Supplemental Digital Content is available in the text
Keywords: Anti-interleukin-1β, ASC, Burkholderia pseudomallei, melioidosis, NLRP3, sepsis
ABSTRACT
Background:
Melioidosis, caused by the gram-negative bacterium Burkholderia pseudomallei, is a common cause of community-acquired sepsis in Southeast Asia and Northern Australia. The NLRP3 inflammasome and its downstream product interleukin-1 beta (IL-1β) have been proposed to play crucial roles in melioidosis. In this study, we characterized the role of IL-1β more closely and we assessed its therapeutic potential.
Methods:
mRNA expression of inflammasome components was determined in isolated leukocytes of 32 healthy controls and 34 patients with sepsis caused by B pseudomallei.
Wild-type (WT), NLRP3-deficient (Nlrp3−/−), and Asc−/− mice were infected with B pseudomallei. In additional experiments, infected WT mice were treated with an anti-IL-1β antibody. After 24, 48, and 72 hours (h) mice were sacrificed and organs were harvested. Furthermore, survival studies were performed.
Results:
Patients with melioidosis exhibited lower mRNA levels of caspase-1, NLRP3, and ASC. Bacterial dissemination and organ damage were increased in B pseudomallei-infected Nlrp3−/− and Asc−/− mice, together with a reduced pulmonary cell influx. Anti-IL-1β treatment of B pseudomallei challenged mice resulted in strongly reduced bacterial counts, organ damage, and pulmonary granulocyte influx together with reduced mortality. Postponement of anti-IL-1β treatment for 24 h postinfection still protected mice during melioidosis.
Conclusion:
Expression of caspase-1, NLRP3, and ASC is altered in melioidosis patients. In mice, both NLRP3 and ASC contribute to the host defense against melioidosis. Anti-IL-1β treatment protects mice against B pseudomallei infection and might be a novel treatment strategy in melioidosis.
INTRODUCTION
The National Select Agent Registry Tier 1 select agent Burkholderia pseudomallei is the causative agent of melioidosis and a frequent cause of community-acquired sepsis in Southeast Asia and northern Australia (1). Melioidosis is characterized by pneumonia and abscess formation in different organ systems. Septic shock occurs in one-fifth of patients (1). Despite adequate use of antibiotics following diagnosis, mortality rates associated with septic melioidosis range from 20 to over 40% depending on the availability of intensive care facilities (1). This, together with first reports on the emergence of antibiotic resistance in B pseudomallei and underreporting (2–4), highlights the importance of expanding our knowledge on the antimicrobial response in melioidosis that can lead to new therapeutic approaches.
In recent years, the importance of the pattern recognition receptor (PRRs) in the host defense against B pseudomallei infection has become apparent. Toll-like receptors (TLR), which recognize conserved microbial structures, known as pathogen-associated molecular patterns (PAMPs), are the most studied PRRs. The inflammasomes, large protein complexes, detect infection and stress-associated signals and represent the most important intracellular PRRs (5). Nod-like receptor (NLR) family, pyrin domain containing (NLRP) 3 is a sensor that functions in a single inflammasome, whereas adaptor apoptosis-associated speck-like protein containing a caspase activation and recruitment domain, ASC, is a common adaptor of several inflammasomes (6). After recognition of PAMPs or damage-associated molecular patterns (DAMPs), the inflammasome platform assembles and proteolytically activates caspase-1. Once activated, caspase-1 cleaves pro-interleukin-1β (IL-1β) and pro-IL18 into their mature forms (7). IL-1β and IL-18 are among the most potent proinflammatory cytokines that are involved in the acute phase response. Caspase-1-activation can also trigger pyroptosis, a form of programmed cell death that effectively restricts intracellular bacterial growth (8). NLRs can be activated by a wide variety of signals, including ATP, uric acid crystals but also bacterial-type three secretion system needles, rod proteins, and flagellin; all major virulence factors of B pseudomallei(1).
We and others have previously shown that IL-1β and IL-18 expression is increased in septic melioidosis patients and both play essential roles in the immune response to B pseudomallei infection (4,9–11). Monocyte IL-1β mRNA expression and plasma IL-18 levels on admission correlate with poor outcome in patients with melioidosis (4,9). The NLRP3 inflammasome is responsible for the production of IL-1β and IL-18 in murine melioidosis, while pyroptosis is thought to be NLRC4-inflammasome dependent (8,10). IL-18 protects against B pseudomallei infection due to its induction of interferon (IFN)-γ (4,10). IL-1β on the other hand has been suggested to play a deleterious role due to excessive recruitment of neutrophils, which may support intracellular growth of B pseudomallei, tissue damage, and inhibition of IFN-γ production (10).
We now expand upon these previous studies by first investigating caspase-1, NLRP3, and ASC expression in patients with culture-proven melioidosis. Having found that these genes are down-regulated during melioidosis, we next aimed to further characterize the role of NLRP3 and ASC in the host defense against pneumonia-derived melioidosis in vivo. B pseudomallei-infected Nlrp3−/− and Asc−/− mice displayed increased bacterial dissemination and organ damage together with a reduced cell influx toward the primary site of infection compared with controls. Last, we evaluated whether treatment with a commercially available monoclonal IL-1β antibody could improve outcome in experimental melioidosis and found that anti-IL-1β treatment conferred marked protection against B pseudomallei induced lethality.
MATERIALS AND METHODS
Ethics statement
All human subjects provided written informed consent. The study was approved by the Ministry of Public Health, Royal Government of Thailand, and the Oxford Tropical Research Ethics Committee, University of Oxford, England.
The Animal Care and Use of Committee of the University of Amsterdam approved all animal experiments (DIX 21AJ and 102327), which adhered to European legislation (Directive 2010/63/EU).
Patients
Thirty-four melioidosis patients were recruited at the Sappasithiprasong Hospital, Ubon Ratchathani, Thailand, as described (9). In short, eligible patients aged 18 to 75 years had culture-proven melioidosis, received active antimicrobial therapy for less than 48 hours (h) (ceftazidime, amoxicillin-clavulanate, meropenem, or imipenem), and had at least three out of four criteria for the systemic inflammatory response syndrome: a core temperature of ≤36°C or ≥38°C; a heart rate of ≥90 beats/min; a respiratory rate of ≥20 breaths/min, a PaCO2 of ≥32 mm Hg, or the use of mechanical ventilation for an acute respiratory process; and a white cell count of ≤4 × 109/L or ≥12 × 109/L or a differential count showing >10% immature neutrophils. Thirty-two healthy blood donors were recruited from the hospital's blood bank and served as controls.
Analysis of mRNA levels by quantitative RT-PCR
Total RNA of human granulocytes and monocytes was isolated using the RNeasy Mini Kit System (Qiagen, Venlo, The Netherlands), treated with RNA-free DNase (Promega, Madison, WI) and reverse transcribed using oligo(dT) primers and Moloney murine leukemia virus RT (Promega). Primers and RT-PCR conditions can be found in the online supplement (see Table S1, Supplemental Digital Content 1, at http://links.lww.com/SHK/A387). Data were analyzed using the comparative Ct method.
Mice
Nlrp3−/− and Asc−/− mice (12) were backcrossed nine times to a C57BL/6 genetic background. Age- and sex-matched pathogen-free 8- to 10-wk-old male wild-type (WT) C57BL/6 mice were purchased from Charles River (Leiden, The Netherlands).
Experimental infection and treatment regiments
Experimental melioidosis was induced by intranasal inoculation with 3 or 5 × 102 colony-forming units (CFU) of B pseudomallei strain 1026b (a clinical isolate) as described (13). At 24 and/or 72 h postinfection, mice were euthanized and sacrificed by bleeding from the inferior vena cava, after which organs were harvested. In some experiments mouse monoclonal antibody directed against mouse IL-1β (α-IL-1β; 10 μg/g bodyweight) with comparable properties to canakinumab in humans (14) was administered intraperitoneally at 6 or 24 h postinfection. In addition, selected animals received ceftazidime 600 mg/kg (GlaxoSmithKline, Brentford, England) intraperitoneally twice daily, starting 6 h after inoculation until sacrifice (13). For survival experiments, mice were observed every 6 h for 11 days and α-IL-1β administration was repeated at day 7. Sample harvesting, determination of bacterial growth, and assays are described in the online supplement (see supplementary data, Supplemental Digital Content 2, at http://links.lww.com/SHK/A388).
Statistical analysis
Values are expressed as mean ± standard error of the mean. Differences between groups were analyzed by Mann–Whitney U test or Kruskal–Wallis analysis followed by separate Mann–Whitney U tests. For survival analysis, Kaplan–Meier analysis followed by log-rank test was performed. Analyses were performed using GraphPad Prism (version 6.00). Values of P <0.05 were considered statistically significant.
RESULTS
Inflammasome mRNA expression in patients with severe melioidosis
Given the importance of NLRs in the release of IL-1β and the orchestration of the immune response, we first determined alterations of NLRP3, ASC, and caspase-1 expression in granulocytes and monocytes of 34 patients with culture-proven septic melioidosis and 32 matched healthy controls. Mortality in this patient cohort was 44%. Caspase-1, NLRP3, and ASC mRNA levels were significantly lower in patients, consistent with the hypothesis that melioidosis alters expression of inflammasome components substantially (Table 1). When compared with controls, caspase-1 expression was markedly reduced in monocytes of patients (P <0.05), while NLRP3 and ASC expression was down-regulated in both granulocytes and monocytes (P <0.05 and P <0.01 respectively; Table 1). No differences in NLR mRNA expression were found between survivors and non-survivors (data not shown).
Table 1.
Relative copy numbers of monocyte and granulocyte mRNA of healthy controls and melioidosis patients
| Controls (n = 32) | Patients (n = 34) | |||
| Granulocytes | Monocytes | Granulocytes | Monocytes | |
| Caspase-1 | 1.04 ± 0.20 | 0.89 ± 0.11 | 0.85 ± 0.43 | 0.54 ± 0.07* |
| NLRP3 | 1.06 ± 0.19 | 1.27 ± 0,25 | 0.60 ± 0.13* | 0.51 ± 0.09** |
| ASC | 1.23 ± 0.25 | 1.28 ± 0.28 | 0.46 ± 0.10* | 0.42 ± 0.06* |
Granulocyte and monocyte mRNA expression levels were determined in healthy controls and patients with culture-proven melioidosis. Thirty-four septic melioidosis patients (17 males) and 32 healthy control subjects (22 males) were enrolled. The mean ages were 52 years (18–86 years) and 41 years (21–59 years) for patients and controls, respectively. Blood cultures were positive for B pseudomallei in 21 patients (61.7%). After inclusion all patients received appropriate antimicrobial therapy. The overall in-hospital patient mortality was 44% (14).
Shown are monocyte and granulocyte mRNA expression levels (normalized for GAPDH). Data are expressed as mean ± SEM and analyzed by Mann–Whitney U test.
*P <0.05.
**P <0.01 compared with controls (Mann–Whitney U test).
Both NLRP3 and ASC contribute to the antibacterial response in murine melioidosis
Having found that major changes occur in the caspase-1 regulatory molecules NLRP3 and ASC in patients, we next studied the involvement of NLRP3 and ASC in our murine melioidosis model. Wild-type, Nlrp3−/−, and Asc−/− mice were intranasally challenged with live B pseudomallei, resulting in a strong increase in IL-1β levels in lung (from 1,660 ± 326 pg/mL (24 h) to 29,571 ± 3,549 pg/mL (72 h); P <0.001) and bronchoalveolair fluid (BALF; from 350 ± 45 pg/mL (24 h) to 2,494 ± 413 pg/mL (72 h); P <0.01) of WT mice. IL-1β levels were strongly reduced in infected Nlrp3−/− and Asc−/− mice (Table 2). First, we evaluated how NLRP3 or ASC deficiency impacts on pulmonary bacterial growth and dissemination toward distant organs. No differences in local bacterial clearance of B pseudomallei were observed between WT, Nlrp3−/− and Asc−/− mice (Fig. 1A). However, at 72 h postinfection bacterial loads in blood were increased in both Nlrp3−/− and Asc−/− mice compared with WTs (P <0.05 and P <0.01 for Nlrp3−/− and Asc−/− mice, respectively; Fig. 1B). Hepatic and splenic bacterial loads were reflective of those in blood: compared with WTs Nlrp3−/− and Asc−/− mice displayed increased bacterial dissemination to spleen (data not shown) and liver (P <0.05–0.001; Fig. 1C). Next, we assessed the pulmonary inflammatory response. Seventy-two hours after infection total leukocyte counts were significantly reduced in BALF of Nlrp3−/− and Asc−/− mice (P <0.01, Fig. 1D), predominantly caused by decreased neutrophil recruitment toward the pulmonary compartment as reflected by myeloperoxidase (MPO; Table 2). In the early phase of the host response KC levels were reduced in both Nlrp3−/− and Asc−/− mice (P <0.05–0.01; Table 2), explaining the reduced leukocyte influx. Thus, ASC and NLRP3 deficiency leads to diminished leukocyte recruitment toward the site of primary infection and facilitates dissemination of B pseudomallei toward distant organs.
Table 2.
Cytokine response in lung homogenates, BALF, and plasma of wild-type (WT), Nlrp3−/− and Asc−/− mice during experimental melioidosis
| T = 24 h | T = 72 h | |||||
| pg/mL | WT | Nlrp3−/− | Asc−/− | WT | Nlrp3−/− | Asc−/− |
| Lung | ||||||
| TNF-α | 806 ± 109 | 645 ± 74 | 482 ± 76 | 1,276 ± 131 | 1,004 ± 157 | 814 ± 80 |
| IL-6 | 841 ± 119 | 783 ± 105 | 743 ± 171 | 8,066 ± 2,164 | 5,735 ± 717 | 12,295 ± ± 1,607 |
| KC | 13,083 ± 2,098 | 5,950 ± 823** | 7,792 ± 1,870* | 41,223 ± 9,319 | 53,639 ± 4,165 | 55,093 ± 4,907 |
| IL-1β | 1,660 ± 326 | 659 ± 56** | 617 ± 12** | 29,571 ± 3,549 | 2,083 ± 667*** | 760 ± 104*** |
| BALF | ||||||
| TNF-α | 1,102 ± 332 | 4,200 ± 774*** | 1,492 ± 273 | 6,518 ± 2,446 | 13,430 ± 2,456 | 13,908 ± 1,899 |
| IL-6 | 340 ± 20 | 439 ± 49 | 458 ± 52 | 14,777 ± 4,636 | 6,265 ± 899 | 23,992 ± 6,707 |
| KC | 5,105 ± 677 | 1,999 ± 283** | 2,958 ± 336* | 36,591 ± 8,648 | 60,000 ± 598* | 60,000 ± 598* |
| IL-1β | 350 ± 45 | 280 ± 22 | 245 ± 13* | 2,494 ± 413 | 1,727 ± 368 | 1,677 ± 115 |
| MPO (ng/mL) | 1,327 ± 397 | 3,293 ± 612 | 3,990 ± 1,000 | 6,757 ± 271 | 5,699 ± 524 | 5,217 ± 334** |
| Plasma | ||||||
| TNF-α | 8 ± 1 | 13 ± 1** | 6 ± 1* | 1,402 ± 1,230 | 2,874 ± 1,556* | 5,343 ± 1,763* |
| IL-6 | 196 ± 28 | 226 ± 28 | 91 ± 8*** | 5,773 ± 1,660 | 10,000 ± 267 | 9,734 ± 377 |
| MCP-1 | 189 ± 21 | 145 ± 10 | 83 ± 13** | 1,728 ± 677 | 4,550 ± 354** | 2,600 ± 394 |
| IFN-γ | 23 ± 2 | 9 ± 1** | 4 ± 1** | 321 ± 173 | 3,051 ± 459*** | 2,301 ± 619** |
| IL-12p70 | 12 ± 2 | 16 ± 2 | 9 ± 2 | 7 ± 1 | 200 ± 25** | 94 ± 25** |
| IL-10 | ND | ND | ND | 11 ± 3 | 7 ± 1 | 31 ± 10* |
| IL-1β | − | − | − | 1133 ± 430 | 252 ± 48 | 150 ± 1* |
Cytokine levels in lung homogenate, broncho-alveolar fluid (BALF), and plasma measured after intranasal infection with 5 × 102 CFU B pseudomallei. Wild-type (WT) and Nlrp3−/− and Asc−/− mice were sacrificed 24 or 72 h after infection. Data are represented as means ± SEM (n = 6–8/group).
IFN-γ indicates interferon-γ; IL, interleukin; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein-1; MPO, myeloperoxidase; ND, not detectable; TNF-α, tumor necrosis factor-α.
*P <0.05.
**P <0.01.
***P <0.001 compared with WT mice.
Fig. 1.
NLRP3 and ASC contribute to bacterial clearance of Burkholderia pseudomallei.
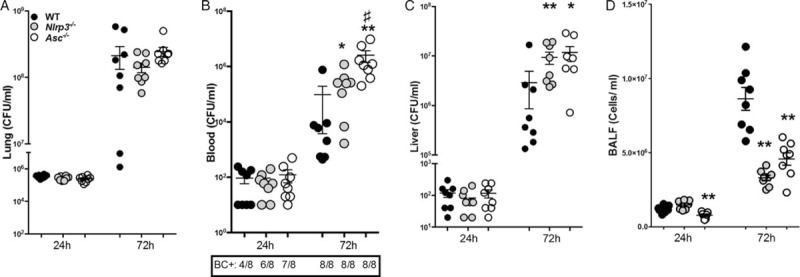
Wild-type (WT) (black circles), Nlrp3−/− (gray circles), and Asc−/− (white circles) mice (n = 8/group) were intranasally infected with B pseudomallei and sacrificed 24 or 72 h postinfection. At these time points bacterial loads were determined in lung homogenate (A), blood (B), and liver (C). Total cell influx was measured in BALF (D). Data are presented as means ± SEM. ∗P <0.05; ∗∗P <0.01, ∗∗∗P <0.001 compared with WT mice, #P <0.05 compared to Nlrp3−/− mice (n = 6–8 mice/group) (Kruskal–Wallis test, followed by separate Mann–Whitney U tests). BC+ denotes blood culture positivity; SEM, standard error of the mean.
NLRP3 and ASC protect against organ damage during experimental melioidosis
To further investigate the impact of NLRP3 and ASC deficiency on the host response against melioidosis, we analyzed the expression of key regulatory cytokines in the systemic compartment of infected mice. TNF-α was elevated in NLRP3- and ASC-deficient mice at 72 h postinfection when compared with controls, corresponding to increased bacterial counts (P <0.05, Table 2). The same trend was observed for systemic IL-6, MCP-1, IL-12p70, and IL-10 levels (Table 2). Early production of IFN-γ, an essential cytokine for the initial host response in melioidosis (11,15), was impaired in Nlrp3−/− and Asc−/−, adding to the observed defective antimicrobial response in these mice (P <0.01 for both groups, Table 2). Last, we examined the contribution of NLRP3 and ASC to multi-organ failure, the primary cause of death in patients with melioidosis (1). B pseudomallei infection resulted in profound pulmonary inflammation as characterized by infiltrates and interstitial inflammation together with necrosis, edema, and thrombi (Fig. 2A; Fig. S1, Supplemental Digital Content 3, at http://links.lww.com/SHK/A389). At both time points, the extent of lung inflammation was significantly greater in Nlrp3−/− and Asc−/− mice when compared with WTs (P <0.05; Fig. 2A). Next, we evaluated distant organ injury. Asc−/− mice displayed more spleen inflammation compared with controls (P <0.05, Fig. 2B); this difference was not significant for Nlrp3−/−. Liver pathology did not differ between mice strains (data not shown); however, evidence for increased hepatic inflammation in Asc−/− and Nlrp3−/− mice was found in elevated ALT (Fig. 2C) and AST (data not shown) levels in both Nlrp3−/− and Asc−/− mice at 72 h postinfection compared with WTs (P <0.05–0.01). Levels of LDH—a general parameter for cell damage—were similarly increased in Nlrp3−/− and Asc−/− mice (P <0.01–0.05; Fig. 2D).
Fig. 2.
Increased organ damage in Nlrp3−/− and Asc−/− mice during experimental melioidosis.
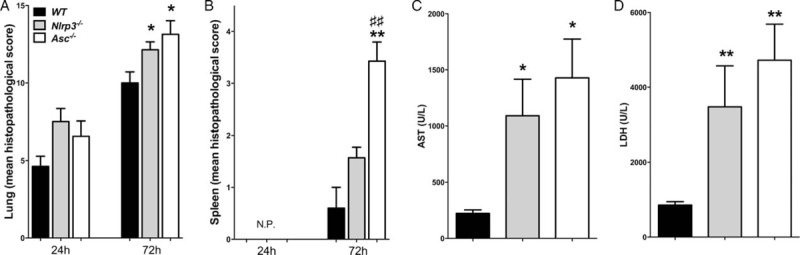
Local and systemic organ damage was assessed by evaluating histopathology in lung (A) and spleen (B) of wild-type (WT) (black bars), Nlrp3−/− (gray bars) and Asc−/− (white bars) mice 24 and 72 h after intranasal infection with B pseudomallei. Nlrp3−/− and Asc−/− mice showed increased liver injury as reflected by elevated concentrations of aspartate aminotransferase (AST; C) and general distant organ injury as reflected by elevated lactate dehydrogenase (LDH) concentrations (D) at 72 h postinfection when organ damage was most profound. Data are presented as means ± SEM. ∗P <0.05; ∗∗P <0.01 compared with WT mice. ##P <0.01 compared with Nlrp3−/− mice (n = 6–8 mice/group) (Kruskal–Wallis test, followed by separate Mann–Whitney U tests). N.P. indicates no detectable pathology; SEM, standard error of the mean.
Anti-IL-1β treatment reduces pulmonary bacterial counts and dissemination toward distant organs
Having established that both NLRP3 and ASC play key roles in the antibacterial host response against melioidosis and the development of organ damage, we next investigated whether therapeutic manipulation of this system could benefit outcome. IL-1β and IL-18 are the main downstream products of the NLRP3 inflammasome. Interestingly, their function in the host defense against B pseudomallei seems to be opposite, since IL-18 is known to protect during B pseudomallei infection, while IL-1β is suggested to play a deleterious role (4,10). We decided to evaluate the therapeutic potential of a commercially available anti-IL-1β antibody (α-IL-1β) in our melioidosis model. To mimic the clinical situation and as a comparison, mice received ceftazidime in selected experiments. Animals treated with α-IL-1β 6 h postinfection showed strongly reduced bacterial counts in the lung already 24 h after infection (P <0.01, Fig. 3A). The protective effect of α-IL-1β in the lung was even more pronounced at 72 h postinfection (P <0.001, Fig. 3A) and resulted in diminished bacterial dissemination toward the systemic compartment (P <0.01 for blood, Fig. 3B) and liver (P <0.05, Fig. 3C). The protective effect of α-IL-1β on bacterial counts was almost comparable to treatment with ceftazidime, which is the antibiotic of choice for melioidosis in most endemic areas (1).
Fig. 3.
IL-1β blockade results in diminished pulmonary bacterial growth and dissemination.
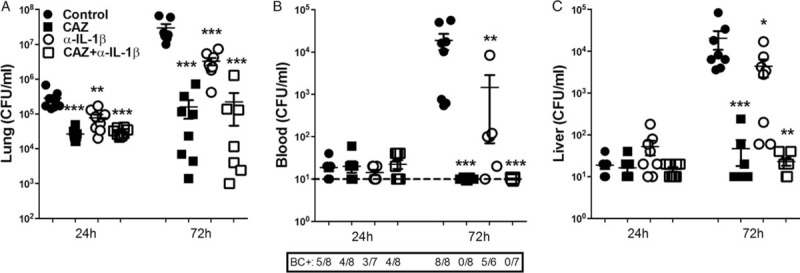
Wild-type mice (n = 7–8/group) were infected with B pseudomallei intranasally and received PBS or were treated postinfection with either ceftazidime (CAZ), anti-IL-1β antibody, or both, starting 6 h postinfection. At 24 and 72 h after bacterial inoculation mice were sacrificed. Blood and organs were harvested and plated on blood-agar plates to determine bacterial counts in lung (A), blood (B), and liver (C). Data are presented as means ± SEM. ∗P <0.05; ∗∗P <0.01; ∗∗∗P <0.001 versus controls. BC+ denotes blood culture positivity (Kruskal–Wallis test, followed by separate Mann–Whitney U tests).
Strong reduction of neutrophil influx toward the primary site of infection in mice treated with α-Il-1β
Although neutrophils are known to play a critical role in the host defense against melioidosis, excessive neutrophil recruitment toward the primary site of infection can contribute to increased inflammation and tissue damage. α-IL-1β administration prevents neutrophil influx as visualized by Ly-6 staining and confirmed by lower MPO concentrations in lung homogenates 72 h postinfection (P <0.01, Fig. 4A and B, Fig. S2, Supplemental Digital Content 4, at http://links.lww.com/SHK/A390). This effect was comparable to ceftazidime treatment (Fig. 4). In correspondence, levels of neutrophil-attracting KC were decreased in α-IL-1β-treated mice 24 h postinfection compared with controls (P <0.05, Table 3). At 72 h after infection lung proinflammatory TNF-α and IL-6 levels were reduced in α-IL-1β-treated mice consistent with decreased bacterial loads (P <0.05, Table 3). In terms of lung pathology, both α-IL-1β and ceftazidime treated animals demonstrated diminished pulmonary inflammation and abscess formation at 72 h postinfection compared with controls (P <0.01) although this difference did not reach statistical significance for the α-IL-1β group (Fig. 4C; Fig. S3, Supplemental Digital Content 5, at http://links.lww.com/SHK/A391).
Fig. 4.
Anti-IL-1β treatment leads to diminished pulmonary neutrophil accumulation and damage in B pseudomallei infection.
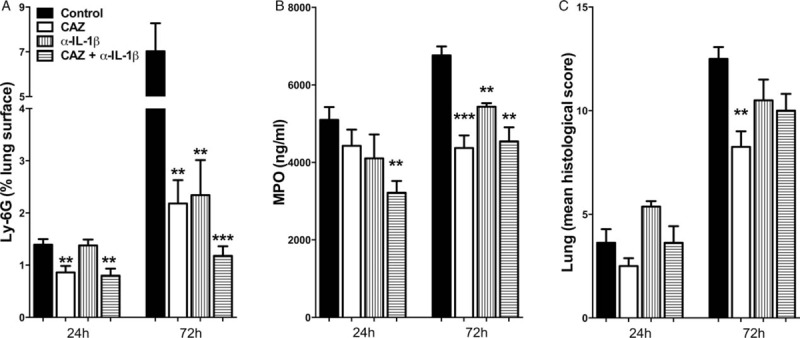
Wild-type mice (n = 7–8/group) were infected with 3 × 102 CFU B pseudomallei intranasally and received PBS or were treated 6 h postinfection with either ceftazidime (CAZ), anti-IL1β antibody, or both. At 24 and 72 h postinfection, mice were sacrificed. Ly6G staining was performed and Ly6G-positive % of total lung surface was calculated (A). Granulocyte influx in lung was additionally determined by MPO assay (B). Local, pulmonary organ damage was assessed by calculating pathology scores (C). Data are presented as means ± SEM. ∗P <0.05. ∗∗P <0.01. ∗∗∗P <0.001 versus controls (Kruskal–Wallis test, followed by separate Mann–Whitney U tests). CFU indicates colony-forming units.
Table 3.
Cytokine response in plasma and lung homogenates of B pseudomallei-infected mice treated either with ceftazidime (CAZ), anti-IL-1β (α-IL-1β), both or PBS
| T = 24 h | T = 72 h | |||||||
| pg/mL | Control | CAZ | α-IL-1β | CAZ + α-IL-1β | Control | CAZ | α-IL-1β | CAZ + α-IL-1β |
| Lung | ||||||||
| TNF-α | 2,689 ± 351 | 1,545 ± 176** | 2,131 ± 159 | 1,901 ± 237 | 8,684 ± 1,285 | 1,587 ± 157** | 5,256 ± 576* | 1,930 ± 317** |
| IL-6 | 3,095 ± 534 | 1,584 ± 131** | 1,986 ± 191 | 1,619 ± 70** | 3,569 ± 478 | 1,220 ± 104*** | 1,965 ± 163* | 1,198 ± 170** |
| KC | 34,132 ± 6,717 | 10,324 ± 1,514** | 13,851 ± 2,673* | 9,376 ± 2,171** | 27,253 ± 5,325 | 3,633 ± 724*** | 15,049 ± 2,944 | 5,027 ± 1,432** |
| IL-1β | − | − | − | − | 34,893 ± 4,467 | 2,362 ± 978** | ND*** | ND*** |
| Plasma | ||||||||
| TNF-α | 12 ± 2 | 12 ± 3 | 49 ± 19** | 15 ± 3 | 123 ± 35 | 17 ± 2*** | 44 ± 6 | 14 ± 2*** |
| IL-6 | 196 ± 27 | 91 ± 19* | 219 ± 30 | 83 ± 10*** | 613 ± 237 | 43 ± 18** | 118 ± 22 | 41 ± 16** |
| MCP-1 | 178 ± 16 | 99 ± 19* | 164 ± 22 | 73 ± 12*** | 440 ± 133 | 99 ± 14*** | 185 ± 15** | 107 ± 18*** |
| IFN-γ | 9 ± 2 | 8 ± 2 | 45 ± 4*** | 11 ± 1 | 166 ± 55 | 25 ± 9** | 112 ± 52 | 34 ± 8** |
| IL-12p70 | 14 ± 2 | ND | ND | ND | 17 ± 4 | 13 ± 2 | ND | ND |
| IL-10 | ND | ND | ND | ND | ND | ND | ND | ND |
Cytokine levels in lung homogenate and plasma measured 24 and 72 h after intranasal infection with 3 × 102 CFU wild-type B pseudomallei. Mice were treated with ceftazidime (CAZ), anti-IL-1β antibody (α-IL-1β), both or were given PBS. Data are represented as means ± SEM (n = 7–8/group).
IFN-γ indicates interferon-γ; IL, interleukin; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-α.
*P <0.05.
**P <0.01.
***P <0.001, when compared with control mice (Kruskal–Wallis test; followed by separate Mann–Whitney U tests).
Anti-IL-1β treatment protects against organ damage and improves survival in melioidosis
The protective effect of α-IL-1β was further demonstrated by diminished liver injury in those mice receiving this treatment. The extent of hepatic inflammation and necrosis was significantly less in α-IL-1β treated mice as examined by liver histology (P <0.05, Fig. 5A, Fig. S3). In accordance, AST levels, reflecting hepatocellular damage, were reduced in mice that were administered α-IL-1β compared with controls (P <0.05, Fig. 5B). ALT (data not shown) and LDH (Fig. 5C) levels tended to be lower in α-IL-1β-treated mice; however, this did not reach statistical significance. Finally, to evaluate whether the beneficial effects of anti-IL-1β treatment improved survival of mice during experimental melioidosis we infected WT mice with 3 × 102 CFU B pseudomallei and administered anti-IL-1β antibody at 6 h postinfection. Mice were subsequently monitored for 11 days. Administration of α-IL-1β resulted in a survival benefit (25% survival in anti-IL-1β treated animals vs. 10% in control mice, P <0.01, Fig. 5D). Since B pseudomallei-challenged animals first become symptomatic at 24 h postinfection, we next evaluated the effectiveness of delayed treatment. Postponement of α-IL-1β treatment for 24 h after infection, which can be of considerable clinical benefit, did not affect the protective effect (P <0.01, Fig. 5D). Taken together, anti-IL-1β treatment protects mice during B pseudomallei infection as reflected in reduced bacterial loads, reduced neutrophil influx, and diminished organ damage resulting in an improved survival.
Fig. 5.
Reduction of organ damage in anti-IL-1β treated mice leads to improved survival.
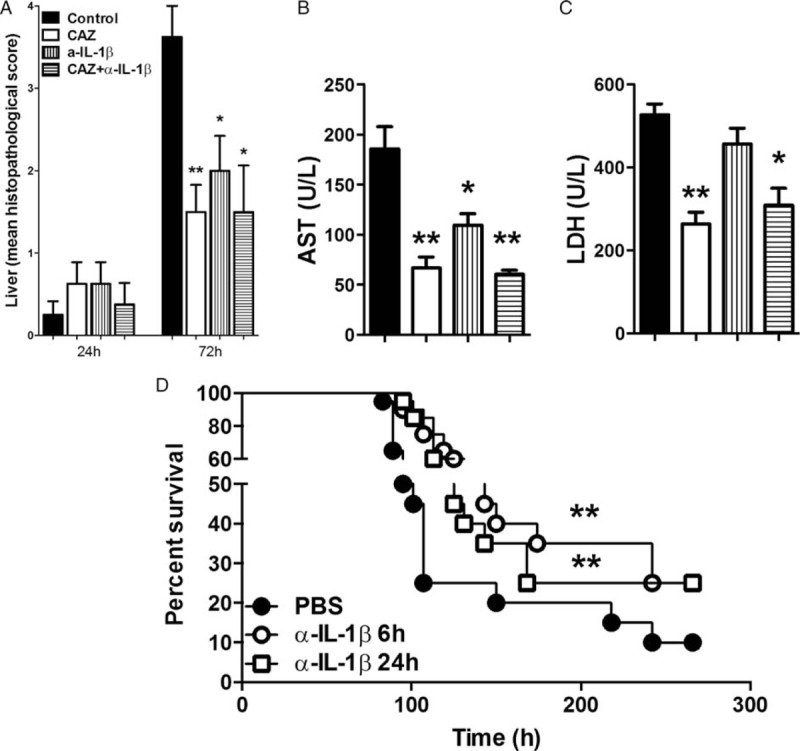
Distant organ damage was assessed by evaluating histopathology in liver (A) 24 and 72 h postinfection with B pseudomallei intranasally. Additionally, plasma levels of aspartate transaminase (AST; B) and lactate dehydrogenase (LDH; C) were determined 72 h postinfection when histopathological damage was most profound. Data are presented as means ± SEM. (n = 7–8/group) ∗P <0.05; ∗∗P <0.01 compared with controls. Survival of B pseudomallei-challenged wild-type mice (n = 20/group) that either received PBS (control, closed circles) or were treated with anti-IL1β antibody (α-IL-1β) at 6 (open circles) or 24 h (open squares) postinfection was monitored (D). After 7 days (168 h) a second dose of α-IL-1β antibody was administered. The infected mice were observed four to six times daily for 11 days (266 h). ∗∗P <0.01 versus controls (Kruskal–Wallis test, followed by separate Mann–Whitney U tests; for the survival experiment, Kaplan–Meier analysis followed by log-rank test was performed).
DISCUSSION
This study is the first to demonstrate that therapeutic blockade of IL-1β protects against experimental melioidosis. Moreover, we demonstrate that mRNA expression of NLRP3 and ASC, key components of the inflammasome receptor complex responsible for IL-18 and IL-1β activation, is down-regulated in patients with septic melioidosis. Using our well-characterized mouse model, we confirmed that both NLRP3 and ASC contribute to the host defense against B pseudomallei; Nlrp3−/− and Asc−/− mice displayed an impaired early local cytokine response and reduced cell influx toward the primary site of infection, when compared with controls, together with increased bacterial organ counts and inflammation at a later stage of infection. In contrast, blocking IL-1β, an essential product of the NLRP3-inflammasome, was beneficial for the host by enhancing bacterial clearance, controlling local neutrophil influx, the inflammatory response and organ damage. Together these data indicate that anti-IL-1β treatment protects mice against B pseudomallei infection by reduction of organ damage resulting in an improved survival.
We here show that the NLRP3 inflammasome is differentially regulated during melioidosis, since NLRP3, ASC, and caspase-1 mRNA expression by monocytes was reduced in melioidosis patients. This finding is in concordance with a study among 26 patient with septic shock admitted to the ICU in which decreased ASC and caspase-1 mRNA expression was found on peripheral monocytes of patients compared with controls (16). Potential regulatory mechanisms responsible for this observation could include inflammasome activation accompanied by autophagy, a lysosome-mediated cytoprotective process that limits inflammasome activity by ASC ubiquitination (17) or IFN-γ production by CD4+ T cells, which is known to inhibit IL-1β production by monocytes. (18). In addition, the observed decreased inflammasome mRNA expression in sepsis could be part of the leukocyte deactivation phenomenon that is a key feature of sepsis (19). This could be due, in part, to the release of anti-inflammatory signals such as IL-10, which are known to down-regulate key inflammasome proteins (20). It has to be underscored that—besides NLRP3 and ASC—NLRP1, AIM2, and NLRC4 are also involved in caspase-1 activation (21). ASC can also regulate the NF-kB pathway, thus linking the inflammasome to the signalosome. A recent meta-analysis that included 18 studies demonstrated associations between IL-1 gene polymorphisms, including IL-1 receptor antagonist (IL-1ra) and IL-1β, and all-cause sepsis risk (22). In patients with melioidosis, a human genetic polymorphism in the NLRC4 gene, a caspase-1-dependent inflammasome that responds to flagellin, has been associated with better outcome (23). No genetic polymorphisms associated with outcome in melioidosis have been described for NLRP3 or ASC.
Both NLRP3 and ASC are important for an appropriate immune response against B pseudomallei. The early inflammatory response was severely impaired in B pseudomallei-challenged Asc−/− and, to a lesser extent, Nlrp3−/− mice. At 72 h however, the increased systemic inflammatory response in Nlrp3−/− and Asc−/− mice was more pronounced, correlating with the increased bacterial loads and organ damage. These data expand on an earlier study, reporting that mice deficient in caspase-1, ASC, and NLRP3 are more susceptible to B pseudomallei-induced pneumonia than WTs in terms of bacterial clearance (10). We could however not confirm the differences in survival that were observed in that study between NLRP3- or ASC-deficient mice and WTs in our model (data not shown).
The current data contribute to the insights previously derived from our group and others that IL-1β and IL-18 play opposing roles in the host defense against B pseudomallei(4,10). IL-18 is protective by stimulating the production of IFN-γ in the presence of IL-12, as well as TNF-α, IL-1β, IL-8, and granulocyte-macrophage-colony-stimulating factor (GM-CSF) leading to neutrophil recruitment to the primary site of infection (24). Melioidosis patients show increased IL-18 levels that correlated with mortality (4,11), while IL-18−/− mice are more susceptible to B pseudomallei infection (4,10). In contrast, we now confirm that IL-1β, one of the most potent proinflammatory cytokines, can play a harmful role in the host response in melioidosis. IL-1β is produced by blood monocytes, tissue macrophages, and dendritic cells at hardly detectable levels in healthy individuals, but production can be induced by microbial stimuli as well as by TNFα, IL-18, IL-1α, or IL-1β itself (25). Activation of alternative IL-1β activating pathways could be one explanation for the increased IL-1β levels despite a potential downregulation of NLRP3 and ASC. Inflammasome- and caspase-1-independent cleavage of IL-1 family precursors is described by neutrophil- and macrophage-derived neutral serine proteases such as proteinase 3 (PR3), elastase, and cathepsin-G and micro-organism proteases (21).
Despite widespread availability of antibiotics, melioidosis remains a debilitating septic disease with a high mortality even in areas with a resource-rich setting such as Northern Australia. Clearly, new adjunctive treatment strategies that modulate the immune response are needed. Reports on the emergence of antibiotic resistance further underscore this (2). Our data now add to the increasing amount of evidence that supports the use of anti-IL-1β as a new treatment strategy in melioidosis. Administration of IL-1 receptor antagonist (IL-1ra) directly after B pseudomallei infection has been shown to work protective in mice (10). Importantly, we previously showed that melioidosis patients taking the antidiabetic drug glyburide (=glibenclamide) have a lower mortality and attenuated inflammatory responses compared with patients not taking glyburide (26). Using our murine melioidosis model, we found that glyburide acts as an anti-inflammatory agent by reducing IL-1β secretion accompanied by diminished cellular influx and reduced bacterial dissemination to distant organs (13).
Anti-IL-1β treatment results in a rapid and sustained reduction in disease severity in patients with autoinflammatory syndromes such as familial Mediterranean fever or TNF receptor-associated periodic syndrome, and systemic inflammatory disorders such as rheumatoid arthritis, systemic juvenile idiopathic arthritis, and Behcet disease (25,27). Several IL-1-targeted agents have been approved for use in patients: anakinra is a IL-1 receptor antagonist (IL-1ra), rilonacept a decoy receptor binding to both IL-1α and 1β, and canakinumab is a neutralizing monoclonal anti-IL-1β antibody (25,27). Contradicting results have been reported on the role of IL-1β during sepsis. Blocking the IL-1β-receptor has proven to be protective during LPS-induced shock (28), while mice lacking Il-β were not protected during (high-dose) endotoxemia (29–31). Another study demonstrated that IL-1β deficiency was only partially protective during LPS- and TNF-induced shock and in a caecal ligation and puncture model (32). One phase III randomized placebo-controlled multicenter trial that included 696 subjects has determined the therapeutic efficacy of recombinant human IL-1ra in the treatment of patients with severe sepsis (33). Although IL-1ra treatment was safe and well tolerated, this trial failed to demonstrate a statistically significant reduction in mortality in patients treated with IL-1ra compared with standard therapy. The large heterogeneity of included patients in terms of origin of sepsis and causative organism has been suggested to be a potential reason for the observed lack of effect (33). For future clinical trials a better homogeneously defined patient population, such as patients with pneumonia-derived sepsis caused by B pseudomallei, will be necessary.
It is important to bear in mind that therapies that are successful in animal models do not always have the same effects in human subjects. For instance, the promising TLR4-antagonist Eritoran was able to tamper the endotoxin-mediated cytokine storm in mice, but did not affect mortality in septic patients (34). And even when an agent, such as activated protein C, remains promising after a phase III trial, this effect may not be reproduced in a follow-up trial (PROWESS vs. PROWESS SHOCK) (35). These findings underline that sepsis is a complicated and rapid developing disease entity, for which single-agent therapy might not be sufficient. It has been suggested the value of animal models for sepsis can be improved by mimicking a more realistic clinical scenario (35), for instance by adding antibiotics as a treatment, as we have done here.
Our study was able to demonstrate a protective effect of anti-IL-1β treatment in a murine melioidosis model, using a homogeneous group of experimental animals in a well-controlled environment. Since melioidosis patients differ in their characteristics, such as genetic background, comorbidities, sex and route of infection, our findings cannot be directly extrapolated to humans. Another limitation of our study is that we have not analyzed NLRP3 and ASC mRNA expression in leukocytes that may have been sequestered intravascularly or into sites of infection or organ injury. Studies addressing tissue leukocyte NLRP3 and ASC expression in relation to their IL-1β release and compared with circulating cells will be of interest. Regardless of these limitations, the present study is the first to describe the possible protective role of specific IL-1β-blockade in a clinically relevant model of melioidosis.
In conclusion, our study shows that NLRP3, ASC, and caspase-1 mRNA expression is down-regulated in monocytes of melioidosis patients. The NLRP3 inflammasome and its adaptor ASC play an important role in melioidosis by inducing a protective early immune response including IFN-γ production, thereby reducing systemic bacterial dissemination and inflammation. In contrast, blocking IL-1β reduces inflammation, local and systemic bacterial loads and improves survival of B pseudomallei-infected mice, even when therapy is initiated at a later time point when disease is already apparent.
Acknowledgments
The authors are grateful to Marieke ten Brink and Joost Daalhuisen for their technical assistance. Professor Tom van der Poll is greatly acknowledged for the fruitful discussions leading toward the work presented in this study.
Footnotes
This work was supported by research grants of the Netherlands Organisation for Health Research and Development (ZonMW; grant nr. 90700424) and The Netherlands Organization for Scientific Research (grant nr: 91610008).
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.shockjournal.com).
REFERENCES
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med 2012; 367:1035–1044. [DOI] [PubMed] [Google Scholar]
- 2.Schweizer HP. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 2012; 7:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Golding N, Dance DAB, Messina JP, Pigott DM, Moyes CL, Rolim DB, Bertherat E, Day NPJ, Peacock SJ, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 2016; 1:15008. [DOI] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, Wieland CW, van der Windt GJ, de Boer A, Florquin S, Dondorp A, Day NP, Peacock SJ, van der Poll T. Endogenous interleukin-18 improves the early antimicrobial host response in severe melioidosis. Infect Immun 2007; 75:3739–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821–832. [DOI] [PubMed] [Google Scholar]
- 6.Bryan NB, Dorfleutner A, Kramer SJ, Yun C, Rojanasakul Y, Stehlik C. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J Inflamm (Lond) 2010; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev 2011; 243:136–151. [DOI] [PubMed] [Google Scholar]
- 8.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 2010; 11:1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiersinga WJ, Dessing MC, Kager PA, Cheng AC, Limmathurotsakul D, Day NP, Dondorp AM, van der Poll T, Peacock SJ. High-throughput mRNA profiling characterizes the expression of inflammatory molecules in sepsis caused by Burkholderia pseudomallei. Infect Immun 2007; 75:3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, F Re. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 2011; 7:e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauw FN, Simpson AJ, Prins JM, Smith MD, Kurimoto M, van Deventer SJ, Speelman P, Chaowagul W, White NJ, van der Poll T. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J Infect Dis 1999; 180:1878–1885. [DOI] [PubMed] [Google Scholar]
- 12.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006; 24:317–327. [DOI] [PubMed] [Google Scholar]
- 13.Koh GC, Weehuizen TA, Breitbach K, Krause K, de Jong HK, Kager LM, Hoogendijk AJ, Bast A, Peacock SJ, van der Poll T, et al. Glyburide reduces bacterial dissemination in a mouse model of melioidosis. PLoS Negl Trop Dis 2013; 7:e2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an Interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine 2008; 44:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santanirand P, Harley VS, Dance DA, Drasar BS, Bancroft GJ. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect Immun 1999; 67:3593–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahy RJ, Exline MC, Gavrilin MA, Bhatt NY, Besecker BY, Sarkar A, Hollyfield JL, Duncan MD, Nagaraja HN, Knatz NL, et al. Inflammasome mRNA expression in human monocytes during early septic shock. Am J Respir Crit Care Med 2008; 177:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 2012; 13:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 2011; 35:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Hart J, Knatz N, Hall MW, Wewers MD. Janus kinase 3 down-regulates lipopolysaccharide-induced IL-1 beta-converting enzyme activation by autocrine IL-10. J Immunol 2004; 172:4948–4955. [DOI] [PubMed] [Google Scholar]
- 21.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol 2015; 33:49–77. [DOI] [PubMed] [Google Scholar]
- 22.Zhang AQ, Pan W, Gao JW, Yue CL, Zeng L, Gu W, Jiang JX. Associations between interleukin-1 gene polymorphisms and sepsis risk: a meta-analysis. BMC Med Genet 2014; 15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West TE, Myers ND, Chantratita N, Chierakul W, Limmathurotsakul D, Wuthiekanun V, Miao EA, Hajjar AM, Peacock SJ, Liggitt HD, et al. NLRC4 and TLR5 each contribute to host defense in respiratory melioidosis. PLoS Negl Trop Dis 2014; 8:e3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahoo M, Ceballos-Olvera I, del Barrio L, Re F. Role of the inflammasome, IL-1beta, and IL-18 in bacterial infections. ScientificWorldJournal 2011; 11:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol 2013; 25:469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh GC, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, Lee SJ, Mahavanakul W, Chaowagul W, Chierakul W, et al. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 2011; 52:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11:633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 1990; 348:550–552. [DOI] [PubMed] [Google Scholar]
- 29.Joosten LA, Van De Veerdonk FL, Vonk AG, Boerman OC, Keuter M, Fantuzzi G, Verschueren I, Van Der Poll T, Dinarello CA, Kullberg BJ, et al. Differential susceptibility to lethal endotoxaemia in mice deficient in IL-1alpha, IL-1beta or IL-1 receptor type I. APMIS 2010; 118:1000–1007. [DOI] [PubMed] [Google Scholar]
- 30.Fantuzzi G, Zheng H, Faggioni R, Benigni F, Ghezzi P, Sipe JD, Shaw AR, Dinarello CA. Effect of endotoxin in IL-1 beta-deficient mice. J Immunol 1996; 157:291–296. [PubMed] [Google Scholar]
- 31.Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 2010; 185:4385–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanden Berghe T, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, Vuylsteke M, Roelandt R, Van Wonterghem E, Vandenbroecke J, et al. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 2014; 189:282–291. [DOI] [PubMed] [Google Scholar]
- 33.Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997; 25:1115–1124. [DOI] [PubMed] [Google Scholar]
- 34.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 2013; 309:1154–1162. [DOI] [PubMed] [Google Scholar]
- 35.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?∗. Crit Care Med 2014; 42:1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]


