IL-7Rα stimulation and similar to cultures containing IL-7 (Fig 1E). These data demonstrate that TSLP can replace IL-7 in providing signals critical for in vitro production and proliferation of human CD19+ PAX5+ pro-B cells and that human B cell production is almost completely blocked in the absence of IL-7Rα stimulation.
Human bone marrow stromal cells express TSLP, as well as IL-7
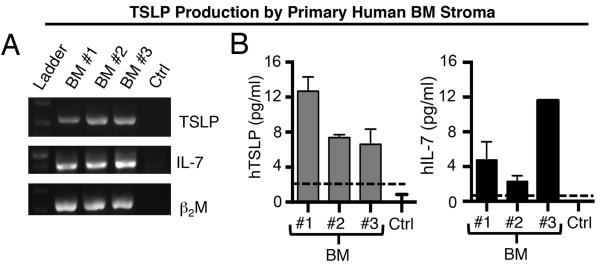
To determine if the BM provides an in vivo source of TSLP we evaluated the ability of primary human BM stroma to produce TSLP. For comparison we evaluated IL-7, which we have previously shown to be expressed by human BM stroma [13]. RT-PCR analysis of stromal cells cultured from healthy donors showed TSLP and IL-7 transcripts (Fig 2A) that resulted in protein production as evidenced by TSLP and IL-7 in supernatants harvested from cultured cells (Fig 2B). These data demonstrate that human BM stroma provide an in vivo source of both cytokines in the normal human BM microenvironment where B cell precursors are generated.
Figure 2. IL-7 and TSLP are produced by human BM stroma.
(A) RT-PCR, was used to detect TSLP, IL-7 or beta-2 microglobulin (β2M, control) transcripts in primary human BM stromal cells from different human donors – pediatric (BM #1 and BM #2) and adult (BM #3) or culture medium as a negative control. Each patient sample was assessed in two or more different PCR reactions. (B) Supernatant from confluent BM stromal cell cultures were assessed by ELISA for TSLP and IL-7 protein production. Data are expressed as mean ± SEM of triplicate values for TSLP, and duplicate values for IL-7. Dashed lines (---) represent ELISA threshold of detection.
IL-7Rα signals induced by TSLP or IL-7 increase the in vivo production of human B cell precursors
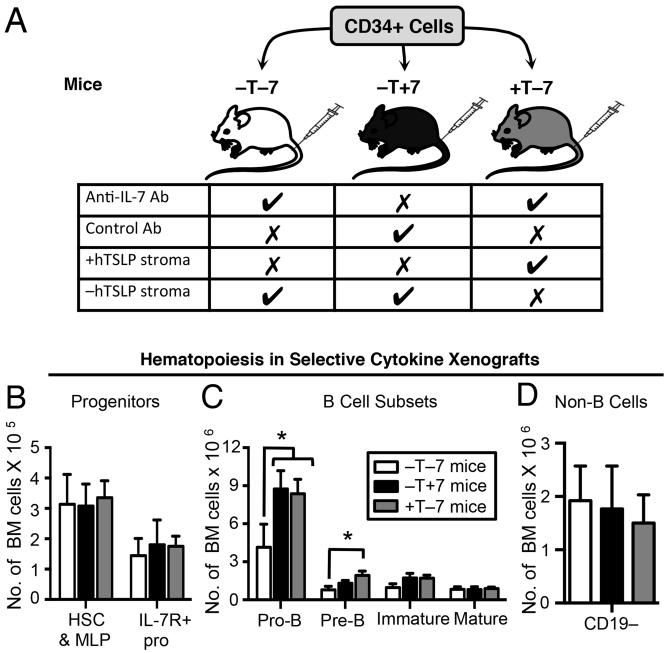
Next we evaluated the in vivo role of IL-7Rα-mediated signals in human B cell development and the ability of physiological levels of TSLP to replace IL-7 in the induction of these signals. Mouse TSLP does not show cross-species activity on human cells [14] although IL-7 does [11, 15]. Thus, classic xenografts provide IL-7, but not TSLP that can stimulate IL-7Rα signals. We used a novel human-mouse xenograft that provides normal serum levels of hTSLP (+T mice) and control (−T mice) that lack hTSLP [14] as an in vivo system for human B cell precursor production. Human IL-7 was not detectable in the serum of +T or −T engineered mice (Milford, unpublished data). To study in vivo human B cell precursor production under selective IL-7 and hTSLP stimulation, we established +T and −T xenografts with CB CD34+ cells and treated for two weeks with antibodies that neutralize mouse and human IL-7 [16] (Fig 3A). This allowed us to compare B lymphopoiesis in mice with 1) no hTSLP and reduced IL-7 (−T−7 mice); 2) no hTSLP and normal IL-7 (−T+7 mice); and 3) physiological hTSLP and reduced IL-7 (+T−7 mice).
Figure 3. IL-7 and TSLP increase the in vivo production of human B cell precursors in human-mouse xenografts.
(A) Immune deficient NSG mice were engineered to express physiological levels of hTSLP (+T mice) or without hTSLP (−T mice) as described [14]. CB CD34+ cells were injected by tail vein into −T and +T mice. Five weeks later, −T mice and +T mice were treated for two weeks with anti-human/mouse IL-7 antibody or isotype-matched control antibody to generate −T−7 mice (no hTSLP and reduced IL-7), −T+7 mice (no hTSLP and normal IL-7), and +T−7 mice (physiological hTSLP and reduced IL-7). At 7 weeks post-transplant, mice were euthanized and BM harvested and stained for human specific markers to identify hematopoietic subsets (for gating see Fig S1 in online Supporting Information.) Graphed are the absolute numbers of cells in (B) progenitor populations, (C) B cell subsets, and (D) non-B cells in the BM of xenograft mice. Data shown were obtained from two independent transplantations of two different CB donors with a total of four mice per group. Statistical analysis was performed using one-tailed, unpaired t-test, *p<0.05 compared to −T−7 mice. Error bars represent mean ± SEM.
Human progenitors and B cell subsets in the bone marrow of xenograft mice were identified by flow cytometry (Fig S2 in online Supporting Information) and cell numbers were compared (Fig 3). No differences in numbers of human hematopoietic stem cells or multi-potential progenitors was observed, including the earliest IL-7R+ progenitors (CD19−CD34+IL-7R+) (Fig 3B). In contrast, mice with normal IL-7 or with physiological levels of hTSLP showed a human pro-B compartment that was increased by 2-3 fold as compared to that observed in −T−7 mice (Fig 3C). Increases observed with physiological hTSLP extended to the pre-B cell stage, although significant increases in pre-B cells were not achieved in −T+7 mice (Fig 3C). Later stages of B cell development were not impacted, which was not surprising, given that the majority of these cells were likely generated prior to the two-week anti-IL-7 treatment period (Fig 3C). No significant differences were detected in the numbers of non-B cells generated in mice with hTSLP or IL-7 as compared to −T−7 xenografts (Fig 3D). T lineage cells are not generated in xenograft mice at this time point and so were not a part of the non-B lineage compartment. These data show that the in vivo production of human pro-B cells under the influence of IL-7 is reduced by anti-IL-7 neutralizing antibodies, and this loss can be restored by hTSLP at physiological levels.
Concluding Remarks
In summary, we used in vitro selective cytokine cultures to show that the production of human B lineage cells beyond the fetal period is dependent on an IL-7Rα-mediated signal that can be provided by either TSLP or IL-7. We demonstrate that both of these cytokines are produced by normal human BM stroma. The validity of these findings for in vivo B lymphopoiesis is supported by the reduction in human B cell precursors observed following treatment with IL-7 neutralizing antibodies in xenografts and the ability of hTSLP to restore this loss.
These studies provide in vivo evidence that IL-7Rα signals positively regulate normal human B cell production and proliferation beyond the fetal period and suggest that TSLP can replace IL-7 in providing these signals. Our data provide a potential explanation for the recent report that B lineage commitment is not blocked in SCID patients who have defects in IL-7 signaling components (common gamma chain and JAK3), but have an intact TSLP receptor signaling pathway [17]. Our studies suggest that therapies to stimulate or block IL-7- and TSLP-mediated IL-7Rα signals are likely to impact B cell production. Consistent with this, data from clinical trials showing that the administration of recombinant human IL-7 can increase newly formed B cells in the periphery and expand the bone marrow lymphoid compartment [18].
Materials and methods
Additional details are available in the online Supporting Information.
Human Samples
Umbilical CB and BM were obtained in accord with Loma Linda University Institutional Review Board (IRB) protocols and the Helsinki Declaration of 1975, as revised in 2008. Isolation of CB CD34+ cells and detection of TSLP production in BM stroma are described in online supplementary methods.
Selective-Cytokine Co-cultures
CB CD34+ cells were seeded on primary human BM stroma and maintained as described [13] with media containing 5% human AB serum (Omega Scientific, Tarzana, CA). All cultures, including control, were supplemented with IL-3 and Flt-3 Ligand. For selective cytokine stimulation, hTSLP and/or IL-7 (R&D Systems Inc., Minneapolis, MN) were added. If neither TSLP nor IL-7 was added, cultures were further supplemented with neutralizing antibodies to counter activity of endogenously produced TSLP and/or IL-7. Cultures without cytokine-specific neutralizing antibody were supplemented with an isotype-matched control. Co-cultures were harvested at 3 weeks. BrdU (Sigma-Aldrich, St. Louis, MO) was added for the final 24 hours in some cultures.
Animal Studies
Studies were performed using NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratory) under protocols approved by the Loma Linda University Institutional Animal Care and Use Committee (IACUC). Mice were engineered by intraperitoneal injection with stroma transduced to express hTSLP (+T mice) or with control vector (−T mice) as described [14]. +T and −T mice were transplanted with CD34+ cells by tail vein injection, after sub-lethal irradiation. Five weeks after CB transplantation, anti-mouse/human IL-7 neutralizing antibody or isotype-matched control (BioXCell, West Lebanon, NH) was injected as described in previous studies that used this anti-IL-7 antibody to inhibit in vivo mouse B lymphopoiesis [16].
Supplementary Material
Acknowledgements
The authors would like to thank Abigail Benitez for assistance in manuscript preparation. This work was supported by NIH R21CA162259, NIH P20 MD006988, NIH 2 R25 GM060507, a St. Baldrick’s Research Grant and Hyundai Hope on Wheels Scholar Hope Grant, the Department of Pathology and Human Anatomy, the Department of Basic Sciences, and the Center for Health Disparities and Molecular Medicine at Loma Linda University School of Medicine (KJP) and by a Grant to Promote Collaborative and Translational Research from Loma Linda University (to KJP and CLM) and a Grant for Research and School Partnerships (to KJP and DJW). This work has been supported by NIH R01 HL095120, a St Baldrick’s Foundation Career Development Award, a Hyundai Hope on Wheels Scholar Grant Award, the Four Diamonds Fund of the Pennsylvania State University, and the John Wawrynovic Leukemia Research Scholar Endowment (to SD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
TMM performed the majority of biological experiments, analyzed and interpreted data, and participated in experimental design and writing the manuscript. RJS, OLF, SRM, and XBZ produced xenografts, IB, AJW, MNC, MCN, and ARB performed laboratory experiments. CLM provided patient samples. XB and SD provided vital reagents. CLM, BTS, JCS, DJW and SD provided conceptual advice. KJP designed experiments, analyzed and interpreted data, wrote the manuscript and was the principal investigator and takes primary responsibility for the paper.
Abbreviations used
- BM
bone marrow
- CB
umbilical cord blood
- FMO
fluorescence minus one
- hTSLP
human thymic stromal lymphopoietin
- TSLP
thymic stromal lymphopoietin
- IL-7Rα
IL-7 receptor alpha
Footnotes
Conflict of Interest
These studies were completed while CLM was at Loma Linda University. CLM now works for Amgen, Thousand Oaks, California; there are no Amgen products relevant to these studies. No other authors have potential conflicts of interest to declare.
References
- 1.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Wang J, Wang Q, Chen G, Zhang J, Chen T, Wan T, Zhang Y, Cao X. Identification of a novel type I cytokine receptor CRL2 preferentially expressed by human dendritic cells and activated monocytes. Biochem Biophys Res Commun. 2001;281:878–883. doi: 10.1006/bbrc.2001.4432. [DOI] [PubMed] [Google Scholar]
- 3.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 4.Sportes C, Gress RE, Mackall CL. Perspective on potential clinical applications of recombinant human interleukin-7. Ann N Y Acad Sci. 2009;1182:28–38. doi: 10.1111/j.1749-6632.2009.05075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin Immunol. 2012;24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 7.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, Ugazio AG, Johnston JA, Candotti F, O'Shea JJ, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 8.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, Migone TS, Noguchi M, Markert ML, Buckley RH, O'Shea JJ, Leonard WJ. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 9.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 10.Giliani S, Mori L, de Saint Basile G, Le Deist F, Rodriguez-Perez C, Forino C, Mazzolari E, Dupuis S, Elhasid R, Kessel A, Galambrun C, Gil J, Fischer A, Etzioni A, Notarangelo LD. Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev. 2005;203:110–126. doi: 10.1111/j.0105-2896.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson SE, Shah N, Panoskaltsis-Mortari A, LeBien TW. Murine and human IL-7 activate STAT5 and induce proliferation of normal human pro-B cells. J Immunol. 2005;175:7325–7331. doi: 10.4049/jimmunol.175.11.7325. [DOI] [PubMed] [Google Scholar]
- 12.Scheeren FA, van Lent AU, Nagasawa M, Weijer K, Spits H, Legrand N, Blom B. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur J Immunol. 2010;40:955–965. doi: 10.1002/eji.200939419. [DOI] [PubMed] [Google Scholar]
- 13.Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, Sahakian E, Kagoda M, Huang G, Hao QL, Sevilla Y, Barsky LW, Zielinska E, Price MA, Wall NR, Dovat S, Payne KJ. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis OL, Milford TM, Martinez SR, Baez I, Coats JS, Mayagoitia K, Concepcion KR, Ginelli E, Beldiman C, Benitez A, Weldon AJ, Arogyaswamy K, Shiraz P, Fisher R, Morris CL, Zhang XB, Filippov V, Van Handel B, Ge Z, Song C, Dovat S, Su RJ, Payne KJ. A novel xenograft model to study the role of TSLP-induced CRLF2 signals in normal and malignant human B lymphopoiesis. Haematologica. 2015 doi: 10.3324/haematol.2015.125336. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barata JT, Silva A, Abecasis M, Carlesso N, Cumano A, Cardoso AA. Molecular and functional evidence for activity of murine IL-7 on human lymphocytes. Exp Hematol. 2006;34:1133–1142. doi: 10.1016/j.exphem.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohn LA, Seet CS, Scholes J, Codrea F, Chan R, Zaidi-Merchant S, Zhu Y, De Oliveira S, Kapoor N, Shah A, Abdel-Azim H, Kohn DB, Crooks GM. Human lymphoid development in the absence of common gamma-chain receptor signaling. J Immunol. 2014;192:5050–5058. doi: 10.4049/jimmunol.1303496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.