Abstract
Background
Aspirin-exacerbated respiratory disease (AERD) differs from aspirin tolerant disease due in part to eosinophilic tissue infiltration and over-expression of arachidonic acid metabolic pathway components that lead to enhanced secretion of cysteinyl leukotrienes (CysLT) and prostaglandin D2 (PGD2) observed constitutively and, paradoxically, in response to aspirin and other cyclooxygenase inhibitors. We have previously demonstrated the capacity of interferon (IFN)-γ to drive CysLT expression and response.
Objective
We investigated eosinophils as a source for PGD2 production in AERD.
Methods
Eosinophils were enriched from tissue and peripheral blood obtained from control, aspirin tolerant, and AERD subjects. mRNA was extracted and evaluated for expression of hematopoietic PGD synthase (hPGDS). Expression of hPGDS protein was confirmed with western hybridization and immunofluorescence staining. Cells were stimulated with aspirin and secretion of PGD2 quantified. CD34+ progenitor cells were isolated and matured into eosinophils in the presence or absence of IFN-γ and hPGDS mRNA and PGD2 release measured.
Results
Gene expression analysis revealed that eosinophils from AERD tissue and blood display increased levels of hPGDS compared with asthmatic and control samples. Western hybridization confirmed the increase in hPGDS mRNA translated to increased protein expression. Immunofluorescence confirmed mast cells and eosinophils from AERD and asthmatic tissue demonstrated hPGDS expression with higher levels in AERD eosinophils. Incubation of eosinophils from blood and tissue with aspirin stimulated PGD2 release. IFN-γ-matured eosinophil progenitors showed enhanced hPGDS expression and increased levels of PGD2 release at baseline and following stimulation with aspirin.
Conclusions
In addition to mast cells, eosinophils represent an important source of PGD2 in AERD and identify a new target for therapeutic intervention.
Clinical Implications
Demonstration of eosinophils as a source of PGD2, that has been shown to be involved in the presence and severity of AERD, identifies a new target for therapeutic intervention.
Keywords: aspirin exacerbated respiratory disease, aspirin tolerant asthma, chronic sinusitis, cytokines, eosinophils, prostaglandin D2, nasal polyps
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a disease of the upper (chronic rhinosinusitis (CRS)/nasal polyposis (NP)) and (usually) the lower (asthma) airways that differs from aspirin tolerant asthma/chronic sinusitis by the unique sensitivity to aspirin and other non-selective cyclooxygenase (COX)-1 inhibitors. AERD comprises as many as 7% of adult-onset asthmatics and up to 12-14% of adult asthmatics with severe asthma 1, 2. During aspirin reactions, many mediators are released including cysteinyl leukotrienes (CysLT), tryptase, ECP and prostaglandin D2 (PGD2) suggesting both mast cell and eosinophil activation 3-5.
In AERD, baseline levels of PGD2 and its metabolites are higher in the blood than in subjects who are aspirin tolerant and following ingestion of aspirin these levels further increase in AERD subjects 5, 6. Aspirin desensitization followed by continual high-dose aspirin therapy is a treatment option for AERD 7 and if the subjects tolerate the desensitization they benefit from improved disease control. However, not all patients with AERD can be desensitized, and this group that fails desensitization is distinguished by their constitutive overproduction and aspirin-stimulated release of PGD2 that correlates with the severity of airflow obstruction 6. In addition to defining a more severe subgroup that fails aspirin desensitization, PGD2 likely contributes to the severity through its myriad of biological activities that includes inducing vasodilation and vascular leakage, bronchoconstriction, and the recruitment and activation of basophils, eosinophils, dendritic cells, and both Th2-like lymphocytes and type 2 innate lymphoid cells 8-11.
While both eosinophils and mast cells are critical to AERD pathogenesis, an important role for eosinophils is supported by observations made by our group 12, 13 and others 14-16 that tissue from AERD subjects express ~3-fold greater numbers of tissue eosinophils and eosinophilcationic protein (ECP) compared to aspirin tolerant subjects. PGD2 has historically been thought of as a mast cell product, however eosinophils can express hematopoietic prostaglandin D2 synthase (hPGDS), the enzyme responsible for PGD2 synthesis, and eosinophils do secrete PGD2 17. Given that eosinophil expression greatly exceeds that of mast cells in AERD it is reasonable to speculate that eosinophils maybe also be an important source of PGD2. Expression of hPGDS has been observed in NP of CRS patients 18 and one report suggested that a subpopulation of eosinophils in the NP were one source of the enzyme 19.
The current studies were therefore performed to assess the production of PGD2 by eosinophils in AERD. In particular, we investigated increased expression of hPGDS in NP of AERD subjects and whether this increase was associated with eosinophils. Recently, aspirin was shown by our research group to directly activate eosinophils and potentiate mediator release 20. We therefore investigated PGD2 release when AERD eosinophils were stimulated with aspirin. In addition, we have demonstrated that interferon (IFN)-γ uniquely contributes to the development and differentiation of eosinophils, enhancing their ability to produce and release mediators including cysteinyl leukotrienes and eosinophil derived neurotoxin 21. We therefore also queried whether a similar result would be observed with expression of hPGDS and subsequent PGD2 release from eosinophil progenitors matured in the presence of this cytokine.
Methods
Subjects
Nasal polyp tissue was obtained from subjects referred to the University of Virginia Health System for sinus surgery under a protocol approved by the University of Virginia Institutional Review Board. Control tissue was harvested from the sinus cavities of patients undergoing surgery that required access to their paranasal sinuses for reasons other than chronic sinusitis (e.g. orbital decompression, cerebrospinal fluid leak repair, or transphenoidal pituitary surgery). Depending upon the quantity of tissue available, specimens were divided and used for subsets of the various experimental procedures outlined below. Eosinophilic sinusitis (chronic hyperplastic eosinophilic sinusitis (CHES)) was histologically defined as previously described based upon the presence of ≥5 eosinophils/400x high powered field 1. AERD was defined by a compelling history involving a hypersensitivity reaction within 2-3 hrs of ingestion of either aspirin or another non-steroidal anti-inflammatory drug. NPs were obtained from 26 subjects including 17 with CHES and 9 with AERD and, additionally, control tissue was obtained from 9 subjects. For studies involving blood eosinophils, 10 AERD, 12 asthmatic and 15 non-asthmatic controls were enrolled though all subjects were not involved with every experiment: see individual figures for exact number. Subject characteristics for those involved in the blood draw are show in the online supplement (Table E1).
Immunofluorescence and anti-tryptase staining
Polyp tissue was fixed in 4% paraformaldehyde, paraffin embedded and sectioned by the Histology Core Laboratory of the University of Virginia. Samples were deparaffinized and hydrated to distilled water. For immunofluorescence, heat-induced antigen retrieval was performed by heating sections for 20 min in citrate buffer (Abcam; Cambridge, MA). Slides were washed and blocked using 1% bovine serum albumin, 10% goat serum (Sigma, St. Louis, MO) and Fc Block 1 μg (BD Pharmingen; Sparks, MD) for 2 hrs. Specific staining for hPGDS was performed using a 1:150 dilution of a rabbit anti-human hPGDS antibody (Abcam ab89709) for 16 hrs at 4° C. Sections were rinsed and then incubated with secondary allophycocyanin goat anti-rabbit IgG (1:200, Life Technologies; Grand Island, NY) for 1 hour at room temperature. Nuclei were stained with 100 ng/ml DAPI (4’, 6-diamidino-2-phenylindole, Sigma) for 30 min at room temperature. Samples were washed in phosphate buffered saline and aqueous mounted with VectaMount AQ (Vector Laboratories; Burlington, CA). The samples were analyzed using a Zeiss AxioImager Z2 equipped with Apotome for optical sectioning (Zeiss; Thornwood, NY) and hPGDS positive cells scored in a blinded fashion. For anti-tryptase staining, rehydrated slides were treated with 3% peroxidase for 5 min followed by antigen retrieval (Dako target retrieval solution pH 9 (Dako, Denmark)). Slides were incubated overnight at 4° C with a 1:500 dilution of anti-human mast cell tryptase clone AA1 (Dako). Development of the slides was performed using the Envision+System-HRP (AEC) (Dako) with labeled polymer added for 30 min and chromogen for 5 min followed by counterstaining with Gills hematoxylin (Sigma) for 3 min. Tryptase positive cells were scored in a blinded fashion taking the average of 10 fields per slide.
Eosinophil Isolation
Eosinophils were enriched from peripheral blood by Ficoll-Hypaque (Sigma) density centrifugation followed by dextran sedimentation and hypotonic lysis. Eosinophils were enriched from granulocytes using negative magnetic affinity column purification (CD16−; Miltenyi Biotec; San Diego, CA) and were greater than 95% pure as measured by flow cytometry 22.
Western hybridization of cellular extracts
Eosinophils were lysed and equal amounts of protein were added to 5X Laemmli Sample Buffer (GeneScript; Piscataway, NJ) and heated for 5 min at 95° C. Samples were electrophoresed on 12% TruPAGE precast gels (Sigma) and transferred to a polyvinylidene difluoride membrane (Millipore; Bilerica, MA) for 18 hrs at 40V. The membrane was blocked with TBST buffer (50 mM Tris-HCl pH 7.6, 150 mM NaCl, 0.05% Tween 20) with 5% non-fat dry milk before addition of anti-hPGDS antibodies (Abcam). The target protein was visualized with the addition of the goat anti-rabbit horseradish peroxidase secondary antibody (Bio-Rad; San Jose, CA), after which blots were developed using the Immobilon Chemiluminescent kit (Millipore). After primary analysis, blots were stripped with 100 mM glycine and reprobed with an antibody to ß-actin. Analysis of blots was performed via densitometry (GS-800 Calibrated Densitometer (Bio-Rad)) using the ratio of hPGDS to ß-actin calculated with ImageQuant TL v2005 (Amersham Biosciences, Pittsburgh, PA).
Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed on NP tissue, peripheral blood eosinophils and on newly differentiated eosinophils for hPGDS. For the NP studies, polyps were minced and digested with Accutase (Innovative Cell Technologies, San Diego, CA) for 1 hr at 37 °C and the leukocyte-containing fraction was collected by passing the cell suspension through a 70 μm nylon mesh strainer (BD Falcon, Bedford, MA). Eosinophils were enriched using magnetic affinity column purification with positive selection for CD15 followed by negative selection for CD16 (Miltenyi Biotec; San Diego, CA) and were >90% pure and viable as measured by flow cytometry. Total RNA was extracted using TRI® reagent (Sigma). Conversion of mRNA to cDNA was performed using a Taqman Reverse Transcription kit (Roche, Branchburg, NJ). Total RNA (200 ng) was added to each reaction along with oligo dT primers, 5.5 mM MgCl2, 2 mM dNTPs, RNasin, and reverse transcriptase. Reactions went through 10 min at 25° C, 30 min at 48° C and 5 min at 95° C in a Bio-Rad iCycler thermocycler (Bio-Rad). The PCR mix consisted of Sensimix (Bioline; Taunton, MA), cDNA and 200 μM of each primer. Data were analyzed as the change in CT of each cytokine transcript in comparison to either EF1α or ß-actin (as appropriate based upon concordance of their CT with that of the gene of interest). Primers for EF1α were purchased (SABiosciences; Frederick, MD). The primers for hPGDS were: sense 5’-GGGCAGAGAAAAACAAGATGT-3’ and antisense 5’-CCCCCCTAAATATGTGTCCAAG-3’ (Integrated DNA Technologies, Inc., Coralville, IA).
Eosinophil progenitor activation
Peripheral blood mononuclear cells (PBMCs) were isolated through Ficoll-Hypaque density centrifugation from blood obtained from 10 healthy volunteers. CD34+ cells were purified (>90%) from PBMCs using positive magnetic affinity column purification (CD34+; Miltenyi Biotec) and eosinophil progenitors were derived using the technique of Hudson et al. 23 by culturing purified CD34+ cells in complete medium (RPMI1640 and 10% FBS) supplemented with stem cell factor (SCF; 25 ng/ml; BD Biosciences), thymopoietin (TPO; 25 ng/ml; R&D System, Minneapolis, MN), Fms-like tyrosine kinase 3(Flt3) ligand (25 ng/ml; BD Biosciences), IL-3 (25 ng/ml; BD Biosciences) and IL-5 (25 ng/ml; BD Biosciences) with or without interferon (IFN)-γ (20 ng/ml; BD Bioscience) for 3 days and then cultured for an additional 3 weeks with just the IL-3 and IL-5 (again ±IFN-γ). Cells were washed and fresh media and cytokines were applied weekly. As previously described 21, these cells are phenotypically and functionally similar to purified blood eosinophils. When examined by flow cytometry, the cells were 3.1% dual positive for CCR3/Siglec-8 without IFN-γ and 17.7% dual positive for CCR3/Siglec-8 with IFN-γ. The cells were examined for expression of FcεRI and CD203c as markers of basophils and these markers were found on less than 3% of the cells. Morphologically the newly differentiated eosinophils had bi-lobed nuclei and granule staining 21. qPCR was performed as described above to measure the expression of hPGDS in the differentiated eosinophils.
Functional behavior of purified blood or newly differentiated eosinophils
Purified blood or eosinophils differentiated with or without IFN-γ were either left in a resting state or stimulated with lysine aspirin (LysASA (Sanofi-Aventis, Athens, Greece)) (1 and 10 mM) for 30 min. For studies involving pretreatment with COX-1 inhibitors, eosinophils were incubated for 30 min with either 10 mM ibuprofen sodium salt (Santa Cruz, Dallas, TX) or 0.1 mM SC-560 (Santa Cruz) after which cells were washed in PBS and stimulated with varying doses of LysASA for an additional 30 min. Supernatants were collected and assayed by enzyme immunoassay (EIA) for PGD2 (Cayman; Ann Arbor, MI) according to the manufacturer's directions: lower limit of detection 55 pg/ml.
Statistical analyses
Data were contrasted between unstimulated and stimulated cells using the Mann Whitney paired t-test for nonparametric data. Control, CHES and AERD cohorts were compared using nonparametric unpaired t-tests. Statistical analyses were performed using GraphPad Prism 6 (La Jolla, CA).
Results
Differential expression of hPGDS in sinus tissue
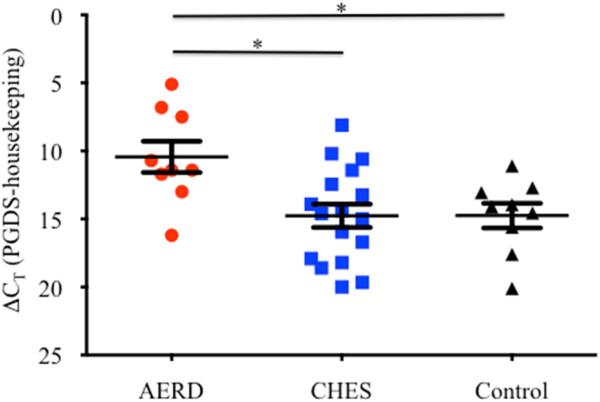
NP tissue from AERD (n=9) and CHES subjects (n=17) along with control sinus tissue (n=9) was digested and RNA extracted. Expression of hPGDS was measured by qPCR (Figure 1) and ΔCT for AERD was 4.34 and 4.33 cycles earlier than the housekeeping gene EF1α for CHES and control groups, respectively, reflecting that hPGDS transcript expression was ~20 fold higher (p<0.02) in the AERD group.
Figure 1. hPGDS gene expression in sinus tissue by qPCR.
Tissue samples were homogenized following surgical removal and RNA isolated. Transcript levels of hPGDS were quantified using PCR with sybr-green detection. Data (mean±SEM) reflect relative expression of each gene in comparison to the housekeeping gene EF1α (ΔCT). Control samples (n=9) are depicted in black triangles, CHES (n=17) in blue squares and AERD (n=9) in red circles. *p<0.02 as compared to CHES and Control.
Immunofluorescence staining of hPGDS in nasal polyps
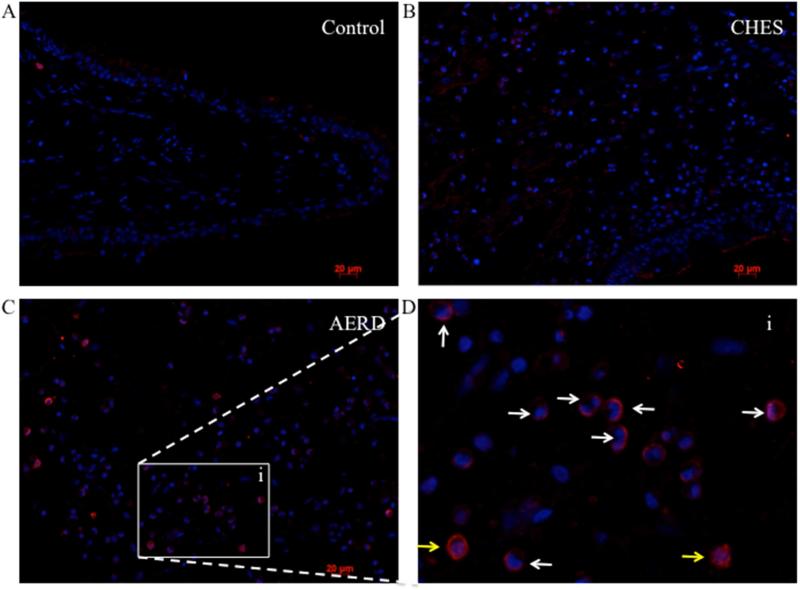
To confirm the post-translational expression of hPGDS and to define the cellular source, we performed immunofluorescence staining on tissue samples from AERD, CHES and control tissue. Representative images are shown in Figure 2 and isotype control is displayed in supplementary Figure E1. Essentially no hPGDS staining was observed in control tissue, largely reflecting the absence of inflammatory cells (Figure 2A). Moderate levels of hPGDS were detected in CHES samples (Figure 2B) with the highest levels found in AERD tissue (Figure 2C). As shown in the insert (Figure 2D), most of these cells were identified as eosinophils by being granulocytes with bi-lobed nuclei (white arrows) or alternatively were mononuclear cells without visualizable granules, most likely mast cells (yellow arrows). The average number of hPGDS expressing cells per high-powered field (hpf) was determined (Figure E2). The highest numbers were found in AERD, with the majority of cells identified as eosinophils consistent with our previous studies 12. To confirm that the majority of cells observed as staining positive for hPGDS were eosinophils, mast cell numbers in tissue sections were counted. Staining for mast cell tryptase was highest in control tissue 13.6±1.6 cells/hpf, followed by CHES 11.1±1.5 cells/hpf and AERD 6.8±2.3 cells/hpf. These results support the immunofluorescence data showing eosinophils as being the most numerous cells expressing hPGDS in polyp tissue.
Figure 2. Immunofluorescence for hPGDS in AERD and CHES polyps.
PGDS staining of paraffin embedded sinus tissue using a primary antibody directed against hPGDS and an APC-labeled secondary antibody (red) with DAPI nuclear stain (blue). A. Control tissue. B. CHES tissue. C. AERD tissue. D. insert (i) of AERD tissue showing a close-up view. White arrows indicate eosinophils and yellow arrows indicate mononuclear cells.
Expression of hPGDS from eosinophils
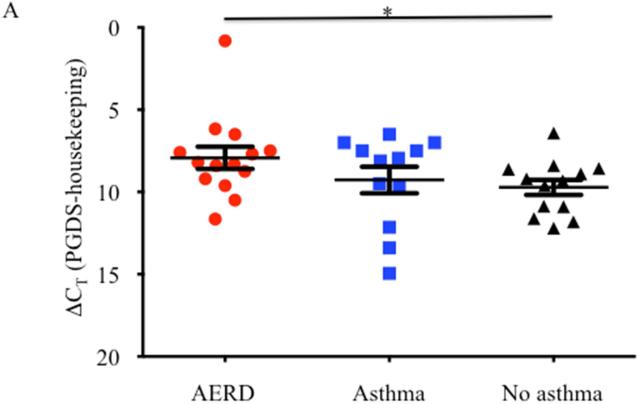
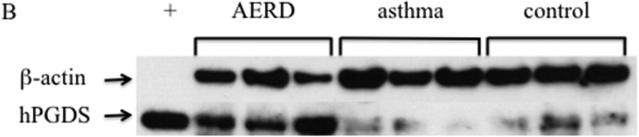
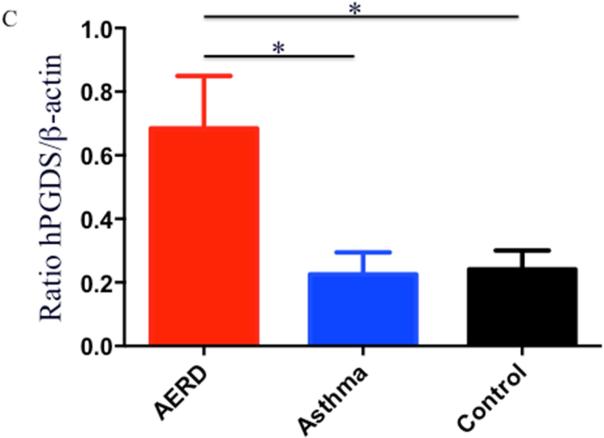
Eosinophils isolated from peripheral blood of AERD subjects (n=14) were examined for expression of hPGDS mRNA and found to have elevated levels (p<0.02) compared to healthy individuals (3.48-fold higher: n=13) or those with asthma (2.55-fold higher: n=12) (Figure 3A). Protein expression for hPGDS was confirmed via Western hybridization (Figure 3B) with higher levels observed in cells from the AERD subjects. Semi-quantitative analysis verified significantly higher levels (p<0.03) of hPGDS protein in eosinophils from AERD subjects as compared to those with asthma or controls without asthma (Figure 3C).
Figure 3. hPGDS expression in eosinophils.
After separation of blood using Ficoll-Hypaque density centrifugation, eosinophils were enriched using magnetic affinity column purification. A. Transcript levels of hPGDS were quantified using PCR with sybr-green detection. Data (mean±SEM) reflect relative expression of each gene in comparison to the housekeeping gene EF1α (ΔCT). Control samples (n=13) are depicted in black triangles, Asthma (n=12) in blue squares and AERD (n=14) in red circles. B. Measurement of hPGDS protein by Western hybridization. Eosinophils from three control, asthmatic and AERD subjects were collected and electrophoresed on a denaturing polyacrylamide gel and probed with rabbit antibody directed against hPGDS and ß-actin as a loading control. C. Semi-quantitative analysis of hPGDS protein levels. (AERD n=9, Asthma n=7, and Control n=10) *p<0.02 as compared to Control.
Release of PGD2 from eosinophils following aspirin activation
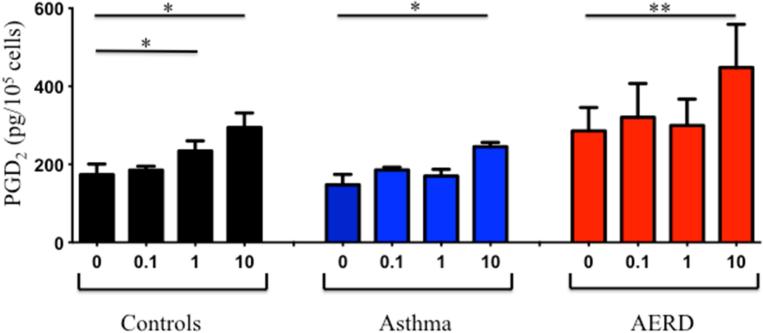
We have previously reported on the ability of aspirin to activate eosinophils directly to release mediators 20, however we did not determine whether or not PGD2 was released under these conditions. Eosinophils were purified from peripheral blood of healthy (n=7), asthmatic (n=6) and AERD subjects (n=9) and stimulated with various concentrations of LysASA. Supernatants were collected and PGD2 measured by ELISA. LysASA stimulated a dose-dependent release of PGD2 from all groups (Figure 4), but the highest levels of PGD2 measured were from AERD eosinophils (p<0.005). There were no between group differences in PGD2 release. The ability of tissue eosinophils to also release PGD2 was confirmed in a small number of samples by purifying eosinophils from sinus tissue and stimulating them ex vivo with LysASA. Similar to the blood eosinophils, AERD tissue eosinophils demonstrated similar trends with higher spontaneous levels of PGD2 release (1513 pg/105 AERD vs. 826 pg/105 CHES eosinophils) and higher levels of PGD2 release following stimulation with 10 mM LysASA (1941 pg/105 AERD vs. 1700 pg/105 CHES eosinophils).
Figure 4. PGD2 release from aspirin-activated eosinophils.
Eosinophils isolated from peripheral blood of control (black: n=7), asthmatic (blue: n=6) and AERD (red: n=9) subjects were activated with various doses of LysASA for 30 minutes. Supernatants were collected and PGD2 levels quantified (pg/105 cells). Data are presented as mean±SEM with *p<0.02 or **p<0.005 in comparison to unstimulated cells.
Influence of IFN-γ on hPGDS expression and PGD2 release following eosinophil differentiation
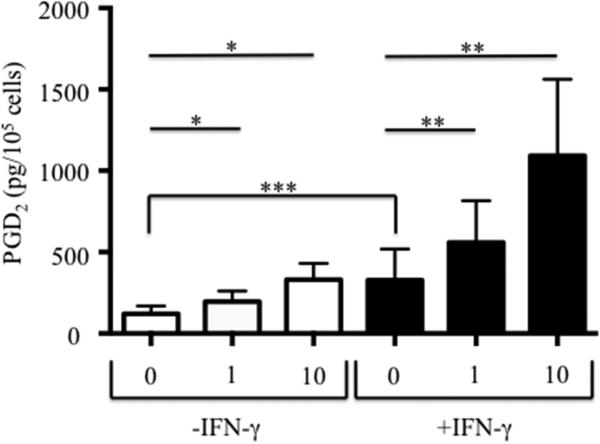
Eosinophils were derived from CD34+ hematopoietic stem cells as described with or without the additional presence of IFN-γ. Expression of mRNA for hPGDS was significantly increased 3.75-fold (p=0.03; n=12) when progenitors were co-incubated with IFN-γ (0.059±0.023 without IFN-γ to 0.220±0.072 with IFN-γ). Having demonstrated an increase in hPGDS, we investigated whether this would result in greater PGD2 release. Spontaneous levels of PGD2 release (Figure 5) were significantly higher in eosinophils (n=10) cultured with IFN-γ than those without (p<0.006) and LysASA stimulation showed a dose-dependent increase in the levels of PGD2 released (p<0.004) in both stimulated and unstimulated conditions.
Figure 5. PGD2 secretion by in vitro-differentiated eosinophils in the presence of IFN-γ.
CD34+-enriched hematopoietic stem cells were differentiated into eosinophils with or without the additional presence of IFN-γ. Newly generated eosinophils were activated for 30 minutes, supernatants collected, and PGD2 levels quantified (pg/105 cells). Data are presented as mean±SEM (n=10). *p<0.004 compared to 0-IFN-γ; **p<0.004 compared to 0+IFN-γ; ***p<0.006 compared to 0-IFN-γ.
Mechanism of PGD2 release following aspirin activation of eosinophils
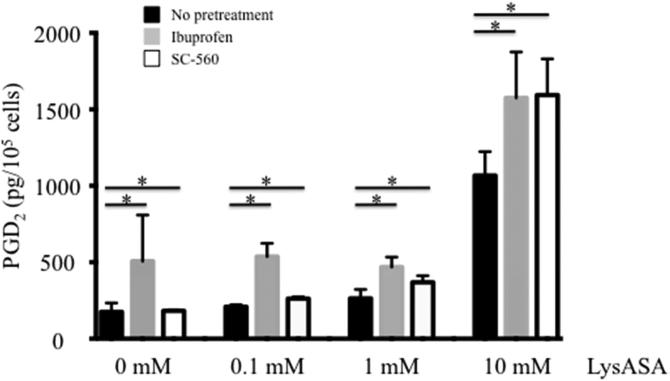
An unanswered question pertained to how PGD2 was being made following activation with LysASA, which should irreversibly inhibit COX-1. To test whether it was through a COX-1-dependent or -independent pathway, eosinophils were incubated with ibuprofen (10 mM) or SC-560 (0.1mM), both COX-1 inhibitors, for 30 min. Cells were washed to remove any excess compound and stimulated with varying doses of LysASA for an additional 30 min. Measurement of PGD2 in the supernatant demonstrated that COX-1 inhibition failed to prevent PGD2 release following LysASA stimulation (Figure 6; p<0.05).
Figure 6. COX-1 inhibition fails to prevent aspirin-induced PGD2 release.
Eosinophils isolated from peripheral blood were incubated with or without the COX-1 inhibitors, ibuprofen or SC-560 for 30 min. Cells were washed and incubated for 30 min with increasing concentrations of LysASA and supernatants collected for measurement of PGD2. Data are presented as mean±SEM (n=3) with *p=NS in comparison to no pretreatment cells.
Discussion
AERD is a disorder characterized by severe eosinophilic infiltrate into the sinus and respiratory tract, a baseline over-production of CysLTs and a unique sensitivity to LTE4 following ingestion of aspirin 1, 15, 24, 25. Expression of LTC4S in eosinophils drives much of the over-production of CysLTs 24 with additional components driven by the activation of mast cells 3, 5, 26, 27 and platelet-adherent neutrophils 28. While contributing to many of the untoward effects of AERD, as evinced by the beneficial effects of leukotriene modifiers 29, increasing attention has been given to contributions from PGD2. PGD2 and its metabolites are higher at baseline in AERD subjects in comparison to aspirin tolerant individuals 30, 31. Within the AERD patients, there exists a subgroup that has particularly enhanced overproduction and release of PGD2 during aspirin challenges that correlates with the severity of AERD and predicts failure of aspirin desensitization 6. In addition to defining a more severe subgroup, PGD2 likely contributes to the severity of AERD through its myriad of biological activities that include inducing vasodilation and vascular leakage as well as bronchoconstriction. Many stromal and inflammatory cells found in AERD tissue express receptors for PGD2, including eosinophils, basophils, mast cells, epithelium, endothelium, and dendritic cells 32-35. In addition to producing PGD2, eosinophils chemotax in response to PGD2 which thereby worsen tissue eosinophilia 32, 34. In a murine study PGD2 in conjunction with CCL11 enhanced eosinophil LTC4 production 36. PGD2 also activates basophils driving upregulated expression of CD203c and CD11b 37. On epithelium, PGD2 acts to alter their differentiation 35. Perhaps the most important potential impact of PGD2 in allergic inflammation is through its ability to recruit, activate, and promote cytokine secretion from both Th2-like lymphocytes and type 2 innate lymphoid cells 8, 9,10, 11.
PGD2 is primarily considered to be a mast cell-specific product, however it has been reported that eosinophils can both express hPGDS and release PGD2 17. Our studies and others demonstrate that eosinophils are more prevalent than mast cells in AERD tissue 12, 16. Thus, even if on a per cell basis mast cells do produce more PGD2 than eosinophils, it is likely that eosinophils may be the more important source of this arachidonate metabolite in AERD. This prompted us to examine the role of PGD2 production by eosinophils in AERD.
To answer the basic question regarding the ability of PGD2 to be produced by polyps, mRNA extracted and investigated for hPGDS expression. Similar to previous reports 18, 19 we found expression of hPGDS in NP of CRS patients (Figure 1). Importantly, the levels of hPGDS transcripts were significantly higher in the subjects with AERD (Figure 1). In addition, based on immunofluorescence staining of AERD polyp tissue, hPGDS protein expression was particularly evident in eosinophils, although with additional expression by a mononuclear cell population, presumably mast cells (Figure 2) and confirmed with anti-tryptase staining. We did observe hPGDS positive cells in the CHES group with again both eosinophils and mononuclear cells staining positive, however the overall numbers were less than in AERD (Figure E2A). Only occasional hPGDS-expressing cells were observed in control tissue and this was confined to mononuclear (presumably mast) cells. Of interest was the finding that mast cell numbers were highest in the control tissue followed by CHES and AERD. Thus, even though mast cells numbers were higher in the control tissue, they did not express high levels of hPGDS: prominent expression only became evident as the disease state increased in severity.
We next analyzed hPGDS expression by peripheral blood eosinophils from AERD, asthmatic and control subjects. On both the mRNA and protein levels, the highest levels of expression were observed in eosinophils from AERD subjects (Figure 3). We have published that eosinophils can be directly activated by aspirin to release CysLTs and ECP 20. This prompted us to query if aspirin activation would also stimulate PGD2 release. In a dose-dependent manner, aspirin triggered the release of PGD2 (Figure 4) with the highest levels released from AERD eosinophils. In addition to levels of expression of PGD2 synthase mRNA transcripts and protein, the capacity for a given eosinophil to secrete PGD2 almost certainly will be further influenced by post-translation mechanisms including the responsiveness of the cell to activation pathways, such as those signaled by aspirin itself. In addition, given the results of our studies 12 and others 14,15 that eosinophils are up to 10-fold more prevalent in AERD than aspirin tolerant sinus and lung tissue, eosinophil number is likely in vivo to be a prominent determinant of the capacity for PGD2 production.
Previously, we demonstrated a role for IFN-γ in accelerating the terminal maturation of eosinophils as indicated by their surface expression of CCR3 and Siglec-8 and release of eosinophil-derived products 21. These studies also demonstrated that IFN-γ was uniquely able to increase expression of LTC4S, an effect not mediated by any of the Th2 signature cytokines studied 21 and we further showed that this upregulation led to increased secretion of CysLTs when cells were stimulated with aspirin 20. We speculated that a similar result would be observed with hPGDS expression and PGD2 secretion from IFN-γ-matured progenitor cells. Our data demonstrated a significant increase in IFN-γ-driven hPGDS expression in eosinophil progenitors. In the absence of IFN-γ, the progenitors displayed a dose-dependent increase in PGD2 release following aspirin stimulation and the level of PGD2 released was augmented when the cells had been matured in the presence of IFN-γ (Figure 5. AERD is a mixed Th1/Th2 phenotype with robust upregulation of IFN-γ 21, 38. This unique environment, that defines the AERD tissue in which eosinophil progenitors are expressed and mature 39-41, generates cells primed for release of mediators that will exacerbate symptoms. Increased production and release of both CysLTs and PGD2 explains in part how ingestion of aspirin can lead to bronchoconstriction and airway hyperreactivity. It is interesting to speculate that the group of subjects reported by Cahill et al. that cannot be desensitized to aspirin due to overproduction of PGD2 may have progenitor and mature eosinophils that express high levels of hPGDS resulting in exaggerated PGD2 release when the cells are stimulated 6. These results again suggest that IFN-γ or STAT1 may be attractive therapeutic targets in AERD as they would target early progenitor cells preventing the conversion to cells capable of synthesizing large quantities of arachidonic metabolites.
While it may seem paradoxical that ingestion of cyclooxygenase inhibitors drives increased production of the cyclooxygenase product PGD2, this can be readily explained by the superseding influences of the direct activating effects of aspirin (and other cox inhibitors) on eosinophils and mast cells 5, 20, 42 combined with the robust influences of releasing these cells from the constraints provided by the anti-inflammatory prostaglandin, PGE2 43-45. It is intriguing to speculate the some of the beneficial influences of aspirin desensitization observed with long-term administration may be linked to inhibition of PGD2 expression. Reactions to aspirin and non-selective NSAIDs in AERD reflect COX-1 inhibition, whereas prostaglandin production by eosinophils (and mast cells) reflects the biological activity of COX-2 16, 46. Therefore, to address whether the mechanism of PGD2 production following aspirin stimulation was driven by COX-1 inhibition, we performed experiments in which eosinophils were preincubated with COX-1 inhibitors prior to aspirin activation and observed no reduction in (the COX-2-mediated) PGD2 release (Figure 6). Thus, the mechanism of aspirin-induced PGD2 secretion is driven by one of the alternative (COX-independent) pathways modulated by aspirin 47.
Despite the use of leukotriene pathway inhibitors and aspirin desensitization, AERD remains a disease with high morbidity 48. While IL-5 is not distinguishing feature of AERD when compared to aspirin-tolerant chronic sinusitis, it is clearly elevated compared to healthy controls and those with chronic sinusitis without polyps, suggesting it may yet be a useful target for eosinophilic sinusitis 49. Multiple biotherapeutic agents are in development or have clinical approval that target the eosinophil including anti-IL-5 monoclonal antibodies (mepolizumab and reslizumab) and anti-IL-5 receptor antibodies (benralizumab) 50-52. Given the particularly robust expression of infiltrating eosinophils in AERD tissue and the potential importance of eosinophil-derived mediators – including PGD2 – in driving the AERD phenotype, our results further support the concept that these agents are likely to be uniquely effective in severe asthmatics with AERD.
In summary, our studies demonstrate that eosinophils can synthesize PGD2 and that, in AERD, eosinophils have a greater capacity to produce and secrete PGD2 following aspirin stimulation than those from healthy individuals. In an environment rich in IFN-γ, eosinophil progenitor cells express increased levels of hPGDS and are hyperresponsive to aspirin stimulation. While on a per cell basis, mast cells can synthesize more PGD2, in AERD there is a massive influx of eosinophils into the sinus and lung tissue making the contribution of PGD2 by eosinophils likely a more substantial contributor of the total amount released. Targeting PGD2 synthesis or binding to its receptors presents an opportunity for therapeutic intervention in this difficult to treat disease and, indeed, this may underlie some of the beneficial effects observed after aspirin desensitization.
Supplementary Material
Capsule Summary.
Increased levels of PGD2 found in AERD come from both mast cells and eosinophils, the release of which likely contributes to the inflammation observed in AERD and severe upper and lower airways symptoms that develop upon ingestion of aspirin.
Acknowledgements
We would like to thank Kodi Ravichandran for use of the Zeiss microscope.
Supported by NIH grants R01-AI057438, R56-AI120055, and U01-AI100799
Abbreviations
- AERD
aspirin exacerbated respiratory disease
- APC
allophycocyanin
- CHES
chronic hyperplastic eosinophilic sinusitis
- COX
cyclooxygenase
- CRS
chronic rhinosinusitis
- CysLT
cysteinyl leukotriene
- DAPI
4’, 6-diamidino-2-phenylindole
- ECP
eosinophil cationic protein
- EIA
enzyme-linked immunoassay
- FBS
fetal bovine serum
- Flt3L
Fms-like tyrosine kinase 3 ligand
- hfp
high-powered field
- hPGDS
hematopoietic prostaglandin D2 synthase
- IFN
interferon
- IL
interleukin
- LT
leukotriene
- NP
nasal polyps
- PBMC
peripheral blood mononuclear cells
- PG
prostaglandin
- PMA
phyorbol myristate acetate
- qPCR
quantitative polymerase chain reaction
- SCF
stem cell factor
- TPO
thymopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Mattos JL, Woodard CR, Payne SC. Trends in common rhinologic illnesses: analysis of U.S. healthcare surveys 1995-2007. Int Forum Allergy Rhinol. 2011;1:3–12. doi: 10.1002/alr.20003. [DOI] [PubMed] [Google Scholar]
- 2.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–81. e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–56. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 4.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of resiratory responses. J Allergy Clin Immunol. 1999;104:559–64. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 5.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. The Journal of allergy and clinical immunology. 2003;111:743–9. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 6.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–52. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson DD. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol. 2003;24:159–68. doi: 10.1385/CRIAI:24:2:159. [DOI] [PubMed] [Google Scholar]
- 8.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 9.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–61. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 11.Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394–403. doi: 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- 12.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. The Laryngoscope. 2011;121:2262–7. doi: 10.1002/lary.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinke JW, Bradley D, Arango P, Crouse CD, Frierson H, Kountakis SE, et al. Cytseinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: Importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–9. doi: 10.1067/mai.2003.67. [DOI] [PubMed] [Google Scholar]
- 14.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–42. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 15.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–46. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Novo CA, Watelet JB, Claeys C, van Cauwenberge P, Bachert C. Prostaglandin, leukotiene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–96. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. Journal of immunology. 2011;187:6518–26. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano M, Fujiwara T, Yamamoto M, Sugata Y, Matsumoto R, Fukushima K, et al. Role of prostaglandin D2 and E2 terminal synthases in chronic rhinosinusitis. Clin Exp Allergy. 2006;36:1028–38. doi: 10.1111/j.1365-2222.2006.02528.x. [DOI] [PubMed] [Google Scholar]
- 19.Hyo S, Kawata R, Kadoyama K, Eguchi N, Kubota T, Takenaka H, et al. Expression of prostaglandin D2 synthase in activated eosinophils in nasal polyps. Arch Otolaryngol Head Neck Surg. 2007;133:693–700. doi: 10.1001/archotol.133.7.693. [DOI] [PubMed] [Google Scholar]
- 20.Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol. 2014;193:41–7. doi: 10.4049/jimmunol.1301753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent Role of Interferon-γ in Aspirin-Exacerbated Respiratory Disease. Journal of Allergy and Clinical Immunology. 2013;132:856–65. e3. doi: 10.1016/j.jaci.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Early SB, Barekzi E, Negri J, Hise K, Borish L, Steinke JW. Concordant modulation of cysteinyl leukotriene receptor expression by IL-4 and IFN-γ on peripheral immune cells. Am J Respir Cell Mol Biol. 2007;36:715–20. doi: 10.1165/rcmb.2006-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson SA, Herrmann H, Du J, Cox P, Haddad el B, Butler B, et al. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J Clin Immunol. 2011;31:1045–53. doi: 10.1007/s10875-011-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–9. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 25.Arm JP, O'Hickey S, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989;140:148–53. doi: 10.1164/ajrccm/140.1.148. [DOI] [PubMed] [Google Scholar]
- 26.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirn-provoked bronchospasm in asthma. Eur Respir J. 1993;6:391–9. [PubMed] [Google Scholar]
- 27.Liu T, Kanaoka Y, Barrett NA, Feng C, Garofalo D, Lai J, et al. Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. J Immunol. 2015 doi: 10.4049/jimmunol.1500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–8. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahlen B, Nizankowska E, Szczeklik A. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Critc Care Med. 1998;157:1187–94. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 30.Sanak M, Gielicz A, Nagraba K, Kaszuba M, Kumik J, Szczeklik A. Targeted eicosanoids lipidomics of exhaled breath condensate in healthy subjects. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1796–800. doi: 10.1016/j.jchromb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Mastalerz L, Celejewska-Wojcik N, Wojcik K, Gielicz A, Januszek R, Cholewa A, et al. Induced sputum eicosanoids during aspirin bronchial challenge of asthmatic patients with aspirin hypersensitivity. Allergy. 2014;69:1550–9. doi: 10.1111/all.12512. [DOI] [PubMed] [Google Scholar]
- 32.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nantel F, Fong C, Lamontagne S, Wright DH, Giaid A, Desrosiers M, et al. Expression of prostaglandin D synthase and the prostaglandin D2 receptors DP and CRTH2 in human nasal mucosa. Prostaglandins Other Lipid Mediat. 2004;73:87–101. doi: 10.1016/j.prostaglandins.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Schratl P, Royer JF, Kostenis E, Ulven T, Sturm EM, Waldhoer M, et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179:4792–9. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 35.Stinson SE, Amrani Y, Brightling CE. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J Allergy Clin Immunol. 2015;135:395–406. doi: 10.1016/j.jaci.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesquita-Santos FP, Vieira-de-Abreu A, Calheiros AS, Figueiredo IH, Castro-Faria-Neto HC, Weller PF, et al. Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin. J Immunol. 2006;176:1326–30. doi: 10.4049/jimmunol.176.3.1326. [DOI] [PubMed] [Google Scholar]
- 37.Monneret G, Boumiza R, Gravel S, Cossette C, Bienvenu J, Rokach J, et al. Effects of prostaglandin D(2) and 5-lipoxygenase products on the expression of CD203c and CD11b by basophils. J Pharmacol Exp Ther. 2005;312:627–34. doi: 10.1124/jpet.104.074823. [DOI] [PubMed] [Google Scholar]
- 38.Hamilos DL, Leung DYM, Wood R, Cunningham L, Bean DK, Yasruel Z, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–44. doi: 10.1016/s0091-6749(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 39.Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer RP, et al. Immunolocalization of CD34 in nasal polyp. Effect of topical corticosteroids. Am J Respir Cell Mol Biol. 1999;20:388–97. doi: 10.1165/ajrcmb.20.3.3060. [DOI] [PubMed] [Google Scholar]
- 40.Denburg JA. Haemopoietic mechanisms in nasal polyposis and asthma. Thorax. 2000;55:S24–S5. doi: 10.1136/thorax.55.suppl_2.S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denburg JA, Keith PK. Eosinophil progenitors in airway diseases: clinical implications. Chest. 2008;134:1037–43. doi: 10.1378/chest.08-0485. [DOI] [PubMed] [Google Scholar]
- 42.Higashi N, Mita H, Ono E, Fukutomi Y, Yamaguchi H, Kajiwara K, et al. Profile of eicosanoid generation in aspirin-intolerant asthma and anaphylaxis assessed by new biomarkers. J Allergy Clin Immunol. 2010;125:1084–91. e6. doi: 10.1016/j.jaci.2009.12.977. [DOI] [PubMed] [Google Scholar]
- 43.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, et al. Inhaled PgE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. American J Respiratory Critical Care Medicine. 1996;153:572–5. doi: 10.1164/ajrccm.153.2.8564100. [DOI] [PubMed] [Google Scholar]
- 44.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16987–92. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014;133:1692–701. e3. doi: 10.1016/j.jaci.2013.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinke JW, Wilson J. Aspirin-exacerbated respiratory disease: pathophysiological insights and clinical advances. J Asthma Allergy. 2016;10:37–43. doi: 10.2147/JAA.S88739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–72. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 48.Ta V, White AA. Survey-Defined Patient Experiences With Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015;3:711–8. doi: 10.1016/j.jaip.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015;192:682–94. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95. e1–8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 51.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 52.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–66. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.