Abstract
Background:
Chinese herbal medicine (CHM) has been used to treat stroke for thousands of years. The objective of the study is to assess the current evidence for bioactive components of CHM as neurogenesis agent in animal models of ischemic stroke.
Methods:
We searched PubMed, China National Knowledge Infrastructure, WanFang Database, and VIP Database for Chinese Technical Periodicals published from the inception up to November 2015. The primary measured outcome was one of neurogenesis biomarker, including Bromodeoxyuridine (BrdU), Nestin, doublecortin (DCX), polysialylated form of the neural cell adhesion molecule (PSA-NCAM), neuronal nuclear antigen (NeuN), and glial fibrillary acidic protein (GFAP).
Results:
Thirty eligible studies were identified. The score of quality assessment ranged from 2 of 10 to 7 of 10. Compared with controls, 10 studies conducting neurobehavioral evaluation showed significant effects on bioactive components of CHM for improving neurological deficits score after ischemic insults (P < 0.01 or P < 0.05); 6 studies in Morris water-maze test showed bioactive components of CHM significantly decreased escape latency and increased residence time (P < 0.05); 5 studies demonstrated that bioactive components of CHM significantly reduced infarct volume after ischemic stroke (P < 0.05); 25 of 26 studies showed that bioactive components of CHM significantly increased the expression of BrdU and/or Nestin markers in rats/mice brain after ischemic injury (P < 0.05, or P < 0.01); 4 of 5 studies for promoting the expression of PSA-NCAM or DCX biomarker (P < 0.05); 5 studies for improving the expression of NeuN biomarker (P < 0.05); 6 of 7 studies for promoting the expression of GFAP biomarker in brain after ischemic stroke (P < 0.05).
Conclusion:
The findings suggest that bioactive components of CHM may improve neurological function, reduce infarct volume, and promote endogenous neurogenesis, including proliferation, migration, and differentiation of neural stem cells after ischemic stroke. However, evidences are supported but limited because only a few studies were available for each descriptive analysis. Further rigor study is still needed.
Keywords: bioactive components, chinese herbal medicine, experimental ischemic stroke, neurogenesis
1. Introduction
Neural stem cells (NSCs) are characterized as having properties of continuous proliferation and multiple differentiation potential. Since NSCs discovered in adult mouse striatum by Reynolds and Weiss in 1992,[1] intensive studies have indicated that neurogenesis can occur in the adult central nervous system (CNS).[2] Persistent neurogenesis mainly occurs in the subventricular zone (SVZ) and the subgranular zone (SGZ) of the dentate gyrus (DG) in the adult brain.[3–5] Neural progenitor cells (NPCs) generated from NSCs in both regions, confining in proliferation and differentiation into neurons or glia cells,[5,6] may offer an endogenous mechanism to brain repair and recovery from injury or disease.[7] Neurogenesis, which involves proliferation of NSCs/NPCs, differentiation of NPCs, and migration of neuroblasts, could be affected not only by multifarious physiological conditions including exercise,[8] enriched living conditions,[9,10] and aging[10,11] but also by various pathological conditions such as stroke,[12,13] psychosocial stress,[14,15] seizure,[16] and neurodegeneration.[17,18] Actively dividing cell population in the SVZ of adult rat is approximately 15% to 21%.[19–21] Previous study indicated that stroke substantially increased dividing SVZ cells up to 31% in mice model.[22] Though supplementing on survival and proliferation of intrinsic NSCs could assist to repair the damaged tissues, the efficacy of this supplementation has been shown to be limited.[23,24] Therefore, enhancing endogenous neurogenesis will have great potential application as a therapeutic strategy for CNS disorders. Neurogenesis markers, including Bromodeoxyuridine (BrdU), Nestin, doublecortin (DCX), polysialylated form of the neural cell adhesion molecule (NCAM), neuronal nuclear antigen (NeuN), and glial fibrillary acidic protein (GFAP) are widely used as the neuroregenerative development of proliferation, migration, and differentiation. BrdU, a synthetic thymidine analog used for measuring cell proliferation, incorporates DNA of dividing cells during the S-phase of the cell cycle.[25] Nestin, a class VI intermediate filament protein, is considered as a NSC/NPC biomarker during development of the CNS.[26] DCX is a microtubule-associated protein expressed by NPCs and immature neurons in embryonic and adult cortical structures, and used increasingly as a migration biomarker for neurogenesis.[27,28] Polysialic acid (PSA) is a linear homopolymer of alpha2–8-N-acetylneuraminic acid and the NCAM is the primary vector for it in vertebrates. PSA-NCAM participates in neural plasticity and neurogenesis, which is particularly considered toward cell migration.[29] NeuN, a homologue to sex-determining genes in Caenorhabditis elegans, is a neuronal nuclear antigen that is commonly used as a hallmark of neuronal differentiation during neurogenesis development.[30,31] GFAP, being described as one of the markers of astrocytic differentiation in vertebrates, is an intermediate-filament protein expressed uniquely in astrocytes and vulnerable to reactive gliosis that follows injuries to the CNS.[32]
Chinese herbal medicine (CHM) has been widely used to treat neurological disorders such as stroke,[33] Alzheimer disease,[34] Parkinson disease,[35] migraine,[36] depression, anxiety, and insomnia[37] in the young and/or the old. In addition, a wealth of active ingredients from herbs has been reported for their benefits to neural repair.[33] Of these bioactive components of CHM, studies showed that they have potential effects of promoting neurogenesis, neurite outgrowth, and synaptogenesis in ischemic stroke.[38,39] Systematic review of preclinical animal data could inform the planning and improve the likelihood of success of future clinical trials, identify where there is a need for further basic research, preclude unnecessary study replication, and contribute to both reduction and refinement in animal experimentation.[40] In addition, it might offer us with credible and solid new evidence on the neurogenesis effect in preclinical experiment to select the optimal requirements for drug administration for clinical trials. Thus, we conducted a preclinical systematic review of bioactive components of CHM as neurogenesis agent in animal models of ischemic stroke.
2. Methods
2.1. Ethical approval
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.2. Database and literature search strategies
The databases, including PubMed, China National Knowledge Infrastructure, WanFang Database, and VIP Database for Chinese Technical Periodicals, were used for the literatures. To identify studies of bioactive components of CHM for neurogenesis after experimental ischemic stroke, we electronically operated each database from the inception up to November 2015. We also hand-searched a list of Chinese and English journals that may publish potentially eligible studies. Our search strategy included the following: (“stroke” OR “ischemia” OR “ischemic injury”) AND (“neurogenesis” OR “neural regeneration” OR “nerve regeneration” OR “neuroregeneration” OR “proliferation”) AND (“herbal” OR “Chinese medicine” OR “nature product” OR “active components” OR “bioactive compounds”). Chinese databases were also searched using the above search terms in Chinese.
2.3. Inclusion criteria
To prevent bias, inclusion criteria were prespecified as the following: experimental study of neurogenesis in ischemic stroke; animal model; the bioactive component of CHM was administered; control group was not administrated with any other CHM; the primary measured outcome was one of neurogenesis markers, including BrdU, Nestin, DCX, PSA-NCAM, NeuN, and GFAP.
2.4. Data extraction
The following details were extracted from each included study: the first author's name, publication year, category of bioactive components of CHM, type of models, the anesthetic method used during the induction of model; individual data for each study, including animal number, species, sex, weight; information on treatment including timing for initial treatment, type and method of treatment procedure, and duration of treatment; outcome measures including BrdU, Nestin, DCX, PSA-NCAM, NeuN, and GFAP, and the time point of outcome assessments; neurobehavioral assessment and infarct volume. We extracted data for mean value, standard deviation, and number of animals per group if appropriate. Meta-analysis was preformed if there were enough data of outcomes. Data extraction was performed by 2 independent authors.
2.5. Quality assessment
Based on the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) 10-item quality checklist[41] and the methodology described by Macleod et al,[42,43] we modified “comparison” as the assessment of outcome in treatment and control groups after treatment with any bioactive components of CHM. The aggregate methodological quality score was calculated by applying a 10-item modified scale as following: publication in a peer-reviewed journal, control of temperature, random allocation to treatment or control, blinded induction of model, blinded assessment of outcome, use of an anesthetic without intrinsic neurogenesis activity, animal model (aged, diabetic, or hypertensive), performing a sample size calculation, compliance with animal welfare regulations, and a statement of potential conflicts of interest. One point was awarded for each item. We resolved any disagreement through discussion or consultation with corresponding author.
3. Results
3.1. Study inclusion
We identified 826 potentially relevant articles from the 5 databases, in which 27 records were excluded because of duplicates. After going through the titles and abstracts, we excluded 727 articles with at least one of following reasons: case report or review; not an animal research; and not the researches on neurogenesis of ischemic stroke. We then screened the remaining 72 articles, which reported the efficacy of bioactive components of CHM for ischemic stroke. Forty-two studies were excluded because of single Chinese herb or formulas or animal trials without disease model. Ultimately, 30 eligible studies were identified. Detailed information was shown in Figure 1.
Figure 1.
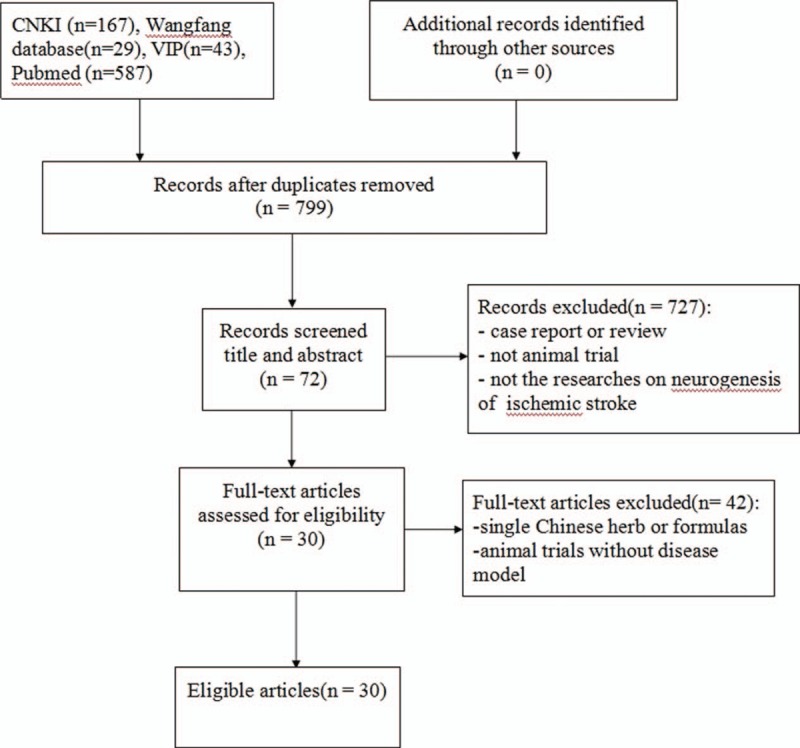
Flow diagram of the article selection for the study.
3.2. Characteristics of included studies
The basic characteristics of included studies are shown in Table 1 . Thirty studies included were published between 2003 and 2015.[44–73] The animal species included Sprague–Dawley rats for 21 studies,[44,45,47–53,56–58,61–65,68–70,73] Westar rats for 7 studies,[46,54,55,59,60,66,67] Mongolian gerbils and Kunming mice in each study.[71,72] The weight of rats varied from 180 to 375 g. Chloral hydrate was used in 17 studies to induce anesthesia,[44,45,48–54,57,58,65,69,70–73] pentobarbital was used in 8 studies,[47,55,56,62–64,66,67] halothane was used in 1 study,[46] and the remaining 4 studies did not report what kind of anesthetic was used.[59–61,68] Sixteen of 22 studies employed middle cerebral artery occlusion (MCAO) as the model of brain ischemia with the occlusion time varying from 1 to 2 hours,[44,45,48–53,55–58,61,65,72,73] whereas the remaining 6 studies utilized permanent MCAO model.[54,59,60,62–64] Seven studies were transient global ischemic models induced by using common carotid arteries occlusion plus irreversibly vertebral arteries occlusion or common carotid arteries occlusion plus hypotension.[46,47,66–70] Transient forebrain ischemic model was induced by using common carotid arteries occlusion in 1 study.[71] Common carotid arteries were completely blocked ranging from 6 to 30 minutes in the 8 studies. The treatment was administrated via intraperitoneal injection in 23 studies,[46–48,50,51,53–56,58–68,70–72] intragastric administration in 6 studies,[44,45,52,57,69,73] and lateral ventricle injection in 1 study.[49] The treatment effect was estimated by using 3 different kinds of neurogenesis outcome measures: 26 studies reported proliferation data as BrdU and/or Nestin markers;[44,45,50–73] 5 studies reported migration data as PSA-NCAM or DCX biomarker;[45,46,48,49,51] and 10 studies reported differentiation data as NeuN or GFAP biomarker.[45,47,48,54,56,57,59,67,70,73] Neurobehavioral assessment was reported in 16 studies.[44–46,48,49,52–55,57,66–69,72,73] Infarct volume was reported in 5 studies.[45,48–50,56] All experiments solely adopted certain kind of bioactive components of CHM in the treatment group and corresponding vehicle in the control group. Twenty-one bioactive components of CHM assessed their effects on neurogenesis after experimental ischemic stroke as follows: ligustrazine-treated in 4 studies,[61–64] ginsenoside Rgl-treated in 3 studies,[59,60,71] curcumin-treated in 2 studies,[50,51] salvianolic acid B used in 2 studies,[58,66] baicalin used in 2 studies,[67,69] L-3-n-butylphthalide used in 2 studies,[72,73] and bilobalide,[44] gypenosides,[45] resveratrol,[46] total saponins of Panax notoginseng,[47] ginsenoside Rd,[48] EGb 761,[49] soy lsoflavones,[52] quercetin,[53] ginseng total saponins,[54] ginsenoside Rb1,[55] tetramethylpyrazine,[56] cornel iridoid glycoside,[57] protoparaxotriol aponins,[65] puerarin,[68] and astragal side IV[70] used in each out of the remaining studies (Table 2).
Table 1.
The characteristic of included studies.
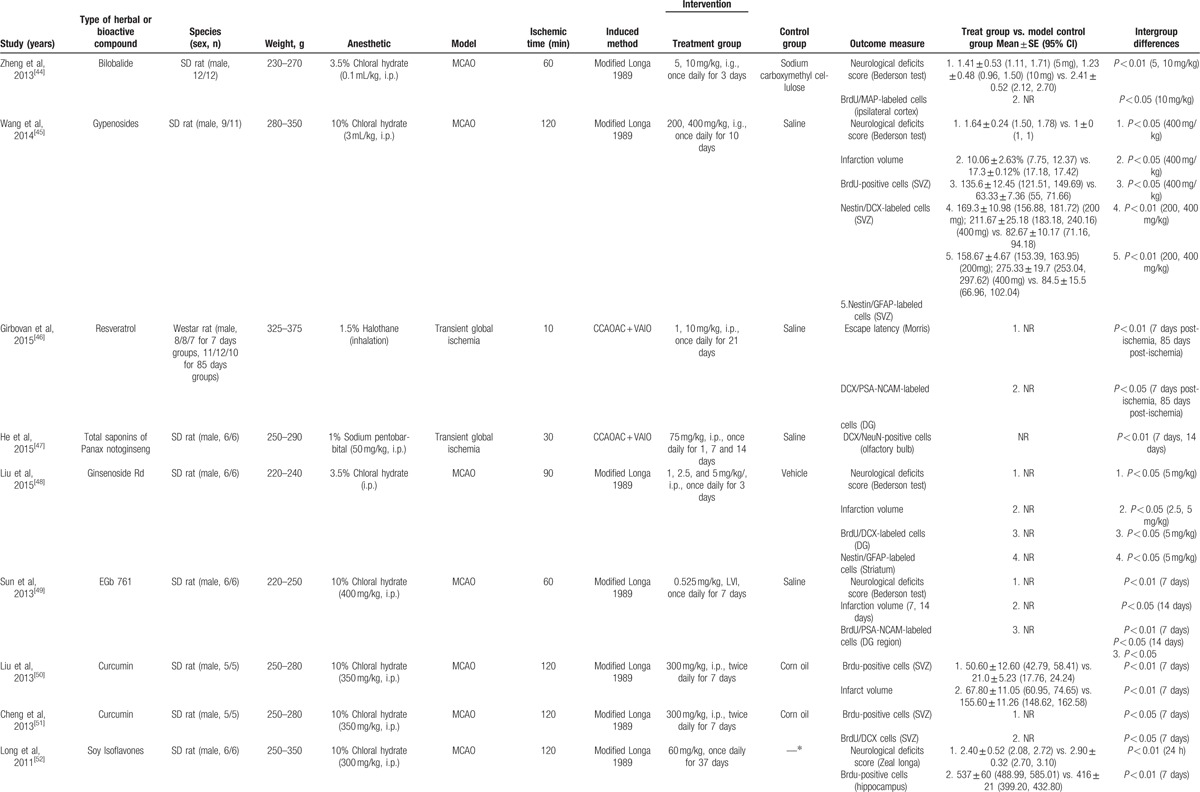
Table 1.
The characteristic of included studies.
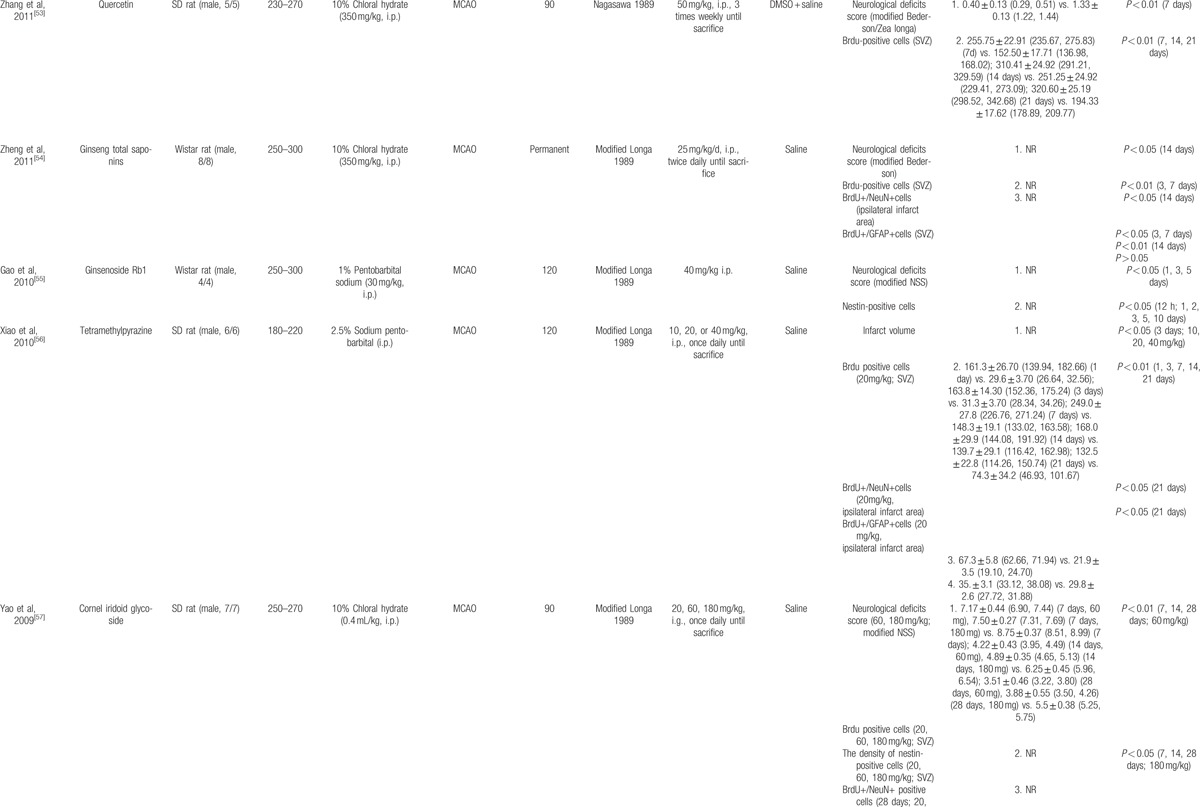
Table 1.
The characteristic of included studies.
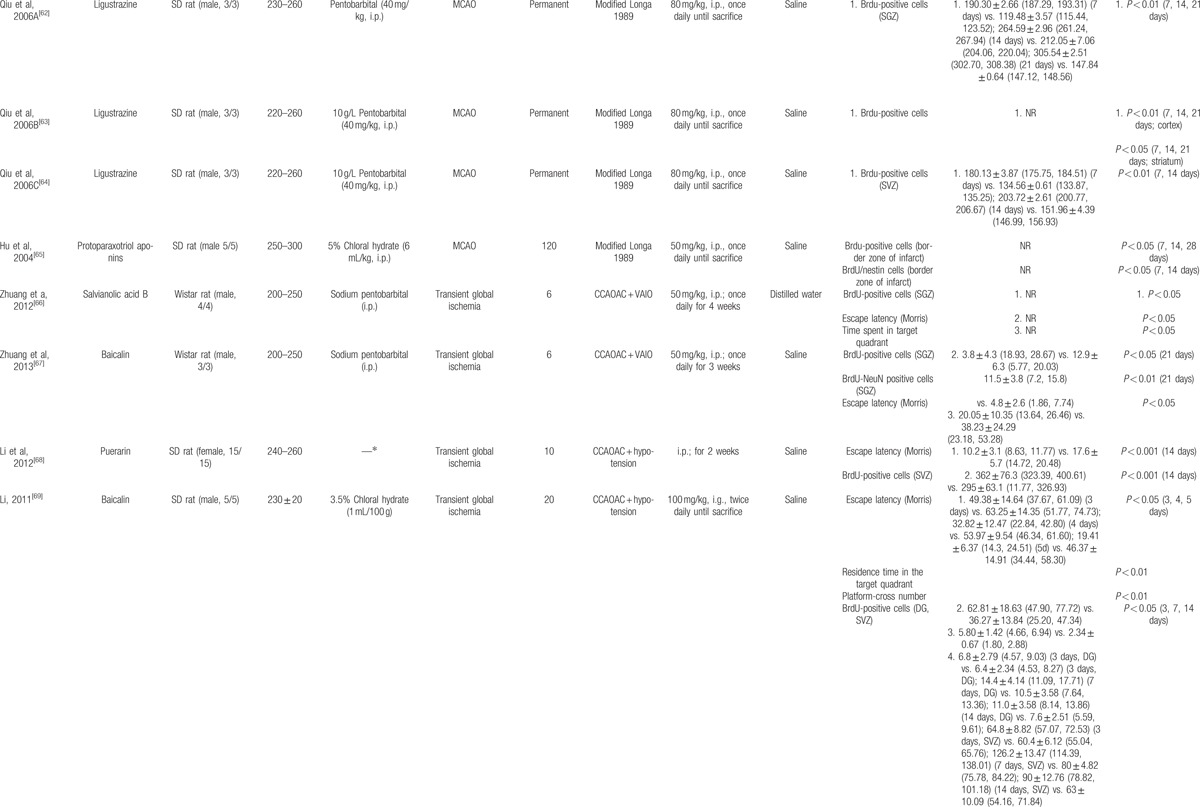
Table 1.
The characteristic of included studies.
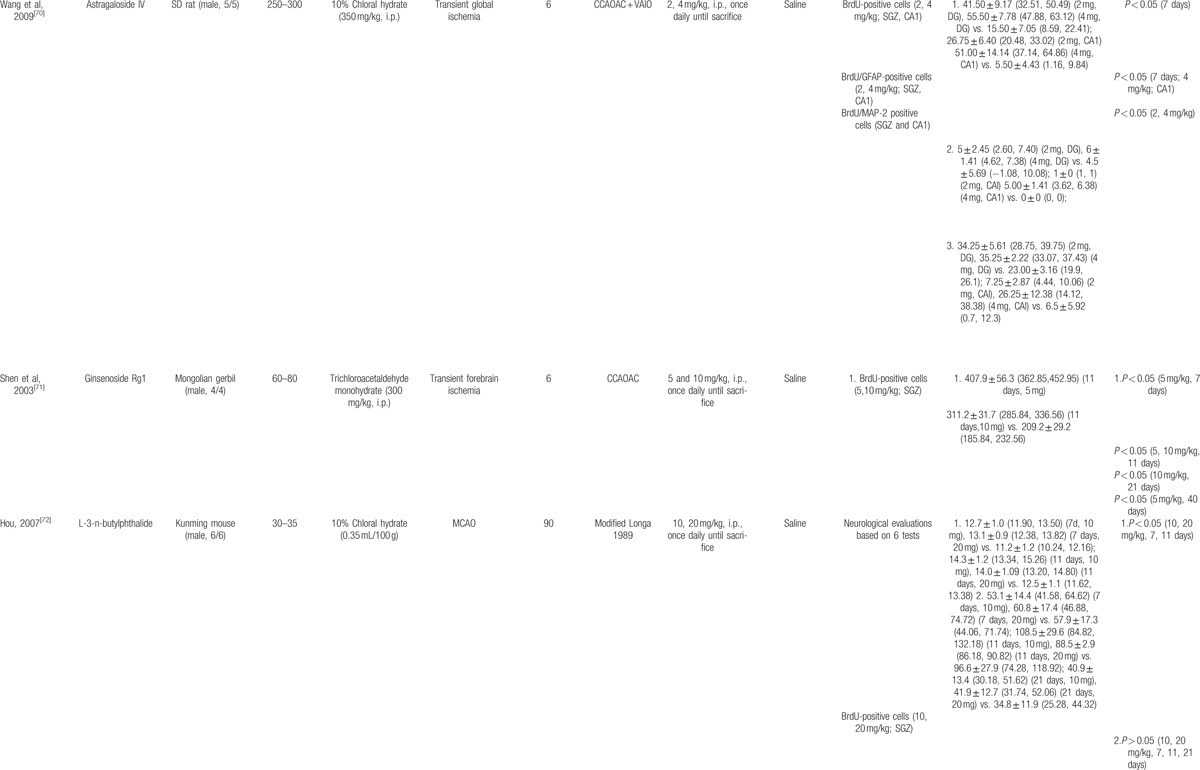
Table 1.
The characteristic of included studies.

Table 2.
Bioactve compounds of included studies.
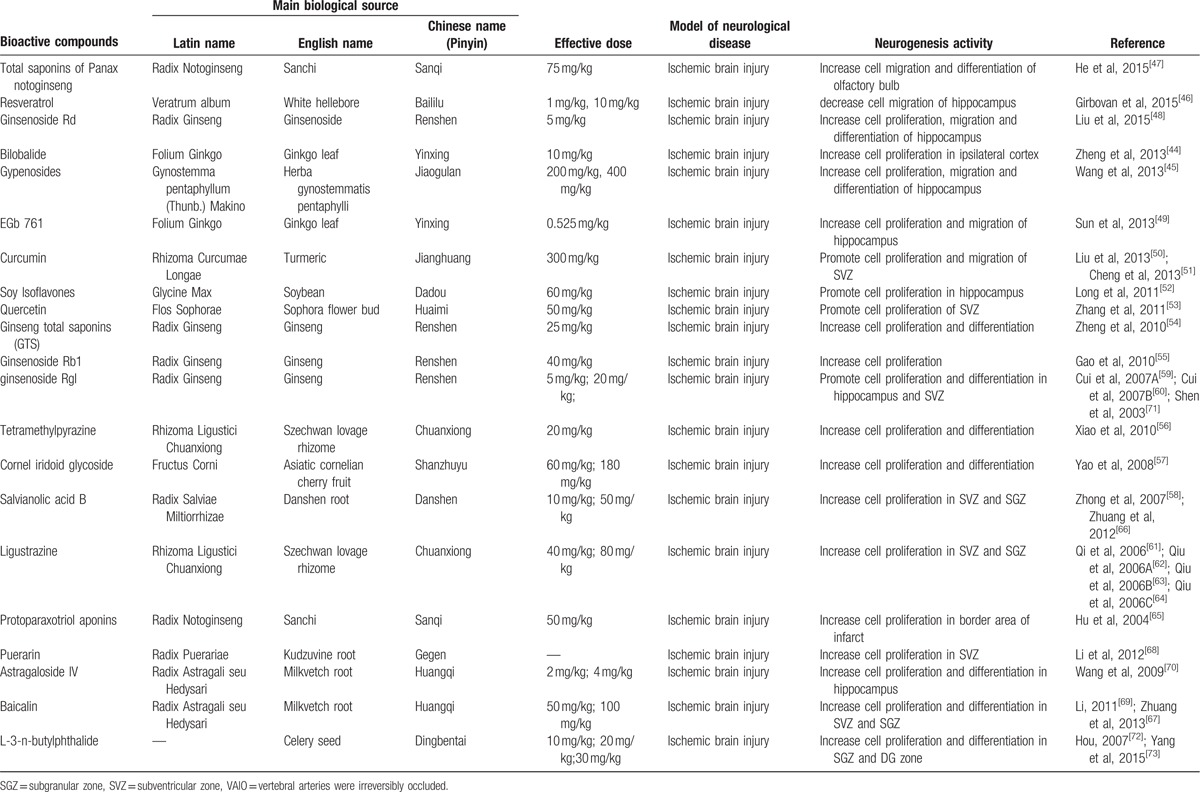
3.3. Study quality
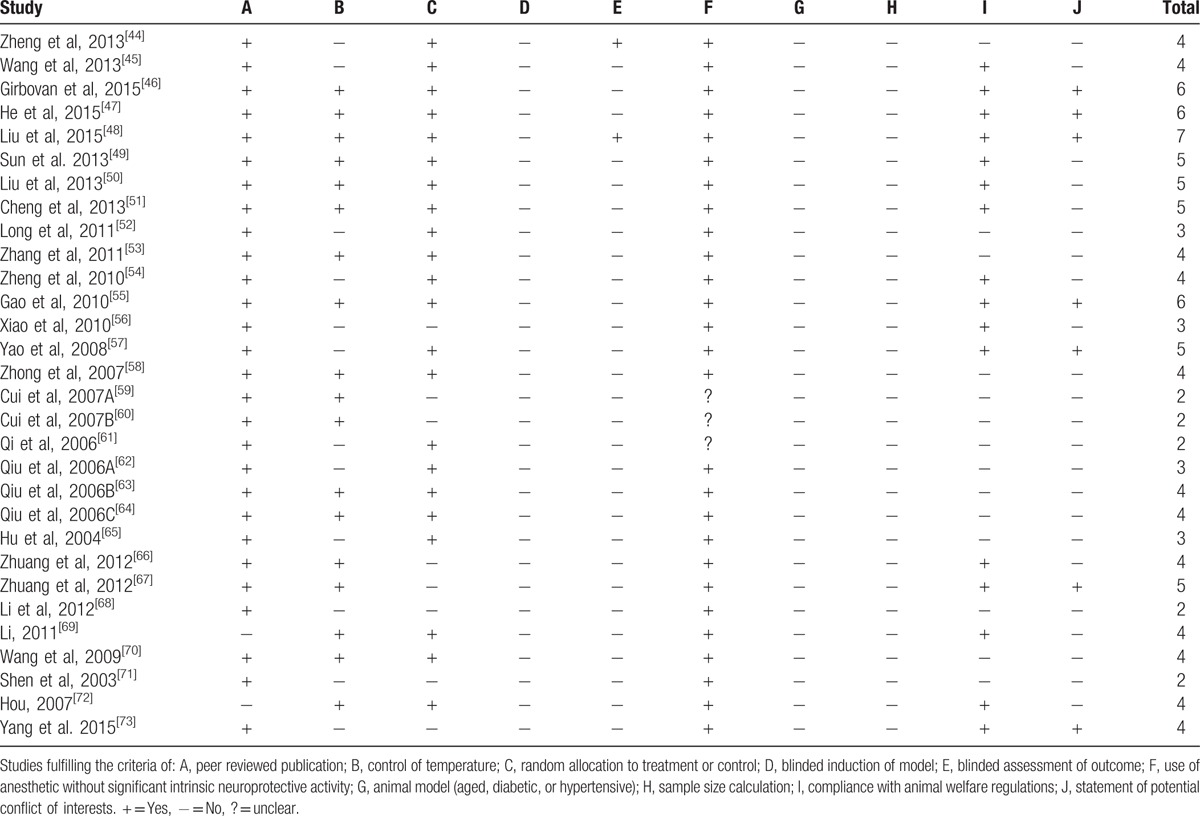
The score of study quality checklist items ranged from 2 of 10 to 7 of 10. Of whom, One study got 7 of 10 points,[48] 3 studies got 6 of 10 points,[46,47,55] 5 studies got 5 of 10 points,[49–51,57,67] 12 studies got 4 of 10 points,[44,45,53,54,58,63,64,66,69,70,72,73] 4 studies got 3 of 10 points,[52,56,62,65] and 5 studies got 2 of 10 points.[59–61,68,71] All the included studies were peer-reviewed and formally published, except 2 studies that were online master's theses.[69,72] Control of temperature as room temperature and rectal temperature of rats was described in 18 studies.[46–51,53,55,58–60,63,64,66,67,69,70,72] Random allocation was described in 22 studies.[44–55,57,58,61–65,69,70,72] Except 4 studies with no-report of anesthetic used,[59–61,68] all the included trials using anesthetics were without significantly intrinsic neuroprotective activity. Two studies declared outcome assessment with blindness.[44,48] None of the included studies described the blindness of model induction and a sample size calculation. An appropriate animal model that is relevant to the clinical situation such as aged animals, hyperglycemia, or hypertension was not used in all the studies. Sixteen studies reported compliance with animal welfare regulations.[45–51,54–57,66,67,69,72,73] Seven studies manifested no potential conflicts of interest.[46–48,55,57,67,73] The methodological quality of each study was summarized in Table 3.
Table 1.
The characteristic of included studies.
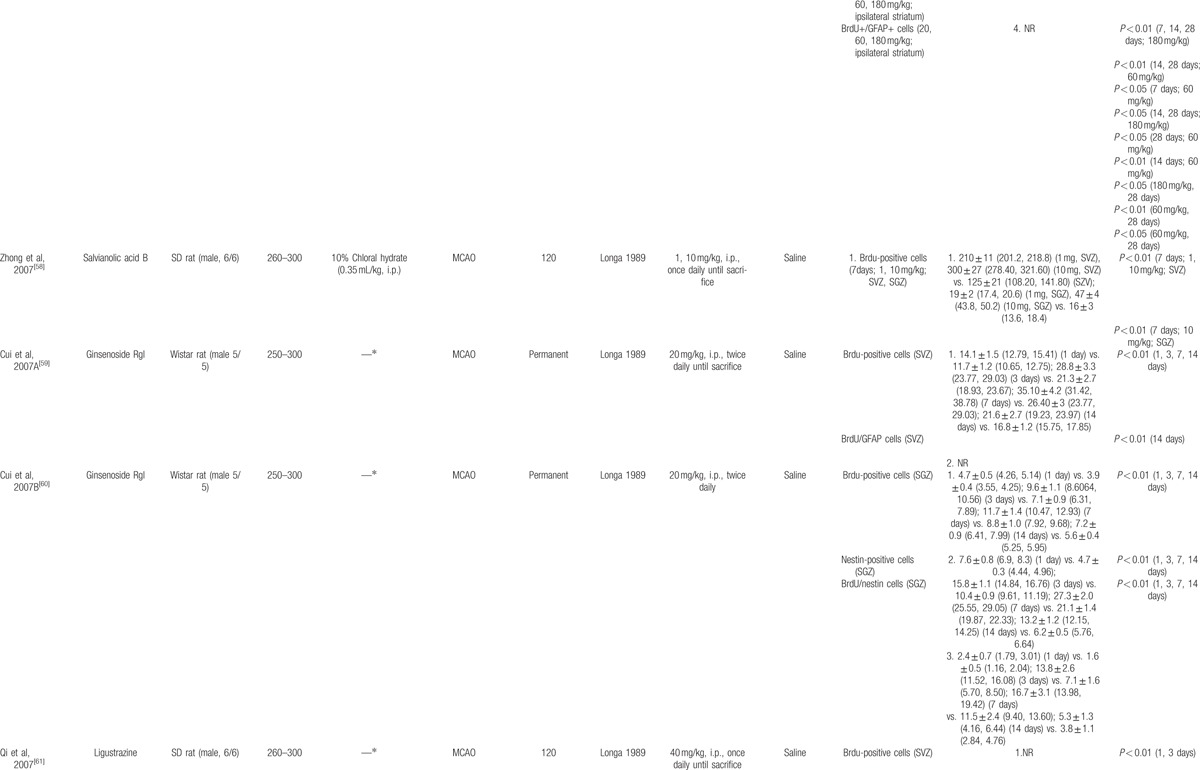
Table 3.
Risk of bias of included studies.
3.4. Effectiveness
3.4.1. Neurobehavioral assessment
Sixteen studies reported neurobehavioral assessment.[44–46,48,49,52–55,57,66–69,72,73] Neurological deficits’ score was assessed in 10 studies by using either Longa criterion, Bederson criterion, or neurological severity score, which indicated that bioactive components of CHM including bilobalide,[44] gypenosides,[45] ginsenoside Rd,[48] EGb 761,[49] soy lsoflavones,[52] quercetin,[53] ginseng total saponins,[54] ginsenoside Rb1,[55] cornel iridoid glycoside,[57] and butylphthalide[72] showed significant lower neurological deficiency (P < 0.01 or P < 0.05). The remaining 6 studies performing Morris water-maze test showed that resveratrol,[44] salvianolic acid B,[66] baicalin,[67,69] puerarin,[68] and butylphthalide[73] significantly decreased escape latency and increased residence time in the target quadrant (P < 0.05).
3.4.2. Infarct volume
Of the 5 studies reporting infarct volume, the bioactive components of CHM including gypenosides,[45] ginsenoside Rd,[48] EGb 761,[49] curcumin,[50] and tetramethylpyrazine[56] in treatment groups significantly reduced infarct volume when compared with the corresponding control groups (P < 0.05).
3.4.3. Neurogenesis outcomes
Twenty-six of all the included studies reporting proliferation data as BrdU and/or Nestin showed that bilobalide,[44] gypenosides,[45] curcumin,[50,51] soy lsoflavones,[52] quercetin,[53] ginseng total saponins,[54] ginsenoside Rb1,[55] tetramethylpyrazine,[56] cornel iridoid glycoside,[57] salvianolic acid B,[58,66] ginsenoside Rgl,[59,60,71] ligustrazine treated in 4 studies,[61–64] protoparaxotriol aponins,[65] baicalin,[67,69] puerarin,[68] astragaloside IV,[70] and L-3-n-butylphthalide[73] significantly increased the expression of BrdU and/or Nestin in rats/mice brain after ischemic injury (P < 0.05, or P < 0.01), with exception in 1 study (P > 0.05).[72]
Of the 5 studies separately adopting PSA-NCAM and DCX markers to assess the migration of NSCs, 4 bioactive components of CHM including gypenosides,[45] ginsenoside Rd,[48] EGb 761,[49] and curcumin[51] significantly promoted the expression of PSA-NCAM and DCX in dentate gyrus, SVZ, or olfactory bulb (P < 0.05). The remaining 1 study reported that resveratrol significantly attenuated the expression of DCX/PSA-NCAM in DG region (P < 0.05).[46]
Of the 10 studies reporting differentiation data as NeuN or GFAP, 5 studies demonstrated that bioactive components of CHM including total saponins of panax notoginseng,[47] ginseng total saponins,[54] tetramethylpyrazine,[56] cornel iridoid glycoside,[57] and baicalin[67] had significant effect on improving the expression of NeuN in ipsilateral infarct area, SGZ, or olfactory bulb (P < 0.05); 7 studies reported that bioactive components of CHM including gypenosides,[45] ginsenoside Rd,[48] tetramethylpyrazine,[56] cornel iridoid glycoside,[57] ginsenoside Rgl,[59] and astragaloside IV[70] had significant effect on promoting the expression of GFAP in SVZ, striatum, ipsilateral infarct area, or SGZ region (P < 0.05), whereas ginseng total saponins showed no significant effect on GFAP expression after ischemic stroke (P > 0.05).[54]
4. Discussion
4.1. Principle finding of the study
To our knowledge, this is the first preclinical systematic review evaluated the efficacy of bioactive components of CHM for neurogenesis. The present study showed that bioactive components of CHM can improve neurological dysfunction, reduce infarct volume, and promote endogenous neurogenesis, including proliferation, migration, and differentiation of NSCs after ischemic stroke.
4.2. Limitations
In the present study, some limitations have been identified. First, in spite of systematic search strategy, other language studies have not been taken into consideration except English and Chinese studies, which may lead to certain degree of selective bias.[74] Furthermore, except the 3 studies of Hou,[72] Girbovan et al,[46] and Zheng et al[54] in respect of proliferation, migration, and differentiation outcome, respectively, all the included studies concluded positive results. Some negative studies missed inevitability, as authors or researchers were unlikely to put effort in publishing negative results and positive ones would be more acceptable in publishing. Thus, the overall effect in this review may be overestimated. Second, study quality was considered as low, which indicated that the results should be explained with caution. The quality of the included studies was a significant predictor of outcome. The dominance of positive studies might imply presence of flaws in randomization and blinding.[75] Third, as high heterogeneities were inherent in the included studies, meta-analyses of the outcome measures can hardly be performed, which effectively pools into single quantitative estimate and summary effect size based on statistical techniques, otherwise.
4.3. Implication for further studies
The most critical step in the fundamental recovery of brain function was reconstruction of neuronal networks, including neuritic regeneration and synaptic reconstruction.[76] None of the included studies investigated whether the newborn neurons integrated into neuronal networks with functional properties. Therefore, further research should pay close attention to the newborns physiological function by electrophysiology and other methodologies.
In the present study, none of the included studies reported the blindness of model induction and a sample size calculation. Randomization was declared in most of the included studies, whereas none of them reported details of how the animals were randomized. Landis et al[77] suggested the core standards of rigorous study design including randomization, blinding, sample-size estimation, and the handling of all data should be depicted in detail. None of models were established on aged, diabetic, or hypertensive animals. The relevance of animal models with normal physiological conditions to human conditions remains dubious.[78] We suggest that the ARRIVE[79] should be used as a guideline when designing and reporting preclinical animal studies.
In conclusion, bioactive components of CHM may improve neurological dysfunction, reduce infarct volume, and promote endogenous neurogenesis, including proliferation, migration, and differentiation of NSCs after ischemic stroke. However, evidences are supported but limited because only a few studies were available for each descriptive analysis. Further research is needed to update supporting the evidence in this area.
Footnotes
Abbreviations: BrdU = Bromodeoxyuridine, CAMARADES = Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies, CHM = Chinese herbal medicine, CNS = central nervous system, DCX = doublecortin, DG = dentate gyrus, GFAP = glial fibrillary acidic protein, MCAO = middle cerebral artery occlusion, NCAM = neural cell adhesion molecule, NeuN = neuronal nuclear antigen, NPCs = Neural progenitor cells, NSCs = Neural stem cells, PSA = Polysialic acid, SGZ = subgranular zone, SVZ = subventricular zone.
JHL, ZXC, and XGZ contributed equally to this work.
This project was supported by the grant of National Natural Science Foundation of China (81573750/81473491/81173395/H2902); the Young and Middle-Aged University Discipline Leaders of Zhejiang Province, China (2013277); Zhejiang Provincial Program for the Cultivation of High-level Health talents (2015).
The authors have no conflicts of interest to disclose.
References
- 1.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992; 255:1707–1710. [DOI] [PubMed] [Google Scholar]
- 2.Temple S. The development of neural stem cells. Nature 2001; 414:112–117. [DOI] [PubMed] [Google Scholar]
- 3.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 1997; 17:5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4:1313–1317. [DOI] [PubMed] [Google Scholar]
- 5.Gage FH. Mammalian neural stem cells. Science 2000; 287:1433–1438. [DOI] [PubMed] [Google Scholar]
- 6.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol 2005; 23:862–871. [DOI] [PubMed] [Google Scholar]
- 8.Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2:266–270. [DOI] [PubMed] [Google Scholar]
- 9.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997; 386:493–495. [DOI] [PubMed] [Google Scholar]
- 10.Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 2003; 17:2042–2046. [DOI] [PubMed] [Google Scholar]
- 11.Moraga A, Pradillo JM, García-Culebras A, et al. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J Neuroinflam 2015; 12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002; 8:963–970. [DOI] [PubMed] [Google Scholar]
- 13.Zhang RL, Chopp M, Roberts C, et al. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. PloS One 2014; 9:e113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould E, McEwen BS, Tanapat P, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 1997; 17:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatr 2000; 5:262–269. [DOI] [PubMed] [Google Scholar]
- 16.Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res 2007; 163:529–540. [DOI] [PubMed] [Google Scholar]
- 17.Bingham B, Liu D, Wood A, et al. Ischemia-stimulated neurogenesis is regulated by proliferation, migration, differentiation and caspase activation of hippocampal precursor cells. Brain Res 2005; 1058:167–177. [DOI] [PubMed] [Google Scholar]
- 18.Ogita K, Nishiyama N, Sugiyama C, et al. Regeneration of granule neurons after lesioning of hippocampal dentate gyrus: evaluation using adult mice treated with trimethyltin chloride as a model. J Neurosci Res 2005; 82:609–621. [DOI] [PubMed] [Google Scholar]
- 19.Schultze B, Korr H. Cell kinetic studies of different cell types in the developing and adult brain of the rat and the mouse: a review. Cell Tissue Kinet 1981; 14:309–325. [DOI] [PubMed] [Google Scholar]
- 20.Smith CM, Luskin MB. Cell cycle length of olfactory bulb neuronal progenitors in the rostral migratory stream. Dev Dynam 1998; 213:220–227. [DOI] [PubMed] [Google Scholar]
- 21.Zhang RL, Zhang ZG, Lu M, et al. Reduction of the cell cycle length by decreasing G1 phase and cell cycle reentry expand neuronal progenitor cells in the subventricular zone of adult rat after stroke. J Cere Blood Flow Met 2006; 26:857–863. [DOI] [PubMed] [Google Scholar]
- 22.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol 1989; 18:311–318. [DOI] [PubMed] [Google Scholar]
- 23.Parent JM, Vexler ZS, Gong C, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 2002; 52:802–813. [DOI] [PubMed] [Google Scholar]
- 24.Abe K, Yamashita T, Takizawa S, et al. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cere Blood Flow Met 2012; 32:1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev 2007; 53:198–214. [DOI] [PubMed] [Google Scholar]
- 26.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell 1990; 60:585–595. [DOI] [PubMed] [Google Scholar]
- 27.Brown JP, Couillard-Després S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 2003; 467:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 2005; 2:1–14. [DOI] [PubMed] [Google Scholar]
- 29.Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 2006; 80:129–164. [DOI] [PubMed] [Google Scholar]
- 30.Mullen RJ, Buck CR, Smith AM, et al. a neuronal specific nuclear protein in vertebrates. Development 1992; 116:201–211. [DOI] [PubMed] [Google Scholar]
- 31.Balaram P, Kaas JH. Towards a unified scheme of cortical lamination for primary visual cortex across primates: insights from NeuN and VGLUT2 immunoreactivity. Front Neuroanat 2014; 8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenner M, Kisseberth WC, Su Y, et al. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci 1994; 14:1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H. Neuroprotective herbs for stroke therapy in traditional eastern medicine. Neurol Res 2005; 27:287–301. [DOI] [PubMed] [Google Scholar]
- 34.Wu JG, Wang YY, Zhang ZL, et al. Herbal medicine in the treatment of Alzheimer's disease. Chin J Integr Med 2015; 21:102–107. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Xie CL, Lu L, et al. Chinese herbal medicine paratherapy for Parkinson's disease: a meta-analysis of 19 randomized controlled trials. Evid Based Complement Alternat Med 2012; 2012:534861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esposito M, Carotenuto M. Ginkgolide B complex efficacy for brief prophylaxis of migraine in school-aged children: an open-label study. Neurol Sci 2011; 32:79–81. [DOI] [PubMed] [Google Scholar]
- 37.Sarris J, Panossian A, Schweitzer I, et al. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol 2011; 21:841–860. [DOI] [PubMed] [Google Scholar]
- 38.Tohda C, Kuboyama T, Komatsu K. Search for natural products related to regeneration of the neuronal network. Neurosignals 2005; 14:34–45. [DOI] [PubMed] [Google Scholar]
- 39.More SV, Koppula S, Kim IS, et al. The role of bioactive compounds on the promotion of neurite outgrowth. Molecules 2012; 17:6728–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy SP, Murphy AN. Pre-clinical systematic review. J Neurochem 2010; 115:805. [DOI] [PubMed] [Google Scholar]
- 41.Sena E, van der Worp HB, Howells D, et al. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 2007; 30:433–439. [DOI] [PubMed] [Google Scholar]
- 42.Macleod MR, O’Collins T, Howells DW, et al. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004; 35:1203–1208. [DOI] [PubMed] [Google Scholar]
- 43.Macleod MR, O’Collins T, Horky LL, et al. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res 2005; 38:35–41. [DOI] [PubMed] [Google Scholar]
- 44.Zheng YQ, Liu JX, Xu L, et al. Study on effect of weinaokang and bilobalide on autophagy and neurogenesis induced by focal cerebral ischemia reperfusion. Chin J Chin Mater Med 2013; 38:2182–2186. [PubMed] [Google Scholar]
- 45.Wang XJ, Sun T, Kong L, et al. Gypenosides pre-treatment protects the brain against cerebral ischemia and increases neural stem cells/progenitors in the subventricular zone. Int J Dev Neurosci 2014; 33:49–56. [DOI] [PubMed] [Google Scholar]
- 46.Girbovan C, Kent P, Merali Z, et al. Dose-related effects of chronic resveratrol administration on neurogenesis, angiogenesis, and corticosterone secretion are associated with improved spatial memory retention following global cerebral ischemia. Nutr Neurosci 2015; 98:2993–3000. [DOI] [PubMed] [Google Scholar]
- 47.He X, Deng FJ, Ge JW, et al. Effects of total saponins of Panax notoginseng on immature neuroblasts in the adult olfactory bulb following global cerebral ischemia/reperfusion. Neural Regen Res 2015; 10:1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu XY, Zhou XY, Hou JC, et al. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol Sin 2015; 36:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, Zhuang W, Xu X, et al. The effect of injection of EGb 761 into the lateral ventricle on hippocampal cell apoptosis and stem cell stimulation in situ of the ischemic/reperfusion rat model. Neurosci Lett 2013; 25:123–128. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Cheng JH, Han Z, et al. Neuroprotection and neurogenesis effect on focal ischemia repercussion injury from curcumin. J Wenzhou Med Coll 2013; 43:171–174. [Google Scholar]
- 51.Cheng JH, Liu S, Han Z, et al. Curcumin promotes proliferation and migration of neural stem cells in cerebral isehemic rats by regulating Notch signaling. Chin J Pathophysiol 2013; 29:878–882. [Google Scholar]
- 52.Long J, Zhang L, Yuan DP, et al. Effects of soy lsoflavones on the Hippocampus neurogenesis of rats with cerebral lschemia and reperfusion. J Nanjing T C M Univ 2011; 27:49–54. [Google Scholar]
- 53.Zhang LL, Cao Q, Hu ZY, et al. Effect of quercetin on neural stem cell proliferation in the subventricular zone of rats after focal cerebral ischemia-reperfusion injury. J South Med Univ 2011; 31:1200–1203. [PubMed] [Google Scholar]
- 54.Zheng GQ, Cheng W, Wang Y, et al. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol 2011; 133:724–728. [DOI] [PubMed] [Google Scholar]
- 55.Gao XQ, Yang CX, Chen GJ, et al. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J Ethnopharmacol 2010; 132:393–399. [DOI] [PubMed] [Google Scholar]
- 56.Xiao X, Liu Y, Qi C, et al. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol Res 2010; 32:547–555. [DOI] [PubMed] [Google Scholar]
- 57.Yao RQ, Zhang L, Wang W, et al. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull 2009; 79:69–76. [DOI] [PubMed] [Google Scholar]
- 58.Zhong J, Tang MK, Zhang Y, et al. Effect of salvianolic acid B on neural cells damage and neurogenesis after brain ischemia-reperfusion in rats. Acta Pharm Sin 2007; 42:716–721. [PubMed] [Google Scholar]
- 59.Cui RT, Pu CQ, Liu JX, et al. Effects of ginsenoside Rgl on proliferation and differentiation of neural stem cells in the subventricular zone in adult rats after focal cerebral ischemia. Chin J Geriatr Heart Brain Vessel Dis 2007; 9:707–709. [Google Scholar]
- 60.Cui RT, Pu CQ, Liu JX, et al. Effects of ginsenoside Rg1 on the proliferation of neural stem cells in rats with focal cerebral ischemia. Med J Chin P L A 2007; 32:842–845. [Google Scholar]
- 61.Qi CF, Zhang JS, Tian YM, et al. Effect of ligustrazine on cell proliferation in subventricular zone in rat brain with focal cerebral ischemia- reperfusion injury. J Centr South Univ (Med Sci) 2007; 32:396–400. [PubMed] [Google Scholar]
- 62.Qiu F, Liu Y, Zhang PB, et al. Effects of ligustrazine on hippocampal dentate gyrus cell proliferation after focal cerebral ischemia in adult rats. J South Med Univ 2006; 26:1400–1403. [PubMed] [Google Scholar]
- 63.Qiu F, Liu Y, Zhang PB, et al. The effect of ligustrazine on cells proliferation in cortex and striatum after focal cerebral ischemia in adult rats. J Chin Med Mater 2006; 29:1196–1200. [PubMed] [Google Scholar]
- 64.Qiu F, Liu Y, Qian YH, et al. Effect of ligustrazine on cell proliferation in subventricular zone of lateral cerebral ventricle after adult rat suffering from focal cerebral ischemia. J Sichuan Univ (Med Sci Ed ) 2006; 37:726–729.780. [PubMed] [Google Scholar]
- 65.Hu XS, Zhou DM, Zhou D, et al. Effect of PTS on expression of cell proliferation following focal cerebral ischemia-reperfusion in rats. West Chin Med J 2004; 19:458–459. [Google Scholar]
- 66.Zhuang P, Zhang Y, Cui G. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One 2012; 7:e35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang PW, Cui GZ, Zhang YJ, et al. Baicalin regulates neuronal fate decision in neural stem/progenitor cells and stimulates hippocampal neurogenesis in adult rats. C N S Neurosci Ther 2013; 19:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li ZW, Mu Y, Guo HN, et al. Effects of puerarin on learning ability and neurogenesis of neural stem cells in adult rats after transient forebrain ischemia. Shanxi Med J 2012; 41:1454.1519. [Google Scholar]
- 69.ZH Li. Effect of baicalin on proliferation of endogenous neural stem cells in global cerebral ischemia/re-perfusion rats (2011). Avaiable at: http://cdmd.cnki.com.cn/Article/CDMD-10631–1011173856.htm(Accessed 9th Mar 2016). [Google Scholar]
- 70.Wang C, Zhang YJ, Feng Y, et al. Effect of astragaloside IV on neurogenesis in adult hippocampus of rats after transient forebrain ischemia. Chin Tradit Herb Drug 2009; 40:754–758. [Google Scholar]
- 71.Shen L, Zhang J. Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci Lett 2003; 344:1–4. [DOI] [PubMed] [Google Scholar]
- 72.TJ Hou. Effects of butylphthalide on endogenous neural stem cells proliferation in mice with cerebral ischemia and reperfusion (Master thesis) 2007. Available at: http://epub.cnki.net/kns/brief/default_result.aspx. [Google Scholar]
- 73.Yang LC, Li J, Xu SF, et al. L-3-n-butylphthalide promotes neurogenesis and neuroplasticity in cerebral ischemic rats. CNS Neurosci Ther 2015; 21:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence-publication bias. J Clin Epidemiol 2011; 64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 75.Jansen Of Lorkeers SJ, Eding JE, Vesterinen HM, et al. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies. Circ Res 2015; 116:80–86. [DOI] [PubMed] [Google Scholar]
- 76.Tohda C, Kuboyama T, Komatsu K. Search for Natural Products Related to Regeneration of the Neuronal Network. Neurosignals 2005; 14:34–45. [DOI] [PubMed] [Google Scholar]
- 77.Landis SC, Amara SG, Asadullah K, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012; 490:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiebers DO, Adams HP, Jr, Whisnant JP. Animal models of stroke: are they relevant to human disease? Stroke 1990; 21:1–3. [DOI] [PubMed] [Google Scholar]
- 79.NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Physiol 2010; 588:2519–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]


