Abstract
This first validation of the International Myeloma Working Group geriatric assessment in 125 newly diagnosed multiple myeloma patients was performed using the International Myeloma Working Group score based on age, the Charlson Comorbidity Index and cognitive and physical conditions (Activities of Daily Living / Instrumental Activities of Daily Living) to classify patients as fit, intermediate-fit or frail. We verified the International Myeloma Working Group score’s impact on outcome, and whether additional tools complement it. Since our prior analyses determined renal, lung and Karnofsky performance impairment as multivariate risks, and the inclusion of frailty, age and cytogenetics complements this, we included the revised myeloma comorbidity index, the Charlson Comorbidity Index, the Hematopoietic Cell Transplantation-Comorbidity Index and the Kaplan-Feinstein Index in this assessment. Multivariate analysis confirmed cytogenetics, Activities of Daily Living, Instrumental Activities of Daily Living and the Charlson Comorbidity Index as risks: 3-year overall survival for fit, intermediate-fit and frail patients was 91%, 77% and 47%, respectively. Using the Charlson Comorbidity Index, the Hematopoietic Cell Transplantation-Comorbidity Index, the Kaplan-Feinstein Index and the revised Myeloma Comorbidity Index allowed us to define fit and frail patients with distinct progression-free and overall survival rates, with the most pronounced differences evidenced via the International Myeloma Working Group score, the Charlson Comorbidity Index and the revised Myeloma Comorbidity Index. Since the Charlson Comorbidity Index is included in the International Myeloma Working Group score, we propose the latter and the revised Myeloma Comorbidity Index for future frailty measurements. Both are useful instruments for identifying myeloma patients with a geriatric risk profile and have a strong prognostic value for functional decline and overall survival. The study was registered as: (clinicaltrials.gov Identifier: 00003686).
Introduction
Treatment concepts and survival of multiple myeloma (MM) patients have substantially changed due to our better understanding of the disease, novel risk-adapted therapies and improved supportive care measures.1–3 MM typically affects elderly patients whose prognosis varies widely and remains more unfavorable than in younger patients. This is shown to be related to a higher frequency of treatment discontinuation and non-hematological adverse events.3–8 Moreover, elderly and frail patients are less frequently included in clinical trials and may receive fewer novel agents.2–4,7–10 This typically occurs because multimorbidity and the interaction of various medications can complicate the treatment of patients, limit their physical condition and impair survival.7,8,10
However, the global population is aging rapidly and the increasing number of elderly patients demands reliable tools to assess their vulnerability as expressed in chronic conditions and limitations in daily activities. Novel risk scores can either rely on MM tumor burden, as postulated with the combined use of the International Staging System (ISS), lactate dehydrogenase (LDH) and high-risk cytogenetics,5,11 and/or the functional condition of patients, which is assessed worldwide.12–16 A functional or geriatric assessment (GA) offers the possible advantage of guiding therapeutic decisions and may prove essential when accounting for treatment compatibility, drug-induced side effects, and mortality.2,14,17,18 This has been acknowledged as relevant, along with competent clinical judgment, apart from those risks generated through the myeloma itself.
GA tools have been postulated to be valuable in different cancers. However, most are not myeloma-specific (such as the Charlson Comorbidity Index [CCI], the Hematopoietic Cell Transplantation-Comorbidity Index [HCT-CI] or the Kaplan-Feinstein [KF] Index). It has not yet been determined which of these is most applicable to myeloma. The International Myeloma Working Group (IMWG) demonstrated, in a pooled analysis of 869 newly diagnosed MM patients, that their IMWG score defined fit, intermediate-fit and frail patients and predicted the risk of mortality. This IMWG score combines age, Activities of Daily Living (ADL), Instrumental Activities of Daily Living (IADL) and CCI. The authors proposed the IMWG score as an additional tool for clinical evaluation, cross-comparison of clinical trials and for the measurement of frailty in designing future trials.4 Indeed, prospective randomized studies constitute a good basis for the development of prognostic scores, since they meet all requirements postulated to be important.2,6–8 However, they bear the additional challenge that patients therein are selected according to strict inclusion criteria. Therefore, an internal and external validation of postulated prognostic scores in unselected patient cohorts is necessary.7,8,10,19
Since the IMWG score was tested, but not validated, the authors encouraged others to substantiate their findings.4,6 In particular, “real world” patients were urged to be assessed, since the IMWG data was based on clinical trial patients who were stringently treated within trial protocols, where the frailest patients were excluded.6 Thus, it was of relevance to assess the external validity of the IMWG score in order to obtain confirmation in “real-world” patients from population-based registries and prospective analyses.6 Splitting the IMWG cohort by the investigators into a test and validation cohort would have resulted in an internal validation, again bearing the limitation of the results being solely based on clinical trial participants.19
Herein, we prospectively and carefully assessed the IMWG score’s impact on clinical outcome, and are the first who have thoroughly validated the IMWG baseline GA in a well-characterized external cohort. Since our prior analyses demonstrated that multivariate risk factors in MM patients include impaired renal function, lung function and Karnofsky performance status (KPS),2,7,8,10,20 and that with a revised Myeloma Comorbidity Index (R-MCI), the inclusion of frailty, age and cytogenetics improves its prediction of fit, intermediate-fit and frail patients,21 we included the R-MCI, CCI, HCT-CI and KF Index in this analysis. Our intention was validation, as diligently performed herein, and not the improvement of the IMWG score.
Methods
Patient population and study design
In analogy to the IMWG analysis,4 we performed a baseline GA in 125 consecutive patients with MM at the time of initial diagnosis and first presentation at our center. Patients received standard antimyeloma treatment according to the institutional MM pathway and current recommendations.2,22 As seen with the IMWG cohort (a.] EMN-01 trial: Rd vs. MPR or CPR, b.] 26866138MMY2069 trial: VP vs. VCP or VMP, and c.] IST-CAR-506 trial: carfilzomib (Cd)), therapy regimens differed, but were those of current antimyeloma treatment: induction mostly consisted of VCD (bortezomib, cyclophosphamide, dexamethasone): patients ineligible for autologous stem cell transplantation (ASCT) received 9 cycles of VCD, and for medically fit patients up to the age of 70 years old, ASCT with VCD induction was performed. If feasible, patients were included in prospective multicenter trials such as the Clarion study, where instead of VCD, patients were randomized to VMP (bortezomib, melphalan, prednisone) or carfilzomib plus MP (melphalan, prednisone), or in the DSMM XII, XIII, XIV trials (n=49, 40%). Briefly, patients in the DSMM XII and XIV trials received RAD (lenalidomide, adriamycin, dexamethasone)23,24 induction, or RAD vs. VRD (bortezomib, lenalidomide, dexamethasone),23,25 respectively. Patients in the DSMM XIII trial were randomized to lenalidomide and dexamethasone (Rd) vs. Rd with ASCT.26 The diversity of different induction regimens in the IMWG cohort vs. ours were seven vs. six, respectively. As treatment differed in both the IMWG and our cohort, both analyses adjusted their univariate and multivariate models for known prognostic factors (ISS, cytogenetics and therapy).
The analysis was carried out according to the guidelines of the Declaration of Helsinki and Good Clinical Practice. All patients gave their written informed consent for institutional-initiated research studies and analyses of clinical outcome studies conforming to the institutional review board guidelines. The study was registered as follows: (clinicaltrials.gov identifier 00003868).
The primary objectives of this analysis were to recapitulate the IMWG score in our MM cohort, to assess additional GA tools to predict fit vs. frail patients and how these geriatric parameters predict overall survival (OS). The secondary objectives included the impact of the IMWG score as compared to the R-MCI, CCI, HCT-CI and KF Index, and to assess their value for OS and progression-free survival (PFS).
Assessment
The GA consisted of 6 tools: the Katz ADL, the Lawton IADL, CCI, HCT-CI, KF Index and R-MCI as described.4,9,18,21,22 The comorbidities assessed in the R-MCI are depicted in the Online Supplementary Table S1, and the CCI, HCT-CI, KF Index and R-MCI in the Online Supplementary Table S2. Online Supplementary Table S1 defines 13 comorbidity factors as mildly, moderately or severely impaired based on the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 and included: renal impairment, lung and KPS impairment, cardiac, liver or gastrointestinal disease, disability, frailty, infection, thromboembolic events, peripheral neuropathy, pain, and secondary malignancies. In addition, age and cytogenetics were assessed: del(17p13), del(13q14), t(4;14), t(14;16); t(14;20), hypodiploidy, c-Myc and chromosome 1 aberrations were defined as unfavorable, and t(11;14), hyperdiploidy and a normal karyotype as favorable cytogenetics. Genetic abnormalities were detected by fluorescence in situ hybridization (FISH). Renal function was determined via estimated glomerular filtration rate ([eGFR] by MDRD), and lung disease via a lung function test. Pulmonary obstruction and/or restriction were distinguished with the aid of parameters such as forced expiratory volume in one second (FEV1), the Tiffeneau-Pinelli index (FEV1/FVC) and total lung capacity (TLC). Respiratory insufficiency was detected through oxygen and carbon dioxide levels in arterial blood gas analysis. Pulmonary obstruction was graded through the impairment of the FEV1: a FEV1 of ≥80% was scored as mild, <80–50% as moderate, and <50% as severe. The KPS was defined as normal (100%), mildly (90%), moderately (80%) or more substantially impaired (≤70%). Frailty and disability were assessed in order to obtain a more precise determination of the physical condition of patients. The Fried definition was utilized for frailty: this takes into account the added presence of weakness, poor endurance, low physical activity, and slow gait speed.27,28 Patient characteristics included age, ISS and treatment (Table 1).
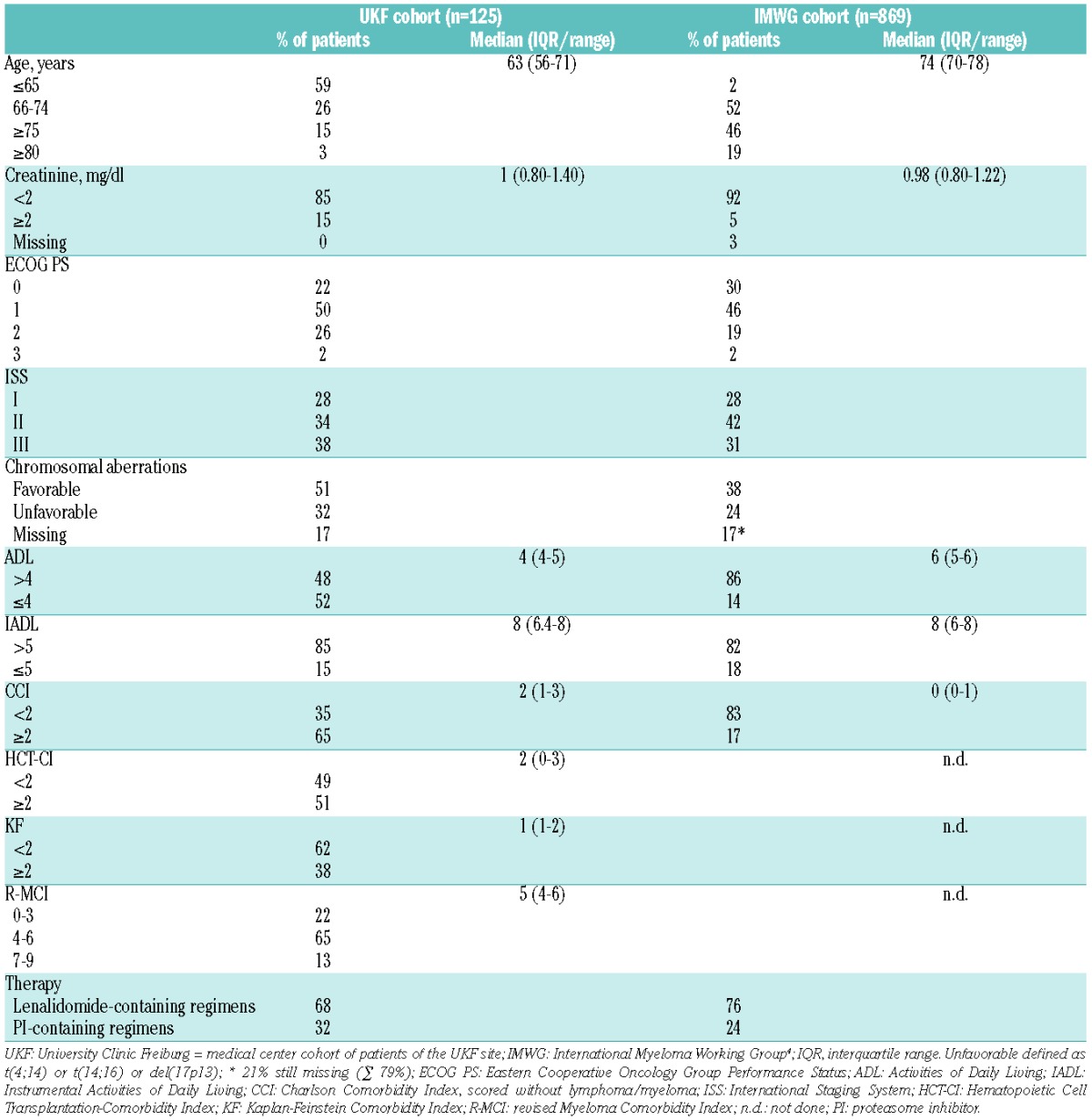
Table 1.
Baseline patient characteristics of University Clinic Freiburg (UKF) and IMWG cohorts.
Statistical analysis
Data were analyzed using SAS 9.2 (SAS Institute Inc., NC, USA). OS was calculated from the date of first presentation at our center until the date of death from any cause, while PFS was calculated from the date of first presentation until the date of progression, relapse or death from any cause. Observations, where the event of interest did not occur, were censored at the time the patient was last seen alive/without a documented event, or at the latest on June 1st, 2015. OS and PFS rates were estimated using the Kaplan-Meier method and compared using the log-rank test.
The IMWG score was assessed and compared to the R-MCI, HCT-CI, CCI and KF Index, evaluating the prognostic role on OS in our cohort with Cox regression models (Table 2). Results were presented as estimated hazard ratios (HRs) with two-sided 95% confidence intervals (CI) and corresponding P-values (Table 2). Cox regression models obtained and displayed in the analysis of the IMWG data were repeated using our data (Tables 2–4) in order to compare the IMWG score and R-MCI, as well as other internationally renowned, but MM unspecific comorbidity scores, such as the CCI, HCT-CI and KF Index (Tables 3 and 4).
Table 2.
Final Cox regression model of University Clinic Freiburg (UKF) and IMWG cohorts.
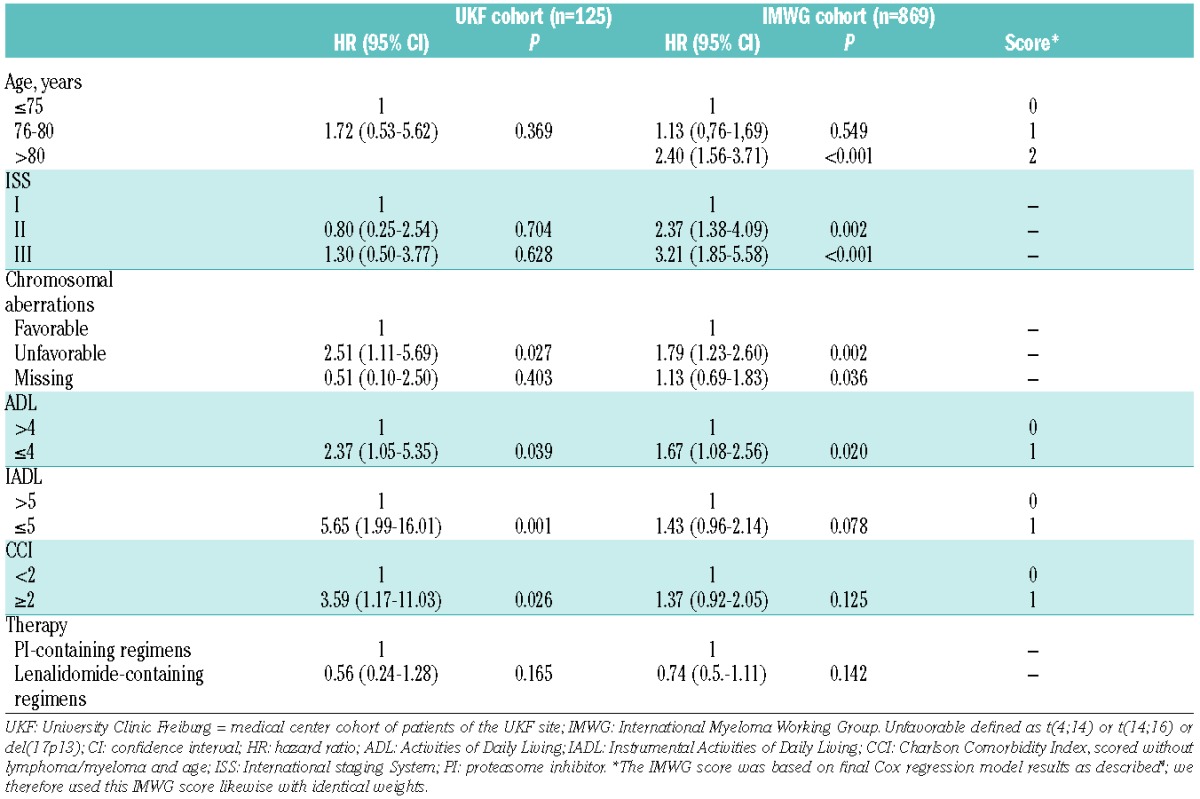
Table 4.
Univariate and multivariate analysis of the impact of frailty on OS and PFS of University Clinic Freiburg (UKF) and IMWG cohort.
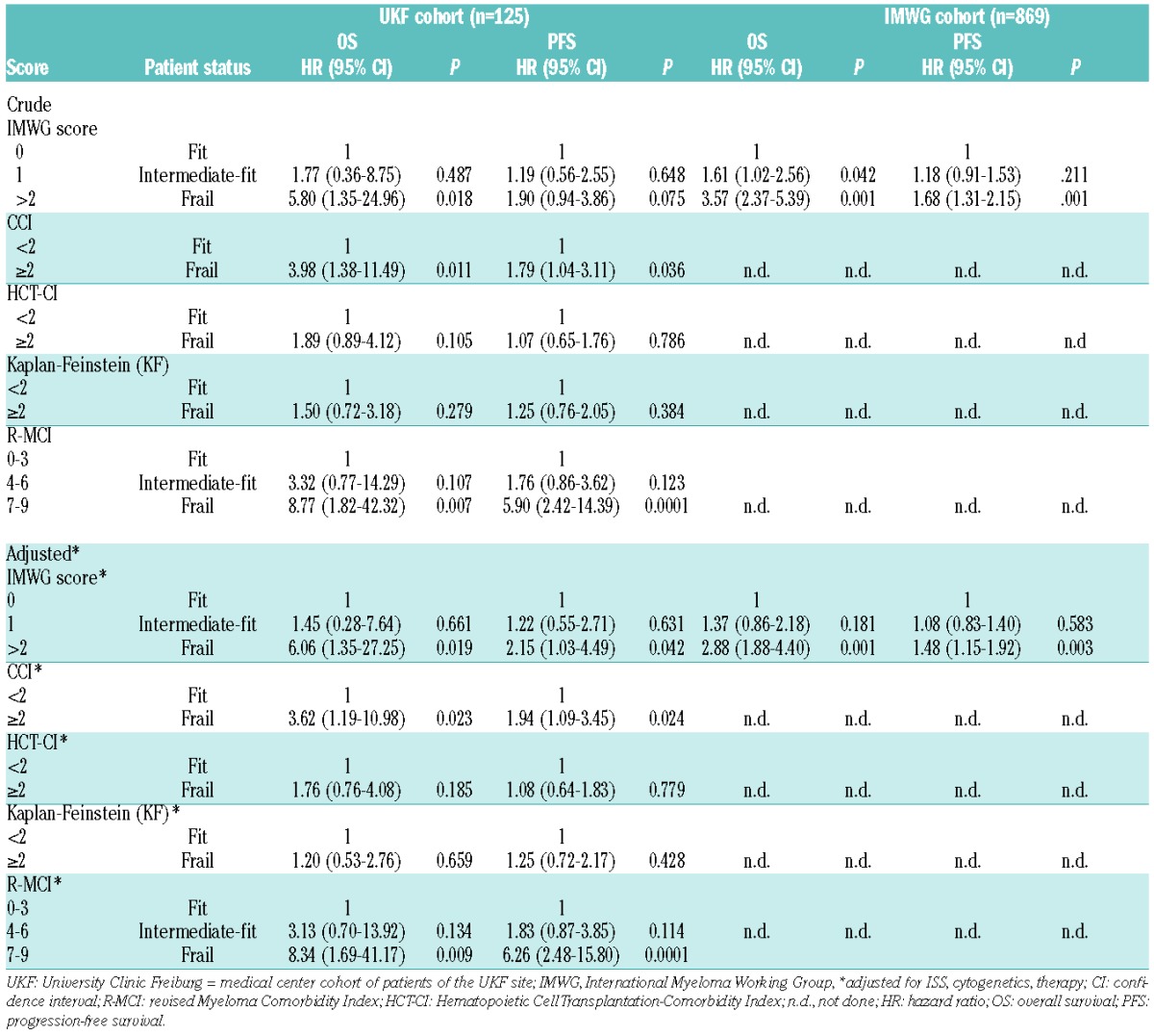
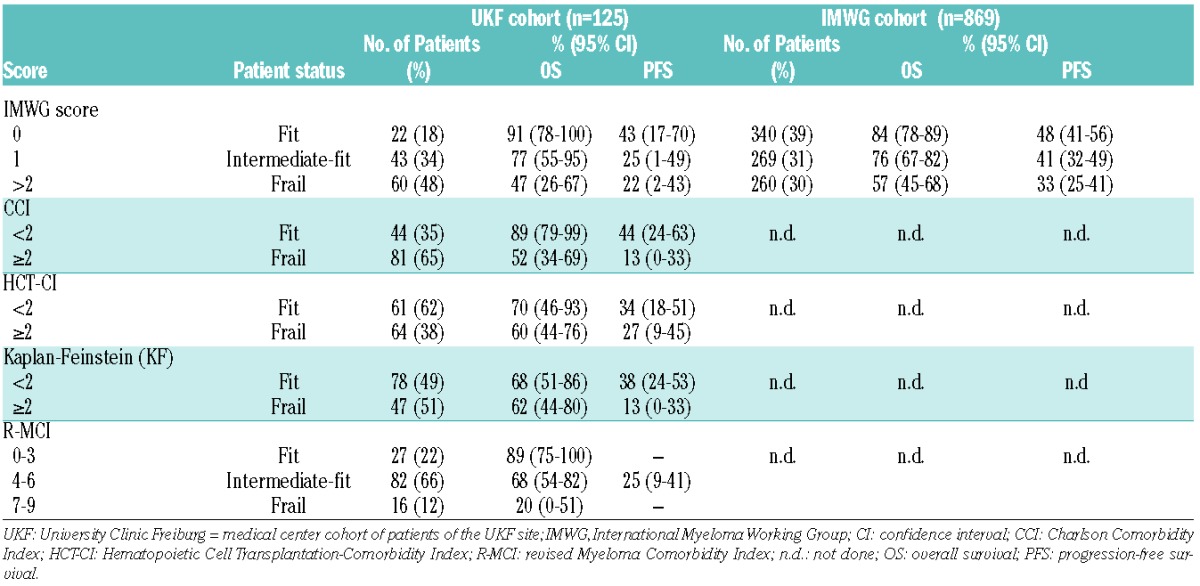
Table 3.
Comorbidity scores and related rates of OS and PFS at 3 years of University Clinic Freiburg (UKF) and IMWG cohort.
Multivariate risks, on which the IMWG score is based,4 were used to score fit, intermediate-fit and frail patients (Table 2). This IMWG score was also applied in order to compare fit vs. frail patients defined by the use of the R-MCI, CCI, HCT-CI and KF Index (Table 3), and via univariate and multivariate models evaluating the prognostic value of the scores, alone and adjusted for known prognostic factors (ISS, cytogenetics, therapy; Table 4): 125 patients with 28 OS events (deaths) and 64 PFS events (death or disease progression) were included in the analyses. Our main results relied on univariate Cox models, namely the presentation of OS and PFS comparisons according to the fitness scores of patients (Table 4), and on multivariate analyses to adjust for 3 additional known prognostic factors, specifically ISS, chromosomal abnormalities and therapy. (Table 4).
Results
Patient characteristics
The analysis included 125 consecutive, prospectively assessed MM patients. The median follow-up was 28 months (interquartile range [IQR] 22–33). The median age was 63 years of age, 26% of patients were 66–74 years old and 15% older than 75 years of age (Table 1), which is typical for tertiary centres.7,8,10,21,22,29 This was in contrast to the IMWG cohort,4 where 46% of patients were older than 75 years of age (Table 1), albeit various other patient characteristics were comparable, e.g. renal function in both showed a median creatinine of 1mg/dl, the Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1 was similarly distributed with 72% and 76%, respectively, patients had mostly ISS II/III stages in 72% and 73%, respectively, and the frequencies of unfavorable, favorable and missing chromosomal aberrations appeared similar. Moreover, the median IADL score in both cohorts was uncompromised at 8 (Table 1).
Since the IMWG cohort consisted exclusively of clinical trial patients4 and ours of consecutive “real world” patients, there were some differences: the number of patients with substantial renal impairment (creatinine >2 mg/dl) was 15% in our cohort (IMWG cohort: 5%), our patients showed an ECOG PS of 2–3 in 28% (IMWG: 21%), ISS III frequencies were higher at 38% (IMWG 31%), and unfavorable cytogenetics in 32% of our patients were higher as compared to 24% in the IMWG cohort. Unfavorable, favorable and missing cytogenetics in the IMWG cohort were 24%, 38% and 17%, respectively, vs. 32%, 51% and 17% in ours, respectively, suggesting with the IMWG cytogenetic data in 79% of patients that another 21% were missing (17+21%=38%). Supporting the characteristics of our “real world” patients vs. the IMWG cohort, our median ADL score was lower with 4 vs. 6, respectively, and the CCI was higher with 2 vs. 0, respectively (Table 1). In agreement with these findings, the median HCT-CI, KF Index and R-MCI in our patients - not objectives of the IMWG analysis - were 2, 1 and 5, respectively, and thus reflected a typical, moderately impaired patient cohort.2,7,8,10,22,29
Identification of prognostic variables in the Cox regression model
The impact of advanced age, functional decline on ADL and IADL, ISS, cytogenetics, therapy and the presence of comorbidities leading to a worsening of OS was investigated in a multivariate Cox regression model (Table 2). The final prognostic model obtained after backward selection in the IMWG data was applied to ours. The results were compared to the IMWG results. Age <80 years showed no significant impact on OS (HR 1.72; P=0.369), which is in agreement with the IMWG score,4 whereas unfavorable cytogenetics (HR 2.51; P=0.027), ADL ≤4 (HR 2.37; P=0.039), IADL ≤5 (HR 5.65; P=0.001) and CCI ≥2 (HR 3.59; P=0.026) significantly reduced OS, the latter three being in accordance with IMWG results (Table 2). Both IADL and CCI in the IMWG cohort increased the HR for impaired OS to 1.43 and 1.37, respectively, but failed to reach significance.4 In our patients, the IADL and CCI revealed a higher HR and reached significance for the CCI, which was likely related to our median CCI of 2 as opposed to 0 in the IMWG trial cohort. Therefore, more CCI-relevant comorbidities were present in our cohort vs. those within the IMWG study4 (Tables 1 and 2). Using the 4 IMWG risk factors: age, impaired ADL, IADL and CCI to define 3 risk groups, patients were stratified into fit (score=0), intermediate-fit (score=1) and frail (score ≥2) patients, displaying substantially different OS and PFS (Tables 3 and 4).
Comorbidity scores and OS and PFS at 3 years for both the University Clinic Freiburg (UKF) and IMWG cohorts
According to the proposed IMWG score that determined fit, intermediate-fit and frail patients with an additive total score of 0, 1 and 2, respectively,4 we had fewer fit (18% vs. 39%), similar intermediate-fit (34% vs. 31%) and more frail patients (48% vs. 30%) than in the IMWG cohort (Table 3): similar to the IMWG data, our 3-year OS was 91% for fit, 77% for intermediate-fit (HR 1.77; 95% CI 0.36–8.75; P=0.487) and 47% for frail patients (HR 5.80; 95% CI 1.35–24.96; P=0.018; Tables 3 and 4). In the multivariate analysis we also confirmed that, when adjusted for staging and the treatment administered, frailty profiles and comorbidity scores were associated with shorter OS (Table 4).
By applying the IMWG score, the 3-year PFS in our cohort was 43% for fit, 25% for intermediate-fit (HR 1.19; 95% CI 0.56–2.55; P=0.648) and 22% for frail patients (HR 1.90; 95% CI 0.94–3.86; P=0.075; Tables 3 and 4). PFS for fit patients was comparable to the IMWG data, albeit lower for intermediate-fit and frail patients, which was likely related to more patients with comorbidities, higher CCI and lower ADL in our cohort (Tables 1 and 3).
Using the 4 other comorbidity scores, namely R-MCI, CCI, HCT-CI and KF Index, also allowed division of fit and frail patients based on previously proposed cutoffs, with substantially different OS and PFS (Table 3). Of note, the proportion of patients via elevated HCT-CI ≥2, KF Index ≥2, CCI ≥2 and R-MCI ≥4, differed substantially, with 38%, 51%, 65% and 78%, respectively (Table 3). Nevertheless, all 4 scores revealed PFS and OS group differences between fit and frail patients, the most pronounced being observed with the use of the IMWG, CCI and R-MCI (Figures 1 and 2).
Figure 1.
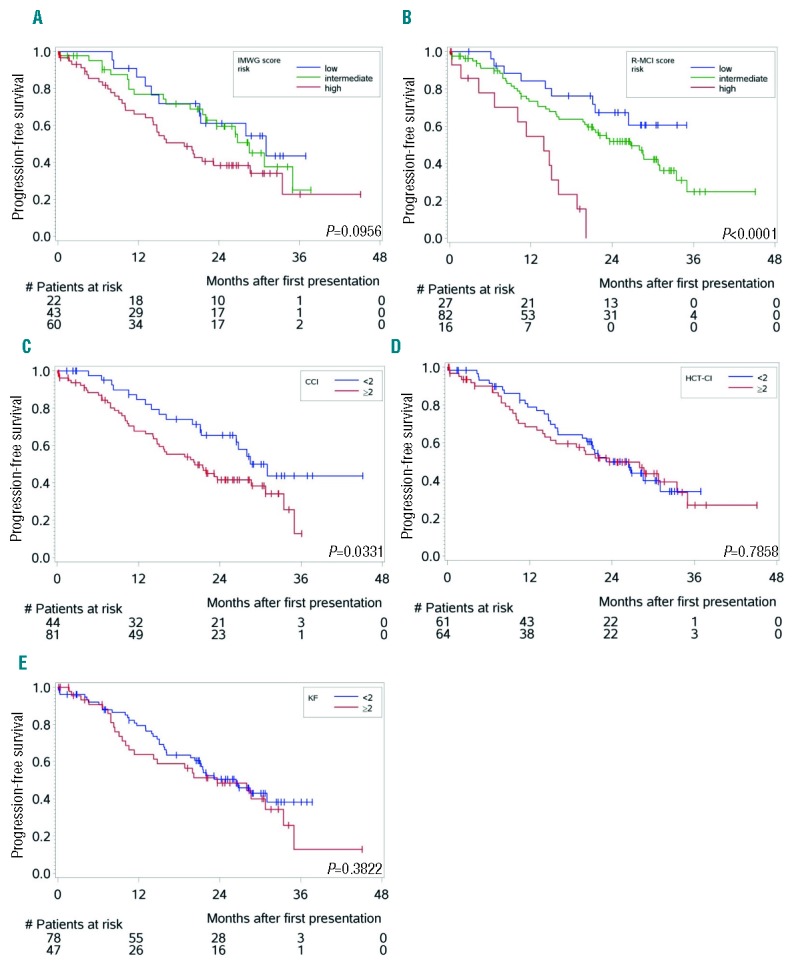
Kaplan-Meier estimates for progression-free survival (PFS) (n=125) according to different comorbidity scores [P-values; log-rank test]. (A) PFS in our patients divided into fit, intermediate-fit and frail patients showed group differences between both fitter patient groups and frail patients using the IMWG score. (B) PFS according to the R-MCI revealed better group distinctions between fit, intermediate-fit and frail patents. (C–E) PFS according to CCI risk groups again showed significant difference, whereas these were undetectable with the use of both HCT-CI (D) and Kaplan-Feinstein (E).
Figure 2.
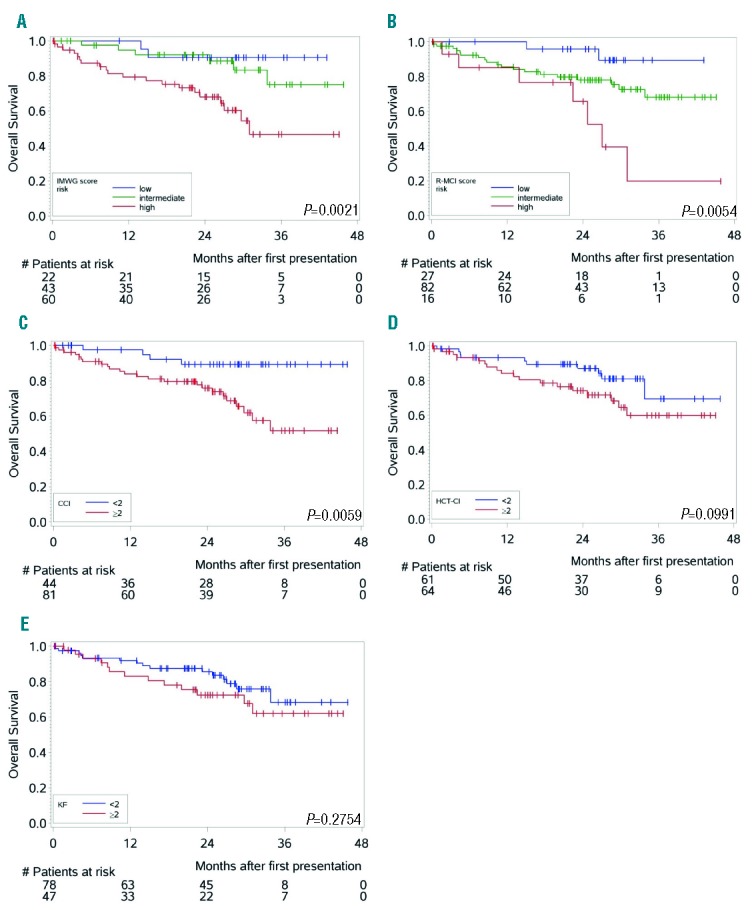
Kaplan-Meier estimates for overall survival (OS) (n=125) according to different comorbidity scores [P-values; log-rank test]. (A) Overall survival (OS) according to the IMWG score assessed with our MM patient cohort showed improved OS for patients classified as fit (low-risk) or intermediate-fit (intermediate-risk) vs. those determined frail (high-risk)4. OS for the fit and intermediate-fit group was slightly, but insignificantly different. (B) OS according to the R-MCI showed improved survival of fit (low-risk) vs. intermediate-fit or frail (high-risk) patients. Differences between these 3 groups of fit, intermediate-fit and frail patients were more distinct compared to the IMWG score.4 (C–E) OS according to the CCI,12 dividing patients into two groups with CCI <2 vs. CCI ≥2 comorbidities, revealed significant OS differences, which were more pronounced than fit vs. frail patients assessed via HCT-CI (D) or Kaplan-Feinstein (E).
Univariate and multivariate analysis of the impact of frailty on OS and PFS for the University Clinic Freiburg (UKF) and IMWG cohorts
In Table 3, 3-year OS and PFS rates are presented with accompanying 95% CI. These comparisons refer to one single time point, where CIs are large due to smaller observation numbers at the end of the observation period. In Table 3, we intended to provide additional descriptive information to Figures 1 and 2, and to provide comparability to 3-year OS and PFS rates reported by the IMWG.4 The proper way to compare groups with respect to OS and PFS over the complete observation period is via Cox models, as presented in Table 4. HRs via multivariate analysis were associated with shorter OS and PFS using the IMWG score, as well as when R-MCI, CCI, HCT-CI and the KF Index were applied. These frailty scores reached significance for OS with the use of the IMWG score for frail vs. both fit and intermediate-fit patients, although the two fittest patient groups clustered together. Similarly, significant OS differences between fit and frail patients were notable with the use of both the R-MCI and CCI. For PFS, the IMWG score showed differences for frail patients vs. the two fitter patient groups, however this difference did not reach significance in our cohort. Significant PFS differences via CCI (<2 vs. ≥2) and R-MCI (≤3 vs. ≥4) were also observed (Table 4, Figures 1 and 2).
These frailty scores continued to be associated with shorter OS and PFS when adjusted for staging and the treatment administered, again with the most pronounced group differences being those between frail vs. fitter patients when these were determined using the IMWG score, CCI and R-MCI (Table 4).
Discussion
MM management strategies continue to evolve, and in the past few decades survival has improved significantly, yet the overall prognosis depends on a variety of disease- and host-related risks.2,18,21,22,29 As individualized treatment aims to balance therapy options against toxic side effects, to preserve quality of life and to further improve survival, there is an obvious rationale for incorporating risk assessment tools into the management of predominantly older patients. This supports the inclusion of additional objective, quantifiable and reproducible information on individual patients beyond the clinical judgment of physicians. Furthermore, this seems relevant in order to avoid applying less effective therapies to older yet fit patients.2,7,16 Therefore, cancer experts have recognized that the consideration of age alone is insufficient for choosing adequate therapy strategies. Nevertheless, age cutoffs continue to exist, and unfortunately older patients are often excluded from clinical trials.7,9,10,18,22,24,30–33
Our prior test and validation analyses have demonstrated the significant influence of impaired organ function, such as renal function and lung function as well as KPS on the PFS and OS of MM patients, thus implementing these factors in a comorbidity tool, named MCI.7,8,10 The more refined use of this MCI, tested and validated in a much larger independent cohort of 801 patients, confirmed the importance of renal function, lung function and KPS, as well as age, frailty and cytogenetics within a refined MCI (R-MCI).21 Other studies have confirmed the relevance of assessing frailty, quality of life and physical activity.18,32,34,35 The assessment of organ function, such as renal and lung impairment, has shown to influence survival rates, treatment toxicity and early death.8,16,36 Moreover, tumor genetics have been reported to relevantly influence the clinical heterogeneity of MM.1,11,14,25,37,38 Although cytogenetics are important for risk appraisal, we and others have demonstrated that physical condition and organ function are likewise crucial.7,8,10,18
We herein assessed the IMWG score4 in an independent external validation cohort. Patient characteristics were comparable, with a median creatinine of 1mg/dl, an ECOG PS of 0–1 which was similarly distributed, primarily ISS II/III stages, and an uncompromised median IADL score of 8. However, there were differences: more of our patients had unfavorable cytogenetics, lower ADL and higher CCI, which is in agreement with the IMWG cohort consisting of clinical trial patients only with strict inclusion criteria. Importantly, we confirmed that an age of <80 years may not substantially increase the risk for MM patients, demonstrating that suitable comorbidity tools and a precisely performed GA are helpful. Unfavorable cytogenetics increase the HR for survival (HR 2.51; 95% CI 1.11–5.69), in addition, functional impairment as determined via ADL, IADL or CCI increased the HRs in our cohort, showing higher HRs in all 4 compared to the IMWG cohort (Table 2).
Our cohort was likewise split into IMWG patient groups with 0, 1 and >2 risk factors showing comparably different OS and PFS groups of fit vs. intermediate-fit and frail patients; this was, however, also possible to perform via R-MCI, CCI, HCT-CI and the KF Index (Table 3). Crude and adjusted HRs of the IMWG score for OS and PFS proved to separate frail, intermediate-fit and fit patients, the latter two of which grouped together. This was confirmed when assessing the IMWG score in a multivariate model adjusting for known prognostic factors (Table 4). Both the CCI and R-MCI, divided into fit vs. frail, showed substantially increased HRs for OS and PFS, whereas less pronounced differences for fit vs. frail patients were obvious with the use of both the KF Index and HCT-CI. Therefore, our detailed comparison of both the crude and adjusted IMWG score with others, suggested some to be of particular value in MM, such as the IMWG score, CCI and R-MCI, whereas others, such as the KF Index and HCT-CI were of lesser significance. The CCI in particular has been tested in several clinical settings and has shown its usefulness.9,12,18,34 However, scoring of the CCI has been modified2,4,7–10,18,39 since it is not specific for MM. Why the IMWG has chosen the CCI to complement their IMWG score is therefore a possible choice, albeit the CCI has been validated in diabetes and its value has also been questioned, all the more as the median CCI in the IMWG cohort was extremely low, with 0. Our results, that all comorbidity tools: IMWG score, R-MCI, CCI, HCT-CI and the KF Index divide patients with largely different OS and PFS, even though only the IMWG score, R-MCI and CCI reached significance, are therefore of value. Of note, both the IMWG score and R-MCI performed comparably well, as shown in other scores, where 2 geriatric screening tools (the G8 questionnaire and the Flemish version of the Triage Risk Screening Tool [fTRST]) were compared.35
This is the first validation study that externally confirmed the IMWG score and other relevant comorbidity tools in real life patients, although our cohort was smaller, it nevertheless provides useful information for a validation analysis. A common procedure is to divide a sample number into 2/3 for score construction and 1/3 for internal validation. In the IMWG analysis,4 smaller subgroup analyses were performed, which also resulted in reduced sample sizes. Therefore, we consider our data valuable, supporting the usefulness of the IMWG score (and others) when applied to different populations. Albeit our patients were typical for a university center, they were younger than the IMWG cohort.4 We have previously shown that stage and age migration may occur29 and that older patients are increasingly seen in university centers: in a previous analysis of 816 MM patients, 3-fold increases for >70-year old patients were observed.29 In this analysis we confirmed that the HR for 60 to 70 and >70-year old MM patients increased from 1.72 to 3.46, respectively.29 The reason why the IMWG cohort did not see an age risk in 76 to 80-year old patients was most likely because: a) age is a lesser risk factor than initially presumed - making simple comorbidity assessments as performed with experience as in our centers the more important, and b) because the IMWG cohort included only trial patients that were much fitter, and age is less relevant in those that fulfill all eligibility criteria. Another criticism might be that antimyeloma treatment differed from those used in the IMWG study.4 Since easily assessable risk scores are important to apply independent of treatment, and previous analyses have shown that heterogeneous therapies are not surrogates for comorbidity risks, we consider our analysis to be of equal importance to prior GA analyses, including that of the IMWG. This is even more evident since we validated and complemented their findings, and all risk scores were assessed as crude and adjusted scores (Table 4).4
The strengths of this analysis were the accurate and prospective assessment of the physical condition of patients with no restriction of validity and information loss based on multicenter data entries.6 Moreover, our cohort reflected typical day to day patients, since ADL and CCI were affected compared to the IMWG cohort, whereas in both the IADL was uncompromised with a median of 8. This suggests this daily activities score to be of lesser importance, and questions the necessity to use both ADL and IADL in a combined risk score. Our thoroughly performed validation of the IMWG score and others, including the R-MCI, was performed within a structured prospective GA and by an experienced group who has been doing these assessments for years.2,7,10,20,21 Moreover, we applied all current state-of-the-art statistics with our renowned statistical team with the important aim to validate easy to assess risk scores that function to improve MM care.40
Currently, the IMWG score consists of: 1. age (3 age groups), 2. ADL (6 self-care tasks), 3. IADL (8 house-hold tasks), and 4. CCI (18 factors and maximum points of 33, plus 1 point per decade of aging from the age of 50). As a sum risk assessment, this constitutes 3+10+8+18=39 risks, instead of 6 within the R-MCI. Thus, within the IMWG score (including age and CCI), age is scored twice; suggesting by the direct comparison of both IMWG score and R-MCI that the former is more challenging than the latter. This can be verified, if the R-MCI is calculated via the website which we designed: www.myelomacomorbidityindex.org. Nevertheless, our intention was pure validation of the IMWG score, which we performed fastidiously. As with the IMWG score, treatment was not modified according to this score. A next step includes prospective randomized clinical studies to design therapeutic approaches with the help of a GA algorithm.2,14,20,41,42 We conclude that both IMWG score and R-MCI are useful instruments in older myeloma patients for identifying those with geriatric risks. The publication of our validation analysis should attract the attention of cancer experts and help in the care of MM patients, the latter being an aim that all of us are enthusiastically persisting in daily.
Acknowledgments
The authors thank Prof. Dr. Hermann Einsele, University of Würzburg, Germany, Prof. Dr. Pieter Sonneveld, Erasmus MC Rotterdam, The Netherlands, Prof. Dr. Christian Straka, Schön Clinic Starnberger See, Germany, Prof. Dr. Keith Stewart, Mayo Clinic Arizona & Rochester, USA, Prof. Dr. Torben Plesner, Center Lillebaelt University of Southern Denmark, Denmark and Prof. Dr. Justus Duyster, University of Freiburg, Germany, for their significant support, fruitful discussion and valuable suggestions. We are also highly obliged to the three anonymous reviewers for their insightful comments that have inspired us to further improve the manuscript and Dr. Marie Follo for proofreading the manuscript, to Hans Schall and Judith Urban for diligent data management using our MM database, and to Dr. Patrique Wolfrum for IT support.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/9/1110
Funding
This work is supported by the Deutsche Krebshilfe (grants 1095969 and 111424 [to ME and RW]).
References
- 1.Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt M, Terpos E, Kleber M, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014; 99(2):232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011; 118(17):4519–4529. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellqvist U-H. New prognostic tools for myeloma. Blood. 2015;125(13):2014–2015. [DOI] [PubMed] [Google Scholar]
- 7.Kleber M, Ihorst G, Gross B, et al. Validation of the Freiburg Comorbidity Index in 466 multiple myeloma patients and combination with the international staging system are highly predictive for outcome. Clin Lymphoma Myeloma Leuk. 2013;13(5):541–551. [DOI] [PubMed] [Google Scholar]
- 8.Kleber M, Ihorst G, Udi J, Koch B, Wäsch R, Engelhardt M. Prognostic risk factor evaluation in patients with relapsed or refractory multiple myeloma receiving lenalidomide treatment: analysis of renal function by eGFR and of additional comorbidities by comorbidity appraisal. Clin Lymphoma Myeloma Leuk. 2012;12(1):38–48. [DOI] [PubMed] [Google Scholar]
- 9.Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleber M, Ihorst G, Terhorst M, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J. 2011;1(9):e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau P, Cavo M, Sonneveld P, et al. Combination of international scoring system 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014; 32(20):2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 13.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. [DOI] [PubMed] [Google Scholar]
- 15.Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013; 98(2):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offidani M, Corvatta L, Polloni C, et al. Assessment of vulnerability measures and their effect on survival in a real-life population of multiple myeloma patients registered at Marche Region Multiple Myeloma Registry. Clin Lymphoma Myeloma Leuk. 2012;12(6):423–432. [DOI] [PubMed] [Google Scholar]
- 17.Ganna A, Ingelsson E. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population-based study. Lancet Lond Engl. 2015;386(9993):533–540. [DOI] [PubMed] [Google Scholar]
- 18.Larocca A, Palumbo A. How I treat fragile myeloma patients. Blood. 2015; 126(19):2179–2185. [DOI] [PubMed] [Google Scholar]
- 19.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453–473. [DOI] [PubMed] [Google Scholar]
- 20.Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network Guidelines for the Management of Multiple Myeloma-related Complications. Haematologica. 2015;100(10):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domm A-S, Hieke S, Ihorst G, et al. Importance and Determinants of Comorbidities, Functional Limitations and Multiple Myeloma (MM)-Specific Risk Factors: Further Development of an Improved and Weighted MM-Risk Score (Freiburg Comorbidity Index [FCI]). Blood. 2014;124(21):733–733. [Google Scholar]
- 22.Engelhardt M, Ihorst G, Landgren O, et al. Large registry analysis to accurately define second malignancy rates and risks in a well-characterized cohort of 744 consecutive multiple myeloma patients followed-up for 25 years. Haematologica. 2015; 100(10):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knop S, Langer C, Engelhardt M, et al. Response to Lenalidomide, Doxorubicin and Dexamethasone (RAD) in Newly Diagnosed Multiple Myeloma Is Independent of Cytogenetic Risk and Retained after Double Stem Cell Transplant. Blood. 2014;124(21):177–177. [Google Scholar]
- 24.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet Lond Engl. 2015; 385(9983):2197–2208. [DOI] [PubMed] [Google Scholar]
- 25.Richardson PG, Bladé J. The comprehensive clinical management of multiple myeloma and related-plasma cell disorders. Expert Rev Hematol. 2014;7(1):1–3. [DOI] [PubMed] [Google Scholar]
- 26.Straka C, Schaefer-Eckart K, Bassermann F, et al. Lenalidomide with Low-Dose Dexamethasone (Rd) Continuously Versus Rd Induction, Tandem MEL140 with Autologous Transplantation and Lenalidomide Maintenance: Planned Interim Analysis of a Prospective Randomized Trial in Patients 60–75 Years of Age with Multiple Myeloma. Blood. 2014;124(21):3969–3969. [Google Scholar]
- 27.Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. Lancet Lond Engl. 2015;385(9968):e7–9. [DOI] [PubMed] [Google Scholar]
- 28.Xue Q-L, Walston JD, Fried LP, Beamer BA. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: the women’s health and aging study. Arch Intern Med. 2011;171(12):1119–1121. [DOI] [PubMed] [Google Scholar]
- 29.Hieke S, Kleber M, König C, Engelhardt M, Schumacher M. Conditional Survival: A Useful Concept to Provide Information on How Prognosis Evolves over Time. Clin Cancer Res. 2015;21(7):1530–1536. [DOI] [PubMed] [Google Scholar]
- 30.Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013;159(10):667–676. [DOI] [PubMed] [Google Scholar]
- 31.Wildes TM, Rosko A, Tuchman SA. Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. 2014;32(24):2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig H, Sonneveld P, Davies F, et al. European perspective on multiple myeloma treatment strategies in 2014. Oncologist. 2014;19(8):829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auner HW, Szydlo R, Hoek J, et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone Marrow Transplant. 2015;50(2):209–215. [DOI] [PubMed] [Google Scholar]
- 34.Maes H, Delforge M. Optimizing quality of life in multiple myeloma patients: current options, challenges and recommendations. Expert Rev Hematol. 2015;8(3):355–366. [DOI] [PubMed] [Google Scholar]
- 35.Kenis C, Decoster L, Van Puyvelde K, et al. Performance of Two Geriatric Screening Tools in Older Patients With Cancer. J Clin Oncol. 2014;32(1):19–26. [DOI] [PubMed] [Google Scholar]
- 36.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219–9226. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper R, van Duin M, van Vliet MH, et al. Prediction of high- and low-risk multiple myeloma based on gene expression and the International Staging System. Blood. 2015; 126(17):1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Donk NWCJ, Sonneveld P. Diagnosis and risk stratification in multiple myeloma. Hematol Oncol Clin North Am. 2014;28(5):791–813. [DOI] [PubMed] [Google Scholar]
- 39.Zweegman S, Palumbo A, Bringhen S, Sonneveld P. Age and aging in blood disorders: multiple myeloma. Haematologica. 2014;99(7):1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ihorst G, Waldschmidt J, Schumacher M, Wäsch R, Engelhardt M. Analysis of survival by tumor response: have we learnt any better? Ann Hematol. 2015; 94(9):1615–1616. [DOI] [PubMed] [Google Scholar]
- 41.Stairmand J, Signal L, Sarfati D, et al. Consideration of comorbidity in treatment decision making in multidisciplinary cancer team meetings: a systematic review. Ann Oncol. 2015;26(7):1325–1332. [DOI] [PubMed] [Google Scholar]
- 42.Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol. 2015; 26(2):288–300. [DOI] [PubMed] [Google Scholar]
- 43.Kristinsson SY, Pfeiffer RM, Björkholm M, Schulman S, Landgren O. Thrombosis is associated with inferior survival in multiple myeloma. Haematologica. 2012;97(10): 1603–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasskarl J, Ihorst G, De Pasquale D, et al. Association of multiple myeloma with different neoplasms: systematic analysis in consecutive patients with myeloma. Leuk Lymphoma. 2011;52(2):247–259. [DOI] [PubMed] [Google Scholar]
- 45.ElSawy M, Storer BE, Pulsipher MA, et al. Multi-centre validation of the prognostic value of the haematopoietic cell transplantation- specific comorbidity index among recipient of allogeneic haematopoietic cell transplantation. Br J Haematol. 2015; 170(4):574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000; 36(4):453–471. [DOI] [PubMed] [Google Scholar]


