Abstract
Neuroimaging genetic studies that associate genetic and epigenetic variation with neural activity or structure provide an opportunity to link genes to psychiatric disorders, often before psychopathology is discernable in behavior. Here we review neuroimaging genetics studies with participants who have Posttraumatic Stress Disorder (PTSD). Results show that genes related to the physiological stress response (e.g., glucocorticoid receptor and activity, neuroendocrine release), learning and memory (e.g., plasticity), mood, and pain perception are tied to neural intermediate phenotypes associated with PTSD. These genes are associated with and sometimes predict neural structure and function in areas involved in attention, executive function, memory, decision-making, emotion regulation, salience of potential threats, and pain perception. Evidence suggests these risk polymorphisms and neural intermediate phenotypes are vulnerabilities toward developing PTSD in the aftermath of trauma, or vulnerabilities toward particular symptoms once PTSD has developed. Work distinguishing between the re-experiencing and dissociative subtypes of PTSD, and examining other PTSD symptom clusters in addition to the re-experiencing and hyperarousal symptoms, will further clarify neurobiological mechanisms and inconsistent findings. Furthermore, an exciting possibility is that genetic associations with PTSD may eventually be understood through differential intermediate phenotypes of neural circuit structure and function, possibly underlying the different symptom clusters seen within PTSD.
Keywords: Intermediate Phenotype, MRI, Neural Circuit, Neurogenetics, Neuroimaging Genetics, PTSD, Risk Polymorphism, Trauma
Posttraumatic Stress Disorder (PTSD) is a debilitating disorder associated with increased suicide risk, educational dropout, unemployment, relationship instability, and the development of comorbid psychiatric disorders (Kessler, 2000). The majority of individuals in the US will experience a traumatic event in their lifetime, yet not all will develop PTSD (Kessler, 2000; Kessler et al., 1995, Koenen et al., 2013). There is evidence that genetic and epigenetic factors account for 30 – 70% of these individual differences (Afifi et al., 2010; Pitman et al., 2012), but the mechanisms by which they exert this influence are not well understood. In the long run, understanding these mechanisms will greatly inform both how to identify those at risk for certain clusters of PTSD symptoms, and how to tailor individual treatments for the best recovery outcomes. Brain structure and function, along with genetics, have emerged as important biological markers of PTSD, helping to identify risk for this disorder and further linking a complex cluster of behaviors to mechanisms of dysfunction and recovery (Greco & Liberzon, 2016; Michopoulos et al., 2015; Peterson et al., 2014; Stark et al., 2015). Combining the two methodologies of neuroimaging and genetics, however, offers an opportunity for an even more nuanced mechanistic understanding of PTSD (Bogdan et al., 2016). In addition to briefly outlining the current findings in the neuroimaging and genetics of PTSD, we focus on how the combination of these techniques has led to further insight into the vulnerabilities and patterns of PTSD dysfunction and recovery.
Classic Findings in the Neurobiology of PTSD
Brain Structure Overview
By examining the structural composition of brain regions we can identify potential dysfunctions. Reductions or increases in volume could point toward under or overuse, respectively, of that brain region or dysregulated communication between regions. PTSD research has consistently implicated abnormal structure in a number of brain regions involved in memory, emotion regulation and production (Figure 1). Several studies have found decreased hippocampal (HP) volume in participants with PTSD (Gurvitis et al., 1996; Stein et al., 1997; Kitayama et al., 2005; Wang et al., 2010; Smith, 2005; Karl, et al., 2006). Reduced volume has also been widely observed in the rostral ventromedial prefrontal cortex (vmPFC) and in the dorsal anterior cingulate cortex (dACC) (Kasai et al., 2008; Kityama, Quinn, & Bremner, 2006; Carrion et al., 2010; Schuff et al., 2008; Karl & Wrner, 2010; Sekiguchi et al., 2013). Work has yet to definitively determine whether these structural abnormalities are risk factors for developing PTSD or a consequence of the disorder or perhaps both. For example, there is evidence to support the hypothesis that lower HP volume is the result of exposure to trauma (Bremner, 2001), as well the hypothesis that it is a risk factor, environmental and/or genetic, for the development of PTSD (Gilbertson et al., 2002; Myslobodsky, 1995). We return to this point in the conclusions section.
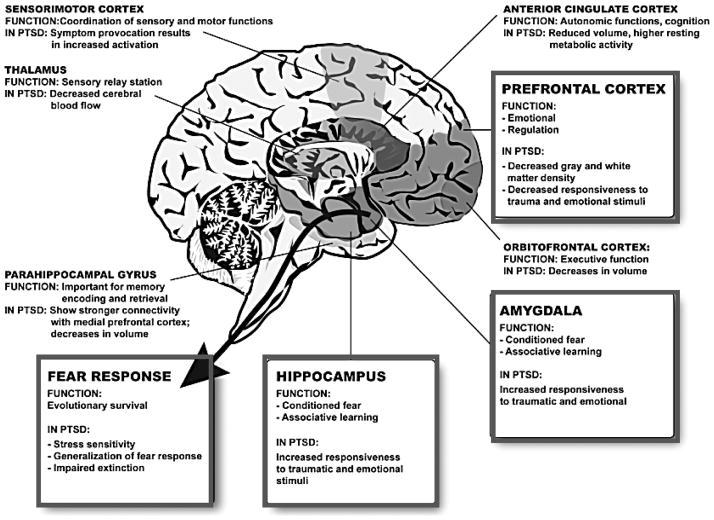
Figure 1. Brain Regions most frequently associated with Posttraumatic Stress Disorder.
This diagram of the human brain illustrates some of the most frequent brain regions associated with PTSD in two decades of work related using fMRI approaches to understand brain activation in PTSD. The prefrontal cortex (PFC) and the hippocampus have strong connections to the amygdala, which is important for conditioned fear and associative emotional learning. The PFC is involved in emotion regulation and is hypoactive in PTSD with some studies showing decreased gray matter density. The hippocampus is thought to play a role in explicit and contextual memories of traumatic events and in mediating extinction of conditioned fear. In PTSD, the hippocampus is decreased in volume. The amygdala is the most well-known area in regulating fear responses, involved in conditioned fear and recovery from fear. Hyperactivation of the amygdala to fearful cues is a robust intermediate phenotype in patients with PTSD. The end result of these neuroanatomical alterations is increased stress sensitivity, generalized fear responses and impaired extinction. Other regions including the anterior cingulate cortex, the orbitofrontal cortex, the parahippocampal gyrus, the thalamus and the sensorimotor cortex also play a secondary role in the regulation of fear and PTSD. (Figure Adapted from Mahan & Ressler, 2012).
Brain Function Overview
Using a non-contrast brain imaging technique, we can measure the concentration of paramagnetic deoxyhemoglobin in a particular area of the brain. Functional magnetic resonance imaging (fMRI) measures changes in the blood oxygen level-dependent (BOLD) signal, which is thought to reflect neural processing in a particular area of the brain (Ogawa et al., 1992). By measuring neural activity in this manner, we can identify potential dysfunctions in a psychological construct of interest as related to PTSD.
Several consistent findings have emerged in functional neuroimaging studies of PTSD. The amygdala, vmPFC, ACC, insula, and HP have been identified as key functional regions involved in PTSD (Figure 1). The amygdala appears to be hyper-responsive in PTSD. Exaggerated amygdala activity has been observed in response to trauma-related stimuli such as sounds (Liberzon et al., 1999; Pissiota et al., 2002), words (Protopopescu et al., 2005), and photographs (Hendler et al., 2003; Shin et al., 1997), trauma-unrelated affective stimuli such as fearful faces (Rauch et al., 2000; Shin et al., 2005; Williams et al., 2006), and during the acquisition of conditioned fear (Bremner et al., 2005). A positive correlation has been reported between amygdala activity and PTSD symptom severity (Rauch et al., 1996; Shin et al., 2004; Protopopescu et al., 2005; Armony et al., 2005). Amygdala hyperactivity may underlie increased salience and attention to threat in PTSD. In addition, PTSD participants often show increased activation in dACC during fear learning and extinction (Rougemont-Bücking et al., 2011; Milad et al., 2009; Bryant et al., 2005; Hayes et al., 2009), and increased insula activity when confronted with potentially aversive stimuli (Pitman et al., 2012; Simmons et al., 2008; Strigo et al., 2010; Aupperle et al., 2012).
In contrast, the vmPFC and rostral vACC are often hypoactive in PTSD. Decreased vmPFC and vACC activation has been reported in response to trauma-related stimuli such as narratives (Shin et al., 2004; Lanius et al., 2001; Shin et al., 1999; Bremner et al., 1999; Britton et al., 2005; Lindauer et al., 2004), photographs, and sounds (Bremner et al., 1999; Yang et al., 2004), as well as in trauma-unrelated stimuli such as fearful faces (Felmingham et al., 2010; Gold et al., 2011; Shin et al., 2005; Williams et al., 2006), and affective words (Bremner et al., 2003). PTSD symptom severity is often negatively correlated with mPFC activity (Shin et al., 2004; Shin et al., 2005; Williams et al., 2006; Britton et al., 2005). Hypoactivation in vmPFC and vACC may contribute to the maintenance of traumatic memories, and may underlie impaired emotion regulation.
Finally, the HP demonstrates mixed hyper- and hypo- activity across studies (Pitman et al., 2012). Inconsistent patterns of activity in HP and other regions (e.g., mPFC and amygdala) across studies could be caused by any number of design differences and symptom heterogeneity. One important potential cause of variation could also be the presence of dissociative symptoms. Often studies do not distinguish between the classic or re-experiencing vs. dissociative subtype of PTSD. The dissociative subtype of PTSD is a diagnosis that is characterized by significant symptoms of depersonalization and derealization, in addition to the classic PTSD symptoms of re-experiencing (e.g., flashbacks), avoidance, negative changes in beliefs and feelings, and hyper-arousal (DSM-5 American Psychiatric Association, 2013). Most generally, depersonalization and derealization are experiences of detachment from one‘s self or surroundings, respectively. Recent research suggests that the re-experiencing subtype of PTSD shows classic amygdala hyper-activity and vmPFC hypo-activity, and in contrast, the dissociative subtype of PTSD shows the opposite pattern (Lanius et al., 2010). For detailed reviews of PTSD neuroimaging studies see Greco & Liberzon (2016), Liberzon and Martis (2006), Pitman et al. (2012), Peterson et al., (2014), and Shin, Rauch, and Pitman (2006).
Genetics Overview
Twin studies of PTSD demonstrate that heritability accounts for approximately 30% of the variance in risk for PTSD, and that genetic factors influence the risk of exposure to traumatic events (True et al., 1993; Xian et al., 2000; Stein et al., 2014; Koenen et al., 2003; Koenen et al., 2005). Both candidate gene and hypothesis-neutral genome-wide association studies (GWAS) have identified several genes that contribute to PTSD risk and symptomatology.
Although replication has been mixed, candidate gene studies have identified that the serotonin transporter gene, COMT, FKBP5, ADCYAP1R1, BDNF, GABARA2, and ApoE2 may play a role in PTSD. Genetic variation (e.g., polymorphisms) in these genes is often associated with PTSD. For example, the polymorphism 5-HTTLPR located in the promoter region of the serotonin transporter gene has been associated with PTSD. Specifically, the short (S) allele of 5-HTTLPR appears to confer risk for PTSD (Kilpatrick et al., 2007; Hans Jörgen Grabe et al., 2009; Koenen et al., 2009; Xie et al., 2009; Kolassa et al., 2010; Wang et al., 2011; Mercer et al., 2012). The MET158 functional polymorphism in the dopaminergic system is associated with a significant reduction in Catechol-O-methyltransferase (COMT) enzyme activity, and has been associated with PTSD (Lachman et al., 1996; Valente et al., 2011; Kolassa et al., 2010). The FKBP5 gene modulates glucocorticoid receptor sensitivity (Scammel et al., 2001) and its genetic variation has been associated with PTSD (Binder et al., 2008; Xie et al., 2010; Mehta et al., 2011; Sarapas et al., 2011; Klengel at al., 2013). Risk alleles of the PAC1 receptor gene (ADCYAP1R1) have been associated with PTSD primarily in African American Women (Ressler et al., 2011; Almli et al., 2013). Brain derived neurotrophic factor (BDNF) encoded by the BDNF gene is involved in fear extinction and recovery from stress, which are impaired in PTSD (Chhatwal et al., 2006; Heldt et al., 2007; Soliman et al., 2010). Finally, the GABA receptor gene (GABARA2) (Nelson et al., 2009) and ApoE2 (Freeman et al., 2014) have also been significantly associated with PTSD.
Genome-wide association studies have linked a number of additional genes and intergenic regions with PTSD: TLL1, RORA, COBL, PRTFDC1, linc01090, and BC036345 (Almli et al., 2015; Guffanti et al., 2013; Logue et al., 2013; Nievergelt et al., 2015; Xie et al., 2013). Several of the single nucleotide polymorphisms (SNPs) identified in these studies, however, did not reach genome-wide significance when replicated. Future studies with larger samples are needed to clarify the relationship between these genes and PTSD. For detailed reviews of PTSD genetics see Almli et al. (2014a), Skelton et al. (2012), and Koenen et al. (2007).
The What and Why of Neuroimaging Genetics
Given the genetic heritability of psychiatric disorders (Afifi et al., 2010; Pitman et al., 2012), understanding genetic vulnerabilities to and protective factors against these conditions is vital. Linking psychopathology and genetic underpinnings directly, however, has not produced stable, robust results. This may be, in part, because genes encode for biological activity within and between cells, and not for heterogeneous psychiatric symptoms (Rasetti & Weinberger, 2011). One way to address this issue is to identify an intermediate phenotype (see also, endophenotype) – a biological marker more directly linked to genetic code compared to the complex patterns of behavior and thought associated with psychiatric disorders (Admon, Milad, & Hendler, 2013; Meyer-Lindenberg & Weinberger, 2006). Brain structure and function are two examples of intermediate phenotypes that can bridge genetics and behavioral psychopathology.
The emerging field of neuroimaging genetics can provide one method for linking genes and psychopathology. Neuroimaging genetics is the examination of genetic variation and its influence on brain structure and function (Meyer-Lindenberg & Weinberger, 2006). Associations between genes and neuroanatomy or function can then be related to the behavioral and psychological symptoms of psychiatric disorders. As a result, there is a greater chance of detecting biological markers related to dysfunction or recovery from psychiatric disorders (Bigos & Weinberger, 2010; Meyer-Lindenberg & Weinberger, 2006; Morey et al., 2011). This technique may also reveal vulnerabilities and emerging physiological consequences of disorders much earlier, before they are perceivable in behavior (Pohlack et al., 2015). Another benefit of this method is that it can begin to distinguish between a vulnerability to a disorder and a consequence of the disorder (Admon, Milad, & Hendler, 2013).
Neuroimaging genetics can be implemented in different populations, healthy controls and those with a psychopathology, to examine the functional relationship between genes and psychiatric disorders. Examining healthy controls allows identification of vulnerabilities before a disorder develops (Rasetti & Weinberger, 2011). Results are not confounded by medication, time course of the disorder, or other comorbid conditions (Rasetti & Weinberger, 2011). Healthy control studies, however, require extra steps to make a clear connection between their results and the disorder of interest before robust conclusions can be made (Rasetti & Weinberger, 2011). Studies conducted with the disordered population possess this direct link with the behavioral and psychological symptoms of the disorder. On the other hand, potential confounds abound with, for example, medication use that interferes with the interpretation of results. Studies with both populations are necessary to provide a complete picture of psychopathology biomarkers.
Neuroimaging Genetics of PTSD
The remainder of this article focuses on neuroimaging genetics studies of participants with PTSD as opposed to healthy control participants. The genes that we focus on are those that have emerged in the PTSD neuroimaging genetics literature as significantly linked to differences in brain structure and function. First, we review genetic polymorphisms, for example, single nucleotide polymorphisms (SNPs), paired with 1) structural and then 2) functional neuroimaging data. The genes in these studies were identified using either a candidate gene or genome-wide association approach. Finally, we end with studies examining epigenetic changes associated with neuroimaging data.
Genetic variation associated with brain structure differences in PTSD as measured by MRI
By examining the structural composition of brain regions and how they relate to genetic variation we can identify potential risk factors in developing PTSD or consequences of the disorder. However, as we will discuss in our conclusions section, it remains unclear in many cases whether the origin of these structural differences is antecedent to trauma or is a change that occurs as a result of PTSD. Here we review findings from three genes, FKBP5, COMT, and BDNF, as they relate to differences in the brain structure of participants with PTSD.
FKBP5
FK506-binding protein 5 gene (FKBP5) modulates glucocorticoid receptor activity (Zannas et al., 2016). Increases in the protein produced by this gene inhibit glucocorticoid signaling either by decreasing the sensitivity or increasing the resistance of the receptor (Zannas et al., 2016). FKBP5 is highly expressed in the hippocampus (Zannas et al., 2016), a structure involved in memory for consciously recalled events (declarative/explicit memory; Kim & Diamond, 2002; Squire, 1992). During memory formation the hippocampus (HP) helps bind together patterns of neural activity throughout the brain that comprise an episodic memory (Kim & Diamond, 2002; Squire, 1992). The SNP rs1360780 risk allele (T) is associated with increased FKBP5 transcription, and therefore increased inhibition of glucocorticoid signaling (Zannas et al., 2016). Interestingly, low glucocorticoid levels and reduced glucocorticoid action have been implicated as vulnerabilities toward developing PTSD (Resnick et al., 1995; Yehuda et al., 1998).
In an adult sample of civilian African American women with a high trauma load, Fani et al (2013) found that FKBP5 SNP rs1360780 risk allele carriers (TT/TC) had altered left HP shape compared to nonrisk allele carriers (CC). This alteration in shape occurred especially in the CA1 region of the HP. Stress blocks neurogenesis in the HP, induces dendritic atrophy, and reduces long-term potentiation (Kim & Diamond, 2002; Sapolsky et al., 1990), which makes it a particularly vulnerable brain structure in PTSD. Approximately half of the sample had current PTSD as measured by the PTSD Symptom Scale, but this diagnosis and symptoms were not significantly different between the risk and nonrisk allele groups. Those with the risk allele (TT/TC), however, had increased attention toward threat cues on an attention task (described in detail in the fMRI section), a hallmark of PTSD symptomatology. One possible interpretation, given this cross-sectional sample, is that HP structural differences associated with increased threat bias in risk allele carries represent a vulnerability toward developing PTSD.
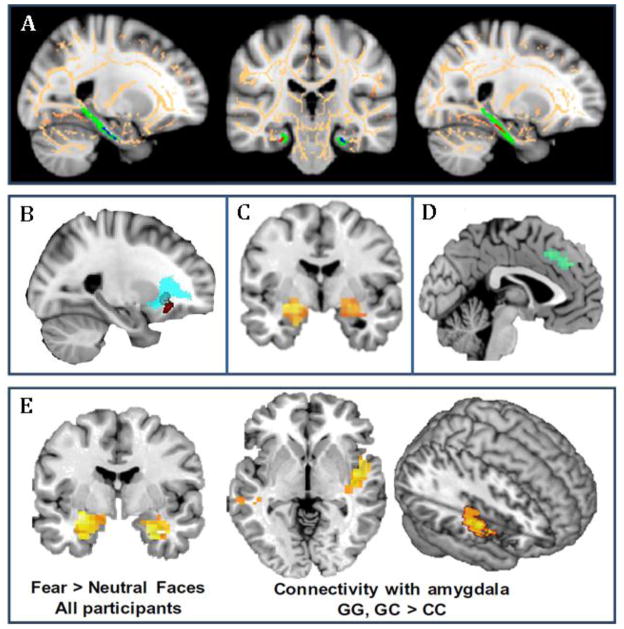
Differential structure of the posterior cingulum has also been linked to FKBP5 SNP rs1360780. The posterior cingulum is a bundle of white matter fibers that runs from the frontal lobe (anterior cingulate) to the temporal lobe (entorhinal cortex). It is a central line of communication between structures in the temporal and frontal lobes (Fani et al., 2014). Abnormalities in the posterior cingulum imply a disruption or dysregulation in communication between these areas. In an adult African American female sample, risk allele carriers (T) had lower fractional anisotropy in the left posterior cingulum, reflecting either lower fiber density, axonal diameter, myelination, or a combination thereof, in this region (Fani et al., 2014; Figure 2A). There was no interaction between genotype, posterior cingulum fractional anisotropy, and PTSD symptoms or diagnosis (approximately half of the sample had a current PTSD diagnosis as measured by the PTSD Symptom Scale). A tentative interpretation to be drawn from this cross-sectional sample is that decreased white matter integrity of the posterior cingulum is a vulnerability toward developing PTSD.
Figure 2. Genetic Associations with Structural and Functional Neural Pathways in PTSD.
Shown are examples of neuroimaging genetic approaches to identifying associations of PTSD-associated SNPs with intermediate phenotypes of neural structure and activation using structural and functional MRI. A) Differential structure of the posterior cingulum connecting hippocampus to cingulate cortex has been linked to FKBP5 SNP rs1360780. Risk allele carriers had lower fractional anisotropy in the left posterior cingulum (from Fani et al., 2014). B) The risk allele carriers of rs406001 (identified in a PTSD GWAS, Xie et al., 2013) demonstrate poorer white matter integrity in the uncinated fasciculus, connecting medial prefrontal with temporal lobe regions, including the amygdala (from Almli et al., 2014b). C) Carriers of the ADCYAP1R1 SNP rs2267735 risk allele show greater activation in amygdala to fearful faces compared to the nonrisk group (from Stevens et al., 2014). D) The number of risk alleles of SNP rs717947 (identified in a GWAS for PTSD) negatively correlated with dmPFC activity to fearful faces compared to neutral faces (from Almli et al., 2015). E) The OPRL1 gene SNP rs6010719 was found to be associated with PTSD and intermediate phenotypes of PTSD. With fMRI, enhanced bilateral amygdala activation in response to fearful versus neutral face stimuli was observed in all participants, irrespective of genotype. However, when viewing fearful faces, risk allele carriers had increased functional connectivity between amygdala and right posterior insula (from Andero et al., 2013).
COMT
The COMT gene codes for Catechol-O-methyltransferase, which acts at both the level of synaptic terminals and the synaptic cleft to catalyze the breakdown of catecholamines such as dopamine (Tunbridge, Harrison, & Weinberger, 2006). COMT helps regulate levels of dopamine, especially in the prefrontal cortex where it is highly expressed (Tunbridge, Harrison, & Weinberger, 2006). Genetic variation in COMT is associated with executive function; for example, polymorphisms on this gene predict differential working memory performance (Tunbridge, Harrison, & Weinberger, 2006). The Val allele of COMT SNP rs4680 (Val158Met) is associated with the increased breakdown of dopamine, and consequently, decreased dopaminergic neurotransmission, decreased cognitive performance as seen behaviorally on neuropsychological tests (Dickinson & Elvevag, 2009), and decreased neural efficiency (i.e., greater activation in PFC during tasks; Mier, Kirsch, & Meyer-Lindenberg, 2010). In contrast, the Met allele is associated with the decreased breakdown of dopamine, and consequently, increased dopaminergic neurotransmission. Perhaps largely as a result of this, individuals demonstrate dysregulated emotional processing, for example, rigidity in emotional responding, and a propensity toward negative affect (Dickinson & Elvevag, 2009). These results highlight that it is important to remember that risk alleles for particular symptoms or psychopathologies may not be “risk” alleles in all contexts. Sometimes these alleles are protective, and the environmental circumstances determine their risk vs. resiliency.
There is evidence that the COMT SNP rs4680 moderates the relationship between neural cortex structure and PTSD. Schulz-Heik et al. (2011) found that a military veteran sample with combat-related PTSD had decreased anterior cingulate cortex (ACC) gray matter volume compared to participants without a PTSD diagnosis (as measured by the Clinician-Administered PTSD Scale, CAPS Blake et al., 1998). This relationship was moderated by COMT SNP rs4680 genotype. Val allele carriers with PTSD had smaller right ACC volume compared to Val allele carriers without PTSD. In contrast, ACC volume was similar in Met allele carriers regardless of PTSD diagnosis. Overall the Val/Val genotype had the smallest right ACC volume compared to all other groups (PTSD and non-PTSD). The ACC is involved in the production and regulation of emotion (Kober et al., 2008). Functional divisions have been identified on the dorsal ventral plane. Dorsal ACC is often involved in the processing of pain, fear learning, response selection, and error detection, and ventral ACC is involved in visceral states, and conflict monitoring during emotional Stroop tasks (Kober et al., 2008; Pitman et al., 2012). As described earlier, PTSD participants often demonstrate hypoactivity in vACC during emotional tasks (e.g., Shin et al., 2001), and in contrast, hyperactivity in dACC during fear learning (e.g., Rougemont–Bücking et al., 2011). Schulz-Heik et al (2011) examined vACC and dACC together in their analyses, which speaks to a structural abnormality that could underlie either (or both) ventral and dorsal functional findings in the PTSD literature. Given that Val allele carriers without PTSD did not have reduced ACC volume, these results suggest the Val allele is a risk factor for developing structural abnormalities associated with PTSD. Reduced ACC volume linked to this genotype may not be a pre-existing vulnerability.
BDNF
The brain-derived neurotrophic factor gene (BDNF) encodes for the BDNF protein, a neurotrophin facilitating the development and survival of neurons. BDNF acts to facilitate the activity-dependent strengthening (e.g., in response to thoughts, emotions, behavior) of connections between neurons (i.e., synaptic plasticity). Because of this, BDNF is heavily involved in learning and memory (Binder & Scharfman, 2004). It is expressed throughout the brain, but plays particularly crucial roles with regard to learning and memory in the HP. Notably, a functional coding SNP in the BDNF gene, SNP rs6265, is associated with increased plasticity (Lyoo et al., 2011). Furthermore, recent data suggests that rs6265 is also associated with altered extinction of fear in humans as well as in a humanized transgenic mouse‘ that contains the same mutation in BDNF (Soliman et al., 2010).
BDNF SNP rs6265 may also play a role in PTSD recovery processes. Lyoo et al. (2011) longitudinally followed an Asian trauma-exposed civilian sample to examine BDNF polymorphisms and their relation to neural structure. Approximately 80% of the trauma-exposed sample was diagnosed with PTSD one-month post trauma (CAPS diagnosed). At one year follow-up assessments, they found that participants exposed to trauma had increased cortical thickness in dlPFC compared to non-trauma controls. This increase in dlPFC cortical thickness appeared most prominent for the Val/Val genotype vs. Met allele carriers at the rs6265 SNP (trend toward significance). The dlPFC thickness was associated with PTSD symptom improvement and recovery, and better performance on neuropsychological tests of executive function. Given the role of dlPFC in emotion regulation, reappraisal, and executive function (Miller & Cohen, 2001; Ochsner, Bunge, Gross, & Gabrieli, 2002; Spreng et al., 2013), this evidence suggests dlPFC may be involved in regulating trauma-related symptoms as individuals recover from PTSD. Furthermore, these data suggest that individuals with Val alleles at the rs6265 locus may have an advantage. Interestingly, BDNF has also been found to interact with glucocorticoid signaling. Higher levels of BDNF are associated with enhanced glucocorticoid receptor signaling, and glucocorticoids are important for neuronal plasticity (Arango-Lievano et al., 2015; Jeanneteau et al., 2012). Therefore, one way the BDNF SNP rs6265 may facilitate plasticity and be associated with improved PTSD-related recovery, is by enhancing glucocorticoid receptor signaling, thus normalizing recovery from stress-related emotional and memory ailments.
GWAS evidence for the COBL gene
Xie and colleagues recently reported a genome-wide significant single nucleotide polymorphism (SNP) (p = 3.97×10−8) rs406001, in a genome-wide association study (GWAS) of PTSD (Xie et al., 2013). The rs406001 SNP is intergenic with no known function; however, the closest gene is COBL, which may be related to actin polymerization, and neuronal development and function. Almli et al., (2014b) sought to replicate the top associations of Xie and colleagues, and to extend these findings with structural MRI to examine potential intermediate neural phenotypes. Although the main effects were not replicated in this secondary cohort, they found a significant genotype by environment interaction (G×E) with childhood trauma in the top SNP, rs406001 (N = 3076, t = 14.98, p = 0.0006). In fact, all three SNPs close to the gene COBL gene reported in Xie et al., (2013), had significant G×E interactions with childhood trauma in the replication cohort. The brain imaging findings indicated that risk allele carriers of rs406001 demonstrate poorer white matter integrity in the uncinate fasciculus, connecting medial prefrontal with temporal lobe regions, including the amygdala (Almli et al., 2014b, Figure 2B). These are regions thought to play a critical role in regulation and extinction of learned fear and trauma associations. These data serve as a partial replication and extension of the first large GWAS for PTSD, suggesting a potential white matter intermediate phenotype underlying PTSD risk.
Genetic variation associated with differential brain activity in PTSD as measured by fMRI
The majority of neuroimaging genetics papers with participants who have PTSD examine BOLD signal associated with attention to threatening stimuli. Given that a core symptom of PTSD is hypervigilance toward threat, this is a key paradigm of interest. Here we review genetic variance associated with four specific genes, FKBP5, SLC6A4, ADCYAP1R1, OPRL1, and the genes encapsulated at chromosome 4p15, in relation to neural activity associated with threat.
FKBP5
As described earlier, FKBP5 modulates glucocorticoid receptor activity. FKBP5 SNP rs1360780 risk allele (T) is associated with increased FKBP5 transcription, and increased inhibition of glucocorticoid signaling (Zannas et al., 2016). In an adult sample of civilian African American women with a high trauma load, Fani et al (2013) found that FKBP5 SNP rs1360780 risk allele carriers (TT/TC) had increased attention toward mild threat cues. In this dot probe paradigm, participants were presented with a pair of faces. The pair was comprised of either two neutral faces or one neutral face and one valenced expression (happy or threatening). At a certain point, an asterisk replaced the faces, and the task was simply to indicate whether the asterisk appeared on the left or right side of the screen. In general, participants with a threat bias attend to the threatening face. With these participants, if the threatening face‘s location matches the location of the asterisk (threat congruent), their left/right responses will be faster vs. if the locations are a mismatch (threat incongruent). Fani et al. (2013) found the risk allele carriers demonstrated this threat bias not only in their behavioral responses on the task, but also in their neural activity. On threat incongruent trials compared to threat congruent trials, participants with the risk allele (TC/TT) had more activity in their HP and parahippocampal gyrus vs. the CC genotype. Given the role of the HP and parahippocampal gyrus in memory encoding and retrieval, these results suggest an increased sensitivity to perceived threat in the environment. Findings were independent of PTSD symptoms or diagnosis. A tentative interpretation of this cross-sectional data suggests this FKBP5 risk allele is associated with increased threat bias (behaviorally and neurally), which in turn may represent a vulnerability toward developing PTSD.
SLC64A
The serotonin transporter gene (SLC64A) encodes a protein that transports serotonin back into presynaptic terminals (i.e., reuptake). Serotonin is linked to mood with decreased serotonin levels associated with lower mood (Young, 2007). Classically studied polymorphisms in this gene include SNPs (e.g., rs16965628, GG genotype and CG genotype) and deletions (e.g., 5-HTTLPR short allele, “S” and long allele, “L” ). The rs16965628 G allele and 5-HTTLPR S allele are both associated with decreased expression of SLC64A (Martin et al., 2007). Both polymorphism types can also be examined in tandem. For example, in one triallelic variation of 5-HTTLPR, the SLA, LGLA genotypes offer a baseline for the expression of SLC64A, the LGLG, SLG, and SS genotypes are low expressing, and the LALA genotype is high expressing.
In a sample of combat-trauma exposed veterans with and without PTSD (as measured by Davidson Trauma Scale, Davidson, 1996), Morey et al. (2011) found the rs16965628 and 5-HTTLPR polymorphisms predicted differential neural activity on a working memory task. Participants were shown a series of faces, a short delay occurred, and then they were shown a subsequent face. Their task was to indicate whether this subsequent face was previously displayed (“old”) or new. During the delay period between initial face exposures and the memory rating (old vs. new), different kinds of distracters were displayed. The distracters were combat-related, non-combat related, or scrambled images, and all were irrelevant to the task at hand. All groups, regardless of PTSD symptom severity or genotype had a similar behavioral performance on this task, but distinct differences emerged between genotype, PTSD diagnosis, and neural activity.
When comparing BOLD signal during the delay period for combat-related distracters vs. non-combat related distracters, rs16965628 genotype affected ventrolateral PFC (vlPFC) activity and 5-HTTLPR genotype affected amygdala activity. In participants with PTSD, those with the rs16965628 GG genotype had more activity in vlPFC to combat distracters compared to trauma-exposed control participants with the GG genotype. CG genotype was not associated with differential vlPFC activity in PTSD and non-PTSD participants. Because all groups performed similarly on the working memory task, this evidence suggests PTSD-positive participants with the GG genotype required more activity in vlPFC to maintain performance when confronted with trauma-related distracters. At the same time, the 5-HTTLPR polymorphism trended toward modulating amygdala activity in response to combat distracters. That is, participants with PTSD and an S allele trended toward greater left amygdala activity compared to S allele carriers without PTSD. There was no difference in amygdala activity between PTSD diagnostic groups in the 5-HTTLPR LL genotype. Although this trend level significance should be interpreted with caution, this result suggests that PTSD-positive participants with an S allele found the threat cues more salient. Taken together, this evidence tentatively suggests that these two polymorphisms are associated with biased neural activity to threat once PTSD has developed.
ADCYAP1R1
Pituitary Adenylate Cyclase-Activating Polypeptide Type I Receptor (PAC1) is encoded by the ADCYAP1R1 gene, and it was previously associated with increased PTSD risk, particularly in women (Ressler et al., 2011; Almli et al., 2013). PAC1 is selective for Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP). The PAC1 receptor is highly expressed in the amygdala, hypothalamus, and HP, among other regions (Kormos & Gaszner, 2013; Shen, Gehlert, & Collier, 2013). PACAP has a diverse set of functions, including both the facilitation and inhibition of neuroendocrine release in response to stressors (Vaudry et al., 2009). The ADCYAP1R1 SNP rs2267735 risk allele (C) is associated with decreased expression of ADCYAP1R1, and less ADCYAP1R1 mRNA (Ressler et al., 2011).
The ADCYAP1R1 SNP rs2267735 may also predict neural activity related to threat processing (Stevens et al., 2014). Urban civilian women with a heavy trauma load passively viewed fearful and neutral faces during fMRI. Participants were divided into two groups based on rs2267735 genotype, risk (CC) and nonrisk (GC/GG). These groups were matched for trauma load and PTSD symptom severity as measured by the modified PSS (Falsetti, 1993). When BOLD signal to fearful faces was compared to neutral faces, the rs2267735 genotype modulated activity in the amygdala and HP. The risk genotype (CC) had greater activation in amygdala and HP to fearful faces compared to the nonrisk group (G allele carriers) (Figure 2C). The number of risk alleles was also positively correlated with activity in these regions. Furthermore, the risk genotype had significantly decreased amygdala hippocampal connectivity compared to the nonrisk allele carriers. Together these results suggest the risk group found the fearful faces more salient, and had more fear reactivity (Stevens et al., 2014). Because this was a cross-sectional sample, and groups were matched on PTSD symptom severity, these results may represent a vulnerability toward developing PTSD.
OPRL1
Opiate Receptor-Like 1 gene (OPRL1) encodes the nociceptin receptor (Andero, 2015). Among many biological roles, this receptor can modulate the perception of pain (Andero et al., 2013). OPRL1 plays a particularly functional role in the amygdala. Evidence suggests polymorphisms of this gene may be related to risk for PTSD. In an urban civilian sample of women with a high trauma load, Andero et al. (2013) found that OPRL1 SNP rs6010719 predicted PTSD symptoms, and behavioral and neural responses to two different fear-related tasks. As described in the previous section, participants passively viewed fearful and neutral faces while they were in the magnetic resonance imaging scanner. Outside of the scanner, participants also completed a fear potentiated startle task. Andero et al (2013) found that as participants’ reported trauma load increased, risk allele carriers (G) had increased PTSD symptoms (as measured by the PSS and CAPS) compared to the CC genotype. Risk allele carriers also had poorer discrimination between safety and danger signals in the fear potentiated startle paradigm compared to the CC genotype. In addition, on the fMRI task, risk allele carriers had increased functional connectivity between amygdala and posterior insula while viewing fearful faces compared to the CC genotype (Figure 2E). The insula is involved in pain perception and the posterior insula especially is involved in visceral state awareness (Craig, 2003). Together these results suggest risk allele carriers have increased PTSD symptom severity, and dysregulated fear responding. The OPRL1 SNP rs6010719 G allele carriers may represent a genotype at potentially higher risk for developing PTSD.
SNP rs717947 at chromosome 4p15
The only GWAS with PTSD participants to use the functional neuroimaging genetics methodology to date identified a SNP (rs717947) at chromosome 4p15 that correlates with neural activity related to threat processing (Almli et al., 2015). The functional role of this SNP is unclear, but it is a methylation quantitative trait locus, suggesting that is may have important roles in influencing the epigenetic regulation of adjacent genetic loci or regulatory regions (Banovich et al., 2014). Participants who completed the neuroimaging genetics portion of this study were urban civilian women with a high trauma load. Participants passively viewed fearful and neutral faces while in the scanner. Risk allele (T) carriers had higher PTSD symptoms compared to the CC genotype. The number of risk alleles negatively correlated with dlPFC and dmPFC activity to fearful faces compared to neutral faces. That is, risk allele carriers had decreased activity in dlPFC and dmPFC to fearful faces (Figure 2D). Given these regions are associated with emotion regulation (Ochsner et al., 2002; Ochsner & Gross, 2005), these results imply that risk allele carriers had increased emotional dysregulation to fearful faces perhaps an inability to regulate fearful emotion or extinguish fear (Almli et al., 2015). Together these results suggest the SNP rs717947 at chromosome 4p15 T allele carriers may represent a genotype at higher risk for developing PTSD.
Epigenetic variation associated with brain structure and function differences in PTSD as measured by MRI and fMRI
Not only can we look at genetic variation as it relates to risk for PTSD, but we can also assess the dynamic regulation of gene expression. One way to measure gene expression is through the methylation status of a gene. For the most part, increased methylation implies decreased gene expression (Jaenisch & Bird, 2003). Here we examine methylation status as it relates to structural and functional differences in the brains of participants with PTSD symptoms. We begin with findings from two genes, SKA2 and FKBP5, as their epigenetic differences relate to brain structure, and then we end with epigenetic findings related to NR3C1 and brain function.
SKA2
Spindle and kinetochore-associated complex subunit 2 (SKA2) encodes for a protein that enhances the activation of glucocorticoid receptors (Rice et al., 2008). In this way it facilitates negative feedback inhibition of the HPA axis, and is protective against the toxic effects of chronic HPA axis activity (Rice et al., 2008). The SKA2 SNP rs7208505 is associated with suicidal behavior (Guintivano et al., 2014), and recent evidence suggests it is also associated with brain structure differences in PTSD (Sadeh et al., 2015). The rs7208505 risk allele (C) can have varied methylation status, but the nonrisk allele (T) cannot be methylated (Guintivano et al., 2014). Because of this, Sadeh et al. (2015) controlled for SKA2 SNP rs7208505 genotype so they could look at the relationship between this gene’s methylation status and PTSD. Participants were white, predominately male military veterans with Criterion A trauma. Over half of the participants had a current PTSD diagnosis as measured by the CAPS. SKA2 methylation (adjusted for genotype) was associated with decreased cortical thickness in frontopolar cortex, superior frontal gyrus (SFG), and orbital frontal cortex (OFC). In turn, reduced cortical thickness in these areas was associated with increased PTSD symptom severity. Frontopolar cortex, SFG, and OFC are involved in diverse cognitive functions, for example, complex decision-making, attention, prospective thinking, introspection, and reward/punishment-related processing (e.g., Christoff & Gabrieli, 2000; Kringelbach & Rolls, 2004; Okuda et al., 2003). Differences in frontopolar cortex, SFG, and OFC structure could point toward dysfunction in their processing, for example, emotional dysregulation, impulsivity, and difficulty picturing the future (Sadeh et al., 2015). A path analysis suggested that SKA2 methylation status mediated the relationship between PTSD and cortical thickness in frontopolar cortex, SFG, and OFC. This indicates that SKA2 methylation (decreased expression of SKA2) may represent a risk for developing PTSD.
FKBP5
As already discussed, FKBP5 modulates glucocorticoid receptor activity. FKBP5 SNP rs1360780 risk allele (T) is associated with increased FKBP5 transcription, and increased inhibition of glucocorticoid signaling (Zannas et al., 2016). Methylation of this gene inhibits its expression, which in turn can cause increased glucocorticoid signaling (Klengel et al., 2012). Klengel et al. (2012) replicated the finding that an interaction between childhood trauma and FKBP5 SNP rs1360780 genotype predicted lifetime PTSD as measured by the CAPS (Binder et al., 2008). Risk allele carriers (T) with childhood trauma were more likely to have had a PTSD diagnosis over their lifetime. Building on these findings, Klengel et al. (2012) found an interaction between FKBP5 SNP rs1360780 genotype and childhood trauma that predicted methylation status of this gene. Participants who experienced childhood abuse and had the risk allele (T) had decreased methylation of this gene (associated with more FKBP5 expression and altered GR sensitivity) compared to participants with the protective genotype (CC). In the risk allele carriers (T), methylation was negatively correlated with overall childhood abuse, especially physical and emotional abuse subscales as measured by the Childhood Trauma Questionnaire (Pennebaker & Susman, 1988). That is, risk allele carriers (T) with greater levels of childhood abuse had demethylation of this gene, and presumably more expression of FKBP5, inhibiting glucocorticoid signaling. FKBP5 SNP rs1360780 methylation also positively correlated with right HP volume. That is, demethylated FKBP5 seen in the risk group was associated with decreased right HP volume, a region especially vulnerable to the effects of chronic stress. Together this evidence suggests that risk allele specific FKBP5 demethylation may mediate the interaction between this genotype and childhood trauma as it relates to PTSD.
NR3C1
The nuclear receptor subfamily 3, group C, member 1 (NR3C1) gene is the glucocorticoid receptor gene. The glucocorticoid receptor is where glucocorticoids, for example, cortisol, bind. Because of this, the glucocorticoid receptor is essential for HPA axis regulation. Increased methylation of NR3C1 is associated with decreased expression of this gene (Yehuda et al., 2013).
Recently methylation status of NR3C1 has been associated with neural activity related to distressing interpersonal interactions and PTSD. Schechter et al (2015) examined NR3C1 methylation in a sample of mothers who had experienced interpersonal violence. One group of mothers had PTSD as measured by the CAPS and PCL-S (Weathers et al., 1993), and the other group of mothers did not meet PTSD diagnosis criteria, although all participants had experienced interpersonal trauma. Both groups watched video clips of mother-child interactions while they were in the magnetic resonance imaging scanner. The interactions included a child playing with their mother and a child being separated from their mother. Some videos were of the participant and their own child; others were of an unfamiliar mother and child. In this sample, decreased methylation of the NR3C1 promoter region was associated with increased PTSD symptom severity. When BOLD signal for child separation videos was compared to the signal for child playing videos, it was also associated with NR3C1 promoter region methylation status. Less methylation of NR3C1 promoter region was associated with decreased activity in vmPFC, dmPFC, dlPFC, precuneus, and thalamus to viewing child separation, and greater PTSD symptom severity. NR3C1 promoter region methylation status was also a significant predictor of activity in dlPFC and vmPFC. These results suggest that increased expression of NR3C1 (demethylation of the promoter region) is associated with increased PTSD symptom severity and decreased activity in areas of the brain related to emotion regulation (e.g., dlPFC, vmPFC) when viewing a distressing interpersonal interaction.
Interestingly, increased expression of glucocorticoid receptors is also associated with enhanced negative feedback inhibition of the HPA axis (Herman et al., 2012). The PFC is heavily populated with glucocorticoid receptors, and actively plays a role in HPA axis negative feedback inhibition (Diorio, Viau, & Meaney, 1993; Herman et al., 2012). Lower levels of glucocorticoids and enhanced HPA axis negative feedback are often understood to be features of PTSD (Yehuda et al., 1990; Yehuda et al., 1993; see Yehuda, 2006 for a discussion of mixed findings). Demethylation of the NR3C1 promoter region, which is expected to increase receptor expression and thus enhance negative feedback of HPA, may therefore represent a risk factor for developing PTSD.
Conclusions and Future Directions
The growing body of research on the neurobiology of PTSD suggests the amygdala, vmPFC, ACC, insula, and HP are consistently implicated, and SLC6A4, COMT, FKBP5, ADCYAP1R1, BDNF, GABARA2, ApoE2, TLL1, RORA, COBL, PRTFDC1, linc01090, and BC036345 are often predictors of dysfunction in the disorder. Neuroimaging genetic studies that associate genetic and epigenetic variation with neural activity or structure related to PTSD provide an even more nuanced layer of mechanistic information on top of each field studied separately. The neuroimaging genetics results reviewed here reveal that genes related to the physiological stress response (e.g., glucocorticoid receptor and activity, neuroendocrine release), learning and memory (e.g., plasticity), mood, and pain perception are tied to neural intermediate phenotypes associated with PTSD. These genes are associated with and sometimes predict neural structure and function in areas involved in diverse functions, for example, attention, executive function, memory, decision making, emotion regulation, salience (e.g., of potential threats), and pain perception. These relationships appear to be vulnerabilities toward developing PTSD, or vulnerabilities toward particular symptoms once PTSD has developed.
However, the specific relationship between neuroimaging genetic results and PTSD is still tentative in most cases. The extent to which identified biological markers represent vulnerability factors that increase the likelihood of developing PTSD following a significant trauma, or are due to the neurotoxic effects of traumatic stress associated with PTSD has yet to be definitely determined. Likely some differences pre-date trauma and PTSD, and others are corollaries of the disorder. For example, there is evidence to suggest that the reduced hippocampal volume often associated with PTSD is a vulnerability toward developing PTSD (Gilberson et al., 2002; Kasai et al., 2008; Pitman et al., 2006). This has been established through studies of monozygotic twins, one of the twins having experienced military combat exposure and one having not. Of the twin pairs, some of the combat exposed individuals developed PTSD and some did not, affording a comparison between PTSD vs. non-PTSD twin pairs. For those who did develop PTSD, both the trauma-exposed twin with PTSD and the unexposed twin without PTSD and smaller hippocampi compared to the twin pairs in which the trauma-exposed twin did not develop PTSD (Gilberson et al., 2002). The reduced HP volume for the unexposed monozygotic twin in the PTSD-twin pair suggests this difference predates combat trauma exposure, and may have been a vulnerability toward developing PTSD.
However, there is strong rodent and human evidence to suggest that the brain, and the HP especially, is subject to structural remodeling due to the effects of stress and glucocorticoids. Thus many structural differences in PTSD may reflect consequences of the disorder. For example, there is evidence to suggest that chronic stress exposure and glucocorticoids can affect the hippocampus by reducing neurogenesis and synaptic plasticity, hindering long-term potentiation and lowering dendritic spine density, among other alterations (see Conrad, 2008 for a review). If not directly causing hippocampal cell death (see Glucocorticoid Cascade Hypothesis, Sapolsky, Krey, & McEwen, 1986), chronic stress exposure and glucocorticoids at the very least make the HP vulnerable to such damage for extended periods of time (see the Glucocorticoid Vulnerability Hypothesis; Conrad, 2008). This evidence for the detrimental effects of stress and glucocorticoid exposure on the HP points towards PTSD potentially causing reductions in HP. Similarly, Kasai et al (2008) found that reductions in pregenual ACC gray matter were a consequence of PTSD in a study of monozygotic twins discordant for combat exposure. The combat exposed twins had smaller pgACC compared to their twins who did not experience combat and did not have PTSD. More longitudinal research that assesses individuals before and after PTSD develops is necessary to definitely delineate pre-existing vulnerabilities and consequences of PTSD.
Similarly on the functional imaging side, for example, it is often unclear whether the neural activity associated with potential threat is a predisposing risk for PTSD or a PTSD-acquired dysfunction. It is perhaps likely that the biomarkers discussed here represent both vulnerability factors and the results of traumatic exposure. A recent review, however, proposes a potential causal model of PTSD risk and corollaries (Admon, Milad, & Hendler, 2013). Based on growing evidence including monozygotic twin studies, Admon et al (2013) suggest increased amygdala activity to emotionally salient stimuli and hyperactivity in the dACC during fear learning appear to be predisposing vulnerabilities toward developing PTSD (e.g., Shin et al., 2011). On the other hand, reduced connectivity between mPFC and HP resulting in reduced capacity to extinguish fear may be an acquired PTSD dysfunction. Combining results from healthy controls, healthy controls whose immediate family members have PTSD, and patients with PTSD themselves will continue to refine this relationship. Admon et al (2013) also suggest a renewed focus on neuroimaging genetics in twin studies and prospective studies that catch participants before trauma occurs.
Neuroimaging genetic approaches may also be valuable in the study of sex differences in PTSD. Males are more likely to experience potentially traumatic events, however, females are more likely to meet criteria for PTSD (Kessler et al., 1995). These differences may in part be accounted for by higher incidences of highly pathogenic types of trauma for females compared to males (e.g., childhood sexual abuse, sexual assault; Tolin & Foa, 2006). However, females are more likely to meet criteria for PTSD even in categories more frequently experienced by males (e.g., non-sexual assault, accidents; Tolin & Foa, 2006), suggesting the relevance of biological mechanisms for PTSD related sex differences. Recent findings on the dynamic regulation of PACAP and the PAC1 receptor gene by estrogen point to genetic variation contributing to these sex differences (Jovanovic et al., 2012; Ressler et al., 2011). The enhanced ability of neuroimaging genetics to identify biological markers related to psychiatric disorders may be especially relevant to the understanding of sex differences in PTSD.
Future research should also aim to distinguish between the re-experiencing and the dissociative subtype of PTSD to help clarify neurobiological mechanisms and inconsistent findings. Notably, the majority of work has focused on the re-experiencing and hyperarousal symptoms associated with PTSD. It may be beneficial to concentrate on other symptom clusters, for example, negative alterations in cognitions and mood, to identify novel neural and genetic mechanisms of PTSD related to self-referential processing. Understanding the neurobiological mechanisms of these symptoms, and how genetics underlies differential neural phenotypes, could prove very fruitful in breaking down barriers to PTSD treatment and recovery.
Acknowledgments
Many thanks to Guia Guffanti and Evan Lebois for their genetic discussions and comments. This work was primarily supported by the National Institutes of Mental Health (MH096764 and MH071537 to KJR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends in cognitive sciences. 2013;17(7):337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Afifi TO, Asmundson GJ, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: A review of twin studies. Clin Psychol Rev. 2010;30:101–12. doi: 10.1016/j.cpr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, Ressler KJ. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013;162(3):262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder. International Journal of Neuropsychopharmacology. 2014a;17(2):355–370. doi: 10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Srivastava A, Fani N, Kerley K, Mercer KB, Feng H, Bradley B, Ressler KJ. Follow-up and extension of a prior genome-wide association study of posttraumatic stress disorder: gene × environment associations and structural magnetic resonance imaging in a highly traumatized African-American civilian population. Biol Psychiatry. 2014b;76(4):e3–4. doi: 10.1016/j.biopsych.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli LM, Stevens JS, Smith AK, Kilaru V, Meng Q, Flory J, Ressler KJ. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015 doi: 10.1002/ajmg.b.32315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R. Nociceptin and the nociceptin receptor in learning and memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, … Ressler KJ. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Science translational medicine. 2013;5(188):188ra73–188ra73. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV, Jeanneteau F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proceedings of the National Academy of Sciences. 2015;112(51):15737–15742. doi: 10.1073/pnas.1509045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. American Journal of Psychiatry. 2005;162(10):1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, … Stein MB. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry. 2012;69(4):360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Banovich NE, Lan X, McVicker G, Van de Geijn B, Degner JF, Blischak JD, … Gilad Y. Methylation QTLs are associated with coordinated changes in transcription factor binding, histone modifications, and gene expression levels. 2014 doi: 10.1371/journal.pgen.1004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Weinberger DR. Imaging genetics—days of future past. Neuroimage. 2010;53(3):804–809. doi: 10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Binder EB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J Am Med Assoc. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Mini review. Growth factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, … Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Charney DS, Keane TM. Clinician-Administered PTSD Scale for DSM-IV, Revised (CAPS) National Center for Posttraumatic Stress Disorder, Medical Center & Neuroscience Division, West Heaven VA, Medical Center; Boston, VA, USA: 1998. [Google Scholar]
- Bogdan R, Pagliaccio D, Baranger DA, Hariri AR. Genetic Moderation of Stress Effects on Corticolimbic Circuitry. Neuropsychopharmacology Reviews. 2016;41(1):275–296. doi: 10.1038/npp.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Hypotheses and controversies related to effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder. Hippocampus. 2001;11(2):75–81. doi: 10.1002/hipo.1023. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999 doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, … Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological medicine. 2005;35(06):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, … Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological psychiatry. 2003;53(10):879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological psychiatry. 2005;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C, … Williams LM. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58(2):111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Richert K, Hoffman BC, Reiss AL. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biological Psychiatry. 2010;68(5):491–493. doi: 10.1016/j.biopsych.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature neuroscience. 2006;9(7):870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JD. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28(2):168–186. [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current opinion in neurobiology. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Davidson J. Davidson trauma scale. Multi-Health Systems Inc; Toronto, ON: 1996. [Google Scholar]
- De Quervain DJF, Kolassa IT, Ackermann S, Aerni A, Boesiger P, Demougin P, … Papassotiropoulos A. PKCα is genetically linked to memory capacity in healthy subjects and to risk for posttraumatic stress disorder in genocide survivors. Proceedings of the National Academy of Sciences. 2012;109(22):8746–8751. doi: 10.1073/pnas.1200857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-5 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Dickinson D, Elvevåg B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164(1):72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. Behaviour Therapist. 1993;16:161–161. [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, Glover E, Jovanovic T, Bradley B, Dinov ID, Zamanyan A, Toga AW, Binder EB, Ressler KJ. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Reiser E, Binder EB, Jovanovic T, Bradley B, Ressler KJ. FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology. 2014;39(5):1206–1213. doi: 10.1038/npp.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of abnormal psychology. 2010;119(1):241. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WST. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. The Journal of neuropsychiatry and clinical neurosciences. 2014 doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, … Pitman RK. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychological medicine. 2011;41(12):2563–2572. doi: 10.1017/S0033291711000730. [DOI] [PubMed] [Google Scholar]
- Greco JA, Liberzon I. Neuroimaging of Fear-Associated Learning. Neuropsychopharmacology Reviews. 2016;41(1):320–334. doi: 10.1038/npp.2015.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti G, Galea S, Yan L, Roberts AL, Solovieff N, Aiello AE, … Koenen KC. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718. 1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology. 2013;38(12):3029–3038. doi: 10.1016/j.psyneuen.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guintivano J, Brown T, Newcomer A, Jones M, Cox O, Maher BS, Kaminsky ZA. Identification and replication of a combined epigenetic and genetic biomarker predicting suicide and suicidal behaviors. American journal of psychiatry. 2014;171(12):1287–1296. doi: 10.1176/appi.ajp.2014.14010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, … Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological psychiatry. 1996;40(11):1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans Jörgen Grabe MD, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, … Rosskopf D. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. The American journal of psychiatry. 2009;166(8):926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Hayes JP, LaBar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Research: Neuroimaging. 2009;172(1):7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular psychiatry. 2007;12(7):656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, … Bleich A. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage. 2003;19(3):587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian journal of medical and biological research. 2012;45(4):292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proceedings of the National Academy of Sciences. 2012;109(4):1305–1310. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry. 2012;18:742–3. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Werner A. The use of proton magnetic resonance spectroscopy in PTSD research—meta-analyses of findings and methodological review. Neuroscience & Biobehavioral Reviews. 2010;34(1):7–22. doi: 10.1016/j.neubiorev.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience & Biobehavioral Reviews. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological psychiatry. 2008;63(6):550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 3–4. [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, … Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. The American journal of psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. Journal of affective disorders. 2006;90(2):171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of affective disorders. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, … Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature neuroscience. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Duncan LE, Liberzon I, Ressler KJ. From Candidate Genes to Genome-wide Association: The Challenges and Promise of Posttraumatic Stress Disorder Genetic Studies. Biological Psychiatry. 2013;74:634–6. doi: 10.1016/j.biopsych.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: Review and recommendations for future studies. Journal of traumatic stress. 2007;20(5):737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. American journal of epidemiology. 2009 doi: 10.1093/aje/kwn397. kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, … Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of general psychiatry. 2005;62(11):1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, … Tsuang MT. A high risk twin study of combat-related PTSD comorbidity. Twin Research. 2003;6(3):218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, Dominique JF. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val 158 Met polymorphism. Biological psychiatry. 2010;67(4):304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Kolassa I, Ertl V, Eckart C, Glöckner F, Kolassa S, Papassotiropoulos A, Elbert T. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. 2010 doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- Kormos V, Gaszner B. Role of neuropeptides in anxiety, stress, and depression: From animals to humans. Neuropeptides. 2013;47:401–19. doi: 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics and Genomics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. The American journal of psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, … Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071(1):87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, … Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biological psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, Uylings HB, Olff M, Carlier IV, … Gersons BP. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biological Psychiatry. 2004;56(11):853–861. doi: 10.1016/j.biopsych.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, … Miller MW. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular psychiatry. 2013;18(8):937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, Kim DJ. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma: longitudinal brain imaging study among survivors of the South Korean subway disaster. Archives of general psychiatry. 2011;68(7):701–713. doi: 10.1001/archgenpsychiatry.2011.70. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neurosci. 2012;35(1):24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen SA, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Molecular psychiatry. 2007;12(5):421–421. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, … Binder EB. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Archives of general psychiatry. 2011;68(9):901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KB, Orcutt HK, Quinn JF, Fitzgerald CA, Conneely KN, Barfield RT, … Ressler KJ. Acute and posttraumatic stress symptoms in a prospective gene× environment study of a university campus shooting. Archives of general psychiatry. 2012;69(1):89–97. doi: 10.1001/archgenpsychiatry.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, Jovanovic T. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol Psychiatry. 2015;78(5):344–53. doi: 10.1016/j.biopsych.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morey R, Hariri A, Gold A, Hauser M, Munger H, Dolcos F, et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC psychiatry. 2011;11:76–89. doi: 10.1186/1471-244X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslobodsky MS, Glicksohn J, Singer J, Stern M, Bar-Ziv J, Friedland N, Bleich A. Changes of brain anatomy in patients with posttraumatic stress disorder: a pilot magnetic resonance imaging study. Psychiatry research. 1995;58(3):259–264. doi: 10.1016/0165-1781(95)02708-5. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, … Madden PA. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Molecular psychiatry. 2009;14(3):234. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Mustapic M, Yurgil KA, Schork NJ, Miller MW, … Baker DG. Genomic predictors of combat stress vulnerability and resilience in US Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in cognitive sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of cognitive neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, … Yamadori A. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19(4):1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Social Science and Medicine. 1988;26:327–332. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- Peterson A, Thome J, Frewen P, Lanius RA. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can J Psychiatry. 2014;59(6):294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A, Frans Ö, Fernandez M, von Knorring L, Fischer H, Fredrikson M. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. European archives of psychiatry and clinical neuroscience. 2002;252(2):68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, … Liberzon I. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, … Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rasetti R, Weinberger DR. Intermediate phenotypes in psychiatric disorders. Current opinion in genetics & development. 2011;21(3):340–348. doi: 10.1016/j.gde.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, … Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, … Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Resnick HS, et al. Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry. 1995;152:1675–1681. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, … May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L, Waters CE, Eccles J, Garside H, Sommer P, Kay P, et al. Identification and functional analysis of SKA2 interaction with the glucocorticoid receptor. J Endocrinol. 2008;198:499–509. doi: 10.1677/JOE-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont–Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron–Milad K, Rodriguez–Romaguera J, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS neuroscience & therapeutics. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, … Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS neuroscience & therapeutics. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Spielberg JM, Logue MW, Wolf EJ, Smith AK, Lusk J, Miller MW. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. The Journal of Neuroscience. 1990;10(9):2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapas C, Cai G, Bierer LM, Golier JA, Galea S, Ising M, … Yehuda R. Genetic markers for PTSD risk and resilience among survivors of the World Trade Center attacks. Disease markers. 2011;30(2–3):101–110. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. General and comparative endocrinology. 2001;124(2):152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Schechter D, Moser D, Paoloni-Giacobino A, Stenz L, Gex-Fabry M, Aue T, et al. Methylation of NR3C1 is related to maternal PTSD, parenting stress and maternal medial prefrontal cortical activity in response to child separation among mothers with histories of violence exposure. Frontiers in Psychology. 2015;6:1–12. doi: 10.3389/fpsyg.2015.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, … Weiner MW. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2008;162(2):147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Heik RJ, Schaer M, Eliez S, Hallmayer JF, Lin X, Kaloupek DG, Woodward SH. Catechol-O-methyltransferase Val158Met polymorphism moderates anterior cingulate volume in posttraumatic stress disorder. Biological psychiatry. 2011;70(11):1091–1096. doi: 10.1016/j.biopsych.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, … Kawashima R. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Molecular psychiatry. 2013;18(5):618–623. doi: 10.1038/mp.2012.51. [DOI] [PubMed] [Google Scholar]
- Shen S, Gehlert DR, Collier DA. PACAP and PAC1 receptor in brain development and behavior. Neuropeptides. 2013;47:421–30. doi: 10.1016/j.npep.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, … Pitman RK. A positron emission tomographic study of symptom provocation in PTSD. Annals of the New York Academy of Sciences. 1997;821(1):521–523. doi: 10.1111/j.1749-6632.1997.tb48320.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. American Journal of Psychiatry. 1999 doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, … Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontalcortex during traumatic imagery in male and female vietnam veterans with ptsd. Archives of general psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071(1):67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, … Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of general psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological psychiatry. 2008;64(8):681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: new pathways and new thinking. Neuropharmacology. 2012;62(2):628–637. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 2005;15(6):798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, … Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of cognitive neuroscience. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992;99(2):195. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stark EA, Parsons CE, Van Hartevelt TJ, Charquero-Ballester M, McManners H, Ehlers A, Stein A, Kringelbach ML. Post-traumatic stress influences the brain even in the absence of symptoms: A systematic, quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2015;56:207–21. doi: 10.1016/j.neubiorev.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological medicine. 1997;27(04):951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, … Ressler KJ. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences. 2014;111(8):3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Simmons AN, Matthews SC, Grimes EM, Allard CB, Reinhardt LE, … Stein MB. Neural correlates of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biological psychiatry. 2010;68(5):442–450. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of general psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val 158 Met and beyond. Biological psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Valente NLM, Vallada H, Cordeiro Q, Bressan RA, Andreoli SB, Mari JJ, Mello MF. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. Journal of molecular neuroscience. 2011;43(3):516–523. doi: 10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. PharmacolRev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Wang Z, Baker DG, Harrer J, Hamner M, Price M, Amstadter A. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depression and anxiety. 2011;28(12):1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, … Schuff N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Archives of general psychiatry. 2010;67(3):296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. Oct, 1993. [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, … Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29(2):347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, … True WR. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug and alcohol dependence. 2000;61(1):95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, … Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of general psychiatry. 2009;66(11):1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, Gelernter J. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biological psychiatry. 2013;74(9):656–663. doi: 10.1016/j.biopsych.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neuroscience letters. 2004;370(1):13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Yehuda R, et al. Predicting the development of posttraumatic stress dis- order from the acute response to a traumatic event. Biol Psychiatry [review] 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the New York Academy of Sciences. 2006;1071(1):137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, Bierer LM. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Frontiers in psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. American Journal of Psychiatry. 1993;150:83–83. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, WAHBY V, GILLER EL, Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. The Journal of nervous and mental disease. 1990;178(6):366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Young SN. How to increase serotonin in the human brain without drugs. Journal of psychiatry & neuroscience: JPN. 2007;32(6):394–399. [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene Stress Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology Reviews. 2016;41(1):261–274. doi: 10.1038/npp.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]