Abstract
Background:
Cabergoline is a long-acting agonist of dopamine, which has a high affinity to dopamine receptors (type 2). Treatment using a dopaminergic agonist reduces hypothalamic stimulation that increases during liver gluconeogenesis, lipids synthesis, and insulin resistance. Our aim was to evaluate the effects of cabergoline on blood glucose levels in patients with type 2 diabetes mellitus (DM).
Methods:
This study was a double-blind, controlled clinical trial in patients with type 2 DM. The patients received treatments of a placebo (control group; n = 20) or cabergoline 0.5 mg (cabergoline group; n = 20) using the sequential method, once per week for 3 months, while using previously prescribed glucose-lowering drugs. All tests, such as levels of fasting blood glucose, 2-hour post-prandial glucose, complete lipid profile, prolactin, alanine amino transferase, aspartate amino transferase, creatinine, blood urea nitrogen, and serum insulin, and homeostasis model assessment insulin resistance were measured at baseline and at 3-month follow-up.
Results:
The fasting blood sugar levels were significantly different between placebo and cabergoline groups after 3 months of treatment (P = 0.004). The prolactin levels were significantly different from beginning of the treatment to 6 months later (P = 0.001). In the cabergoline group, there was a significant decrease in glycosylated hemoglobin (HbA1C) levels after 3 months (P = 0.003). Overall, 65%and 45% patients in the cabergoline and control groups, respectively, responded to treatment (HbA1C<7%).
Conclusion:
Cabergoline may be useful as a long-acting antidiabetic agent in patients with type 2 diabetes mellitus.
Keywords: blood glucose levels, cabergoline, type 2 diabetic
1. Introduction
Type 2 diabetes mellitus (DM) is the most common type of DM. The prevalence of type 2 DM has remarkably increased with the growing rate of obesity during the past decade, and half of these patients are unaware of their disease.[1] In 2015, approximately 8.8% of people had diabetes worldwide and this percentage will likely increase by10.4% in 2040.[2] Approximately 5 million people aged between 20 and 79 years died from diabetes in 2015, equivalent to 1 death every 6 seconds.[2] Diabetes accounted for 14.5% of global all-cause mortality among people in this age group. There are about 4.6 million diabetic people living in Iran in 2015. Moreover, the percentage of diabetic patients is predicted to increase by 122% and 42% in developing and developed countries, respectively, from 1995 to 2025. The treatment costs for retinopathy, nephropathy, and cardiovascular disease may be equivalent to half the public health costs in the United States.[2] Due to increased treatment costs, researchers suggest that achieving treatment goals can noticeably reduce the above-mentioned complications. However, only 50% to 70% of patients with type 2 DM will achieve these goals despite available treatments. Therefore, researchers have provided special attention to the use of novel drugs and treatment protocols in the recent years.[3]
Dopamine and dopaminergic signals control some metabolic pathways, such as those in the central nervous system (CNS).[4] Based on these metabolic pathways, we can claim that glucose metabolism is accurately controlled by the CNS and medioventral area of the hypothalamus.[5] The CNS controls liver gluconeogenesis via sympathetic nervous system; however, this pathway is not functional in obese diabetic patients who have impaired responses to the pathway signals. These patients have increased liver gluconeogenesis, insulin resistance, and β-cell disorders in the pancreas.[6] It has also been reported that agents, which interfere with dopaminergic activity (i.e., antipsychotics), will lead to side effects, such as metabolic disorders, weight gain, insulin resistance, and dyslipidemia.[7]
Bromocriptine is a dopaminergic agonist type 2, which can affect serotonin turnover in the CNS.[7,8] The main indications of bromocriptine during the past 25 years are Parkinson's disease, prolactinoma, acromegaly, infertility, and galactorrhea.[9] Bromocriptine can also be used to treat many metabolic disorders associated with insulin resistance and obesity.[10] Furthermore, it is observed that treatment by a dopaminergic agonist reduces hypothalamic stimulation that increases during liver gluconeogenesis, lipids synthesis, and insulin resistance.[11,12] This agent such as bromocriptine has been substantiated for use in type 2 DM patients by the Food and Drug Administration (FDA). In contrast to other glucose-lowering agents, bromocriptine is beneficial for controlling blood sugar levels via its effect on the CNS and by reducing glycogenesis.[13,14] Cycloset is a rapid-releasing variant of bromocriptine, which reduces insulin resistance, hyperlipidemia, and hyperglycemia in obese or type 2 DM patients, and considered an antidiabetic agent.[14]
Cabergoline is a long-acting agonist of dopamine, which has a high affinity to dopamine receptors (type 2), and it is the first treatment choice for hyperprolactinemic disorders. Compared to bromocriptine, cabergoline is more long-acting, administered in lower doses, has fewer side effects, and more easily accepted by patients. However, there is a lack of studies concerning the effects of cabergoline for reducing blood sugar levels and insulin resistance.[15,16] Considering the issues mentioned above, this study was designed to evaluate the effects of cabergoline on blood sugar levels in patients with type 2 DM.
2. Methods
This was a double-blind clinical trial; a placebo was used as control to evaluate the effects of cabergoline on blood glucose levels in patients who were referred to our diabetes clinic center. Twenty patients were randomly placed in each group (i.e., control and cabergoline groups) based on calculating power (85%) and index of assurance (95%) with a significant difference (P < 0.05) in glycosylated hemoglobin (HbA1C). Each group consisted of 4 men (20%) and 16 women (80%). This study was approved by the Committee of Ethics (90–91) of Mazandaran University of Medical Sciences, Iran and registered in the Iranian Registry of Clinical Trial (IRCT code: 201205053176N2). All patients in this study provided signed informed consent before entering the study.
The inclusion criteria were as follows: patients with type 2 DM, which was diagnosed at least 3 months prior to the study, who received treatment by ≥2 oral glucose-lowering drugs with at least 50% of maximum dose; patients were aged between 20 and 80 years; and the level of HbA1C in patients were ≥7% and <10%. In addition, women who were of reproductive age had to use a reliable contraceptive method during the time of the study. The exclusion criteria were as follows: unwillingness of the patients; pregnancy and lactation; uncontrolled thyroid diseases; serum creatinine level >2 mg/dL; patients who were currently treated with agents that affect blood sugar levels (i.e., glucocorticoid, thiazide, antipsychotic antagonist, and ergot); severe weight loss (at least 10% during last 6 months prior to the study); and history of immunodeficiency disorders, cardiovascular diseases, diabetic ketoacidosis, sever hepatic diseases, allergy to cabergoline, proliferative retinopathy, and psychological disorders.
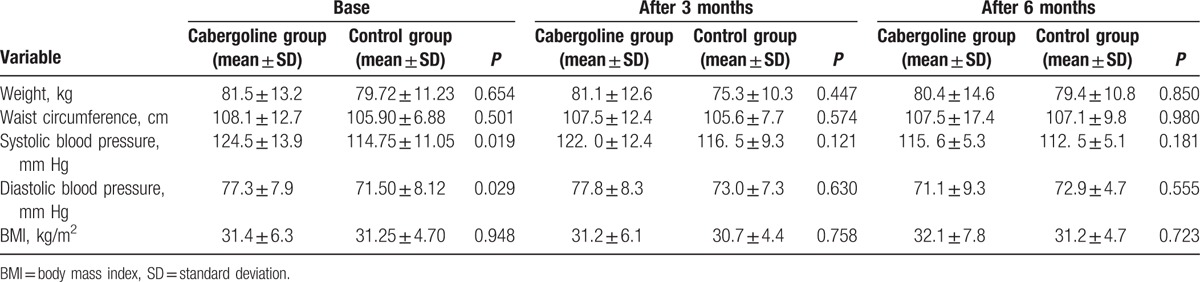
The demographic information of the patients, including age, sex, weight, height, waist circumference, duration of diabetes, and drugs (i.e., previously prescribed glucose-lowering drugs), was recorded. In this treatment, 10 patients (25%) received 2 types of drugs so that 7 patients (17.5%) got Glibenclamide and Metformin and 3 (7.5%) Metformin and Acarbose. Thirty other patients (75%) got three types of drugs, 25 (62.5%) received Metformin, Glibenclamide, and Pioglitazone and finally 5 (12.5%) used Glibenclamide, Metformin, and Acarbose. Results showed that there was no statistical significant difference between 2 groups (P = 0.792). The patients also received at least 50% of maximum dose of their previous medications. Weight was measured using the SECA scale with an accuracy of 100 g. The waist circumference was measured in the umbilical region using fabric meters. The blood pressure was measured by a skilled nurse using a Microlife blood pressure monitor (Riester, Ri-San, Germany). Patients’ weight, blood pressure, and waist circumference were measured at 3 and 6 months of treatment.
The basic laboratory tests of the patients, including fasting blood sugar (FBS), 2-hour post-prandial glucose (2HPP), complete lipid profile (after 12 hours of fasting), serum insulin, prolactin, hemoglobin (Hb), alanine amino transferase (ALT), aspartate amino transferase (AST), creatinine (Cr), and blood urea nitrogen (BUN), were conducted at treatment outset and at 3 and 6 months. HbA1C was measured by NycoCard kits with covariate of variation 3.5% NycoCard kits and device (Italy). Patients with HbA1C levels ≤7% after 3 months were withdrawn from the study. Patients with an HbA1C level between 7% and 10% (i.e., 7%< HbA1C<10%) at 3 months received treatments of cabergoline (0.5 mg) or placebo twice per week for the next 3 months. Blood glucose levels were measured by Pars-Azmoon kits (Iran) and the device of Prestigezui (prestigezui 24 I Japan). Insulin and prolactin levels were evaluated by ELISA in order to analyze the effects of cabergoline on insulin sensitivity and changes in prolactin levels. Venous blood samples were obtained from all patients and placed in the centrifuge for 10 minutes with a round speed of 3000 r/min. It was later evaluated by an automated analyzer (Hitachi 9/7, Germany); serum insulin levels were measured by ELISA and Monobind screening test kits. Insulin resistance was calculated by the formula of homeostasis model assessment insulin resistance (HOMA-IR).
The drug (cabergoline) and placebo used in this study, which should be similar in shape, size, color, and packaging, were designed by the Department of Pharmacology at the University of Medical Sciences of Mazandaran, Iran. The pharmacologists in this department were responsible for naming and coding the drugs and placebos, but had no role in choosing or prescribing them to the patients. Finally, the coded drugs and placebos were delivered randomly to the patients by a nurse who assisted the researchers in this project. The patients received oral treatments of either placebo or cabergoline (0.5 mg) using the sequential method once per week for 3 months while using previously prescribed glucose-lowering drugs.
Study data were analyzed using the SPSS Statistic Software version 19 (SPSS Inc., Chicago, IL). A Student's t-test was conducted to quantitatively analyze the data in both groups and paired t-test was used to compare the before and after treatment results in each group. The P-value was calculated at a level of significance of 0.05.
3. Results
The mean age of patients was 53.8 ± 6.5 years in cabergoline group and 53.9 ± 7.9 years in control group (P = 0.983). The duration of DM was not significantly different between two group (11.7 ± 4.3 and 11.0 ± 6.8 years in case and control groups respectively, P = 0.701). The demographic characteristics of the patients during study initiation are shown in Table 1. No side effects were reported in any of the patients.
Table 1.
Demographic information of the study population.
Ten patients (50%) in the cabergoline group and 13 patients (70%) in control group had to continue their treatment for an additional 3 months because they had an HbA1C level between 7% and 10%. Overall, 65%and 45% of patients in the cabergoline and control groups, respectively, responded to the treatment (HbA1C<7%) (Fig. 1).
Figure 1.
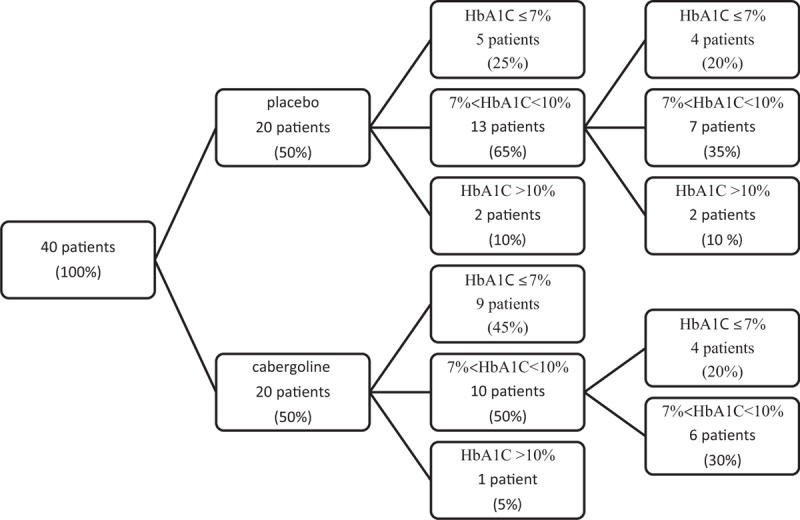
Consort flowchart of patients.
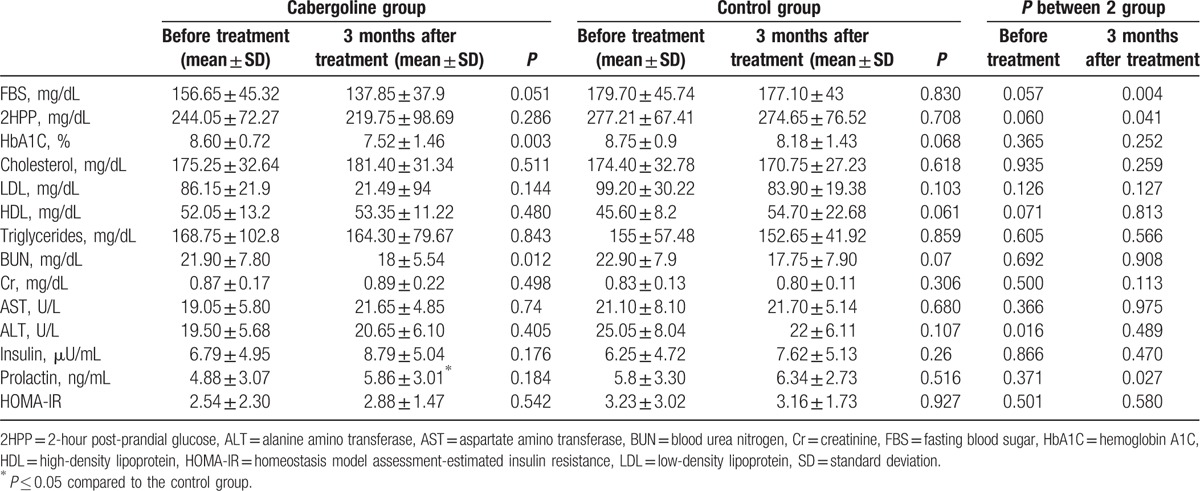
The laboratory characteristics of the patients in the control and cabergoline groups at the treatment outset and at 3 months are shown in Table 2. The FBS levels were significantly different between the groups after 3 months of treatment (P = 0.004). As before intervention, 3 and 6 months after it, HbA1C level difference reached no statistical significance between 2 groups (P = 0.252, P = 0.231, respectively). In the cabergoline group, a significant decrease in HbA1C levels was observed after 3 months of treatment (P = 0.003) and 6 months of treatment (P = 0.017). After 6-month follow-up, HDL cholesterol was significantly higher in the case group as compared with control (60.0 ± 9.2 and 48.6 ± 6.8, respectively, P = 0.003). Total cholesterol, triglyceride, LDL cholesterol were not significantly different between 2 groups after 3 and 6 months. In case and control groups, HOMA-IR index was 2.88 ± 1.47 and 3.16 ± 1.73, respectively, after 3 months and 3.04 ± 2.37 and 3.44 ± 2.22, respectively, after 6 months so that there was no statistical difference (P = 0.580, and P = 0.683, respectively). The prolactin levels were statistically significant in the cabergoline group (P = 0.001) at the beginning of treatment and 6 months later on; however, no significant difference was observed in the control group (P = 0.846) .
Table 2.
Laboratory characteristics of the patients before and after 3 months of treatment.
4. Discussion
According to our study, treatment with cabergoline decreases FBS, HBA1C, and prolactin levels in type 2 diabetic patients with persistent hyperglycemia. Taghavi et al showed that cabergoline decreased FBS in patients with type 2 diabetes with persistent hyperglycemia.[17] Saadat et al observed a significant decrease in fasting plasma glucose and prolactin levels (P < 0.001) after 4 months of treatment with cabergoline in prediabetic patients.[18] Although Reis et al conducted a study in mice in 1997 and revealed that the increase of prolactin hormone exacerbated insulin resistance,[19] no significant difference in insulin resistance (HOMA-IR) was observed after treatment with cabergoline (P = 0.389) in our study, which was similar to the finding by Gibson et al (P = 0.97).[15] In contrast, Saadat et al showed significant reductions in HOMA-IR (P = 0.04) after cabergoline treatment in prediabetic patients.[18] Cabergoline was reported to have a weight loss effect in some studies,[15] but Santos Silva et al and Gibson et al were unable to observe this effect after 6 months of cabergoline treatment in their studies.[15,20] Similarly, we were able to show that there was no significant difference in weight between the cabergoline and control groups 3 months after treatment (P = 0.447). Our patients in the cabergoline group showed a significant decrease in prolactin levels from the beginning of treatment to the end of study compared with the control group (P = 0.000), which is consistent with other studies.[18,21,22] The decrease in prolactin levels is an expected finding in patients treated with cabergoline.
Cabergoline is a long-acting agonist of dopamine, which has a high affinity with dopamine receptor type 2. This agent has a remarkable effect on the receptors of a2B, 5-HT2c, 5HT2A, 5-HT1A, D4, and D3, and dopaminergic receptor type 2.[15] Although the effect of dopamine on pancreatic β-cell secretion is not strongly obvious, dopaminergic receptor type 2 has been reported to play a role in this process in a study conducted in animals.[23] The use of this agent has been reported to increase the level of dopamine and decrease the level of noradrenergic in the hypothalamus, resulting in enhanced insulin sensitivity in peripheral tissues and reduced liver gluconeogenesis.[24] Moreover, the decline of serum prolactin levels will likely justify this effect in the agent. In another animal study, administration of prolactin hormone resulted in positive effects on carbohydrate metabolism and adipose tissues (i.e., reduced production of adiponectin from adipose tissue).[25] Regarding the lower level of prolactin in the cabergoline group, it seems that the antidiabetic effect of cabergoline is through dopamine and prolactin metabolism. Currently, the various effects of cabergoline are claimed to be due to a lack of treatment response in some patients.[25]
One of the limitations of our study includes short duration of follow-up. In addition, although there was no statistical significance for the HbA1c values after intervention between 2 groups, the values of glycosylated hemoglobin significantly decreased after treatment in cabergoline group. It is possible that lower statistical power (small sample size) affects this nonsignificance.
5. Conclusion
Cabergoline is a long-acting dopamine agonist that may be useful as a long-acting antidiabetic agent in patients with type 2 DM. More studies are needed to clarify the mechanism of the dopamine agonist effect on glucose metabolism.
Footnotes
Abbreviations: 2HPP = 2-hour post-prandial glucose, ALT = alanine amino transferase, AST = aspartate amino transferase, BUN = blood urea nitrogen, CNS = central nervous system, Cr = creatinine, DM = diabetes mellitus, FBS = fasting blood sugar, FDA = Food and Drug Administration, Hb = hemoglobin, HbA1C = glycosylated hemoglobin, HDL = high-density lipoproteins, HOMA-IR = homeostasis model assessment insulin resistance, LDL = low-density lipoproteins.
This study was conducted as part of Dr Ezzatossadat Daneshpour's postgraduate thesis.
This study was supported by a grant from Mazandaran University of Medical Sciences.
The authors have no conflicts of interest to disclose.
References
- 1.Johnson WD, Kroon JJ, Greenway FL, et al. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001-2006. Arch Pediatr Adolesc Med 2009; 163:371–377. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas. 7th ed.Brussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes: 2015. Diabetes Care 2015; 33:S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cincotta AH, Meier AH, Southern LL. Bromocriptine alters hormone rhythms and lipid metabolism in swine. Ann Nutr Metab 1989; 33:305–314. [DOI] [PubMed] [Google Scholar]
- 5.Kvernmo T, Härtter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther 2006; 28:1065–1078. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschoner A, Engl J, Laimer M, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract 2007; 61:1356–1370. [DOI] [PubMed] [Google Scholar]
- 8.S Hynie. Ergot compounds and brain function; Neuroendocrine and neuropsychiatric aspects: advances in biochemical psychopharmacology: Volume 23, Series Editors: Erminio Costa and Paul Greengard, Volume Editors: Menek Goldstein, Donald B. Calne, Abraham Lieberman and Michael O. Thorner, Raven Press, New York 1980, xvii, 413 pp, illus. Neuropharmacology 1982; 21:103. [Google Scholar]
- 9.Sowell MO, Mukhopadhyay N, Cavazzoni P, et al. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. J Clin Endocrinol Metab 2002; 87:2918–2923. [DOI] [PubMed] [Google Scholar]
- 10.Luo S, Luo J, Cincotta AH. Association of the antidiabetic effects of bromocriptine with a shift in the daily rhythm of monoamine metabolism within the suprachiasmatic nuclei of the Syrian hamster. Chronobiol Int 2000; 17:155–172. [DOI] [PubMed] [Google Scholar]
- 11.Luo S, Meier AH, Cincotta AH. Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology 2008; 68:1–10. [DOI] [PubMed] [Google Scholar]
- 12.Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology 2000; 71:68–78. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JL, Timpe EM, Petkewicz KA. Bromocriptine mesylate for glycemic management in type 2 diabetes mellitus. Ann Pharmacother 2010; 44:1777–1785. [DOI] [PubMed] [Google Scholar]
- 14.Scranton RE, Gaziano JM, Rutty D, et al. A randomized, double-blind, placebo-controlled trial to assess safety and tolerability during treatment of type 2 diabetes with usual diabetes therapy and either Cycloset or placebo. BMC Endocr Disord 2007; 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson CD, Karmally W, McMahon DJ, et al. Randomized pilot study of cabergoline, a dopamine receptor agonist: effects on body weight and glucose tolerance in obese adults. Diabetes Obes Metab 2012; 14:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korner J, Lo J, Freda PU, et al. Treatment with cabergoline is associated with weight loss in patients with hyperprolactinemia. Obes Res 2003; 11:311–312. [DOI] [PubMed] [Google Scholar]
- 17.Taghavi SM, Fatemi SS, Rokni H. Cabergoline effect on blood sugar in type 2 diabetic patients with oral agent failure. Med J Malaysia 2012; 67:390–392. [PubMed] [Google Scholar]
- 18.Saadat N, EsmailyF H, Abbasinazari M, et al. Does twice-weekly cabergoline improve anthropometrical and biochemical profiles in prediabetes? A randomized double-blind clinical trial pilot study. Iran J Pharm Res 2015; 14 (Suppl):77–86. [PMC free article] [PubMed] [Google Scholar]
- 19.Reis F, Reis A, Coimbra C. Effects of hyperprolactinaemia on glucose tolerance and insulin release in male and female rats. J Endocrinol 1997; 153:423–428. [DOI] [PubMed] [Google Scholar]
- 20.SantosSilva CM, Barbosa FR, Lima GA, et al. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 2011; 19:800–805. [DOI] [PubMed] [Google Scholar]
- 21.Biller B, Molitch ME, Vance ML, et al. Treatment of prolactin-secreting macroadenomas with the once-weekly dopamine agonist cabergoline. J Clin Endocrinol Metab 1996; 81:2338–2343. [DOI] [PubMed] [Google Scholar]
- 22.Cozzi R, Attanasio R, Lodrini S, et al. Cabergoline addition to depot somatostatin analogues in resistant acromegalic patients: efficacy and lack of predictive value of prolactin status. Clin Endocrinol 2004; 61:209–215. [DOI] [PubMed] [Google Scholar]
- 23.García-Tornadú I, Ornstein AM, Chamson-Reig A, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology 2010; 151:1441–1450. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Jonathan N, Hugo ER, Brandebourg TD, et al. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab 2006; 17:110–116. [DOI] [PubMed] [Google Scholar]
- 25.Doknic M, Pekic S, Zarkovic M, et al. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol 2002; 147:77–84. [DOI] [PubMed] [Google Scholar]


