Abstract
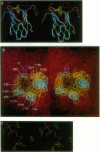
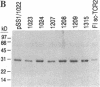
A computer-generated model of the single-chain variable V alpha V beta fragment of the RFL3.8 T-cell receptor (TCR) specific for fluorescein served as a starting point for mutagenesis aimed at identification of its antigen-contacting residues. Selected backbone segments of the model representing regions of prominent sequence similarity between antibodies and TCRs were least-squares superimposed onto the corresponding segments of the crystallographically resolved 4-4-20 antibody complexed with its antigen, fluorescein. The superimposition placed the antibody-bound fluorescein molecule close to a cavity on the surface of the TCR model formed by the complementarity-determining region (CDR) loops. Some of the TCR cavity forming loops displayed sequence motifs related to canonical CDR loops previously found in antibodies. Six putative amino acid contacts were identified and single-chain TCRs with mutations at each of these positions were expressed in Escherichia coli, purified, refolded, and assayed for fluorescein binding. Five of the six mutations resulted in a loss of detectable binding. These RFL3.8 antigen combining site residues are distributed among the beta 3, alpha 1, and alpha 2 CDR loops and show striking chemical similarity to the known fluorescein contact residues on 4-4-20. Thus, antibodies and TCRs are similar both in their overall architecture and in the chemical details of specific antigen recognition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Ashwell J. D., Klusner R. D. Genetic and mutational analysis of the T-cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Levitt M., Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. The predicted structure of immunoglobulin D1.3 and its comparison with the crystal structure. Science. 1986 Aug 15;233(4765):755–758. doi: 10.1126/science.3090684. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Novotný J., Bruccoleri R., Karplus M. Domain association in immunoglobulin molecules. The packing of variable domains. J Mol Biol. 1985 Dec 5;186(3):651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Connolly M. L. Solvent-accessible surfaces of proteins and nucleic acids. Science. 1983 Aug 19;221(4612):709–713. doi: 10.1126/science.6879170. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Jhurani P., Ng P., Wells J. A. Receptor and antibody epitopes in human growth hormone identified by homolog-scanning mutagenesis. Science. 1989 Mar 10;243(4896):1330–1336. doi: 10.1126/science.2466339. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Wells J. A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989 Jun 2;244(4908):1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- Dao-Pin S., Baase W. A., Matthews B. W. A mutant T4 lysozyme (Val 131----Ala) designed to increase thermostability by the reduction of strain within an alpha-helix. Proteins. 1990;7(2):198–204. doi: 10.1002/prot.340070208. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Goodnow C. C., Dudzik K. I., Oi V. T., Davis M. M. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc Natl Acad Sci U S A. 1987 May;84(9):2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire C., Rebaï N., Schweisguth F., Necker A., Mazza G., Auphan N., Millward A., Schmitt-Verhulst A. M., Malissen B. Engineered secreted T-cell receptor alpha beta heterodimers. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8077–8081. doi: 10.1073/pnas.88.18.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M. H., Sauer R. T. Phage lambda repressor revertants. Amino acid substitutions that restore activity to mutant proteins. J Mol Biol. 1985 Nov 5;186(1):53–63. doi: 10.1016/0022-2836(85)90256-6. [DOI] [PubMed] [Google Scholar]
- Herron J. N., He X. M., Mason M. L., Voss E. W., Jr, Edmundson A. B. Three-dimensional structure of a fluorescein-Fab complex crystallized in 2-methyl-2,4-pentanediol. Proteins. 1989;5(4):271–280. doi: 10.1002/prot.340050404. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Esser U., Fazekas de St Groth B., Reay P. A., Davis M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992 Jan 16;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Mariuzza R. A., Winter G. Secretion of a homodimeric V alpha C kappa T-cell receptor-immunoglobulin chimeric protein. J Biol Chem. 1989 May 5;264(13):7310–7316. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hercend T., Schlossman S. F., Reinherz E. L. The human T-cell receptor. Annu Rev Immunol. 1984;2:23–50. doi: 10.1146/annurev.iy.02.040184.000323. [DOI] [PubMed] [Google Scholar]
- Nalefski E. A., Wong J. G., Rao A. Amino acid substitutions in the first complementarity-determining region of a murine T-cell receptor alpha chain affect antigen-major histocompatibility complex recognition. J Biol Chem. 1990 May 25;265(15):8842–8846. [PubMed] [Google Scholar]
- Novotny J., Bruccoleri R. E., Kourilsky P. On the molecular nature of "restrictive" antigenic elements present on major histocompatibility complex (MHC) proteins. Res Immunol. 1989 Feb;140(2):145–158. doi: 10.1016/0923-2494(89)90079-5. [DOI] [PubMed] [Google Scholar]
- Novotny J., Ganju R. K., Smiley S. T., Hussey R. E., Luther M. A., Recny M. A., Siliciano R. F., Reinherz E. L. A soluble, single-chain T-cell receptor fragment endowed with antigen-combining properties. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8646–8650. doi: 10.1073/pnas.88.19.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotný J., Tonegawa S., Saito H., Kranz D. M., Eisen H. N. Secondary, tertiary, and quaternary structure of T-cell-specific immunoglobulin-like polypeptide chains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):742–746. doi: 10.1073/pnas.83.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J. Hypothesis: a molecular model for the MHC-restricted recognition of antigens by the T-cell receptor. Ann Inst Pasteur Immunol. 1987 Mar-Apr;138(2):175–180. doi: 10.1016/s0769-2625(87)80069-7. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Wu T. T., Kabat E. A. Subgroups of variable region genes of beta chains of T-cell receptors for antigen. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4461–4463. doi: 10.1073/pnas.83.12.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano R. F., Hemesath T. J., Pratt J. C., Dintzis R. Z., Dintzis H. M., Acuto O., Shin H. S., Reinherz E. L. Direct evidence for the existence of nominal antigen binding sites on T cell surface Ti alpha-beta heterodimers of MHC-restricted T cell clones. Cell. 1986 Oct 24;47(2):161–171. doi: 10.1016/0092-8674(86)90439-3. [DOI] [PubMed] [Google Scholar]
- Strominger J. L. Developmental biology of T cell receptors. Science. 1989 May 26;244(4907):943–950. doi: 10.1126/science.2658058. [DOI] [PubMed] [Google Scholar]
- Strong R. K., Campbell R., Rose D. R., Petsko G. A., Sharon J., Margolies M. N. Three-dimensional structure of murine anti-p-azophenylarsonate Fab 36-71. 1. X-ray crystallography, site-directed mutagenesis, and modeling of the complex with hapten. Biochemistry. 1991 Apr 16;30(15):3739–3748. doi: 10.1021/bi00229a022. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Chothia C., Lesk A. M. Framework residue 71 is a major determinant of the position and conformation of the second hypervariable region in the VH domains of immunoglobulins. J Mol Biol. 1990 Sep 5;215(1):175–182. doi: 10.1016/S0022-2836(05)80102-0. [DOI] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992 Apr 30;356(6372):793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]