Abstract
The objective of this study was to identify the initial value of blood lactate that best correlates with 28-day mortality in resuscitated septic shock patients. This was a retrospective cohort study including 443 patients admitted to an intensive care unit (ICU) with severe sepsis or septic shock from the emergency department. A receiver-operating characteristic (ROC) curve was drawn to obtain the best cutoff value for initial blood lactate associated with 28-day mortality. Patients were then dichotomized according to the chosen lactate cutoff, and sensitivity, specificity, and positive and negative predictive values were calculated. Baseline blood lactate level more than 2.5 mmol/L showed the largest area under the ROC curve to predict 28-day mortality (ROC area, 0.70; 95% confidence interval [CI], 0.62–0.79), with sensitivity, specificity, and negative predictive value of 67.4%, 61.7%, and 94.2%, respectively. Mortality at 28 days was 16.9% (31/183) in patients with initial lactate more than 2.5 mmol/L and 5.8% (15/260) in patients with initial lactate at most 2.5 mmol/L (relative risk, 2.93; 95% CI, 1.63–5.28; P < 0.001). Initial blood lactate levels more than 2.5 mmol/L (hazard ratio [HR], 2.86; 95% CI, 1.53–5.33; P = 0.001) and Sepsis-related Organ Failure Assessment score at ICU admission (HR, 1.18; 95% CI, 1.09–1.27; P < 0.001) were associated with increased 28-day mortality in the adjusted Cox regression. In this retrospective cohort study, a lactate level more than 2.5 mmol/L was the best threshold to predict 28-day mortality among severe sepsis and septic shock patients. Further prospective studies should address the impact on morbidity and mortality of this threshold as a trigger to resuscitation in this population of critically ill patients.
Keywords: Biological markers, cohort, lactic acid, mortality, prognosis, ROC curve, sepsis, septic shock
INTRODUCTION
Severe sepsis and septic shock represent leading causes of morbidity and death worldwide, with mortality rates approaching 20% to 30% in the most recent clinical trials (1–3). Incidence of severe sepsis and septic shock has been increasing over the years, despite efforts to improve early recognition and treatment (4, 5).
Increased blood lactate levels in severe sepsis and septic shock most commonly indicate impaired oxidative phosphorylation secondary to decreased oxygen availability to the cells (hypoxic hypoxia) and/or tissue hypoperfusion (stagnant hypoxia) (6). Because blood lactate levels can be easily and quickly determined, these have been used as a surrogate of tissue hypoperfusion in critically ill patients admitted to the emergency department (ED) or to intensive care unit (ICU) (7–13). Indeed, increased blood lactate levels have been used to identify critically ill patients at high risk of death even before the development of hemodynamic instability, i.e. cryptic shock, as well as to trigger resuscitation (14).
Current guidelines for severe sepsis and septic shock resuscitation recommend that patients with severe sepsis or septic shock with an initial blood lactate level twice above the normal limit (≥4 mmol/L) should be promptly resuscitated (15). Nevertheless, an increasing number of studies have been suggesting that lower elevations of blood lactate levels are also associated with increased risk of death (9–11, 13). Therefore, the optimal lactate cutoff that should trigger resuscitation in this population of critically ill patients remains unclear (11).
Our objective was to identify a cutoff value for initial blood lactate level that best correlates with 28-day mortality among medical patients with severe sepsis or septic shock admitted to the ICU from the ED.
PATIENTS AND METHODS
Study design and setting
This was a retrospective cohort study performed at a 41-bed, mixed ICU, in a private tertiary care hospital in São Paulo, Brazil. The study protocol was approved, and informed consent was waived by Hospital Israelita Albert Einstein institutional review board (protocol number 11057312.6.0000.0071).
Patients
All adult (≥18 years) medical patients admitted to the ICU from the ED with severe sepsis or septic shock, diagnosed between January 2008 and December 2012, were included in this study. Patients admitted to the ICU from other hospitals, from the ward, step-down unit, and operating room were excluded from this analysis. Patients without an initial blood lactate measure were also excluded.
Definitions
Sepsis was defined as a systemic inflammatory response syndrome (SIRS) secondary to a confirmed or suspected infection (15). SIRS was defined by the presence of two or more of the following criteria: body temperature more than 38°C or less than 36°C, heart rate more than 90 bpm, respiratory rate more than 20 ipm or partial arterial pressure of CO2 less than 32 mmHg, white blood count more than 12,000/mm3 or less than 4,000/mm3, or the presence of more than 10% rods (16). Severe sepsis was defined as sepsis associated with organ dysfunction, and septic shock was defined as hypotension refractory to fluid infusion with the need for vasopressors (15).
According to our institutional protocol for severe sepsis and septic shock resuscitation, patients admitted to the ED who fulfilled criteria for severe sepsis and septic shock were submitted to blood sampling for lactate measurement and blood cultures, received a first dose of broad-spectrum antibiotics within 1 h from the admission, received a fluid load with 20 to 30 mL/kg of crystalloids, and were admitted to the ICU (17, 18).
Goal-directed therapy was applied to patients with severe sepsis associated with arterial lactate levels at least 4.0 mmol/L or those who remained hypotensive (systolic blood pressure <90 mmHg or mean arterial blood pressure [MAP] <65 mmHg) despite fluid resuscitation (17, 18). The following therapeutic goals were targeted during the first 6 h of resuscitation: central venous pressure between 8 and 12 mmHg (12–15 mmHg in mechanically ventilated patients), MAP at least 65 mmHg, central venous oxygen saturation (ScvO2) at least 70%, and diuresis at least 0.5 mL/kg/h (17, 18).
Collected variables
The following data were collected and recorded: demographic characteristics, comorbidities, admission diagnosis, Acute Physiology and Chronic Health Evaluation II (APACHE II) score (19), Sepsis-related Organ Failure Assessment (SOFA) score (20), site of infection, hemodynamic parameters, administered treatments (fluids, vasopressors, blood transfusion, steroids, and antibiotics) during the first 24 h of ICU admission, the need for mechanical ventilation, mortality at day 28, and length of ICU and hospital stays.
Lactate clearance time (time span between the first two blood lactate measurements; hours), absolute lactate clearance (initial lactate value minus delayed value; mmol/L), relative lactate clearance (absolute clearance divided by initial value and the result multiplied by 100; %), and lactate clearance rate (relative clearance divided by clearance time; % per hour) were determined as previously described (21). Blood lactate levels were obtained after severe sepsis, and septic shock diagnosis at the ED and after ICU admission was measured by catalyzed lactate oxidase (VITROS; Jonhson & Johnson, New Brunswick, NJ) and expressed in mmol/L.
Statistical analysis
Categorical variables were expressed as absolute and relative frequencies and continuous variables as median and interquartile range (IQR). Binary variables were compared with chi-square test or with Fisher exact test when appropriate. Continuous variables were compared with Mann-Whitney U test.
A receiver-operating characteristic (ROC) curve was constructed to assess the best blood lactate level cutoff related to 28-day mortality. Subsequently, patients were dichotomized according to the lactate cutoff chosen by ROC curve analysis. We calculated sensitivity, specificity, and positive and negative predictive value for this cutoff value. We also constructed ROC curves to test the ability of initial lactate levels, absolute lactate clearance, relative lactate clearance, and lactate clearance rate to predict 28-day mortality in the subgroup of patients who had more than one blood lactate measurement performed during the first 24 h of ED admission.
Survival analysis was performed using the Kaplan-Meier method with the dichotomized values of initial lactate levels, and the log-rank test was used to compare the groups. Unadjusted analysis was performed using the Cox proportional hazards regression model, using as censored variable, 28-day mortality, and time in days (up to 28 days), including sex, age, presence of comorbidities, severe sepsis or septic shock diagnosis, APACHE II score, SOFA score at ICU admission, vasopressor use, need for mechanical ventilation, and site of infection. Variables with P < 0.20 in the unadjusted analysis were included in the adjusted Cox regression and those with P < 0.05 were included in the final model. The hazard ratios (HRs) with their respective 95% confidence intervals (95% CIs) were estimated for each covariate.
Two-tailed tests were used, and when P < 0.05, the test was considered statistically significant. Stata version 13.0 (StataCorp, College Station, Tex) and SPSS version 21.0 (IBM Statistical Package for the Social Science) were used for statistical analyses.
RESULTS
Study patients and clinical presentation
Four hundred forty-three patients were included in this analysis. Demographics and clinical characteristics of studied patients are shown in Table 1. The median (IQR) age was 70 (55–82) years. Severe sepsis patients accounted for 58.2% (258/443) of patients and septic shock for 41.8% (185/443) of patients. The most prevalent comorbidities were systemic hypertension (32.7%) and diabetes mellitus (20.8%). The most common site of infection was the respiratory tract (50.6%), followed by the urinary tract (20.3%) (Table 1).
Table 1.
Baseline characteristics of 443 severe sepsis and septic shock patients admitted to the intensive care unit
| Characteristics | All (N = 443) | Lactate ≤2.5 mmol/L (N = 260) | Lactate >2.5 mmol/L (N = 183) | P* |
| Age, year, median (IQR) | 70 (55–82) | 71 (54–83) | 70 (58–82) | 0.845 |
| Male, n (%) | 273 (61.6) | 156 (60.0) | 117 (63.9) | 0.428 |
| Comorbidities, n (%) | ||||
| Systemic hypertension | 145 (32.7) | 80 (30.8) | 65 (35.5) | 0.305 |
| Diabetes mellitus | 92 (20.8) | 51 (19.6) | 41 (22.4) | 0.478 |
| Transplant | 48 (10.8) | 29 (11.2) | 19 (10.4) | 0.877 |
| Oncologic | 46 (10.4) | 25 (9.6) | 21 (11.5) | 0.531 |
| Chronic kidney failure | 30 (6.8) | 20 (7.8) | 10 (5.5) | 0.443 |
| Congestive heart failure | 28 (6.3) | 19 (7.4) | 9 (4.9) | 0.329 |
| Liver cirrhosis | 14 (3.2) | 4 (1.5) | 10 (5.5) | 0.026 |
| COPD | 14 (3.2) | 5 (1.9) | 9 (4.9) | 0.098 |
| Source of sepsis, n (%) | ||||
| Respiratory system | 224 (50.6) | 146 (56.2) | 78 (42.6) | 0.005 |
| Urinary system | 90 (20.3) | 44 (16.9) | 46 (25.1) | 0.041 |
| Abdominal | 73 (16.5) | 34 (13.1) | 39 (21.3) | 0.027 |
| Skin and soft tissues | 17 (3.8) | 11 (4.2) | 6 (3.3) | 0.803 |
| Bloodstream | 10 (2.3) | 6 (2.3) | 4 (2.2) | 1.000 |
| Unknown | 21 (4.7) | 14 (5.4) | 7 (3.8) | 1.000 |
| Other | 8 (1.8) | 5 (1.9) | 3 (1.6) | 1.000 |
| Diagnosis at ED, n (%) | ||||
| Severe sepsis | 258 (58.2) | 166 (63.8) | 92 (50.3) | 0.005 |
| Septic shock | 185 (41.8) | 94 (36.2) | 91 (49.7) | 0.005 |
| APACHE II score, median (IQR) | 20 (17–24) | 19 (16–25) | 20 (17–24) | 0.542 |
| SOFA score, median (IQR) | 4 (3–7) | 4 (3–6) | 5 (3–8) | 0.033 |
| Initial lactate, mmol/L, median (IQR) | 2.1 (1.3–3.3) | 1.44 (1.11–1.89) | 3.66 (3.00–5.77) | <0.001 |
| ScvO2, %, median (IQR) | 75 (71–81) | 75 (70–81) | 75 (72–81) | 0.406 |
| Vasopressor use on day 1, n (%) | 185 (42.3) | 92 (37.5) | 93 (52.0) | 0.001 |
| Mechanical ventilation on day 1, n (%) | 83 (18.9) | 48 (18.5) | 35 (19.4) | 0.806 |
*P values were provided by chi-square test or Fisher exact test for binary variables and Mann-Whitney U test for continuous variables.
APACHE II, Acute Physiology and Chronic Health Evaluation II (varies from 0 to 71, higher values indicate greater severity); COPD, chronic obstructive pulmonary disease; ED, emergency department; IQR, interquartile range; ScvO2, central venous oxygen saturation; SOFA score, Sequential Organ Failure Score (range 0–24, higher values indicate greater number of organ dysfunction) at ICU admission.
Values represent median (IQR) or n (%).
The median (IQR) APACHE II score was 20 (17–24), and the median SOFA score at ICU admission was 4 (3–7). The median (IQR) initial blood lactate level was 2.1 mmol/L (1.3–3.3 mmol/L) and median (IQR) ScvO2 was 75% (71%–81%). Approximately 42% of patients (185/443) were on vasopressor drip and 19% (83/443) required mechanical ventilation during the first 24 h of ICU admission (Table 1).
Initial lactate levels as a predictor of death
The blood lactate cutoff of 2.5 mmol/L showed the largest area under the ROC curve (ROC area = 0.70) related to 28-day mortality (Fig. 1). The sensitivity, specificity, and negative predictive value of initial lactate levels more than 2.5 mmol/L for 28-day mortality were 67.4%, 61.7%, and 94.2%, respectively (Table 2).
Fig. 1.
ROC curve addressing the association between admission blood lactate and 28-day mortality.
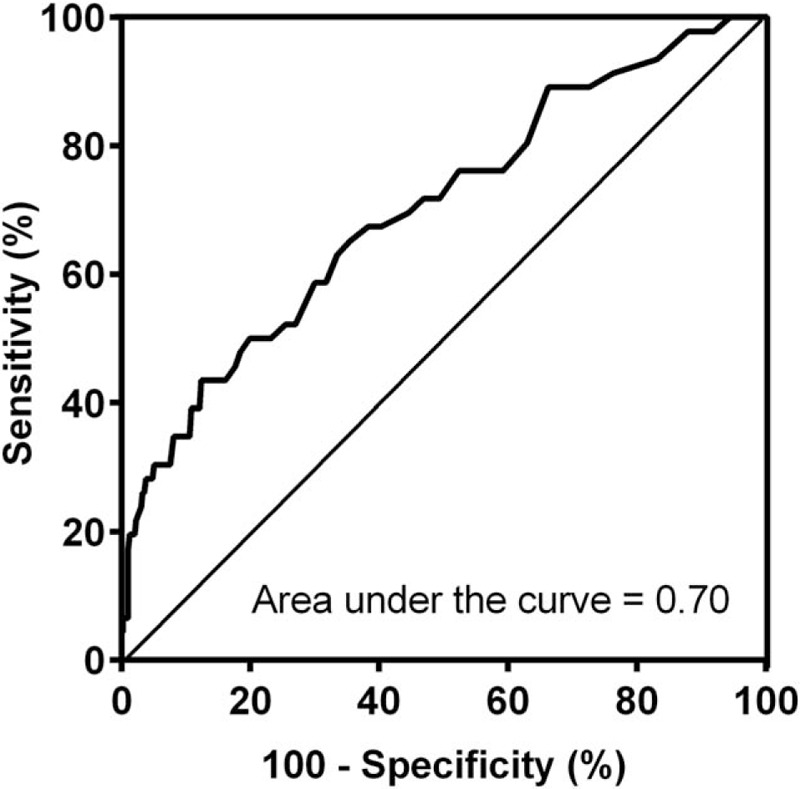
ROC, receiver-operating characteristic.
Table 2.
Diagnostic assessment of initial blood lactate more than 2.5 mmol/L to predict 28-day mortality
| Measurement | Estimate | 95% CI |
| Area under the ROC Curve | 0.70 | 0.62–0.79 |
| Sensitivity | 67.4 | 52.0–80.5 |
| Specificity | 61.7 | 56.7–66.5 |
| Positive predictive value | 16.9 | 11.8–23.2 |
| Negative predictive value | 94.2 | 90.7–96.7 |
CI, confidence interval; ROC, receiver-operating characteristic.
Mortality at 28 days was 16.9% (31/183) in patients with initial lactate more than 2.5 mmol/L and 5.8% (15/260) in patients with initial lactate at most 2.5 mmol/L (absolute difference, 11.1% [95% CI, 5.0%–17.3%]; relative risk, 2.93 [95% CI, 1.63–5.28]; P < 0.001). Initial blood lactate levels more than 2.5 mmol/L were significantly associated with increased 28-day mortality (HR, 3.22; 95% CI, 1.74–5.98; P < 0.001) (Fig. 2).
Fig. 2.
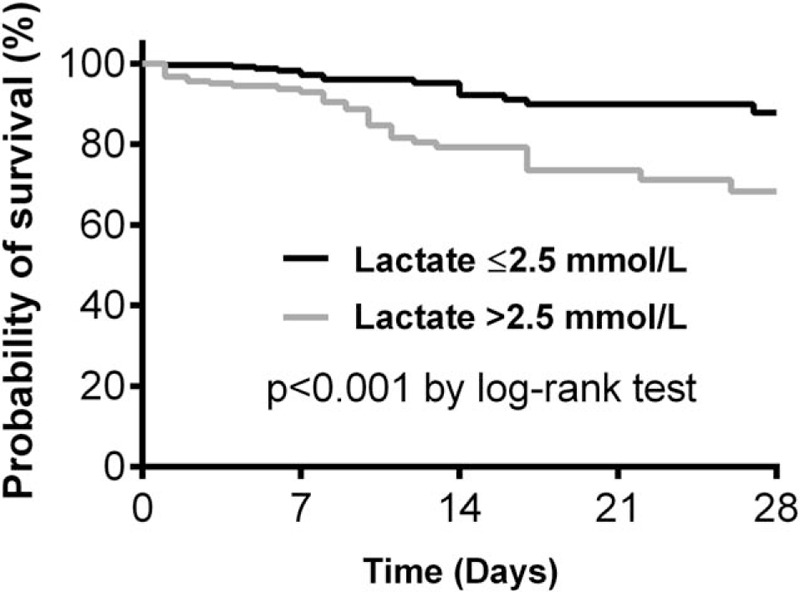
Kaplan-Meier survival curves for 28-day survival according to lactate levels.
Lactate kinetics as a predictor of death
Out of the 443 patients included in the primary analysis, 260 (58.7%) had more than one lactate measurement performed during the first 24 h of ED admission. Of those 260 patients, 33 (12.7%) died, whereas 227 (87.3%) were alive at day 28 (Table 3). Lactate clearance time, absolute lactate clearance, relative lactate clearance, and lactate clearance rate did not differ between survivals and nonsurvivals (Table 3). The AUC was highest for the initial lactate levels (0.664) followed by absolute lactate clearance (0.571), clearance rate (0.550), and relative lactate clearance (0.515).
Table 3.
Measures of lactate kinetics and their association with 28-day mortality
| Characteristics | Dead at day 28, 33/260 (12.7%) | Alive at day 28, 227/260 (87.3%) | P* |
| Initial lactate, mmol/L | 3.66 (2.00–7.10) | 2.33 (1.44–3.44) | 0.002 |
| Second lactate, mmol/L | 2.78 (1.56–5.22) | 1.78 (1.22–2.67) | 0.001 |
| Lactate clearance time, h | 6 (4–14) | 6 (5–11) | 0.481 |
| Absolute lactate clearance, mmol/L | 0.55 (0.00–1.88) | 0.33 (−0.22 to 1.44) | 0.186 |
| Relative lactate clearance, % | 17.5 (−0.11 to 39.1) | 17.7 (−11.2 to 45.4) | 0.778 |
| Lactate clearance rate, %/h | 2.84 (−0.01 to 6.64) | 1.90 (−1.48 to 6.79) | 0.353 |
*P values were provided by Mann-Whitney U test.
Values represent median (IQR).
Predictors of 28-day mortality
From the initial model containing 10 predictors (Table 4), the backward elimination Cox regression analysis yielded a final model containing lactate more than 2.5 mmol/L (HR, 2.86; 95% CI, 1.53–5.33; P = 0.001) and SOFA score at ICU admission (HR, 1.18; 95% CI, 1.09–1.27; P < 0.001) significantly associated with increased 28-day mortality. The presence of diabetes mellitus was an independent protective factor (HR, 0.25; 95% CI, 0.08–0.84; P = 0.024) (Table 4).
Table 4.
Univariate and multivariate Cox proportional hazards regression models to assess which variables were independently associated with 28-day mortality
| Univariate analysis | Multivariate analysis | |||||
| Characteristics | HR | 95% CI | P | HR | 95% CI | P |
| Lactate >2.5 mmol/L | 3.22 | 1.74–5.98 | <0.001 | 2.86 | 1.53–5.33 | 0.001 |
| Systemic hypertension | 0.54 | 0.26–1.11 | 0.095 | |||
| Diabetes mellitus | 0.27 | 0.08–0.87 | 0.028 | 0.26 | 0.08–0.84 | 0.024 |
| Liver cirrhosis | 2.34 | 0.84–6.53 | 0.105 | |||
| Abdominal infection | 1.98 | 1.03–3.83 | 0.042 | |||
| APACHE II score | 1.04 | 0.90–1.10 | 0.124 | |||
| SOFA score | 1.20 | 1.12–1.29 | <0.001 | 1.18 | 1.09–1.27 | <0.001 |
| Septic shock | 2.17 | 1.18–3.96 | 0.013 | |||
| Vasopressor use | 1.94 | 1.05–3.56 | 0.034 | |||
| Mechanical ventilation | 1.79 | 0.98–3.26 | 0.059 | |||
CI, confidence interval; HR, hazard ratio; SOFA, Sequential Organ Failure Score (range 0–24, higher values indicate greater number of organ dysfunction); APACHE II score, Acute Physiology and Chronic Health Evaluation II (varies from 0 to 71, higher values indicate greater severity).
DISCUSSION
In our retrospective single-center cohort study with 443 severe sepsis and septic shock patients admitted to the ICU, initial lactate levels more than 2.5 mmol/L exhibited the highest sensitivity, specificity, and negative predictive value for 28-day mortality. Patients with initial lactate levels above this cutoff had a mortality rate 3.2 times higher than patients with initial lactate lower or equal to 2.5 mmol/L.
Clinical studies have emphasized tissue hypoxia, characterized by supply-dependent oxygen consumption, as a leading cause of increased lactate levels in septic patients (22, 23). During the acute phase, hyperlactatemia is often considered a marker of tissue hypoperfusion (15). Although the current guidelines for severe sepsis and septic shock resuscitation recommend that patients with severe sepsis or septic shock with an initial blood lactate level of at least 4.0 mmol/L must be promptly resuscitated (15), recent studies have shown that less expressive elevations in lactate levels have also been associated with poor outcomes (9–13), regardless of the presence of hepatic dysfunction (12).
Several other studies are consistent with our findings of mild hyperlactatemia as a predictor of mortality in septic patients (9, 10, 13). A prospective single-center cohort study involving 1,287 patients admitted to the ED with suspected infection showed that initial venous lactate levels between 2.5 and 4.0 mmol/L were independently associated with an increased risk of 28-day in-hospital death (9). Another single-center cohort study including 830 patients with severe sepsis and septic shock admitted to the ED showed that initial venous lactate levels between 2.0 and 3.9 mmol/L, compared with initial lactate levels less than 2.0 mmol/L, were associated with increased mortality at day 28, regardless of the presence of shock (10).
A post hoc analysis of the Vasopressin in Septic Shock Trial, including 665 patients, showed that patients with lactate levels between 1.4 and 2.3 mmol/L had a significantly increased risk of 28-day mortality and organ dysfunction compared with those with lactate at most 1.4 mmol/L (13). In this study, baseline lactate values of 2.3 mmol/L exhibited 60% sensitivity and 55% specificity for 28-day mortality (13), which is similar to our findings. Therefore, initial blood lactate levels measure, commonly available in most EDs and ICUs, is a valuable tool to help clinicians at the bedside identify high-risk septic patients in need of additional resources of care, such as ICU admission, invasive hemodynamic monitoring, and varying degrees of organ support. Another post hoc analysis of a multicenter, noninferiority trial showed that initial venous lactate levels had an area under the ROC curve of 0.64 to predict in-hospital survival (21). In contrast to our study, only patients with initial venous lactate levels at least 2.0 mmol/L were included, and the authors did not show the exact lactate threshold associated with the highest area under the ROC curve (21).
Along with our results, available evidence suggests that the current guidelines might be too conservative when recommending that resuscitation should be only reserved for those septic patients with blood lactate concentrations at least 4.0 mmol/L (15). Considering the increased risk of unfavorable outcomes reported in septic patients presenting to the ED with intermediate hyperlactatemia (9–13), aggressive resuscitation may be advisable and might improve morbidity and mortality. Nevertheless, a mild hyperlactatemia must be placed in the appropriate clinical context to prove value as a prognostication in sepsis (24). Indeed, a recent randomized controlled trial involving 348 critically ill patients used a lower cutoff of blood lactate concentration (≥3.0 mmol/L) to trigger resuscitation (25). In this study, patients undergoing lactate-guided therapy exhibited a lower risk of in-hospital mortality than the control group (25).
We found diabetes mellitus to be a protective factor for 28-day mortality in patients with severe sepsis and septic shock. Although patients with diabetes have an increased risk of infections and sepsis (26, 27) due to depressed humoral and cellular immunity (28, 29), conflicting data exist on whether the outcomes of septic patients are affected by the presence of diabetes (30). Although some authors reported increased mortality among patients with diabetes with infection (31, 32), others reported improved survival (33–35). The exact mechanisms responsible for these controversial findings remain unclear (30, 36). It was demonstrated that administration of exogenous insulin was associated with improvement on host immunity and decreased production of proinflammatory mediators, such as tumor necrosis factor alpha (37), decreased production of macrophage migration inhibitory factor, and intranuclear factor kappa B and reactive oxygen species generation by mononuclear cells in obese patients (38). Therefore, we can hypothesize that blood glucose control with exogenous insulin administration, which is routinely used in ICU patients (39), could explain, at least partially, the mechanism related to the observed protective effect of diabetes on 28-day mortality in our study population. In addition, besides the effect of insulin on the immune and inflammatory response, diabetes has been associated with reduced risk of acute respiratory dysfunction, which may have positively affected our population of critically ill patients (40).
Our study has limitations. First, this was a single-center study. Therefore, our results may have limited external validity. Second, because of the retrospective nature of our study, we are subject to selection and information bias. We analyzed variables that were routinely collected and documented as part of patient care. Third, patients admitted to the ED after 2010 could have received fluids, vasopressors, inotropes, and red blood cells transfusion guided by lactate clearance (41). Nevertheless, because all patients received their first dose of broad-spectrum antibiotics within 1 h from the admission and received an initial fluid load (20–30 mL/kg of crystalloids), it is unlikely that additional therapeutic interventions guided by the lactate clearance or ScvO2 would have biased our results. Fourth, survival bias could have undervalued the observed association between lactate clearance and 28-day mortality in our study. Thus, our results must be interpreted with caution. Finally, patients recovering from surgery and those with a higher risk of delayed resuscitation (ward and hospital referrals) were not included in this analysis, which might have had an impact on our observed mortality rate and death prediction.
CONCLUSION
In our retrospective cohort study, severe sepsis or septic shock patients admitted to the ICU from the ED with initial blood lactate more than 2.5 mmol/L were at increased risk of death. Further prospective multicenter studies should address the impact of lower serum lactate cutoffs as a trigger to resuscitation on morbidity and mortality in this population of critically ill patients.
Acknowledgments
The authors thank James Hesson for proofreading this manuscript.
Footnotes
RRF, LLR, and TDC equally contributed to this work.
The authors report no conflicts of interest.
REFERENCES
- 1.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370 18:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371 16:1496–1506. [DOI] [PubMed] [Google Scholar]
- 3.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372 14:1301–1311. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29 7:1303–1310. [DOI] [PubMed] [Google Scholar]
- 5.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care 2009; 13 1:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014; 18 5:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med 2005; 45 5:524–528. [DOI] [PubMed] [Google Scholar]
- 8.Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, Hollenberg SM, Parrillo JE. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med 2007; 33 6:970–977. [DOI] [PubMed] [Google Scholar]
- 9.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med 2007; 33 11:1892–1899. [DOI] [PubMed] [Google Scholar]
- 10.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009; 37 5:1670–1677. [DOI] [PubMed] [Google Scholar]
- 11.Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care 2010; 14 1:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang YR, Um SW, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ, Jeon K. Initial lactate level and mortality in septic shock patients with hepatic dysfunction. Anaesth Intensive Care 2011; 39 5:862–867. [DOI] [PubMed] [Google Scholar]
- 13.Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock 2012; 38 1:4–10. [DOI] [PubMed] [Google Scholar]
- 14.Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med 1996; 14 2:218–225. [DOI] [PubMed] [Google Scholar]
- 15.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41 2:580–637. [DOI] [PubMed] [Google Scholar]
- 16.Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20 6:864–874. [PubMed] [Google Scholar]
- 17.Assuncao MS, Teich V, Shiramizo SC, Araujo DV, Carrera RM, Serpa NA, Silva E. The cost-effectiveness ratio of a managed protocol for severe sepsis. J Crit Care 2014; 29 4:692–696. [DOI] [PubMed] [Google Scholar]
- 18.Palomba H, Correa TD, Silva E, Pardini A, Assuncao MS. Comparative analysis of survival between elderly and non-elderly severe sepsis and septic shock resuscitated patients. Einstein (Sao Paulo) 2015; 13 3:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13 10:818–829. [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, Willatts S, De MA, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22 7:707–710. [DOI] [PubMed] [Google Scholar]
- 21.Puskarich MA, Trzeciak S, Shapiro NI, Albers AB, Heffner AC, Kline JA, Jones AE. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013; 143 6:1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronco JJ, Fenwick JC, Tweeddale MG, Wiggs BR, Phang PT, Cooper DJ, Cunningham KF, Russell JA, Walley KR. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 1993; 270 14:1724–1730. [PubMed] [Google Scholar]
- 23.Friedman G, De BD, Shahla M, Vincent JL. Oxygen supply dependency can characterize septic shock. Intensive Care Med 1998; 24 2:118–123. [DOI] [PubMed] [Google Scholar]
- 24.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315 8:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen TC, van BJ, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med 2010; 182 6:752–761. [DOI] [PubMed] [Google Scholar]
- 26.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003; 26 2:510–513. [DOI] [PubMed] [Google Scholar]
- 27.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005; 41 3:281–288. [DOI] [PubMed] [Google Scholar]
- 28.Alexiewicz JM, Kumar D, Smogorzewski M, Klin M, Massry SG. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med 1995; 123 12:919–924. [DOI] [PubMed] [Google Scholar]
- 29.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med 1997; 14 1:29–34. [DOI] [PubMed] [Google Scholar]
- 30.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med 1999; 341 25:1906–1912. [DOI] [PubMed] [Google Scholar]
- 31.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996; 275 2:134–141. [PubMed] [Google Scholar]
- 32.Thomsen RW, Hundborg HH, Lervang HH, Johnsen SP, Schonheyder HC, Sorensen HT. Diabetes mellitus as a risk and prognostic factor for community-acquired bacteremia due to enterobacteria: a 10-year, population-based study among adults. Clin Infect Dis 2005; 40 4:628–631. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen RW, Hundborg HH, Lervang HH, Johnsen SP, Sorensen HT, Schonheyder HC. Diabetes and outcome of community-acquired pneumococcal bacteremia: a 10-year population-based cohort study. Diabetes Care 2004; 27 1:70–76. [DOI] [PubMed] [Google Scholar]
- 34.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care 2009; 13 1:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham BB, Keniston A, Gajic O, Trillo Alvarez CA, Medvedev S, Douglas IS. Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit Care Med 2010; 38 1:16–24. [DOI] [PubMed] [Google Scholar]
- 36.Schuetz P, Castro P, Shapiro NI. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care 2011; 34 3:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das UN. Is insulin an antiinflammatory molecule? Nutrition 2001; 17 5:409–413. [DOI] [PubMed] [Google Scholar]
- 38.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 2001; 86 7:3257–3265. [DOI] [PubMed] [Google Scholar]
- 39.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345 19:1359–1367. [DOI] [PubMed] [Google Scholar]
- 40.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med 2000; 28 7:2187–2192. [DOI] [PubMed] [Google Scholar]
- 41.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 2010; 303 8:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]


