Abstract
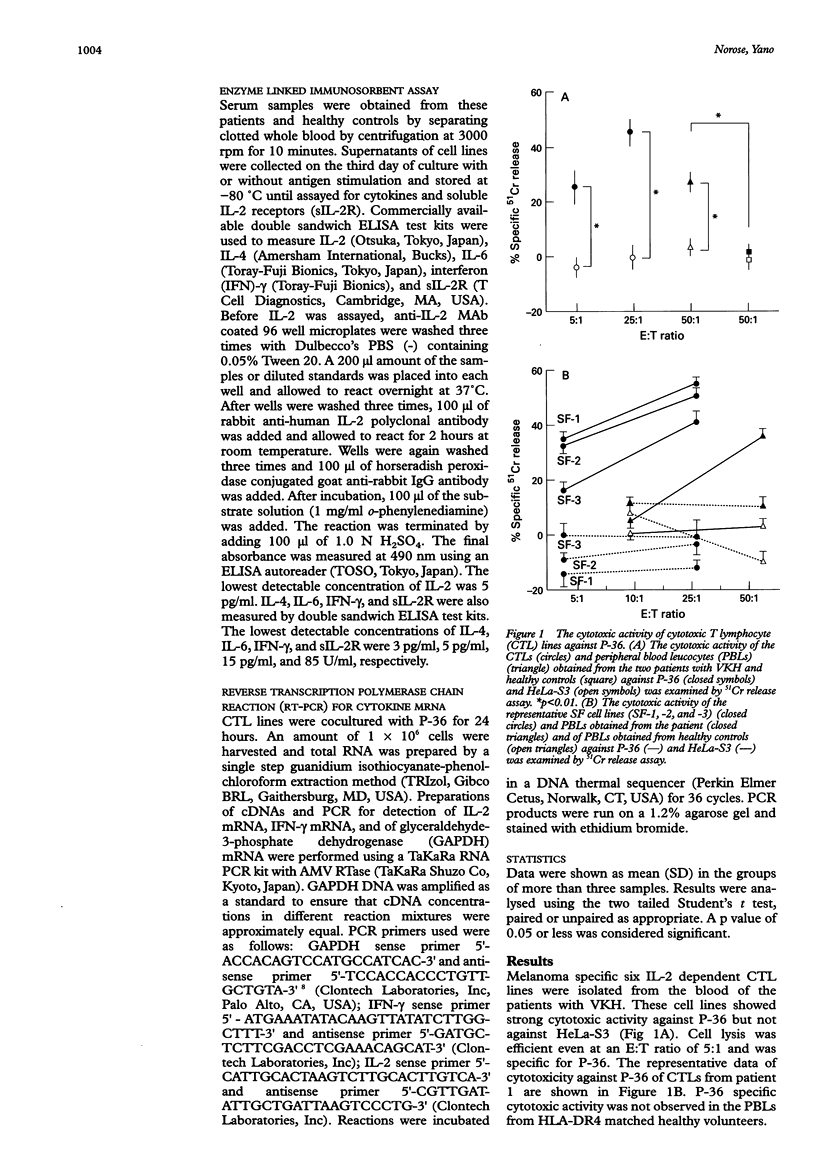
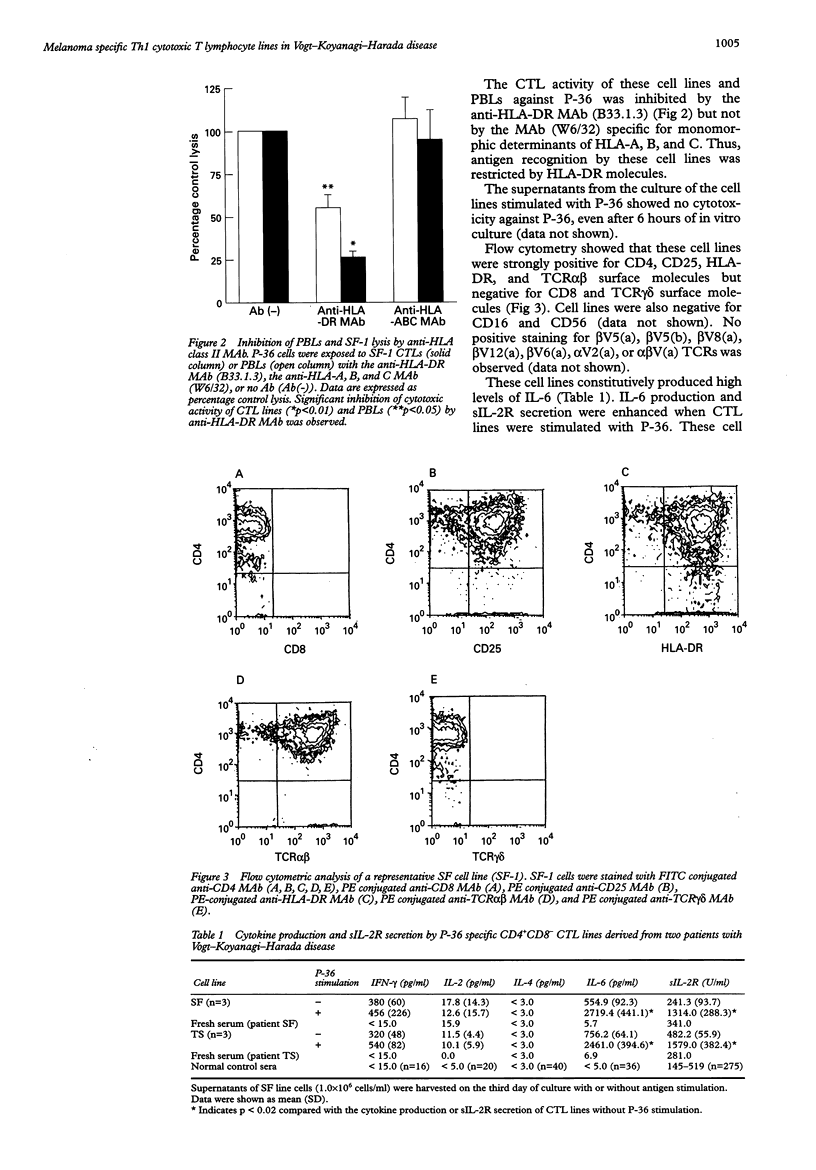
AIMS/BACKGROUND: To determine the functional properties and cytokine production profiles of melanoma specific cytotoxic T lymphocytes (CTLs) induced from peripheral blood leucocytes of two patients with Vogt-Koyanagi-Harada disease (VKH). METHODS: Melanoma specific CTL lines were established by long term coculture with a human melanoma cell line (P-36). Cytotoxic activity against P-36 was measured by 51Cr release. The involvement of human leucocyte antigen (HLA) class I or class II molecules in the cytotoxicity of the CTL lines against P-36 was analysed using anti-HLA class I or anti-HLA class II monoclonal antibody (MAb). Surface molecules of CTL lines were analysed by flow cytometry using MAbs specific for CD4, CD8, CD16, CD25, CD56, HLA-DR, T cell antigen receptor (TCR) alpha beta and TCR gamma delta. Cytokine production and soluble interleukin 2 receptor (sIL-2R) secretion were determined by enzyme linked immunosorbent assays. mRNAs of cytokines were analysed using reverse transcription polymerase chain reaction (RT-PCR). RESULTS: CTLs showed strong cytotoxic activity against P-36. The CTL activity of the cell lines against P-36 was inhibited by the anti-HLA-DR MAb, whereas the MAb specific for monomorphic determinants of HLA-A, B, and C failed to block lytic activity. Flow cytometry identified the following surface molecules: CD4+, CD8-, CD16-, CD25+, CD56-, HLA-DR+, TCR alpha beta +, and TCR gamma delta-. CTLs constitutively produced a high level of IL-6. IL-6 production and sIL-2R secretion of CTLs were enhanced when CTLs were stimulated with P-36. CTLs also produced high levels of interferon gamma (IFN-gamma) and IL-2, but not IL-4. mRNAs of IL-2 and IFN-gamma were detected by RT-PCR in the CTLs. CONCLUSIONS: Melanoma specific HLADR restricted T helper 1 (Th1) CTLs may play a role in the immunopathogenesis of VKH.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcari P., Martinelli R., Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984 Dec 11;12(23):9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalla L., Linker-Israeli M., Steinman L., Rao N. A. Inhibition of autoimmune uveitis by anti-CD4 antibody. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1264–1270. [PubMed] [Google Scholar]
- Chen Q., Hersey P. MHC-restricted responses of CD8+ and CD4+ T-cell clones from regional lymph nodes of melanoma patients. Int J Cancer. 1992 May 8;51(2):218–224. doi: 10.1002/ijc.2910510209. [DOI] [PubMed] [Google Scholar]
- Darrow T. L., Slingluff C. L., Jr, Seigler H. F. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. Evidence for shared tumor antigens. J Immunol. 1989 May 1;142(9):3329–3335. [PubMed] [Google Scholar]
- De Vos A. F., Hoekzema R., Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992 Jun;11(6):581–597. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984 Mar 22;308(5957):365–367. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- Hayward A., Boylston A., Beverley P. Lysis of CD3 hybridoma targets by cloned human CD4 lymphocytes. Immunology. 1988 May;64(1):87–92. [PMC free article] [PubMed] [Google Scholar]
- Hersey P., MacDonald M., Schibeci S., Burns C. Clonal analysis of cytotoxic T lymphocytes (CTL) against autologous melanoma. Classification based on phenotype, specificity and inhibition by monoclonal antibodies to T cell structures. Cancer Immunol Immunother. 1986;22(1):15–23. doi: 10.1007/BF00205711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., McAllister C. G., Mittal K. K., Vistica B. P., Shinohara T., Gery I. A cell line and clones of lymphocytes from a healthy donor, with specificity to S-antigen. Invest Ophthalmol Vis Sci. 1988 Nov;29(11):1636–1641. [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Kawakami Y., Zakut R., Topalian S. L., Stötter H., Rosenberg S. A. Shared human melanoma antigens. Recognition by tumor-infiltrating lymphocytes in HLA-A2.1-transfected melanomas. J Immunol. 1992 Jan 15;148(2):638–643. [PubMed] [Google Scholar]
- Krensky A. M., Clayberger C., Reiss C. S., Strominger J. L., Burakoff S. J. Specificity of OKT4+ cytotoxic T lymphocyte clones. J Immunol. 1982 Nov;129(5):2001–2003. [PubMed] [Google Scholar]
- Maccalli C., Mortarini R., Parmiani G., Anichini A. Multiple sub-sets of CD4+ and CD8+ cytotoxic T-cell clones directed to autologous human melanoma identified by cytokine profiles. Int J Cancer. 1994 Apr 1;57(1):56–62. doi: 10.1002/ijc.2910570111. [DOI] [PubMed] [Google Scholar]
- Maezawa N., Yano A. Two distinct cytotoxic T lymphocyte subpopulations in patients with Vogt-Koyanagi-Harada disease that recognize human melanoma cells. Microbiol Immunol. 1984;28(2):219–231. doi: 10.1111/j.1348-0421.1984.tb00673.x. [DOI] [PubMed] [Google Scholar]
- Mauri D., Wyss-Coray T., Gallati H., Pichler W. J. Antigen-presenting T cells induce the development of cytotoxic CD4+ T cells. I. Involvement of the CD80-CD28 adhesion molecules. J Immunol. 1995 Jul 1;155(1):118–127. [PubMed] [Google Scholar]
- McClellan K. A., MacDonald M., Hersey P., Billson F. A. Vogt-Koyanagi-Harada syndrome--isolation of cloned T cells with specificity for melanocytes and melanoma cells. Aust N Z J Ophthalmol. 1989 Nov;17(4):347–352. doi: 10.1111/j.1442-9071.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Kuwabara T., McAllister C., Nussenblatt R. B., Gery I. Adoptive transfer of experimental autoimmune uveoretinitis in rats. Immunopathogenic mechanisms and histologic features. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):1–9. [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Norose K., Yano A., Aosai F., Segawa K. Immunologic analysis of cerebrospinal fluid lymphocytes in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1210–1216. [PubMed] [Google Scholar]
- Paul W. E., Sredni B., Schwartz R. H. Long-term growth and cloning of non-transformed lymphocytes. Nature. 1981 Dec 24;294(5843):697–699. doi: 10.1038/294697a0. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98(4):279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Murata T., Inomata H. Class II major histocompatibility complex on melanocytes of Vogt-Koyanagi-Harada disease. Arch Ophthalmol. 1991 Sep;109(9):1270–1274. doi: 10.1001/archopht.1991.01080090096030. [DOI] [PubMed] [Google Scholar]
- Skibber J. M., Lotze M. T., Uppenkamp I., Ross W., Rosenberg S. A. Identification and expansion of human lymphokine-activated killer cells: implications for the immunotherapy of cancer. J Surg Res. 1987 Jun;42(6):613–621. doi: 10.1016/0022-4804(87)90004-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Zeh H. J., 3rd, Maeurer M. J., Salter R. D., Lotze M. T. Identification of human melanoma peptides recognized by class I restricted tumor infiltrating T lymphocytes. J Immunol. 1993 Oct 1;151(7):3719–3727. [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Rosenberg S. A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989 May 15;142(10):3714–3725. [PubMed] [Google Scholar]
- Vose B. M., Bonnard G. D. Human tumour antigens defined by cytotoxicity and proliferative responses of cultured lymphoid cells. Nature. 1982 Mar 25;296(5855):359–361. doi: 10.1038/296359a0. [DOI] [PubMed] [Google Scholar]
- Wang X. C., Norose K., Yano A., Ohta K., Segawa K. Two-color flow cytometric analysis of activated T lymphocytes in aqueous humor of patients with endogenous vs. exogenous uveitis. Curr Eye Res. 1995 Jun;14(6):425–433. doi: 10.3109/02713689509003752. [DOI] [PubMed] [Google Scholar]
- Wölfel T., Klehmann E., Müller C., Schütt K. H., Meyer zum Büschenfelde K. H., Knuth A. Lysis of human melanoma cells by autologous cytolytic T cell clones. Identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J Exp Med. 1989 Sep 1;170(3):797–810. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. H., Aosai F., Norose K., Ueda M., Yano A. Differential regulation of HLA-DR expression and antigen presentation in Toxoplasma gondii-infected melanoma cells by interleukin 6 and interferon gamma. Microbiol Immunol. 1996;40(6):443–449. doi: 10.1111/j.1348-0421.1996.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Yang T. H., Aosai F., Norose K., Ueda M., Yano A. Enhanced cytotoxicity of IFN-gamma-producing CD4+ cytotoxic T lymphocytes specific for T. gondii-infected human melanoma cells. J Immunol. 1995 Jan 1;154(1):290–298. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- de Smet M. D., Yamamoto J. H., Mochizuki M., Gery I., Singh V. K., Shinohara T., Wiggert B., Chader G. J., Nussenblatt R. B. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am J Ophthalmol. 1990 Aug 15;110(2):135–142. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- de Waard-Siebinga I., Creyghton W. M., Kool J., Jager M. J. Effects of interferon alfa and gamma on human uveal melanoma cells in vitro. Br J Ophthalmol. 1995 Sep;79(9):847–855. doi: 10.1136/bjo.79.9.847. [DOI] [PMC free article] [PubMed] [Google Scholar]



