Abstract
Purpose
Receptor kinases Discoidin Domain Receptors (DDRs) 1 and 2 are emerging as new therapeutic targets in breast cancer (BC). However, the expression of DDR proteins during BC progression and their association with BC subtypes remain poorly defined. Herein we report the first comprehensive immunohistochemical analyses of DDR protein expression in a wide range of breast tissues.
Methods
DDR1 and DDR2 expression was investigated by immunohistochemistry in 218 samples of normal breast (n=10), ductal carcinoma in situ (DCIS, n=10), and invasive carcinomas (n=198), arrayed in tissue microarrays with comprehensive clinical and follow-up information. Staining was evaluated for cell type, subcellular localization, and percentage and intensity (scores 1–4) and association with disease subtype and outcome.
Results
In normal epithelium and DCIS, DDR1 was highly expressed, while DDR2 was negative in normal epithelium and in DCIS it localized to cells at the epithelial-stromal interface. Of the 198 invasive carcinomas, DDR1 was high in 87 (44%) and low in 103 (52%), and DDR2 was high in 110 (56%) and low in 87 (44%). High DDR2 was associated with high tumor grade (p=0.002), triple negative subtype (TNBC) (p<0.0001), and worse survival (p=0.037). We discovered a novel concordant deregulation of DDR expression, with a DDR1Low/DDR2High profile significantly associated with TNBC, compared to luminal tumors (p=0.012), and with worse overall survival.
Conclusions
DDR2 upregulation occurs in DCIS, before stromal invasion, and may reflect epithelial-stromal cross talk. A DDR1Low/DDR2High protein profile is associated with TNBC, and may identify invasive carcinomas with worse prognosis.
INTRODUCTION
There is accumulating evidence that collagen plays a key role in breast cancer development and progression. In normal breast tissues, high mammographic density, which is partly due to increased fibrillar collagen deposition, is associated with a two-fold increased risk of breast cancer development [1,2]. Recently, stromal signatures have been shown to predict prognosis in invasive breast carcinomas [3]. The presence of dense fibrosis is one of the histopathological features of basal-like triple negative breast carcinomas (TNBC) [4], a biologically aggressive subtype of invasive carcinoma of the breast. The tumor-associated, altered peritumoral stroma is particularly enriched in fibrillar collagens, which have been shown to provide a path for breast cancer cell invasion, and thus is associated with increased metastasis [5]. Moreover, the enzymes involved in collagen biosynthesis have been implicated in breast cancer metastasis [6,7]. Collectively, this evidence points to a critical role for collagen in breast cancer progression. The mechanism by which collagen elicits its pro-malignant effects on breast cancer cells are ill-defined but may include activation of signaling pathways mediated by the action of specific collagen receptors. In the last few years, it has become evident that the Discoidin Domain Receptors (DDRs), a family of receptor tyrosine kinases (RTKs) that are activated in response to collagen, are part of the arsenal of cell surface receptors that mediate tumor cell-collagen interactions [8,9].
The DDR family comprises two highly homologous receptors, DDR1 and DDR2, which upon binding to collagens undergo receptor autophosphorylation leading to the activation of intracellular signaling pathways [10,11]. However, evidence also suggests that DDRs can alter cell behavior via collagen-independent effects [8]. Both DDR receptors recognize, and are activated by fibrillar and non-fibrillar collagens. However, only DDR1 can be activated by basement membrane collagen IV, and thus DDR1 mediates the interactions of epithelial cells with the underlying basement membrane. The DDR1 subfamily comprises five isoforms, with DDR1a and DDR1b being the most common variants expressed in cells [8]. DDR2 is a single receptor variant and is mostly expressed in mesenchymal cells. However, evidence suggests that DDR2 is induced during epithelial-to-mesenchymal transition (EMT) [8,12].
DDRs play key roles in mammary gland differentiation and breast cancer progression. DDR1 knockdown in mice causes abnormal mammary gland development and leads to lactation deficiency, suggesting a key role for DDR1 in mammary gland function [13,14]. DDR1 activation has been implicated in Wnt5-mediated regulation of mammary gland development in mice [15]. In breast cancer cells, DDR1 transcription is negatively regulated by the epithelial to mesenchymal transition (EMT) transcription factor Zeb1 [16]. In contrast to DDR1, no evidence of an abnormal or dysfunctional mammary gland phenotype has been reported in DDR2 deficient mice [17–19]. DDR2 plays a role in the maintenance of EMT by stabilizing Snail1 and enhances migration, invasion, and metastasis of breast cancer cells [12]. Kinome reprograming as a consequence of MEK inhibition in triple negative breast cancer (TNBC) has been shown to induce expression and activation of DDR1 and DDR2. Consistently, targeting these receptors restored MEK inhibitor sensitivity [20]. These findings suggest that DDRs are promising targets in TNBC patients who develop drug resistance in response to certain targeted therapies. In this scenario, identifying TNBC patients with DDR overexpression may improve therapeutic efficacy. Thus, it is important to examine in detail the expression of DDRs in specimens of various breast cancer subtypes.
Previous immunohistochemical studies examined the individual expression of DDR1 or DDR2 in breast cancer with emphasis in invasive ductal and lobular carcinomas [21,22,12,23]. These studies showed that DDR2 is elevated in invasive ductal breast cancer [23,12]. In contrast, DDR1 displayed variable levels, and thus a clear consensus on DDR1 expression in breast cancer is still lacking [21,16,23,22]. Importantly, breast cancers can express both DDR1 and DDR2 within the same tumor [24]. Indeed, a cohort of 476 samples of breast cancer specimens displayed concomitant DNA amplification of DDR1 (19%) and DDR2 (67%) genes when compared to normal breast tissues [24]. DDR2 mRNA levels were found to be associated with shorter survival in breast cancer patients [23]. However, the association between DDR protein expression levels with the histopathological features of breast cancer and patient survival in different breast cancer subtypes was not examined. Therefore, there is still a significant lack of information on the expression of both DDR proteins in breast cancer and their association with disease subtype and progression. Here we conducted the first comprehensive immunohistochemical study of DDR1 and DDR2 expression using a defined and well-characterized set of breast tissue samples including normal breast, ductal carcinoma in situ (DCIS), and invasive carcinomas of different subtypes and clinicopathological characteristics. DDR expression was also evaluated for any significant association with EMT and stem cell markers. We report a differential expression of DDR proteins during breast cancer progression that may aid in defining the potential of DDRs as biomarkers and/or drug targets in specific breast cancer subtypes.
MATERIALS AND METHODS
Selection of Patients and Tissue Samples
Triplicate samples of 198 invasive breast carcinomas were arrayed in high-density tissue microarrays (TMAs) in our laboratory (n=198 tumors, and 594 tissue microarray samples) [25]. Clinical information and pathological features including patient age, tumor stage, hormonal receptor status and HER-2/neu overexpression, lymph node and distant metastasis were recorded from the surgical pathology reports. Additionally, we obtained whole tissue sections of normal breast tissues from reduction mammoplasties from healthy women (n=10) and of ductal carcinoma in situ (n=10). All samples were obtained from the Surgical Pathology files at the University of Michigan with Institutional Review Board approval (IRB#HUM00050330).
Immunohistochemistry and Scoring
The expression of DDR1 and DDR2 proteins was analyzed in consecutive sections of all whole tissues and TMAs. Formalin fixed, paraffin embedded tissue blocks were sectioned at 5μ and placed on charged slides. Slides were deparaffinized in xylene and rehydrated through graded alcohols. For DDR1 Heat Induced Epitope Retrieval was performed in the Decloaking Chamber (Biocare Medical) with Borg, (Biocare Medical). Slide pretreatment for DDR2 was with Proteinase K, prediluted, (Dakocytomation) for 10 minutes at room temperature. All slides were incubated in Peroxidase (Biocare Medical) for 5 minutes to quench endogenous peroxidases. Slides were incubated for 1.5 hours at room temperature with one of the following antibodies: anti-DDR1 (Santa Cruz Biotechnology, Cat#SC-532, 1:425), anti-DDR2 (R&D Systems, Cat#MAB2538, 1:75), anti-Snail1, anti-Slug (Cell Signaling, Cat# 3895, 1:800, and Cat#9585, 1:100), anti- E-Cadherin (BD Biosciences, Cat# 610182, 1:5000), an antibody cocktail containing anti-CD44 (Abcam, Cat# AB51037, 1:500) and anti-CD24 (Biocare Medical, Cat# CM323, 1:120), or anti-ALDH-1 (BD Biosciences, Cat# 611194, 1:6000). Antibodies were detected with either anti-rabbit or anti-mouse Envision+ HRP Labeled Polymer (DakoCytomation) for 30 minutes at room temperature. Negative controls were performed by replacing the primary antibodies with an IgG1, Universal Polymer Negative Control Serum (Biocare Medical) (Supplementary Figure 1). Slides were counterstained in hematoxylin, and mounted with Permount.
Expression of DDR1 and DDR2 proteins was evaluated for: 1. Cell type and intracellular localization; 2. Intensity of staining (scores 1–4); and 3. Pattern of staining (focal vs. diffuse). Because the expression of DDR proteins has not been systematically categorized in breast tissues previously, to assess intensity of staining we applied a published scoring schema used to evaluate breast cancer biomarker expression [25]. Based on this system, cytoplasmic DDR1 (and DDR2) expression was scored as negative (score = 1, no staining); weak (score = 2, < 25% of epithelial cells); moderate (score = 3, 25–75% of epithelial cells); and strong (score = 4, >75% of epithelial cells) [25]. High DDR1 (or 2) was defined as scores 3 and 4; low DDR1 (or 2) was defined as scores 1 and 2 [25]. The pattern of DDR1 and DDR2 expression was categorized as focal or diffuse based on previous studies [26]. Using this system, focal epithelial cell staining was defined when less than 20% of cells expressed DDR1 (or 2), and diffuse epithelial cell staining was defined when 20% or more of the epithelial cells expressed the protein. We applied the same criteria for the stromal cells.
Expression of Slug and Snail1 were considered positive when nuclear expression was detected in the cancer cells, or negative [27]. E-cadherin staining was considered as normal when expressed at the cytoplasmic membranes with crisp complete pattern, or aberrant when membranous staining was reduced or absent, following previously validated studies [28]. Dual immunostaining for CD44 and CD24 proteins, and the presence of ALDH-1 expression was evaluated as positive or negative based on detection of each protein in the cancer cells, as described previously [29,30]. CD44 was visualized as red and CD24 as brown. All slides were evaluated by C.G.K. and K.A.T. blinded for clinical and pathological information.
Statistical analysis
The association between DDR expression and the clinicopathological and biomarker expression features of the invasive carcinomas was analyzed by using chi-square or Fisher’s exact test. Overall survival was calculated from the date of surgical excision of the primary tumor to the date of death. Patients who died of or with the disease were included in the analysis. Overall survival curves were constructed by the Kaplan–Meier method. Univariate analyses were performed by using a two-sided log-rank test to evaluate stage, grade, tumor size, nodal status, histology, ER status, PR status, HER-2/neu status, Snail1, Slug, CD44/CD24, ALDH-1, and DDR1 and 2. To assess the influence of several variables simultaneously, a multivariable Cox proportional hazards model of statistically significant covariates was developed by removing non significant parameters in a step-wise manner. A P value of <0.05 was considered statistically significant. Statistical analyses were performed by the biostatistician in the study (K.M.K.).
RESULTS
Expression Patterns of DDR in Normal Breast Tissues, Ductal Carcinoma in situ, and Invasive Carcinomas
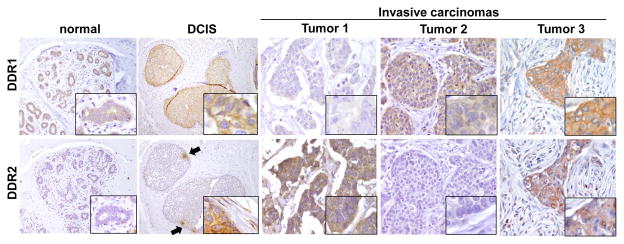
We studied ten cases of normal breast parenchyma and fibrocystic changes obtained from reduction mammoplasties from healthy women. DDR1 was diffusely expressed in the cytoplasm and cytoplasmic membrane of epithelial cells (Figure 1) but was undetected in the surrounding stroma. This is consistent with the notion that DDR1 is a major collagen receptor of epithelial cells, and with its role in normal mammary gland differentiation, as observed in DDR1 deficient mice [13]. In DCIS, DDR1 was expressed in the cellular membranes of cancer cells (Figure 1). In contrast to DDR1, DDR2 was negative in normal breast cells. Interestingly, in DCIS we observed cytoplasmic DDR2 expression in groups of DCIS cancer cells facing the stromal compartment and in the adjacent fibroblasts (Figure 1). This pattern of DDR2 staining in DCIS was not observed in the DCIS cells located towards the center for the involved ducts, suggesting that DDR2 upregulation is limited to cells closely interacting with the stromal microenvironment.
FIGURE 1. Expression of DDR1 and DDR2 in normal breast lobules, ductal carcinoma in situ (DCIS) and invasive carcinomas.
In normal lobules, DDR1 is expressed in the cytoplasm and membranes of normal epithelial cells. No DDR1 staining is observed in the stromal fibroblasts. DDR2 is not detectable in normal lobules. In DCIS, DDR1 is expressed in the cytoplasm and membrane of cancer cells. DDR2 is expressed focally in groups of cells at the periphery of the ducts (black arrows). The cancer cells located in the center of the DCIS are negative for DDR2. Inset shows specific cytoplasmic staining for DDR2 in DCIS cells adjacent to the stroma (asterisk). Note that the stromal fibroblasts also express DDR2 in the cytoplasm (asterisk). Representative invasive ductal carcinomas with different levels of DDR expression. Tumor 1 exhibits high DDR1 and low DDR2, Tumor 2 exhibits low DDR1 and high DDR2, and Tumor 3 has high expression of DDR1 and DDR2. Magnification 400x, insets 600x.
We next investigated the expression of DDR proteins in a cohort of 198 invasive carcinomas of different pathological subtypes and clinical stages (Table 1). DDR1 protein expression was high in 87 (43.9%), low in 103 (52%), and unavailable in 8 (4%) tumors, while DDR2 was highly expressed in 110 (55.6%), low in 87 (43.9%), and unavailable in 1 case (Table 1, Figure 1). High DDR2 expression in the invasive carcinoma cells was accompanied by DDR2 upregulation in the stromal fibroblasts adjacent to the invasive cancer cells (Figure 2). We also noted upregulation of DDR2 in blood vessels present in the vicinity of the invasive carcinoma (Figure 2).
Table 1.
Characteristics of Patients in Our Study (n=198)
| Characteristics | N (%) |
|---|---|
| Race | |
| White | 161 (81.3) |
| Black | 24 (12.1) |
| Other/Unknown | 13 (6.6) |
| Menopausal Status | |
| Pre | 43 (21.7) |
| Peri | 16 (8.1) |
| Post | 125 (63.1) |
| Unknown | 14 (7.1) |
| Tumor Size (cm) | |
| ≤2 | 101 (51.0) |
| >2 | 77 (38.9) |
| Unknown | 20 (10.1) |
| Tumor Grade | |
| I | 13 (6.6) |
| II | 69 (34.8) |
| III | 104 (52.5) |
| Unknown | 12 (6.1) |
| Estrogen Receptor | |
| Positive | 91 (46.0) |
| Negative | 96 (48.5) |
| Unknown | 11 (5.6) |
| Progesterone Receptor | |
| Positive | 74 (37.4) |
| Negative | 114 (57.6) |
| Unknown | 10 (5.1) |
| HER2/neu Status | |
| Overexpressed | 21 (10.6) |
| Not overexpressed | 177 (89.4) |
| Lymphovascular Invasion | |
| Present | 61 (30.8) |
| Absent | 125 (63.1) |
| Unknown | 12 (6.1) |
| Lymph Nodes | |
| Negative | 89 (44.9) |
| Positive | 76 (38.4) |
| Unknown | 33 (16.7) |
| Breast Cancer Subtype | |
| Luminal A | 88 (44.4) |
| Luminal B | 7 (3.5) |
| HER2 | 12 (6.1) |
| Triple Negative | 80 (40.4) |
| Unknown | 11 (5.6) |
| Adjuvant Hormonal Therapy | |
| Yes | 66 (33.3) |
| No | 120 (60.6) |
| Unknown | 12 (6.1) |
| Radiation Therapy | |
| Yes | 94 (47.5) |
| No | 90 (45.5) |
| Unknown | 14 (7.1) |
| Death | |
| Yes | 105 (53.0) |
| No | 85 (42.9) |
| Unknown | 8 (4.0) |
| DDR1 Status | |
| High | 87 (43.9) |
| Low | 103 (52.0) |
| Unknown | 8 (4.0) |
| DDR2 Status | |
| High | 110 (55.6) |
| Low | 87 (43.9) |
| Unknown | 1 (0.1) |
| DDR1 Low/DDR2 High | |
| Yes | 63 (31.8) |
| No | 126 (63.6) |
| Unknown | 9 (4.5) |
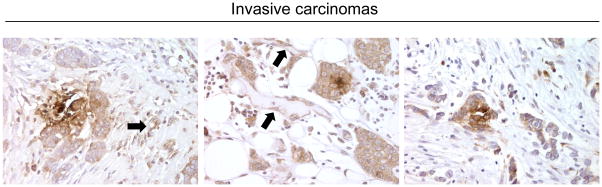
FIGURE 2. DDR2 is upregulated in a group of invasive carcinomas in the cytoplasm of cancer cells and in stromal fibroblasts.
Three representative invasive ductal carcinomas showing upregulation of DDR2 in the cytoplasm of cancer cells, stromal fibroblasts, and vessels (arrows). 400x.
DDRs have been differentially associated with expression of EMT markers in breast cancer cells with DDR1 downregulated and DDR2 upregulated during EMT [12,16]. Therefore we examined our cohort of breast cancer specimens for any association between DDR1 and DDR2 expression and a variety of EMT markers including E-cadherin, Slug, and Snail1. In addition, we examined whether DDR expression was associated with established markers of cancer stem cells including CD44/CD24, and ALDH-1. These analyses showed no significant associations with levels of either DDR1 and/or DDR2 in our samples.
DDR1 and DDR2 are Concordantly Deregulated in Triple Negative Breast Carcinomas
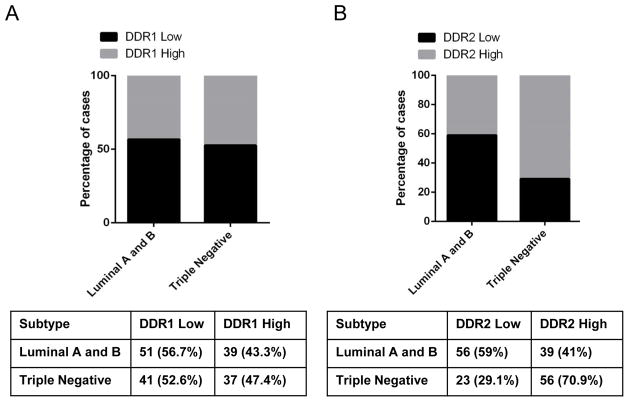
The role of the tumor microenvironment in promoting breast cancer invasion is well-established. However, reliable microenvironment-based biomarkers that provide information on the biological behavior of carcinomas are needed. To this end, we investigated whether expression of DDR1 and DDR2 is associated with clinical and pathological features in invasive carcinomas. The clinical and pathological characteristics of the patients, whose tumor samples were used in this study, are summarized in Table 1. The median age of the study population was 57.3 years (range 28.6–96.0) and the median total follow up time was 6.4 years (range 0.04–20.4 years). No significant associations were found between DDR1 expression and clinicopathological features. Moreover, we found equal distribution of high and low DDR1 expressing tumors within the luminal and TNBC subtypes (Figure 3). In contrast, high DDR2 expression was significantly associated with higher histological grade (p=0.002), negative estrogen and progesterone receptor (ER and PR) status, negative HER2/neu overexpression (p<0.0001, p=0.0005, and p=0.01, respectively, Table 2), and with the TNBC subtype in which the majority of cases displayed high DDR2 expression (Figure 3, Chi Square test p<0.0001). Simultaneous analyses of DDR1 and DDR2 proteins revealed a previously unknown concordant deregulation of DDRs across breast cancer subtypes. DDR1Low/DDR2High expression is significantly associated with TNBC compared to luminal and HER2 subtypes (p=0.012). Of the 77 TNBC cases available for immunostaining, 34 had DDR1Low/DDR2High compared to other expression patterns (Table 3). Taken together these data show that DDR2 alone or in combination with DDR1 is associated with breast cancer subtype, and that DDR1 and DDR2 are coordinately deregulated in TNBCs.
FIGURE 3. Relationship between DDR1 and DDR2 expression with breast cancer subtype.
A. Graph shows equal distribution of high and low DDR1 expressing tumors within the luminal and TNBC subtypes. B. DDR2 expression is significantly higher in TNBC compared to Luminal tumors (Chi Square test p<0.0001).
Table 2.
Association of DDR1 and DDR2 (low 1–2, high 3–4) with clinical and pathologic characteristics
| Characteristic | DDR1 (N=190)
|
DDR2 (N=197)
|
||||
|---|---|---|---|---|---|---|
| LOW | HIGH | p-value* | LOW | HIGH | p-value* | |
| Menopausal Status | 0.17 | 0.63 | ||||
| Pre | 20 (46.5) | 23 (53.5) | 19 (44.2) | 24 (55.8) | ||
| Peri | 6 (37.5) | 10 (62.5) | 5 (33.3) | 10 (66.7) | ||
| Post | 68 (58.1) | 49 (41.9) | 58 (46.4) | 67 (53.6) | ||
| Tumor Size (cm) | 0.60 | 0.39 | ||||
| ≤2 | 53 (53.5) | 46 (46.5) | 48 (48.0) | 52 (52.0) | ||
| >2 | 42 (57.5) | 31 (42.5) | 32 (41.6) | 45 (58.4) | ||
| Tumor Grade | 0.15 | 0.002 | ||||
| I or II | 47 (61.8) | 29 (38.2) | 46 (56.1) | 36 (43.9) | ||
| III | 52 (51.0) | 50 (49.0) | 34 (33.0) | 69 (67.0) | ||
| Estrogen Receptor | 0.78 | |||||
| Positive | 48 (55.8) | 38 (44.2) | 54 (59.3) | 37 (40.7) | <0.0001 | |
| Negative | 50 (53.8) | 43 (46.2) | 27 (28.4) | 68 (71.6) | ||
| Progesterone Receptor | 0.13 | 0.0005 | ||||
| Positive | 43 (61.4) | 27 (38.6) | 44 (59.5) | 30 (40.5) | ||
| Negative | 55 (50.0) | 55 (50.0) | 38 (33.6) | 75 (66.4) | ||
| HER2/neu Status | 0.18 | 0.011 | ||||
| Overexpressed | 8 (40.0) | 12 (60.0) | 4 (19.0) | 17 (81.0) | ||
| Not overexpressed | 95 (55.9) | 75 (44.1) | 83 (47.2) | 93 (52.8) | ||
| LVI | 0.49 | 0.93 | ||||
| Present | 30 (50.8) | 29 (49.2) | 26 (43.3) | 34 (56.7) | ||
| Absent | 67 (56.3) | 52 (43.7) | 55 (44.0) | 70 (56.0) | ||
| Lymph Nodes | 0.82 | 0.32 | ||||
| Negative | 45 (52.3) | 41 (47.7) | 38 (43.2) | 50 (56.8) | ||
| Positive | 39 (54.2) | 33 (45.8) | 27 (35.5) | 49 (64.5) | ||
Chi-square test of independence or Fisher’s exact without unknown category
Table 3.
Association of DDR1 low and DDR2 high expression in Triple Negative Breast Cancer (n=77)
| DDR1 | DDR2 | ||
|---|---|---|---|
| Low | High | p | |
| Low | 6 (26.1) | 34 (63.0) | 0.003 |
| High | 17 (73.9) | 20 (37.0) | |
DDR1Low/DDR2 High Protein Expression Predicts Worse Survival in Breast Cancer
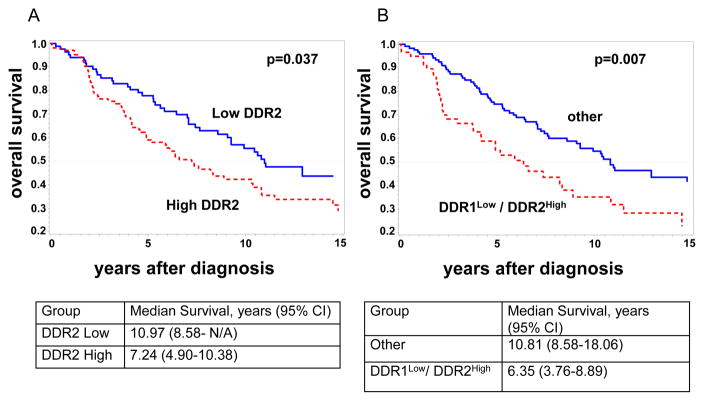
DDR2 mRNA has been shown to be associated with outcome in publicly available microarray databases of breast cancer, [23] but whether DDR2 protein levels can predict survival is unknown. Kaplan Meier survival analyses showed that patients with tumors expressing high DDR2 had a significantly worse overall survival than those expressing low DDR2 after initial surgical treatment (Kaplan Meier, log rank p=0.037, Figure 4A). The median survival for patients with tumors expressing high and low DDR2 levels was 7.2 years vs. 11.0 years (Log rank p=0.037). The 10-year overall survival for patients with high DDR2 was 42% compared to 56%, for those with low DDR2. When DDR1 was considered in the analysis, DDR1Low/DDR2High expression was associated with a significantly shorter overall survival compared to other DDR expression (log rank p=0.007) (Figure 4B). The median survival for patients with tumors expressing DDR1Low/DDR2High levels was 6.4 years and, by contrast 10.8 years for patients with tumors without this phenotype (Log rank p=0.007). The 10-year overall survival for patients with DDR1Low/DDR2High tumors was 35% compared to 55%, for those without this phenotype.
FIGURE 4. Kaplan-Meier analysis of DDR expression and disease outcome.
A. Kaplan-Meier analysis of overall survival shows that patients with invasive carcinomas with high DDR2 protein alone (red) have a significant worse survival than those with low DDR2 (blue) (n=189, log rank p = 0.037). B. Patients with invasive carcinomas with DDR1Low/DDR2High (red) phenotype have a significantly worse survival than any other combination of DDR expression (blue) (n=181, log rank p = 0.007).
Multivariable Cox regression models showed that only DDR1Low/DDR2High but not DDR2 alone, was an independent predictor of survival (Table 4). DDR1Low/DDR2High was able to predict survival independently of tumor size, TNBC phenotype, and lymphovascular invasion, with a hazard ratio of 1.73 and a 95% confidence interval of 1.13–2.63, p=0.011. Collectively, these data show that DDR2 protein is associated with survival in patients with breast cancer and that when considered in association with DDR1 protein levels, the DDR1Low/DDR2 High profile may be an independent predictor of outcome.
Table 4.
DDR1Low/DDR2High is significantly associated with survival in Multivariable Cox Regression Model (n=181)
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Lymphovascular Invasion (vs. Negative) | 0.17 | ||
| Positive | 1.47 | 0.96–2.25 | 0.078 |
| Unknown | 0.79 | 0.26–2.33 | 0.66 |
| Breast Cancer Type (vs. Luminal) | 0.79 | ||
| Triple Negative | 1.17 | 0.74–1.83 | 0.51 |
| Unknown | 1.00 | 0.50–2.00 | 0.99 |
| Tumor Size (vs. ≤2) | 0.45 | ||
| >2 cm | 1.07 | 0.70–1.65 | 0.75 |
| unknown | 1.54 | 0.79–3.01 | 0.20 |
| DDR1Low/DDR2 High (vs. other) | 1.73 | 1.13–2.63 | 0.011 |
DISCUSSION
The unique collagen-binding RTKs DDR1 and DDR2 have emerging roles in cancer progression and are being considered as therapeutic targets for breast and other carcinomas [31,12,32,8,20,33]. However, comprehensive analyses of both DDR proteins in breast cancer tissues remained to be conducted. In the present study, we characterized for the first time the expression pattern of DDR1 and DDR2 proteins in a wide spectrum of normal breast and breast cancer tissue samples, and assessed their utility as prognostic markers in patients with breast cancer.
We report that in normal breast tissues and in DCIS, DDR1 was expressed in the plasma membrane and cytoplasm of the epithelial cells and was undetectable in stromal components. The invasive carcinomas in our cohort displayed almost an equal distribution of tumors expressing either high or reduced DDR1 protein. Our statistical analyses did not reveal any significant association between high or low DDR1 protein expression and the histopathological features of the tumors. Based on the reported experimental evidence, it is possible that variations in DDR1 expression may be a reflection of the complex effects that DDR1 elicits in breast cancer cells, from tumor suppressive to tumor promoter in a context dependent manner. On one hand, reduction or loss of DDR1 protein expression in tumor cells may affect cell-cell interactions to promote invasion, in particular during the transition from DCIS to invasive carcinoma. Indeed, evidence from cultured normal epithelial cells demonstrated that DDR1 localizes predominantly to regions of cell-cell contact, where it interacts with and stabilizes E-cadherin [34,35,31,16]. Our group has also reported that the pro-invasive MT1-MMP sheds DDR1, but not DDR2, in breast cancer cells, a process that results in reduced DDR1 phosphorylation [36]. Thus, MT1-MMP-mediated cleavage of the receptor ectodomain may hinder the tumor suppressive effects of DDR1 during breast cancer progression. At the transcriptional level, downregulation of DDR1 mRNA by EMT transcription factors may also contribute to progression [24,16]. On the other hand, high expression or re-expression of DDR1 in invasive carcinoma cells residing within the interstitial collagen matrix may be required for collective cell migration [35] and induction of MMP expression [32]. Indeed, DDR1 is also activated by interstitial collagen I [10,11]. High DDR1 expression may also play a role in breast cancer cell proliferation and/or survival [20,37]. Thus, in a subset of invasive carcinomas DDR1 may contribute to disease aggressiveness. It is important to note that there are five structurally different DDR1 isoforms, which may activate different signal transduction pathways [10,8]. However, there are currently no available antibodies to detect the different DDR1 isoforms. Thus, based on the antibodies used here, which recognize DDR1a, b, and c, we cannot rule the interesting possibility that a DDR1 isoform switch takes place during breast cancer progression. For instance, the subset of invasive breast carcinomas displaying high levels of DDR1 found here may represent tumors with a different DDR1 isoform. If so, it will be important to elucidate whether the biological effects (tumor suppressive or promoter) of DDR1 in breast cancer progression are isoform dependent. Future studies are warranted to elucidate the complexity of DDR1 regulation and function in breast cancer.
We found that DDR2 expression was undetectable in normal breast lobules, as previously reported [12]. Interestingly, we noticed that DDR2 was upregulated in DCIS, especially in clusters of cancer cells localized towards the basal portion of the duct including, but not restricted to myoepithelial cells adjacent to the stromal compartment, suggesting that DDR2 upregulation may play a role in epithelial-stromal interactions. This hypothesis is supported by studies showing that ducts involved by DCIS exhibit a discontinuous and attenuated basement membrane and a discontinuous layer of myoepithelial cells [38,39], which may allow direct contact between cancer cells and the interstitial collagen, a ligand for DDR2 [10,8]. In our breast cancer cohort, DDR2 protein was upregulated in 55.6% of invasive carcinomas, supporting the immunohistochemical data of Zhang et al [12]. However, we did not find a statistical association between EMT markers and DDR2 expression. We found that high DDR2 protein levels were significantly associated with high tumor grade, a measure of tumor differentiation and indicator of poor patient outcome [40], TNBC, and with worse survival compared to low DDR2 protein. These data further validate the reported association between high levels of DDR2 messenger RNA with higher risk of relapse in breast cancer [23], as well as the reported association between DDR2 gene amplified copy number and worse survival [12].
As far as we know, our study is the first to examine the co-expression of DDR1 and DDR2 proteins in the same cohort of breast tissue specimens. These analyses uncovered a novel association between low DDR1 and high DDR2 protein expression in invasive carcinomas predominantly of the TNBC subtype. Although it has not been reported in human samples before, low DDR1 expression has been found in several TNBC basal cell lines [41]. We found that patients with invasive carcinomas expressing a combination of DDR1Low/DDR2 High protein expression exhibited a significantly worse survival when compared to patients that are negative for this DDR profile. Importantly, in multivariable analyses DDR1Low/DDR2 High, but not DDR2 alone, was independently associated with survival. Further studies are warranted to validate the prognostic significance of DDR1Low/DDR2 High in breast cancer patients and to define the cross talk between DDR types in breast cancer subtypes, and their role in regulating disease progression. Since DDRs share some of the same ligands (collagen I for both DDR1 and DDR2), it will be important to explore how the signaling pathways activated by each DDR type and isoform in invasive breast cancer cells are coordinated to elicit the proper cellular response and tumor outcome at various stages of disease.
In conclusion, our findings advance the current understanding of DDR1 and DDR2 expression in normal breast and breast cancer paving the way to further investigate the roles of DDR1 in breast cancer progression, DDR2 as a novel target in TNBCs, and the potential prognostic value of the DDR1low/DDR2high expression profile in breast cancer patients.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 CA107469, CA125577, and U01 CA154224 (to CGK), and P30 CA46592 (NIH University of Michigan’s Cancer Center Support Grant) to C.K., and R01 CA61986 and SRIG funds from the Karmanos Cancer Institute to R.F.
Footnotes
Author contributions: R.F., R.R.V, and C.G.K. designed research; K.A.T., and F.N. performed experiments; K.M.K. performed statistical analyses; and C.G.K., R.F., R.R.V, K.A.T. wrote the manuscript.
CONFLICT OF INTEREST STAMENT: The authors declare they have no conflicts of interest.
References
- 1.Maskarinec G, Pagano IS, Little MA, Conroy SM, Park SY, Kolonel LN. Mammographic density as a predictor of breast cancer survival: the Multiethnic Cohort. Breast Cancer Res. 2013;15(1):R7. doi: 10.1186/bcr3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign Breast Disease, Mammographic Breast Density, and the Risk of Breast Cancer. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck AH, Espinosa I, Gilks CB, van de Rijn M, West RB. The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab Invest. 2008;88(6):591–601. doi: 10.1038/labinvest.2008.31. labinvest200831 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulford LG, Easton DF, Reis-Filho JS, Sofronis A, Gillett CE, Lakhani SR, Hanby A. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathology. 2006;49(1):22–34. doi: 10.1111/j.1365-2559.2006.02453.x. HIS2453 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12(8):540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 7.Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, Hubbi ME, Wirtz D, Semenza GL. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73(11):3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31(1–2):295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 10.Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, Mahasenan KV, Mobashery S, Huang P, Agarwal G, Fridman R. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288(11):7430–7437. doi: 10.1074/jbc.R112.444158. R112.444158 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leitinger B. Transmembrane collagen receptors. Annual review of cell and developmental biology. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15(6):677–687. doi: 10.1038/ncb2743. ncb2743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraci-Orf E, McFadden C, Vogel WF. DDR1 signaling is essential to sustain Stat5 function during lactogenesis. Journal of cellular biochemistry. 2006;97(1):109–121. doi: 10.1002/jcb.20618. [DOI] [PubMed] [Google Scholar]
- 15.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134(21):3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 16.Koh M, Woo Y, Valiathan RR, Jung HY, Park SY, Kim YN, Kim HR, Fridman R, Moon A. Discoidin domain receptor 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-Ras-induced epithelial to mesenchymal transition. Int J Cancer. 2014 doi: 10.1002/ijc.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kano K, Kitamura A, Matsuwaki T, Morimatsu M, Naito K. Discoidin domain receptor 2 (DDR2) is required for maintenance of spermatogenesis in male mice. Molecular reproduction and development. 2010;77(1):29–37. doi: 10.1002/mrd.21093. [DOI] [PubMed] [Google Scholar]
- 18.Kano K, Marin de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Molecular endocrinology. 2008;22(8):1866–1880. doi: 10.1210/me.2007-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martinez C, Klein R. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2(5):446–452. doi: 10.1093/embo-reports/kve094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, Johnson NL, Granger DA, Jordan NV, Darr DB, Usary J, Kuan PF, Smalley DM, Major B, He X, Hoadley KA, Zhou B, Sharpless NE, Perou CM, Kim WY, Gomez SM, Chen X, Jin J, Frye SV, Earp HS, Graves LM, Johnson GL. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149(2):307–321. doi: 10.1016/j.cell.2012.02.053. S0092-8674(12)00350-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, Landberg G, Andersson T. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. 2005;11(2 Pt 1):520–528. 11/2/520 [pii] [PubMed] [Google Scholar]
- 22.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J, Srovnal J, Hajduch M, Murray P, Kolar Z. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. 1471-2407-7-55 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren T, Zhang J, Liu X, Yao L. Increased expression of discoidin domain receptor 2 (DDR2): a novel independent prognostic marker of worse outcome in breast cancer patients. Med Oncol. 2013;30(1):397. doi: 10.1007/s12032-012-0397-3. [DOI] [PubMed] [Google Scholar]
- 24.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. nature11412 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100(20):11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volante M, Sperone P, Bollito E, Frangipane E, Rosas R, Daffara F, Terzolo M, Berruti A, Papotti M. Matrix metalloproteinase type 2 expression in malignant adrenocortical tumors: Diagnostic and prognostic significance in a series of 50 adrenocortical carcinomas. Mod Pathol. 2006;19(12):1563–1569. doi: 10.1038/modpathol.3800683. 3800683 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci U S A. 2012;109(41):16654–16659. doi: 10.1073/pnas.1205822109. 1205822109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez ME, Moore HM, Li X, Toy KA, Huang W, Sabel MS, Kidwell KM, Kleer CG. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc Natl Acad Sci U S A. 2014;111(8):3098–3103. doi: 10.1073/pnas.1308953111. 1308953111 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh YC, Wu CC, Wang YK, Tang MJ. DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol Biol Cell. 2011;22(7):940–953. doi: 10.1091/mbc.E10-08-0678. mbc.E10-08-0678 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro-Sanchez L, Soto-Guzman A, Guaderrama-Diaz M, Cortes-Reynosa P, Salazar EP. Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin Exp Metastasis. 2011;28(5):463–477. doi: 10.1007/s10585-011-9385-9. [DOI] [PubMed] [Google Scholar]
- 33.Richters A, Nguyen HD, Phan T, Simard JR, Grutter C, Engel J, Rauh D. Identification of type II and III DDR2 inhibitors. J Med Chem. 2014;57(10):4252–4262. doi: 10.1021/jm500167q. [DOI] [PubMed] [Google Scholar]
- 34.Eswaramoorthy R, Wang CK, Chen WC, Tang MJ, Ho ML, Hwang CC, Wang HM, Wang CZ. DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. Journal of cellular physiology. 2010;224(2):387–397. doi: 10.1002/jcp.22134. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13(1):49–58. doi: 10.1038/ncb2133. ncb2133 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu HL, Sohail A, Valiathan RR, Wasinski BD, Kumarasiri M, Mahasenan KV, Bernardo MM, Tokmina-Roszyk D, Fields GB, Mobashery S, Fridman R. Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J Biol Chem. 2013;288(17):12114–12129. doi: 10.1074/jbc.M112.409599. M112.409599 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcotte R, Brown KR, Suarez F, Sayad A, Karamboulas K, Krzyzanowski PM, Sircoulomb F, Medrano M, Fedyshyn Y, Koh JL, van Dyk D, Fedyshyn B, Luhova M, Brito GC, Vizeacoumar FJ, Vizeacoumar FS, Datti A, Kasimer D, Buzina A, Mero P, Misquitta C, Normand J, Haider M, Ketela T, Wrana JL, Rottapel R, Neel BG, Moffat J. Essential gene profiles in breast, pancreatic, and ovarian cancer cells. Cancer Discov. 2012;2(2):172–189. doi: 10.1158/2159-8290.CD-11-0224. 2159-8290.CD-11-0224 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damiani S, Ludvikova M, Tomasic G, Bianchi S, Gown AM, Eusebi V. Myoepithelial cells and basal lamina in poorly differentiated in situ duct carcinoma of the breast. An immunocytochemical study. Virchows Arch. 1999;434(3):227–234. doi: 10.1007/s004280050332. [DOI] [PubMed] [Google Scholar]
- 39.Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10(3):231–247. doi: 10.1007/s10911-005-9584-6. [DOI] [PubMed] [Google Scholar]
- 40.Rosen PP. Rosen’s Breast Pathology. In: Rosen PP, editor. Rosen’s Breast Pathology. 3. I. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 41.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/−) stem cell phenotype in human breast cancer. Journal of mammary gland biology and neoplasia. 2010;15(2):235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.