Abstract
The practice of medicine was profoundly transformed by the introduction of the antibiotics (compounds isolated from Nature) and the antibacterials (compounds prepared by synthesis) for the control of bacterial infection. As a result of the extraordinary success of these compounds over decades of time, a timeless biological activity for these compounds has been presumed. This presumption is no longer. The inexorable acquisition of resistance mechanisms by bacteria is retransforming medical practice. Credible answers to this dilemma are far better recognized than they are being implemented. In this perspective we examine (and in key respects, reiterate) the chemical and biological strategies being used to address the challenge of bacterial resistance.
Introduction
Franklin’s choice of the inevitability of “death and taxes” was surely not meant as an exclusive list. The modern observer of the diminishing efficacy of the chemotherapeutic armamentarium against bacterial infection would not hesitate to add “resistance” to his list. Bacterial resistance was, is, and forever shall be.1–4 It is found in the hospital, in the microbiome;5 in the animal manure of farms;6,7 in the soil;8–12 in remote caves;13 in the permafrost;14,15 in a centuries-old mummy;16 and in the remote reaches of the oceans.17 Moreover, given the impossibility of removing antibiotics from the environment—that is, removal of the evolutionary pressure—the evolution of the resistome cannot be reversed.18 We are compelled to live with the evolutionary legacy of antibiotics, both as ultimate pollutants in the environment and as persistent genes throughout the microbiome,19 even as we confront the consequences of this legacy. The question is less what we are to do, but how this confrontation must be done. The multi-factorial answers to this question are well known, and have been expressed in a chorus of different voices.20–38 Some of the answers to the question of what to do—such as limiting antibiotics in food sources; improving scientific,39 environmental,40 and clinical stewardship;41–46 and revising clinical trial design and creating post-registration financial incentives to reward the commercial investment in antibacterial discovery and development)47,48—transcend science. The importance and the inseparability of each of these aspects are well recognized. Nonetheless, the slow translation of many of these aspects into the societal and political spheres raises the question whether the full value of the chemical arsenal against bacterial infection—the (isolated from Nature) antibiotics, the semi-synthetic antibiotics, and the synthetic antibacterials—can be preserved. Until this translation occurs, how can society, and how can science, sustain the future of all—both old and new—antibacterial entities? The immediate task for society is the impeccable husbandry of the antibacterials that we already possess, as cogently argued by The Center for Disease Dynamics, Economics, and Policy.49 Nonetheless, their world-wide assessment shows that we remain well short of this standard. The task for science is to contribute to the technologies that will contribute to this stewardship, while simultaneously preparing for the possibility that stewardship will not be enough. The antibacterial future will include the pragmatic resurrection (or repurposing) of existing structures,20,50 identifying advantageous structural permutations of these same structures, and the discovery of new antibacterial strategies and structures. The chemistry, biochemistry, microbiology, and medical aspects of resistance are inseparable. In this brief perspective we exemplify, using for the most part recent discoveries, how scientists are confronting resistance in order to secure an antibiotic future as endless as that of resistance. These discoveries are discussed first from a chemical, and then a biological, perspective.
Endless antibiotics: a chemical perspective
The most fundamental chemical perspective is the antibacterial as a composition of matter. The simplest proposal to address the diminishing ability of existing antibacterials is the discovery of better ones. If only this discovery was the simple matter of wishing! The daunting challenge of the discovery of new chemical matter that is efficacious, as a result of the compromise of one of the limited number of validated bacterial targets, is the theme of two separate lamentations from two separate industrial discovery teams.51,52 Is antibacterial discovery so exceptionally challenging? The chemical structures useful against bacterial infection are sourced from two realms. The first realm is the synthetic: structures that are unprecedented, or are only partly precedented, in Nature. The fluoroquinolones are an important example of exceptionally useful antibacterials having (for the most part) origins in the synthetic realm.53,54 The second realm is the antibiotics discovered by Nature, as exemplified by the incredible array of natural products found during the “Golden Age” of antibiotic isolation and thereafter.55 The interface between the two realms comprises the structures synthetically elaborated from the antibiotics of Nature. This transformation is robustly exemplified by the structure-activity development of the sub-classes (including the penicillins, cephalosporins, carbapenems, monobactams, monocarbams, and monosulfactams) of the β-lactam antibacterials.56 The challenge with respect to the chemistry of antibiotic discovery thus coincides with two inquiries. Is there opportunity for structural development within the “golden age” antibiotic structures? Are there unrecognized compounds with antibacterial activity in the synthetic structures found in chemical libraries, or to be found in Nature?
The answer to both questions is “yes”, notwithstanding the daunting difficulty of realizing chemical answers.51,52 A sense of the answer to the first question is given by Scheme 1, where recent structures represent some of the key structural and mechanistic frontiers for the grand classes of the antibacterials of the golden age of discovery (the aminoglycosides, the β-lactams, the tetracyclines, the macrolides, and the quinolones). The focus of the remainder of this review is the answer to the second question: finding new antibacterials from chemical libraries and from Nature.
Scheme 1.
Structure–activity development within the “Golden Age” classes of the antibiotics continues, and certainly will address the future need for new antibacterials. This Scheme displays structures (identified by their generic name and/or their registry number) that exemplify (but by no means define) the research frontiers for these classes. The Aminoglycoside class (Column 1) is represented by the late-stage clinical candidate plazomicin,57 having broad-spectrum activity, low toxicity, and good evasion of the aminoglycoside-modifying enzymes of resistance.58,59 Structure [1626394-79-3] is an exploratory monosulfonamide-modified derivative of sisomicin with excellent Gram-negative activity and (in mice) exceptionally reduced ototoxicity.60 Structure [1620221-11-5], a second exploratory aminoglycoside, combines selective deletion of alcohol functional groups (to avoid resistance enzyme modification) with a fluoro-dependent reduction of the basicity of the neighboring amine with a consequent reduction in toxicity.61 Among the recent developments in the β-Lactam antibiotics (Column 2) is the reevaluation of older β-lactam structures such as temocillin62 and aztreonam. These latter two structures were perceived at time of their entry into the clinic as undesirably narrow-spectrum. With the proliferation of β-lactamase resistance enzymes over the past two decades, the ability of these older structures to resist hydrolysis by many β-lactamases is now regarded as advantageous. The antibacterial spectrum of aztreonam is further improved upon combination with β-lactamase inhibitors.63 The new β-Lactamase Inhibitor class (Column 2) includes the diazabicyclooctane avibactam.64,65 Other members of the diazabicyclooctane class under active investigation include the MK-7615 structure66 and the OP-0595 structure.67 A second new exploratory β-lactamase inhibitor, the cyclic boronic acid RPX-7009, restores carbapenem activity to bacteria expressing the KPC β-lactamase.68 Macrolides within the new Ketolide class (Column 3) are represented by telithromycin and by the newer fluoro-substituted solithromycin.69 The characterization of the rRNA methylase that confers ketolide resistance to the producing Streptomyces strains will facilitate the structure-activity development of this class, given the probable eventual transition of this activity as a resistance mechanism.70 New structures within the Tetracycline class (Column 4) include the clinically-approved tigecyline69 and the exploratory structure omadacycline. Although the structural difference between the two is subtle, the latter has oral activity.71 A second exploratory class of new tetracyclines is the hexacyclines.72 Resurgent interest in bacterial Gyrase/Topoisomerase Inhibitors (Column 4) is driven both by the proven clinical value of the fluoroquinolones and the consequent resistance development.73 A comparison of the structures of moxifloxacin, a recent generation fluoroquinolone, with that of the new exploratory structure ETX-0914 (having both Gram-positive and Gram-negative activity) shows superficial similarity (both have a modified fluoroquinoline core). Notwithstanding this similarity and the shared target, the mechanistic difference between the two is distinct.74,75 ETX-0914 is a clinical candidate targeting Neisseria gonorrhoeae. It is discussed in this review as an outstanding example of the possible value that synthetic chemical libraries may have for the discovery of new antibacterials.
Part of the reason for the perception of a scarceness of antibacterial matter in chemical libraries is the poor alignment between the desirable chemical properties of drugs that address bacterial, compared to eukaryotic, targets, in such libraries.76–78 Recognition of this difference has led to the design of chemical libraries that are biased toward the generally more polar character of known antibacterials. These efforts have attained promising results.79 Aligning yet further the libraries to coincide with the different antibacterial sub-classes (such as attention to the requirements for optimal inhibition of cell wall targets compared to the ribosome as a target) may improve success.80 Nonetheless—and notwithstanding the reality that successful antibacterial discovery requires more than optimization of the physicochemical properties of the chemical library81—there are antibacterials waiting to be found in chemical libraries. For example, the interplay of library screening, synthesis, and the use of reverse genomics identified the spiropyrimidinetrione inhibitors of the bacterial topoisomerases.82, 83 Subsequent structure-activity effort yielded a lead structure (EXT-0914) with excellent in vitro activity, and promising in vivo activity,84 against inter alia Neisseria gonorrhoeae.85–87 Likewise, library screening has had exceptional success in identifying potential antibacterials targeting Mycobacterium tuberculosis, as evidenced by both the imidazopyrimidine88–95 and benzothiazinone96–103 classes. As with any such structure, whether natural or synthetic, it remains to be seen whether it has the robustness (most emphatically, with respect to resistance development) needed to progress. But the structures are there. The new Community for Open Antimicrobial Drug Discovery effort is an opportunity to identify these structures.104,105 This objective of this effort is to expand the chemical diversity of antibacterial (and antifungal) compositions of matter through a free screening program (http://www.co-add.org), requiring submission of only 1 mg of a pure, water- or DMSO-soluble compound for the purpose of screening. The results of the screening are “free” (ownership is retained by the submitters).
Virtual chemical libraries in principle should not have the limitation of (for example) mismatched physical properties, but in fact virtual screening shares this same encumbrance since every virtual structure must have a physical counterpart. The treatment of disease (alas) will never be virtual. Nonetheless, the dramatically improved structural understanding of validated bacterial targets has provided examples of successful virtual screening.106 A recent example of successful virtual screening include the identification of inhibitors of the Penicillin-Binding Proteins having promising in vitro Gram-positive activity.107–109 Other examples include efforts directed toward the cytoskeletal protein FtsZ;110,111 the sortase transpeptidases;112,113 the GlmU uridyltransferase (from a lead identified by high-throughput screening);114,115 and the Mur enzymes of peptidoglycan biosynthesis.116–118 The qualification of “promising” for all of these structures (again) refers to the momentous difficulty of taking a structure from in vitro activity to clinical efficacy. But this is a difficulty that is universal for all of drug discovery. A chemist’s reflection on the ungainly heterocyclic structures that are approved inhibitors against the kinases of human cancer119 leads to the conclusion that however momentous the difficulty, the difficulty can be surmounted if the resources and incentives are in place. Antibiotic discovery is challenging. But it is not evident that its requirements for resources and incentives are any greater than for any other target, or any other disease. Rather, it is simply that neither the correct resources nor the correct incentives are fully in place.
The conclusion that antibacterial drug discovery is a surmountable challenge also stands on the ingenuity of Nature. If the golden age of antibiotic discovery has passed, a new era of natural product discovery is dawning.120–123 The antibiotics identified in the “golden age” of discovery originated from extraordinary scientific perspicacity. While this requirement has not lessened with time—the challenge of finding and then isolating (or replicating by total synthesis) a natural product, on the mass scale required for antibiotic activity, cannot be understated—the chemist today has an unprecedented ability not just to manipulate secondary biosynthesis, but also to interrogate a vastly greater diversity of bacterial and fungal species.124–137 Many of the genes for secondary metabolism are under epigenetic control.138–140 One can now pair the sequencing of a genome in order to identify the genes dedicated to secondary metabolite biosynthesis, with epigenetic activation of what often are silent biosynthetic pathways. For example, application of this strategy to a filamentous fungus, exploiting histone deacetylase inhibition to alter gene expression, activated 75% of the genes involved in secondary biosynthesis and resulted in the expression of ten secondary metabolites, four of which were new.141 There is no reason to believe that this strategy is not general. It is a strategy that could diversify access to both exploratory antibacterials, such as the nybomycin class of gyrase inhibitors142,143 as well as proven antibiotics such as the glycopeptides.144–149 Nor will it necessarily require a small molecule epigenetic modifier: interspecies communication within the microbial “interactome” has the same ability.150–153 Future natural product discovery will not be limited to the particular proteins encoded by the genes of an organism.124 We now have such a grasp on the modular organization of polyketide assembly that manipulation of the modules is feasible.55, 154–156 Future natural-product discovery will not be limited to the genome of a single organism,132,133,157–160 nor to the multimodular synthases they may encode, as evidenced by the emerging ability to reprogram the biosynthetic function of these synthases.161,162 A compelling example of the future possibilities for the discovery of antibiotics that were previously hidden, is the use of microbial co-culture to elicit antibiotic expression by the so-called “dark” or “uncultivatable” bacteria.145,163–166 This strategy culminated in the discovery of the Gram-positive active, cell-wall biosynthesis-targeting depsipeptide, teixobactin.167
The transformation of a new structure from Nature into a clinically useful antibiotic follows in many cases combined empirical synthetic tailoring with property-based and structure-based design.69,168,169 Recent exemplifications (from among many) include structural development of the tetracyclines,69 the glycopeptides,170,171 and the antifolates.172–175 The selection of a clinical candidate among chemical structures with similar in vitro characteristics will be facilitated by an emerging new pharmacological criterion, a long residence time for drug engagement of its target.176–182 Structures that possess this ability achieve an advantageous kinetic selectivity, wherein off-target interactions are minimized. The relevance of this criterion was exemplified recently during the assessment of inhibitors of LpxC, a key enzyme in the biosynthetic pathway to the lipopolysaccharides of the outer membrane of the Gram-negative bacteria. LpxC is a validated antibacterial target. However, its conformational mobility as a protein enables it to accommodate resistance mutations.183,184 Incorporation of kinetic performance, measured as the on-rate for formation and off-rate for breakdown within a series of LpxC inhibitors, yielded a pharmacodynamic model wherein the dose-response curves for these inhibitors in a Pseudomonas aeruginosa animal model of infection was predicted.185 The use of comparative kinetic data in compound evaluation will facilitate the pre-clinical assessment of exploratory antibacterial structures.
Endless antibiotics: a biological perspective
Notwithstanding the fact that antibiotic resistance in Nature coincided with the discovery of the antibiotics, the conceptualization of “resistance” continues to evolve.186–188 This evolution is multi-dimensional. Its directions include not just the mechanism(s) of the resistance against a particular antibacterial, but the spectrum of thought ranging from reflection on the ecological purpose of the antibiotics, to the criteria necessary to attain the multi-agent synergy against infectious disease that the future may demand. Here we offer a concise perspective on the eclectic breadth of the conceptualization of bacterial resistance, with emphasis on (and acknowledgement of) the most recent contributions defining its directions.
The emerging methodologies to probe the relationships among bacterial pathways and antibacterial structures will prove transformative for antibacterial discovery.189,190 Both the “Comprehensive Antibiotic Resistance Database” (“CARD”: http://arpcard.mcmaster.ca)191,192 and the NCBI National Database for Antimicrobial Resistant Organisms (http://www.ncbi.nlm.nih.gov/projects/pathogens/) address the bioinformatic aspects of these relationships. One example of a new methodology to complement bioinformatics analysis is the use of sub-μm fluorescence to attain resolution within the dimensions of the bacterial cell. With this resolution, an immediate visual assignment of the antibacterial mechanism by examination of the cytological profile of fluorescent reporters as a result of the presence of the antibacterial.193,194 A second example is the use of imaging mass spectrometry to evaluate specialized metabolite synthesis by Streptomyces coelicolor as the result of interspecies interaction.150,151 This methodology has the promise of improving our understanding of bacterial communication, especially as it relates to antibiotic synthesis, mechanism, and resistance responses. With respect to these mechanistic aspects, it is prudent for us to appreciate how poor is our understanding, even for the “golden age” antibiotics. The venerable class of β-lactam antibiotics exemplifies our ignorance. We are reminded that there is an enormous breadth of structure around the β-lactam core among the sub-families of this class. Individual β-lactam structures show differential affinity for their Penicillin-Binding Protein (PBP) enzyme targets. Each bacterium has a family of PBPs, and each of these PBPs uniquely contributes—some essentially, others much less so—to the growth and shape of the bacterium. Hence, each β-lactam structure uniquely profiles the PBP family of a bacterium.195,196 This uniqueness explains why particular β-lactams are clinically efficacious for infections by particular bacteria, but does not reveal the mechanistic interconnection between PBP inactivation and subsequent bactericidal cell lysis. While recent profiling of the relationship among β-lactam structure, PBP inactivation, and MIC value confirms the importance of selective PBP inactivation, it also identifies particular β-lactams for which the MIC does not coincide with PBP inactivation. The answer to the “bactericidal mechanism of the β-lactams” is remarkably incomplete.197–199
A further context around this incompleteness is our equally rudimentary understanding of the ecological purpose in Nature for the antibiotics. The conception that many natural products produced by microbes are messengers, a molecular realm termed by Davies as the “parvome”,200–204 is now well accepted. But what is their message? The traditional explanation for the antibiotics as defensive molecules to secure and preserve an ecological niche is supported by recent experiments.205,206 Nonetheless, the antibiotic concentrations used in chemotherapy vastly exceed the concentrations attained in ecological niches, and accordingly we must be mindful of understanding the bacterial responses to sub-MIC (sub-lethal) antibiotic exposure.207,208 The proven relationships among quorum sensing,209–212 biofilm formation,213,214 and virulence establish communication as a key role for the antibiotics of Nature.215–217 Antibiotics, even if simplistically conceptualized as weaponry, communicate. Their communication ability is intimate to the complexity of bacterial tolerance218 and persistence219–227 within the diversity of the ecological microbiomes.5,228–231 Chemical communication among bacteria is understandably an evolutionary force for genetic transformation19,208,232–234 and thus is one and the same with resistance.
Here we return to the mysterious depth of the resistome, and the underestimated ability of bacteria to adapt to what we naively might believe to be an even more than decimating assault by the antibacterial concentrations attained during clinical use. We cannot be surprised that while we know that antibiotics are powerful evolutionary forces, we do not understand the relative pressure of these forces in particular ecological niches235–237 and within the universe of resistance mechanisms.238–241 Both new mechanisms for antibacterial invention (such as the discovery that the allosteric regulation of the essential PBP2a enzyme of methicillin-resistant Staphylococcus aureus can be disrupted)109,242–244 and new mechanisms to secure resistance (as just demonstrated for the tetracyclines)245,246 will be found. We face the dilemma that while the micobiome is heterogeneous247 and the natural state for bacteria is a surface-bound community,248 there are compelling arguments to study the behavior of individual bacteria,249,250 even for the determination of the MIC for an antibacterial.251
How is this labyrinth to be confronted for antibacterial discovery? The obvious answer is the use of the antibiotics themselves, as superlative chemical probes, to provide both understanding and opportunity with respect to the confluence of targets and pathways. Two complementary themes exemplify efforts toward such identification. One is the relationship of antibiotic activity to metabolism (defined in the broadest sense). The second is the ability of antibacterial pairs to synergize their respective antibiotic activities. A direct relationship between metabolism and antibiotic activity is well recognized.252–257 Less understood is why the relationship directly correlates for some antibiotics, but indirectly for others.258 The ability to correlate the mechanism of ribosome-directed antibiotics, their antibiotic efficacy, and the rate of growth of E. coli provides promise that an understanding of the key aspects of this relationship, in terms of clinical strategies, will be forthcoming.259 A more demanding question is for which bacteria, and for which circumstances, the generation of reactive-oxygen species260 contributes253, 261–265 or does not contribute266–268 to antibacterial efficacy. An answer to this question may contribute (for example) to a mechanistic understanding of how the new antibiotic lysocin acts through interference with the menaquinone of the bacterial membrane;269 and whether the role of glutamate dehydrogenase activity in coordinating FtsZ-dependent cell division in Caulobacter crescentus270 represents a potentially synergistic pathway confluence in bacteria that have FtsZ-dependent bacterial division. Such synergism—collateral sensitivity—is a central theme to the future discovery of antibiotics.271–281 Exploration of this concept with respect to the β-lactam antibiotics in S. aureus has identified synergistic confluence with inhibitors of its cell division pathway,282 of its peptidoglycan biosynthesis pathway,283,284 and of its wall-teichoic acid biosynthesis pathway.285–290 This latter correlation is especially interesting, as the wall-teichoic acids are important contributors to colonization.291,292 Collateral synergy is now also demonstrated between the teichoic acids and the undecaprenyl lipid biosynthesis pathway.205 The undecaprenyl lipids are attractive targets as bacteria have a small pool of these lipids to support cell-wall biosynthesis.293–296. Understanding just how to achieve this sensitivity is incomplete,281,297,298 as evidenced by other studies on the undecaprenyl pathway. Incomplete inhibition of undecaprenyl biosynthesis in B. subtilis induced a stress response that resulted in increased resistance to other cell-wall-active antibiotics.299 Lastly, even when the outcome for the pairing of an antibiotic with a synergistic inhibitor is decisively advantageous in pharmacological models, validating this outcome in the clinic is especially challenging. Not only must safety be proven for the pairing of the both entities, but for optimal efficacy for the structures of the pair, and dosing choices for the pair, should correspond to matched pharmacokinetics of the two entities. The current cluster of β-lactam/β-lactamase inhibitors in clinical evaluation for Gram-negative bacterial infection reflects not just their promise of efficacy as a pairing, but the ability to bring to the clinical design the established clinical experience of the β-lactam partner. The task is simpler when one of the entities is known.
Conclusion
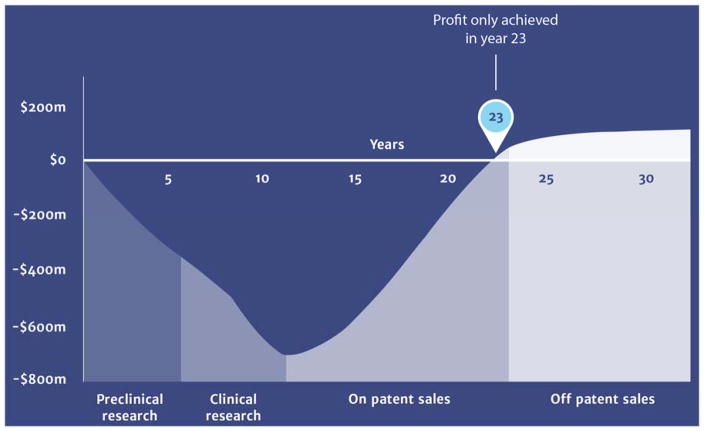
The title of this perspective pairs a declaration with a question. The declaration is irrefutable. This perspective has addressed the question, but without separation of the question into its two very different contexts. New antibiotics will be discovered. In this context of the question, our answer is resoundingly positive. Indeed, the basis for this opinion is the central theme of this perspective. There is, however, a second context for this same question. Will these new antibiotics achieve clinical impact? The answer to this question is less positive. The enthusiastic optimism and resoundingly positive answer to the question in the first context, and reserved (even deeply reserved) pessimism for the answer in the second context, is not cognizant dissonance. At this time good progress is being made with respect to the 10 × 20 initiative of the Infectious Diseases Society of America.300,301 New antibiotics (especially new β-lactamase-inhibitor combinations)65,302–310 are reaching the clinic. Yet over this same decade a fundamental transition has occurred with respect to early antibiotic discovery. This task has transitioned from major pharma companies to smaller biotechnology companies, and to academic centers. An excellent example of success from such collaboration is SMT-19969, now in Phase II clinical trials for Clostridium difficile infection.311,312 SMT-19969 is a member of the synthetic, DNA-interacting bis-benzimidazole class of structures. Its mechanism is not gyrase inhibition, as is the case for other members of this class.311 SMT-19969 shows good selectivity for C. difficile. As it is less active against other Gram-positive anaerobes and the Gram-negative anerobes, and only weakly active against both Gram-positive aerobes it has potential as a selective, microbiome-sparing entity. Although the transition of early discovery away from major pharma certainly does not itself mean that antibacterial discovery and development will falter, there is reason for concern. The urgency for the preservation of existing antibacterials, and the discovery of new antibacterials, is such that Nathan has argued for an open discovery initiative embracing academic, industrial, foundational, and governmental collaboration.313 Given the present requirements for the size and scope of clinical evaluation (although these requirements are changing for the better) within the unchanging cost structure for the antibiotic market, there are credible reasons for such a proposal. Absolute ownership of intellectual property is a necessity for all new drug entities. Acquisition of this ownership requires tight integration of the timing of the patent prosecution with clinical development. The financial return on the investment required to bring an antibacterial to market, even using optimistic estimates, is predicted to occur only in the final years of the patent life. This sobering reality is illustrated (Figure 1). The necessity for a fundamental change in how the necessary investment in the discovery and development of anti-infective drugs is made appears inescapable.28,47,313,314 As we stated emphatically a decade ago, bringing a new drug to market (nor even the discovery of a new antibiotic) is not an instantaneous event.315 Achieving intellectual property ownership while sustaining credible progression to the return on investment, in a development model where early discovery does not tightly transition to clinical development, is a profound challenge. The financial incentives for antibiotic discovery must change if clinically relevant antibiotic discovery is to be sustainable. The interim solutions of reconsidering old antibiotics316,317 or synergistic pairing of existing antibacterials (as we have just discussed) are essential. But neither solution, alone or together, offers the prospect of endless antibiotics.
Figure 1.
A simulation of the cost to discover and develop an antibacterial (preclinical and clinical research) compared to the return on the cost of the investment (on-patent and off-patent sales). This Figure is taken from p. 11 of the 2015 document “Securing new drugs for future generations: The pipeline of antibiotics” of the Wellcome Trust.48 The factual basis for this figure is provided in the appendix to this document. (Acknowledgement: ‘Review on Antimicrobial Resistance. Securing new drugs for future generations: the pipeline of antibiotics. 2015’).
Contributor Information
Jed F. Fisher, Email: jfisher1@nd.edu.
Shahriar Mobashery, Email: mobashery@nd.edu.
References
- 1.D’Costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding GB, Poinar HN, Wright GD. Nature. 2011;477:457. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 2.Wright GD, Poinar H. Trends Microbiol. 2012;20:157. doi: 10.1016/j.tim.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Finley RL, Collignon P, Larsson DGJ, McEwen SA, Li XZ, Gaze WH, Reid-Smith R, Timinouni M, Graham DW, Topp E. Clin Infect Dis. 2013;57:704. doi: 10.1093/cid/cit355. [DOI] [PubMed] [Google Scholar]
- 4.Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL. Nat Rev Microbiol. 2015;13:310. doi: 10.1038/nrmicro3439. [DOI] [PubMed] [Google Scholar]
- 5.Sommer MOA, Dantas G, Church GM. Science. 2009;325:1128. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson C. Science. 2015;347:704. doi: 10.1126/science.347.6223.704. [DOI] [PubMed] [Google Scholar]
- 7.Price LB, Koch BJ, Hungate BA. Proc Natl Acad Sci USA. 2015;112:5554. doi: 10.1073/pnas.1505312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Nat Rev Microbiol. 2010;8:251. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MOA, Dantas G. Science. 2012;337:1107. doi: 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths BS, Philippot L. FEMS Microbiol Rev. 2013;37:112. doi: 10.1111/j.1574-6976.2012.00343.x. [DOI] [PubMed] [Google Scholar]
- 11.Nesme J, Cécillon S, Delmont TO, Monier JM, Vogel TM, Simonet P. Curr Biol. 2014;24:1096. doi: 10.1016/j.cub.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Nesme J, Simonet P. Environ Microbiol. 2015;17:913. doi: 10.1111/1462-2920.12631. [DOI] [PubMed] [Google Scholar]
- 13.Bhullar K, Waglechner N, Pawlowski A, Koteva K, Banks ED, Johnston MD, Barton HA, Wright GD. PLoS One. 2012;7:e34953. doi: 10.1371/journal.pone.0034953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrova M, Kurakov A, Shcherbatova N, Mindlin S. Microbiology. 2014;160:2253. doi: 10.1099/mic.0.079335-0. [DOI] [PubMed] [Google Scholar]
- 15.Perron GG, Whyte L, Turnbaugh PJ, Goordial J, Hanage WP, Dantas G, Desai MM. PLoS One. 2015;10:e0069533. doi: 10.1371/journal.pone.0069533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago-Rodriguez TM, Fornaciari G, Luciani S, Dowd SE, Toranzos GA, Marota I, Cano RJ. PLoS One. 2015;10:e0138135. doi: 10.1371/journal.pone.0138135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves MS, Pereira A, Araújo SM, Castro BB, Correia AC, Henriques I. Front Microbiol. 2014;5:426. doi: 10.3389/fmicb.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson DI, Hughes D. Nat Rev Microbiol. 2010;8:260. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 19.Gillings MR. Front Microbiol. 2013;4:4. doi: 10.3389/fmicb.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischbach MA, Walsh CT. Science. 2009;325:1089. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJ, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. Nat Rev Microbiol. 2011;9:894. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver LL. Clin Microbiol Rev. 2011;24:71. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett JG, Gilbert DN, Spellberg B. Clin Infect Dis. 2013;56:1445. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 24.Lewis K. Nat Rev Drug Discov. 2013;12:371. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 25.O’Connell KMG, Hodgkinson JT, Sore HF, Welch M, Salmond GPC, Spring DR. Angew Chem Int Ed. 2013;52:10706. doi: 10.1002/anie.201209979. [DOI] [PubMed] [Google Scholar]
- 26.Dantas G, Sommer MOA. Am Scientist. 2014;102:42. [Google Scholar]
- 27.Fisher JF, Johnson JW, Mobashery S. In: Handbook of Antimicrobial Resistance. Götte M, Berghuis A, Matlashewski G, Wainberg M, Sheppard D, editors. Chapt. 12. Springer Science+Business Media; New York: 2014. p. 29. [Google Scholar]
- 28.Metz M, Shlaes DM. Antimicrob Agents Chemother. 2014;58:4253. doi: 10.1128/AAC.02623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan C, Cars O. N Engl J Med. 2014;371:1761. doi: 10.1056/NEJMp1408040. [DOI] [PubMed] [Google Scholar]
- 30.Oldfield E, Feng X. Trends Pharmacol Sci. 2014;35:664. doi: 10.1016/j.tips.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piddock LJV. Microbiology. 2014;160:2366. doi: 10.1099/mic.0.082412-0. [DOI] [PubMed] [Google Scholar]
- 32.Rossolini GM, Arena F, Pecile P, Pollini S. Curr Opin Pharmacol. 2014;18:56. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Singh SB. Bioorg Med Chem Lett. 2014;24:3683. doi: 10.1016/j.bmcl.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 34.Spellberg B, Gilbert DN. Clin Infect Dis. 2014;59(Suppl 2):S71. doi: 10.1093/cid/ciu392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perros M. Science. 2015;347:1062. doi: 10.1126/science.aaa3048. [DOI] [PubMed] [Google Scholar]
- 36.Tillotson G. Lancet Infect Dis. 2015;15:758. doi: 10.1016/S1473-3099(15)00081-X. [DOI] [PubMed] [Google Scholar]
- 37.Wright GD. ACS Infect Dis. 2015;1:80. doi: 10.1021/id500052s. [DOI] [PubMed] [Google Scholar]
- 38.Bush K. ACS Infect Dis. 2015;1 doi: 10.1021/acsinfecdis.5b00100. in press. [DOI] [PubMed] [Google Scholar]
- 39.Bowater L. J Antimicrob Chemother. 2015;70:1925. doi: 10.1093/jac/dkv071. [DOI] [PubMed] [Google Scholar]
- 40.Jechalke S, Heuer H, Siemens J, Amelung W, Smalla K. Trends Microbiol. 2014;22:536. doi: 10.1016/j.tim.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Charani E, Cooke J, Holmes A. J Antimicrob Chemother. 2010;65:2275. doi: 10.1093/jac/dkq357. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien DJ, Gould IM. Curr Opin Infect Dis. 2013;26:352. doi: 10.1097/QCO.0b013e3283631046. [DOI] [PubMed] [Google Scholar]
- 43.File TM, Jr, Srinivasan A, Bartlett JG. Clin Infect Dis. 2014;59(Suppl 3):S93. doi: 10.1093/cid/ciu543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livermore DM. Int J Antimicrob Agents. 2014;43:319. doi: 10.1016/j.ijantimicag.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Tamma PD, Holmes A, Ashley ED. Curr Opin Infect Dis. 2014;27:348. doi: 10.1097/QCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 46.Metlay JP. Clin Infect Dis. 2015;60:1317. [Google Scholar]
- 47.Shlaes DM. ACS Infect Dis. 2015;1:232. doi: 10.1021/acsinfecdis.5b00048. [DOI] [PubMed] [Google Scholar]
- 48.O’Neill J. Review on Antimicrobial Resistance. 2015 http://amr-review.org/sites/default/files/SECURING%20NEW%20DRUGS%20FOR%20FUTURE%20GENERATIONS%20FINAL%20WEB_0.pdf.
- 49.Gelband H, Miller-Petrie M, Pant S, Gandra S, Levinson J, Barter D, White A, Laxminarayan R. The Center for Disease Dynamics, Economics, and Policy (Global Antibiotic Resistance Partnership) 2015 http://cddep.org/publications/state_worlds_antibiotics_2015.
- 50.Cox G, Wright GD. Int J Med Microbiol. 2013;303:287. doi: 10.1016/j.ijmm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Nat Rev Drug Discov. 2007;6:29. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 52.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA. Nat Rev Drug Discov. 2015;14:529. doi: 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- 53.Bisacchi GS, Manchester JI. ACS Infect Dis. 2015;1:4. doi: 10.1021/id500013t. [DOI] [PubMed] [Google Scholar]
- 54.Bisacchi GS. J Med Chem. 2015;58:4874. doi: 10.1021/jm501881c. [DOI] [PubMed] [Google Scholar]
- 55.Walsh CT, Wencewicz TA. J Antibiot. 2013;67:7. doi: 10.1038/ja.2013.49. [DOI] [PubMed] [Google Scholar]
- 56.Testero SA, Fisher JF, Mobashery S. Burger’s Medicinal Chemistry, Drug Discovery and Development, Seventh Edition. 2010;7:259. [Google Scholar]
- 57.Karaiskos I, Souli M, Giamarellou H. Expert Opin Investig Drugs. 2015 doi: 10.1517/13543784.2015.1095180. in press. [DOI] [PubMed] [Google Scholar]
- 58.Becker B, Cooper MA. ACS Chem Biol. 2013;8:105. doi: 10.1021/cb3005116. [DOI] [PubMed] [Google Scholar]
- 59.Fosso MY, Li Y, Garneau-Tsodikova S. Med Chem Comm. 2014;5:1075. doi: 10.1039/C4MD00163J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huth ME, Han KH, Sotoudeh K, Hsieh YJ, Effertz T, Vu AA, Verhoeven S, Hsieh MH, Greenhouse R, Cheng AG, Ricci AJ. J Clin Invest. 2015;125:583. doi: 10.1172/JCI77424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maianti JP, Kanazawa H, Dozzo P, Matias RD, Feeney LA, Armstrong ES, Hildebrandt DJ, Kane TR, Gliedt MJ, Goldblum AA, Linsell MS, Aggen JB, Kondo J, Hanessian S. ACS Chem Biol. 2014;9:2067. doi: 10.1021/cb5003416. [DOI] [PubMed] [Google Scholar]
- 62.Giske CG. Clin Microbiol Infect. 2015;21:899. doi: 10.1016/j.cmi.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Estabrook M, Jacoby GA, Nichols WW, Testa RT, Bush K. Antimicrob Agents Chemother. 2015;59:1789. doi: 10.1128/AAC.04191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drawz SM, Papp-Wallace KM, Bonomo RA. Antimicrob Agents Chemother. 2014;58:1835. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olsen I. Eur J Clin Microbiol Infect Dis. 2015;34:1303. doi: 10.1007/s10096-015-2375-0. [DOI] [PubMed] [Google Scholar]
- 66.Blizzard TA, Chen H, Kim S, Wu J, Bodner R, Gude C, Imbriglio J, Young K, Park YW, Ogawa A, Raghoobar S, Hairston N, Painter RE, Wisniewski D, Scapin G, Fitzgerald P, Sharma N, Lu J, Ha S, Hermes J, Hammond ML. Bioorg Med Chem Lett. 2014;24:780. doi: 10.1016/j.bmcl.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 67.Morinaka A, Tsutsumi Y, Yamada M, Suzuki K, Watanabe T, Abe T, Furuuchi T, Inamura S, Sakamaki Y, Mitsuhashi N, Ida T, Livermore DM. J Antimicrob Chemother. 2015;70:2779. doi: 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]
- 68.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D, Sabet M, Tarazi Z, Clifton MC, Atkins K, Raymond A, Potts KT, Abendroth J, Boyer SH, Loutit JS, Morgan EE, Durso S, Dudley MN. J Med Chem. 2015;58:3682. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 69.Wright PM, Seiple IB, Myers AG. Angew Chem Int Ed. 2014;53:8840. doi: 10.1002/anie.201310843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almutairi MM, Park SR, Rose S, Hansen DA, Vázquez-Laslop N, Douthwaite S, Sherman DH, Mankin AS. Proc Natl Acad Sci USA. 2015;112:12956. doi: 10.1073/pnas.1512090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, Chen J, Mechiche R, Ohemeng K, Verma AK, Cannon EP, Macone A, Tanaka SK, Levy S. Antimicrob Agents Chemother. 2015;59:7044. doi: 10.1128/AAC.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun C, Hunt DK, Chen CL, Deng Y, He M, Clark RB, Fyfe C, Grossman TH, Sutcliffe JA, Xiao XY. J Med Chem. 2015;58:4703. doi: 10.1021/acs.jmedchem.5b00262. [DOI] [PubMed] [Google Scholar]
- 73.Aldred KJ, Kerns RJ, Osheroff N. Biochemistry. 2014;53:1565. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basarab GS, Doig P, Galullo V, Kern G, Kimzey A, Kutschke A, Newman JP, Morningstar M, Mueller J, Otterson L, Vishwanathan K, Zhou F, Gowravaram M. J Med Chem. 2015;58:6264. doi: 10.1021/acs.jmedchem.5b00863. [DOI] [PubMed] [Google Scholar]
- 75.Kern G, Palmer T, Ehmann DE, Shapiro AB, Andrews B, Basarab GS, Doig P, Fan J, Gao N, Mills SD, Mueller J, Sriram S, Thresher J, Walkup GK. J Biol Chem. 2015;290:20984. doi: 10.1074/jbc.M115.663534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Shea R, Moser HE. J Med Chem. 2008;51:2871. doi: 10.1021/jm700967e. [DOI] [PubMed] [Google Scholar]
- 77.Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ. Ann NY Acad Sci. 2010;1213:5. doi: 10.1111/j.1749-6632.2010.05828.x. [DOI] [PubMed] [Google Scholar]
- 78.Barelier S, Eidam O, Fish I, Hollander J, Figaroa F, Nachane R, Irwin JJ, Shoichet BK, Siegal G. ACS Chem Biol. 2014;9:1528. doi: 10.1021/cb5001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fleeman R, LaVoi TM, Santos RG, Morales A, Nefzi A, Welmaker GS, Medina-Franco JL, Giulianotti MA, Houghten RA, Shaw LN. J Med Chem. 2015;58:3340. doi: 10.1021/jm501628s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mugumbate G, Overington JP. Bioorg Med Chem. 2015;23:5218. doi: 10.1016/j.bmc.2015.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown DG, May-Dracka TL, Gagnon MM, Tommasi R. J Med Chem. 2014;57:10144. doi: 10.1021/jm501552x. [DOI] [PubMed] [Google Scholar]
- 82.Miller AA, Bundy GL, Mott JE, Skepner JE, Boyle TP, Harris DW, Hromockyj AE, Marotti KR, Zurenko GE, Munzner JB, Sweeney MT, Bammert GF, Hamel JC, Ford CW, Zhong WZ, Graber DR, Martin GE, Han F, Dolak LA, Seest EP, Ruble JC, Kamilar GM, Palmer JR, Banitt LS, Hurd AR, Barbachyn MR. Antimicrob Agents Chemother. 2008;52:2806. doi: 10.1128/AAC.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruble JC, Hurd AR, Johnson TA, Sherry DA, Barbachyn MR, Toogood PL, Bundy GL, Graber DR, Kamilar GM. J Am Chem Soc. 2009;131:3991. doi: 10.1021/ja808014h. [DOI] [PubMed] [Google Scholar]
- 84.Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, Alm RA, Barvian K, Doig P, Galullo V, Gardner H, Gowravaram M, Huband M, Kimzey A, Morningstar M, Kutschke A, Lahiri SD, Perros M, Singh R, Schuck VJA, Tommasi R, Walkup G, Newman JV. Sci Rep. 2015;5:11827. doi: 10.1038/srep11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobsson S, Golparian D, Alm RA, Huband M, Mueller J, Jensen JS, Ohnishi M, Unemo M. Antimicrob Agents Chemother. 2014;58:5585. doi: 10.1128/AAC.03090-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alm RA, Lahiri SD, Kutschke A, Otterson LG, McLaughlin RE, Whiteaker JD, Lewis LA, Su X, Huband MD, Gardner H, Mueller JP. Antimicrob Agents Chemother. 2015;59:1478. doi: 10.1128/AAC.04456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Unemo M, Ringlander J, Wiggins C, Fredlund H, Jacobsson S, Cole M. Antimicrob Agents Chemother. 2015;59:5220. doi: 10.1128/AAC.00786-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moraski GC, Markley LD, Hipskind PA, Boshoff H, Cho S, Franzblau SG, Miller MJ. ACS Med Chem Lett. 2011;2:466. doi: 10.1021/ml200036r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moraski GC, Markley LD, Chang M, Cho S, Franzblau SG, Hwang CH, Boshoff H, Miller MJ. Bioorg Med Chem. 2012;20:2214. doi: 10.1016/j.bmc.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moraski GC, Markley LD, Cramer J, Hipskind PA, Boshoff H, Bailey M, Alling T, Ollinger J, Parish T, Miller MJ. ACS Med Chem Lett. 2013;4:675. doi: 10.1021/ml400088y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moraski GC, Miller PA, Bailey MA, Ollinger J, Parish T, Boshoff HI, Cho S, Anderson JR, Mulugeta S, Franzblau SG, Miller MJ. ACS Infect Dis. 2015;1:85. doi: 10.1021/id500008t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abrahams KA, Cox JA, Spivey VL, Loman NJ, Pallen MJ, Constantinidou C, Fernández R, Alemparte C, Remuiñán MJ, Barros D, Ballell L, Besra GS. PLoS One. 2012;7:e52951. doi: 10.1371/journal.pone.0052951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim SA, Nam J, Kang H, Kwon H, Oh CT, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SP, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han SJ, No Z, Lee J, Brodin P, Cho SN, Nam K, Kim J. Nat Med. 2013;19:1157. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 94.Kang S, Kim RY, Seo MJ, Lee S, Kim YM, Seo M, Seo JJ, Ko Y, Choi I, Jang J, Nam J, Park S, Kang H, Kim HJ, Kim J, Ahn S, Pethe K, Nam K, No Z, Kim J. J Med Chem. 2014;57:5293. doi: 10.1021/jm5003606. [DOI] [PubMed] [Google Scholar]
- 95.Kim MS, Jang J, Rahman NBAb, Pethe K, Berry EA, Huang LS. J Biol Chem. 2015;290:14350. doi: 10.1074/jbc.M114.624312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makarov V, Manina G, Mikusova K, Möllmann U, Ryabova O, Saint-Joanis B, Dhar N, Pasca MR, Buroni S, Lucarelli AP, Milano A, De Rossi E, Belanova M, Bobovska A, Dianiskova P, Kordulakova J, Sala C, Fullam E, Schneider P, McKinney JD, Brodin P, Christophe T, Waddell S, Butcher P, Albrethsen J, Rosenkrands I, Brosch R, Nandi V, Bharath S, Gaonkar S, Shandil RK, Balasubramanian V, Balganesh T, Tyagi S, Grosset J, Riccardi G, Cole ST. Science. 2009;324:801. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neres J, Pojer F, Molteni E, Chiarelli LR, Dhar N, Boy-Röttger S, Buroni S, Fullam E, Degiacomi G, Lucarelli AP, Read RJ, Zanoni G, Edmondson DE, De Rossi E, Pasca MR, McKinney JD, Dyson PJ, Riccardi G, Mattevi A, Cole ST, Binda C. Sci Transl Med. 2012;4:150ra121. doi: 10.1126/scitranslmed.3004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batt SM, Jabeen T, Bhowruth V, Quill L, Lund PA, Eggeling L, Alderwick LJ, Futterer K, Besra GS. Proc Natl Acad Sci USA. 2012;109:11354. doi: 10.1073/pnas.1205735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tiwari R, Moraski GC, Krchnak V, Miller PA, Colon-Martinez M, Herrero E, Oliver AG, Miller MJ. J Am Chem Soc. 2013;135:3539. doi: 10.1021/ja311058q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grover S, Alderwick LJ, Mishra AK, Krumbach K, Marienhagen J, Eggeling L, Bhatt A, Besra GS. J Biol Chem. 2014;289:6177. doi: 10.1074/jbc.M113.522623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mikusová K, Makarov V, Neres J. Curr Pharm Des. 2014;20:4379. doi: 10.2174/138161282027140630122724. [DOI] [PubMed] [Google Scholar]
- 102.Makarov V, Lechartier B, Zhang M, Neres J, van der Sar AM, Raadsen SA, Hartkoorn RC, Ryabova OB, Vocat A, Decosterd LA, Widmer N, Buclin T, Bitter W, Andries K, Pojer F, Dyson PJ, Cole ST. EMBO Mol Med. 2014;6:372. doi: 10.1002/emmm.201303575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tiwari R, Miller PA, Cho S, Franzblau SG, Miller MJ. ACS Med Chem Lett. 2015;6:128. doi: 10.1021/ml5003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blaskovich MAT, Zuegg J, Elliott AG, Cooper MA. ACS Infect Dis. 2015;1:285. doi: 10.1021/acsinfecdis.5b00044. [DOI] [PubMed] [Google Scholar]
- 105.Cooper MA. Nat Rev Drug Discov. 2015;14:587. doi: 10.1038/nrd4706. [DOI] [PubMed] [Google Scholar]
- 106.Agarwal AK, Fishwick CWG. Ann NY Acad Sci. 2010;1213:20. doi: 10.1111/j.1749-6632.2010.05859.x. [DOI] [PubMed] [Google Scholar]
- 107.O’Daniel PI, Peng Z, Pi H, Testero SA, Ding D, Spink E, Leemans E, Boudreau MA, Yamaguchi T, Schroeder VA, Wolter WR, Llarrull LI, Song W, Lastochkin E, Kumarasiri M, Antunes NT, Espahbodi M, Lichtenwalter K, Suckow MA, Vakulenko S, Mobashery S, Chang M. J Am Chem Soc. 2014;136:3664. doi: 10.1021/ja500053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spink E, Ding D, Peng Z, Boudreau MA, Leemans E, Lastochkin E, Song W, Lichtenwalter K, O’Daniel PI, Testero SA, Pi H, Schroeder VA, Wolter WR, Antunes NT, Suckow MA, Vakulenko S, Chang M, Mobashery S. J Med Chem. 2015;58:1380. doi: 10.1021/jm501661f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bouley R, Kumarasiri M, Peng Z, Otero LH, Song W, Suckow MA, Schroeder VA, Wolter WR, Lastochkin E, Antunes NT, Pi H, Vakulenko S, Hermoso JA, Chang M, Mobashery S. J Am Chem Soc. 2015;137:1738. doi: 10.1021/jacs.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan FY, Sun N, Neves MA, Lam PC, Chung WH, Wong LK, Chow HY, Ma DL, Chan PH, Leung YC, Chan TH, Abagyan R, Wong KY. J Chem Inf Model. 2013;53:2131. doi: 10.1021/ci400203f. [DOI] [PubMed] [Google Scholar]
- 111.Chan FY, Sun N, Leung YC, Wong KY. J Antibiot. 2015;68:253. doi: 10.1038/ja.2014.140. [DOI] [PubMed] [Google Scholar]
- 112.Chan AH, Wereszczynski J, Amer BR, Yi SW, Jung ME, McCammon JA, Clubb RT. Chem Biol Drug Des. 2013;82:418. doi: 10.1111/cbdd.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang J, Liu H, Zhu K, Gong S, Dramsi S, Wang YT, Li J, Chen F, Zhang R, Zhou L, Lan L, Jiang H, Schneewind O, Luo C, Yang CG. Proc Natl Acad Sci USA. 2014;111:13517. doi: 10.1073/pnas.1408601111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larsen NA, Nash TJ, Morningstar M, Shapiro AB, Joubran C, Blackett CJ, Patten AD, Boriack-Sjodin PA, Doig P. Biochem J. 2012;446:405. doi: 10.1042/BJ20120596. [DOI] [PubMed] [Google Scholar]
- 115.Doig P, Boriack-Sjodin PA, Dumas J, Hu J, Itoh K, Johnson K, Kazmirski S, Kinoshita T, Kuroda S, Sato T, Sugimoto K, Tohyama K, Aoi H, Wakamatsu K, Wang H. Bioorg Med Chem. 2014;22:6256. doi: 10.1016/j.bmc.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 116.Tomasic T, Kovac A, Klebe G, Blanot D, Gobec S, Kikelj D, Masic LP. J Mol Model. 2012;18:1063. doi: 10.1007/s00894-011-1139-8. [DOI] [PubMed] [Google Scholar]
- 117.Hrast M, Sosič I, Šink R, Gobec S. Bioorg Chem. 2014;55:2. doi: 10.1016/j.bioorg.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 118.Perdih A, Hrast M, Barreteau H, Gobec S, Wolber G, Solmajer T. J Chem Inf Model. 2014;54:1451. doi: 10.1021/ci500104m. [DOI] [PubMed] [Google Scholar]
- 119.Wu P, Nielsen TE, Clausen MH. Trends Pharmacol Sci. 2015;36:422. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 120.Clardy J, Fischbach MA, Walsh CT. Nat Biotechnol. 2006;24:1541. doi: 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]
- 121.Demain AL, Sanchez S. J Antibiot. 2009;62:5. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li JWH, Vederas JC. Science. 2009;325:161. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 123.Wright GD. Can J Microbiol. 2014;60:147. doi: 10.1139/cjm-2014-0063. [DOI] [PubMed] [Google Scholar]
- 124.Corre C, Challis GL. Nat Prod Rep. 2009;26:977. doi: 10.1039/b713024b. [DOI] [PubMed] [Google Scholar]
- 125.Fischbach MA. Curr Opin Microbiol. 2009;12:520. doi: 10.1016/j.mib.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walsh CT, Fischbach MA. J Am Chem Soc. 2010;132:2469. doi: 10.1021/ja909118a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bologa CG, Ursu O, Oprea TI, Melançon CE, III, Tegos GP. Curr Opin Pharmacol. 2013;13:678. doi: 10.1016/j.coph.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu H-W, Begley T. Curr Opin Chem Biol. 2013;17:529. doi: 10.1016/j.cbpa.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hayashi MA, Bizerra FC, Da Silva PI., Jr Front Microbiol. 2013;4:195. doi: 10.3389/fmicb.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor PW. Int J Antimicrob Agents. 2013;42:195. doi: 10.1016/j.ijantimicag.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 131.Kirst HA. Expert Opin Drug Discov. 2013;8:479. doi: 10.1517/17460441.2013.779666. [DOI] [PubMed] [Google Scholar]
- 132.Charlop-Powers Z, Owen JG, Reddy BV, Ternei MA, Brady SF. Proc Natl Acad Sci USA. 2014;111:3757. doi: 10.1073/pnas.1318021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charlop-Powers Z, Milshteyn A, Brady SF. Curr Opin Microbiol. 2014;19:70. doi: 10.1016/j.mib.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seyedsayamdost MR, Clardy J. ACS Synth Biol. 2014;3:745. doi: 10.1021/sb400025p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stallforth P, Clardy J. Proc Natl Acad Sci USA. 2014;111:3655. doi: 10.1073/pnas.1400516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Antoraz S, Santamaría RI, Díaz M, Sanz D, Rodríguez H. Front Microbiol. 2015;6:461. doi: 10.3389/fmicb.2015.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim E, Moore BS, Yoon YJ. Nat Chem Biol. 2015;11:649. doi: 10.1038/nchembio.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cherblanc FL, Davidson RWM, Di Fruscia P, Srimongkolpithak N, Fuchter MJ. Nat Prod Rep. 2013;30:605. doi: 10.1039/c3np20097c. [DOI] [PubMed] [Google Scholar]
- 139.Unkles SE, Valiante V, Mattern DJ, Brakhage AA. Chem Biol. 2014;21:502. doi: 10.1016/j.chembiol.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 140.Mattern DJ, Valiante V, Unkles SE, Brakhage AA. Front Microbiol. 2015;6:775. doi: 10.3389/fmicb.2015.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mao XM, Xu W, Li D, Yin WB, Chooi YH, Li YQ, Tang Y, Hu Y. Angew Chem Int Ed. 2015;54:7592. doi: 10.1002/anie.201502452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hiramatsu K, Igarashi M, Morimoto Y, Baba T, Umekita M, Akamatsu Y. Int J Antimicrob Agents. 2012;39:478. doi: 10.1016/j.ijantimicag.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 143.Parkinson EI, Bair JS, Nakamura BA, Lee HY, Kuttab HI, Southgate EH, Lezmi S, Lau GW, Hergenrother PJ. Nat Commun. 2015;6:6947. doi: 10.1038/ncomms7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thaker MN, Wang W, Spanogiannopoulos P, Waglechner N, King AM, Medina R, Wright GD. Nat Biotechnol. 2013;31:922. doi: 10.1038/nbt.2685. [DOI] [PubMed] [Google Scholar]
- 145.Thaker MN, Waglechner N, Wright GD. Nat Protoc. 2014;9:1469. doi: 10.1038/nprot.2014.093. [DOI] [PubMed] [Google Scholar]
- 146.Yim G, Thaker MN, Koteva K, Wright G. J Antibiot. 2014;67:31. doi: 10.1038/ja.2013.117. [DOI] [PubMed] [Google Scholar]
- 147.Yim G, Kalan L, Koteva K, Thaker MN, Waglechner N, Tang I, Wright GD. ChemBioChem. 2014;15:2613. doi: 10.1002/cbic.201402179. [DOI] [PubMed] [Google Scholar]
- 148.Thaker MN, Wright GD. ACS Synth Biol. 2015;4:195. doi: 10.1021/sb300092n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Butler MS, Hansford KA, Blaskovich MAT, Halai R, Cooper MA. J Antibiot. 2014;67:631. doi: 10.1038/ja.2014.111. [DOI] [PubMed] [Google Scholar]
- 150.Traxler MF, Watrous JD, Alexandrov T, Dorrestein PC, Kolter R. mBio. 2013;4:e00459/13. doi: 10.1128/mBio.00459-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hopwood DA. mBio. 2013;4:e00612/13. doi: 10.1128/mBio.00612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Charusanti P, Fong NL, Nagarajan H, Pereira AR, Li HJ, Abate EA, Su Y, Gerwick WH, Palsson BO. PLoS One. 2012;7:e33727. doi: 10.1371/journal.pone.0033727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Traxler MF, Kolter R. Nat Prod Rep. 2015;32:956. doi: 10.1039/c5np00013k. [DOI] [PubMed] [Google Scholar]
- 154.Lowry B, Walsh CT, Khosla C. Synlett. 2015;26:1008. doi: 10.1055/s-0034-1380264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walsh CT. Nat Chem Biol. 2015;11:620. doi: 10.1038/nchembio.1894. [DOI] [PubMed] [Google Scholar]
- 156.Rutledge PJ, Challis GL. Nat Rev Microbiol. 2015;13:509. doi: 10.1038/nrmicro3496. [DOI] [PubMed] [Google Scholar]
- 157.Banik JJ, Brady SF. Curr Opin Microbiol. 2010;13:603. doi: 10.1016/j.mib.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Feng Z, Chakraborty D, Dewell SB, Reddy BV, Brady SF. J Am Chem Soc. 2012;134:2981. doi: 10.1021/ja207662w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Milshteyn A, Schneider JS, Brady SF. Chem Biol. 2014;21:1211. doi: 10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cohen LJ, Kang HS, Chu J, Huang YH, Gordon EA, Reddy BV, Ternei MA, Craig JW, Brady SF. Proc Natl Acad Sci USA. 2015;112:E4825. doi: 10.1073/pnas.1508737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kapur S, Lowry B, Yuzawa S, Kenthirapalan S, Chen AY, Cane DE, Khosla C. Proc Natl Acad Sci USA. 2012;109:4110. doi: 10.1073/pnas.1118734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Khosla C, Herschlag D, Cane DE, Walsh CT. Biochemistry. 2014;53:2875. doi: 10.1021/bi500290t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lok C. Nature. 2015;522:270. doi: 10.1038/522270a. [DOI] [PubMed] [Google Scholar]
- 164.von Nussbaum F, Süssmuth RD. Angew Chem Int Ed. 2015;54:6684. doi: 10.1002/anie.201501440. [DOI] [PubMed] [Google Scholar]
- 165.Hunter P. EMBO Rep. 2015;16:563. doi: 10.15252/embr.201540385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Piddock LJV. J Antimicrob Chemother. 2015;70:2679. doi: 10.1093/jac/dkv175. [DOI] [PubMed] [Google Scholar]
- 167.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schaberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. Nature. 2015;517:455. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cain R, Narramore S, McPhillie M, Simmons K, Fishwick CWG. Bioorg Chem. 2014;55:69. doi: 10.1016/j.bioorg.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 169.Hong J. Chem—Eur J. 2014;20:10204. doi: 10.1002/chem.201402804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Okano A, Nakayama A, Schammel AW, Boger DL. J Am Chem Soc. 2014;136:13522. doi: 10.1021/ja507009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Okano A, Nakayama A, Wu K, Lindsey EA, Schammel AW, Feng Y, Collins KC, Boger DL. J Am Chem Soc. 2015;137:3693. doi: 10.1021/jacs.5b01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou W, Scocchera EW, Wright DL, Anderson AC. Med Chem Commun. 2013;4:908. [Google Scholar]
- 173.Lamb KM, Dayanandan NG, Wright DL, Anderson AC. Biochemistry. 2013;52:7318. doi: 10.1021/bi400852h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lamb KM, Lombardo MN, Alverson J, Priestley ND, Wright DL, Anderson AC. Antimicrob Agents Chemother. 2014;58:7484. doi: 10.1128/AAC.03555-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Keshipeddy S, Reeve SM, Anderson AC, Wright DL. J Am Chem Soc. 2015;137:8983. doi: 10.1021/jacs.5b01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lu H, Tonge PJ. Curr Opin Chem Biol. 2010;14:467. doi: 10.1016/j.cbpa.2010.06.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dahl G, Akerud T. Drug Discov Today. 2013;18:697. doi: 10.1016/j.drudis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 178.Liu L. J Med Chem. 2014;57:2843. doi: 10.1021/jm5003815. [DOI] [PubMed] [Google Scholar]
- 179.Bradshaw JM, McFarland JM, Paavilainen VO, Bisconte A, Tam D, Phan VT, Romanov S, Finkle D, Shu J, Patel V, Ton T, Li X, Loughhead DG, Nunn PA, Karr DE, Gerritsen ME, Funk JO, Owens TD, Verner E, Brameld KA, Hill RJ, Goldstein DM, Taunton J. Nat Chem Biol. 2015;11:525. doi: 10.1038/nchembio.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Copeland RA. Nat Chem Biol. 2015;11:451. doi: 10.1038/nchembio.1831. [DOI] [PubMed] [Google Scholar]
- 181.Cusack KP, Wang Y, Hoemann MZ, Marjanovic J, Heym RG, Vasudevan A. Bioorg Med Chem Lett. 2015;25:2019. doi: 10.1016/j.bmcl.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 182.Zhang R. Nat Chem Biol. 2015;11:382. doi: 10.1038/nchembio.1795. [DOI] [PubMed] [Google Scholar]
- 183.Lee CJ, Liang X, Chen X, Zeng D, Joo SH, Chung HS, Barb AW, Swanson SM, Nicholas RA, Li Y, Toone EJ, Raetz CRH, Zhou P. Chem Biol. 2011;18:38. doi: 10.1016/j.chembiol.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee CJ, Liang X, Gopalaswamy R, Najeeb J, Ark ED, Toone EJ, Zhou P. ACS Chem Biol. 2014;9:237. doi: 10.1021/cb400067g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Walkup GK, You Z, Ross PL, Allen EKH, Daryaee F, Hale MR, O’Donnell J, Ehmann DE, Schuck VJA, Buurman ET, Choy AL, Hajec L, Murphy-Benenato K, Marone V, Patey SA, Grosser LA, Johnstone M, Walker SG, Tonge PJ, Fisher SL. Nat Chem Biol. 2015;11:416. doi: 10.1038/nchembio.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perry JA, Westman EL, Wright GD. Curr Opin Microbiol. 2014;21:45. doi: 10.1016/j.mib.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 187.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Nat Rev Microbiol. 2015;13:42. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 188.Culyba MJ, Mo CY, Kohli RM. Biochemistry. 2015;54:3573. doi: 10.1021/acs.biochem.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wright GD. Chem Biol. 2012;19:3. doi: 10.1016/j.chembiol.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 190.Farha MA, Brown ED. Ann NY Acad Sci. 2015 doi: 10.1111/nyas.12803. in press. [DOI] [Google Scholar]
- 191.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, Kalan L, King AM, Koteva K, Morar M, Mulvey MR, O’Brien JS, Pawlowski AC, Piddock LJV, Spanogiannopoulos P, Sutherland AD, Tang I, Taylor PL, Thaker M, Wang W, Yan M, Yu T, Wright GD. Antimicrob Agents Chemother. 2013;57:3348. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McArthur AG, Wright GD. Curr Opin Microbiol. 2015;27:45. doi: 10.1016/j.mib.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 193.Nonejuie P, Burkart M, Pogliano K, Pogliano J. Proc Natl Acad Sci USA. 2013;110:16169. doi: 10.1073/pnas.1311066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Penwell WF, Shapiro AB, Giacobbe RA, Gu R-F, Gao N, Thresher J, McLaughlin RE, Huband MD, DeJonge BLM, Ehmann DE, Miller AA. Antimicrob Agents Chemother. 2015;59:1680. doi: 10.1128/AAC.04808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kocaoglu O, Carlson EE. Antimicrob Agents Chemother. 2015;59:2785. doi: 10.1128/AAC.04552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kocaoglu O, Tsui H-CT, Winkler ME, Carlson EE. Antimicrob Agents Chemother. 2015;59:3548. doi: 10.1128/AAC.05142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yao Z, Kahne D, Kishony R. Mol Cell. 2012;48:705. doi: 10.1016/j.molcel.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cho H, Uehara T, Bernhardt TG. Cell. 2014;159:1300. doi: 10.1016/j.cell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kjeldsen TSB, Sommer MOA, Olsen JE. BMC Microbiol. 2015;15:63. doi: 10.1186/s12866-015-0399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davies J, Davies D. Microbiol Mol Biol Rev. 2010;74:417. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Davies J. Curr Opin Chem Biol. 2011;15:5. doi: 10.1016/j.cbpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 202.Davies J, Ryan KS. ACS Chem Biol. 2012;7:252. doi: 10.1021/cb200337h. [DOI] [PubMed] [Google Scholar]
- 203.Davies J. J Antibiot. 2013;66:361. doi: 10.1038/ja.2013.61. [DOI] [PubMed] [Google Scholar]
- 204.Davies J. The origin and evolution of antibiotics. Springer-Verlag; Berlin-Heidelberg: 2014. [Google Scholar]
- 205.Farha MA, Czarny TL, Myers CL, Worrall LJ, French S, Conrady DG, Wang Y, Oldfield E, Strynadka NC, Brown ED. Proc Natl Acad Sci USA. 2015;112:11048–53. doi: 10.1073/pnas.1511751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cornforth DM, Foster KR. Proc Natl Acad Sci USA. 2015;112:10827. doi: 10.1073/pnas.1513608112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Andersson DI, Hughes D. Nat Rev Microbiol. 2014;12:465. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 208.Baquero F, Coque TM. mBio. 2014;5:e02270/14. doi: 10.1128/mBio.02270-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Platt TG, Fuqua C. Trends Microbiol. 2010;18:383. doi: 10.1016/j.tim.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.LaSarre B, Federle MJ. Microbiol Mol Biol Rev. 2013;77:73. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gerdt JP, Blackwell HE. ACS Chem Biol. 2014;9:2291. doi: 10.1021/cb5004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rampioni G, Leoni L, Williams P. Bioorg Chem. 2014;55:60. doi: 10.1016/j.bioorg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 213.Blackledge MS, Worthington RJ, Melander C. Curr Opin Pharmacol. 2013;13:699. doi: 10.1016/j.coph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Beloin C, Renard S, Ghigo J-M, Lebeaux D. Curr Opin Pharmacol. 2014;18:61. doi: 10.1016/j.coph.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 215.Worthington RJ, Melander C. Angew Chem Int Ed. 2012;51:6314. doi: 10.1002/anie.201202440. [DOI] [PubMed] [Google Scholar]
- 216.Stacy AR, Diggle SP, Whiteley M. Curr Opin Microbiol. 2012;15:155. doi: 10.1016/j.mib.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 217.Yajima S. Tetrahedron Lett. 2014;55:2773. [Google Scholar]
- 218.Meredith HR, Srimani JK, Lee AJ, Lopatkin AJ, You L. Nat Chem Biol. 2015;11:182. doi: 10.1038/nchembio.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lewis K. Annu Rev Microbiol. 2010;64:357. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 220.Vega NM, Allison KR, Khalil AS, Collins JJ. Nat Chem Biol. 2012;8:431. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Balaban NQ, Gerdes K, Lewis K, McKinney JD. Nat Rev Microbiol. 2013;11:587. doi: 10.1038/nrmicro3076. [DOI] [PubMed] [Google Scholar]
- 222.Cohen NR, Lobritz MA, Collins JJ. Cell Host Microbe. 2013;13:632. doi: 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Helaine S, Kugelberg E. Trends Microbiol. 2014;22:417. doi: 10.1016/j.tim.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 224.Maisonneuve E, Gerdes K. Cell. 2014;157:539. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 225.Levin BR, Concepción-Acevedo J, Udekwu KI. Curr Opin Microbiol. 2014;21:18. doi: 10.1016/j.mib.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ayrapetyan M, Williams TC, Oliver JD. Trends Microbiol. 2015;23:7. doi: 10.1016/j.tim.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 227.Holden DW. Science. 2015;347:30. doi: 10.1126/science.1262033. [DOI] [PubMed] [Google Scholar]
- 228.Sommer MOA, Dantas G. Curr Opin Microbiol. 2011;14:556. doi: 10.1016/j.mib.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 229.Triggle DJ. Biochem Pharmacol. 2012;84:1543. doi: 10.1016/j.bcp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 230.Riley MA, Robinson SM, Roy CM, Dorit RL. Future Med Chem. 2013;5:1231. doi: 10.4155/fmc.13.79. [DOI] [PubMed] [Google Scholar]
- 231.Waldor MK, Tyson G, Borenstein E, Ochman H, Moeller A, Finlay BB, Kong HH, Gordon JI, Nelson KE, Dabbagh K, Smith H. PLoS Biol. 2015;13:e1002050. doi: 10.1371/journal.pbio.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gullberg E, Cao S, Berg OG, Ilback C, Sandegren L, Hughes D, Andersson DI. PLoS Pathog. 2011;7:e1002158. doi: 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Blázquez J, Couce A, Rodriguez-Beltrán J, Rodriguez-Rojas A. Curr Opin Microbiol. 2012;15:561. doi: 10.1016/j.mib.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 234.Gullberg E, Albrecht LM, Karlsson C, Sandegren L, Andersson DI. mBio. 2014;5:e01918/14. doi: 10.1128/mBio.01918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Forsberg KJ, Patel S, Gibson MK, Lauber CL, Knight R, Fierer N, Dantas G. Nature. 2014;509:612. doi: 10.1038/nature13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Perry JA, Wright GD. BioEssays. 2014;36:1179. doi: 10.1002/bies.201400128. [DOI] [PubMed] [Google Scholar]
- 237.Sommer MOA. Nature. 2014;509:567. doi: 10.1038/nature13342. [DOI] [PubMed] [Google Scholar]
- 238.Gibson MK, Forsberg KJ, Dantas G. ISME J. 2015;9:207. doi: 10.1038/ismej.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Martínez JL, Coque TM, Baquero F. Nat Rev Microbiol. 2015;13:116. doi: 10.1038/nrmicro3399. [DOI] [PubMed] [Google Scholar]
- 240.Martínez JL, Coque TM, Baquero F. Nat Rev Microbiol. 2015;13:396. doi: 10.1038/nrmicro3399-c2. [DOI] [PubMed] [Google Scholar]
- 241.Bengtsson-Palme J, Larsson DGJ. Nat Rev Microbiol. 2015;13:396. doi: 10.1038/nrmicro3399-c1. [DOI] [PubMed] [Google Scholar]
- 242.Otero LH, Rojas-Altuve A, Llarrull LI, Carrasco-López C, Kumarasiri M, Lastochkin E, Fishovitz J, Dawley M, Hesek D, Lee M, Johnson JW, Fisher JF, Chang M, Mobashery S, Hermoso JA. Proc Natl Acad Sci USA. 2013;110:16808. doi: 10.1073/pnas.1300118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fishovitz J, Rojas-Altuve A, Otero LH, Dawley M, Carrasco-Lopez C, Chang M, Hermoso JA, Mobashery S. J Am Chem Soc. 2014;136:9814. doi: 10.1021/ja5030657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fishovitz J, Taghizadeh N, Fisher JF, Chang M, Mobashery S. J Am Chem Soc. 2015;137:6500. doi: 10.1021/jacs.5b01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Forsberg KJ, Patel S, Wencewicz TA, Dantas G. Chem Biol. 2015;22:888. doi: 10.1016/j.chembiol.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Graham DW. Chem Biol. 2015;22:805. doi: 10.1016/j.chembiol.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 247.Ackermann M. Nat Rev Microbiol. 2015;13:497. doi: 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- 248.Persat A, Nadell CD, Kim MK, Ingremeau F, Siryaporn A, Drescher K, Wingreen NS, Bassler BL, Gitai Z, Stone HA. Cell. 2015;161:988. doi: 10.1016/j.cell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ackermann M, Schreiber F. Environ Microbiol. 2015;17:2193. doi: 10.1111/1462-2920.12877. [DOI] [PubMed] [Google Scholar]
- 250.Wang Y, Ran M, Wang J, Ouyang Q, Luo C. PLoS One. 2015;10:e0127115. doi: 10.1371/journal.pone.0127115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Artemova T, Gerardin Y, Dudley C, Vega NM, Gore J. Mol Syst Biol. 2015;11:822. doi: 10.15252/msb.20145888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Allison KR, Brynildsen MP, Collins JJ. Nature. 2011;473:216. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CTY, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ. Proc Natl Acad Sci USA. 2014;111:E2100. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Peng B, Su YB, Li H, Han Y, Guo C, Tian YM, Peng XX. Cell Metab. 2015;21:249. doi: 10.1016/j.cmet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 255.Bhargava P, Collins JJ. Cell Metab. 2015;21:154. doi: 10.1016/j.cmet.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 256.Su Y, Peng B, Han Y, Li H, Peng X. J Proteome Res. 2015;14:1612. doi: 10.1021/pr501285f. [DOI] [PubMed] [Google Scholar]
- 257.Dwyer DJ, Collins JJ, Walker GC. Annu Rev Pharmacol Toxicol. 2015;55:313. doi: 10.1146/annurev-pharmtox-010814-124712. [DOI] [PubMed] [Google Scholar]
- 258.Palmer AC, Kishony R. Nat Commun. 2014;5:4296. doi: 10.1038/ncomms5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Greulich P, Scott M, Evans MR, Allen RJ. Mol Syst Biol. 2015;11:796. doi: 10.15252/msb.20145949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fang FC. Nat Biotechnol. 2013;31:415. doi: 10.1038/nbt.2574. [DOI] [PubMed] [Google Scholar]
- 261.Brynildsen MP, Winkler JA, Spina CS, Macdonald IC, Collins JJ. Nat Biotechnol. 2013;31:160. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mosel M, Li L, Drlica K, Zhao X. Antimicrob Agents Chemother. 2013;57:5755. doi: 10.1128/AAC.00754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lobritz MA, Belenky P, Porter CBM, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ. Proc Natl Acad Sci USA. 2015;112:8173. doi: 10.1073/pnas.1509743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhao X, Drlica K. Curr Opin Microbiol. 2014;21:1. doi: 10.1016/j.mib.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhao X, Hong Y, Drlica K. J Antimicrob Chemother. 2015;70:639. doi: 10.1093/jac/dku463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Science. 2013;339:1213. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 267.Liu Y, Imlay JA. Science. 2013;339:1210. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kuhnert N. Angew Chem Int Ed. 2013;52:10946. doi: 10.1002/anie.201304548. [DOI] [PubMed] [Google Scholar]
- 269.Hamamoto H, Urai M, Ishii K, Yasukawa J, Paudel A, Murai M, Kaji T, Kuranaga T, Hamase K, Katsu T, Su J, Adachi T, Uchida R, Tomoda H, Yamada M, Souma M, Kurihara H, Inoue M, Sekimizu K. Nat Chem Biol. 2015;11:127. doi: 10.1038/nchembio.1710. [DOI] [PubMed] [Google Scholar]
- 270.Beaufay F, Coppine J, Mayard A, Laloux G, De Bolle X, Hallez R. EMBO J. 2015;34:1786. doi: 10.15252/embj.201490730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Roemer T, Davies J, Giaever G, Nislow C. Nat Chem Biol. 2012;8:46. doi: 10.1038/nchembio.744. [DOI] [PubMed] [Google Scholar]
- 272.Imamovic L, Sommer MOA. Sci Transl Med. 2013;5:204ra132. doi: 10.1126/scitranslmed.3006609. [DOI] [PubMed] [Google Scholar]
- 273.Lázár V, Pal Singh G, Spohn R, Nagy I, Horváth B, Hrtyan M, Busa-Fekete R, Bogos B, Méhi O, Csörgő B, Pósfai G, Fekete G, Szappanos B, Kégl B, Papp B, Pál C. Mol Syst Biol. 2013;9:700. doi: 10.1038/msb.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Roemer T, Boone C. Nat Chem Biol. 2013;9:222. doi: 10.1038/nchembio.1205. [DOI] [PubMed] [Google Scholar]
- 275.Munck C, Gumpert HK, Wallin AIN, Wang HH, Sommer MOA. Sci Translation Med. 2014;6:262ra156. doi: 10.1126/scitranslmed.3009940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bollenbach T. Curr Opin Microbiol. 2015;27:1. doi: 10.1016/j.mib.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 277.Chevereau G, Bollenbach T. Mol Syst Biol. 2015;11:807. doi: 10.15252/msb.20156098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gill EE, Franco OL, Hancock REW. Chem Biol Drug Design. 2015;85:56. doi: 10.1111/cbdd.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pál C, Papp B, Lázár V. Trends Microbiol. 2015;23:401. doi: 10.1016/j.tim.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Pettus K, Sharpe S, Papp JR. Antimicrob Agents Chemother. 2015;59:2443. doi: 10.1128/AAC.04127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rodriguez de Evgrafov M, Gumpert H, Munck C, Thomsen TT, Sommer MOA. Mol Biol Evol. 2015;32:1175. doi: 10.1093/molbev/msv006. [DOI] [PubMed] [Google Scholar]
- 282.Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, Lebeau-Jacob C, Benton-Perdomo L, Monteiro JM, Pereira PM, Elsen NL, Wu J, Deschamps K, Petcu M, Wong S, Daigneault E, Kramer S, Liang L, Maxwell E, Claveau D, Vaillancourt J, Skorey K, Tam J, Wang H, Meredith TC, Sillaots S, Wang-Jarantow L, Ramtohul Y, Langlois E, Landry F, Reid JC, Parthasarathy G, Sharma S, Baryshnikova A, Lumb KJ, Pinho MG, Soisson SM, Roemer T. Sci Transl Med. 2012;4:126ra35. doi: 10.1126/scitranslmed.3003592. [DOI] [PubMed] [Google Scholar]
- 283.Roemer T, Schneider T, Pinho MG. Curr Opin Microbiol. 2013;16:538. doi: 10.1016/j.mib.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 284.Mann PA, Muller A, Xiao L, Pereira PM, Yang C, Lee SH, Wang H, Trzeciak J, Schneeweis J, Dos Santos MM, Murgolo N, She X, Gill C, Balibar CJ, Labroli M, Su J, Flattery A, Sherborne B, Maier R, Tan CM, Black T, Onder K, Kargman S, Monsma FJ, Pinho MG, Schneider T, Roemer T. ACS Chem Biol. 2013;8:2442. doi: 10.1021/cb400487f. [DOI] [PubMed] [Google Scholar]
- 285.Campbell J, Singh AK, Swoboda JG, Gilmore MS, Wilkinson BJ, Walker S. Antimicrob Agents Chemother. 2012;56:1810. doi: 10.1128/AAC.05938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Brown S, Santa Maria JP, Jr, Walker S. Annu Rev Microbiol. 2013;67:313. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang H, Gill CJ, Lee SH, Mann P, Zuck P, Meredith TC, Murgolo N, She X, Kales S, Liang L, Liu J, Wu J, Santa Maria J, Su J, Pan J, Hailey J, Mcguinness D, Tan CM, Flattery A, Walker S, Black T, Roemer T. Chem Biol. 2013;20:272. doi: 10.1016/j.chembiol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Farha MA, Koteva K, Gale RT, Sewell EW, Wright GD, Brown ED. Bioorg Med Chem Lett. 2014;24:905. doi: 10.1016/j.bmcl.2013.12.069. [DOI] [PubMed] [Google Scholar]
- 289.Santa Maria JP, Jr, Sadaka A, Moussa SH, Brown S, Zhang YJ, Rubin EJ, Gilmore MS, Walker S. Proc Natl Acad Sci USA. 2014;111:12510. doi: 10.1073/pnas.1404099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sewell EWC, Brown ED. J Antibiot. 2014;67:43. doi: 10.1038/ja.2013.100. [DOI] [PubMed] [Google Scholar]
- 291.Kohler T, Weidenmaier C, Peschel A. J Bacteriol. 2009;191:4482. doi: 10.1128/JB.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Winstel V, Kühner P, Salomon F, Larsen J, Skov R, Hoffmann W, Peschel A, Weidenmaier C. mBio. 2015;6:e00632/15. doi: 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Barreteau H, Magnet S, El Ghachi M, Touzé T, Arthur M, Mengin-Lecreulx D, Blanot D. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:213. doi: 10.1016/j.jchromb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 294.Johnson JW, Fisher JF, Mobashery S. Ann N Y Acad Sci. 2013;1277:54. doi: 10.1111/j.1749-6632.2012.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kingston AW, Zhao H, Cook GM, Helmann JD. Mol Microbiol. 2014;93:37. doi: 10.1111/mmi.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Manat G, Roure S, Auger R, Bouhss A, Barreteau H, Mengin-Lecreulx D, Touzé T. Microb Drug Resist. 2014;20:199. doi: 10.1089/mdr.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Day T, Huijben S, Read AF. Trends Microbiol. 2015;23:126. doi: 10.1016/j.tim.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Palmer AC, Kishony R. Nat Rev Genet. 2013;14:243. doi: 10.1038/nrg3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lee YH, Helmann JD. Antimicrob Agents Chemother. 2013;57:4267. doi: 10.1128/AAC.00794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gilbert DN, Guidos RJ, Boucher HW, Talbot GH, Spellberg B, Edwards JE, Scheld WM, Bradley JS, Bartlett JG. Clin Infect Dis. 2010;50:1081. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 301.Boucher HW, Talbot GH, Benjamin DKJ, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. Clin Infect Dis. 2013;56:1685. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Page MGP, Bush K. Curr Opin Pharmacol. 2014;18:91. doi: 10.1016/j.coph.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 303.Harris PN, Tambyah PA, Paterson DL. Lancet Infect Dis. 2015;15:475. doi: 10.1016/S1473-3099(14)70950-8. [DOI] [PubMed] [Google Scholar]
- 304.King DT, King AM, Lal SM, Wright GD, Strynadka NCJ. ACS Infect Dis. 2015;1:175. doi: 10.1021/acsinfecdis.5b00007. [DOI] [PubMed] [Google Scholar]
- 305.Lee JH, Lee JJ, Park KS, Lee SH. Lancet Infect Dis. 2015;15:876. doi: 10.1016/S1473-3099(15)00143-7. [DOI] [PubMed] [Google Scholar]
- 306.Liscio JL, Mahoney MV, Hirsch EB. Int J Antimicrob Agents. 2015;46:266. doi: 10.1016/j.ijantimicag.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 307.Pitart C, Marco F, Keating TA, Nichols WW, Vila J. Antimicrob Agents Chemother. 2015;59:3059. doi: 10.1128/AAC.05136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shiber S, Yahav D, Avni T, Leibovici L, Paul M. J Antimicrob Chemother. 2015;70:41. doi: 10.1093/jac/dku351. [DOI] [PubMed] [Google Scholar]
- 309.Winkler ML, Papp-Wallace KM, Bonomo RA. J Antimicrob, Chemother. 2015;70:2279. doi: 10.1093/jac/dkv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Winkler ML, Papp-Wallace KM, Taracila MA, Bonomo RA. Antimicrob Agents Chemother. 2015;59:3700. doi: 10.1128/AAC.04405-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mann J, Taylor PW, Dorgan CR, Johnson PD, Wilson FX, Vickers R, Dale AG, Neidle S. Med Chem Commun. 2015;6:1420. doi: 10.1039/c5md00238a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Vickers R, Robinson N, Best E, Echols R, Tillotson G, Wilcox M. BMC Infect Dis. 2015;15:91. doi: 10.1186/s12879-015-0759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nathan C. Nat Rev Microbiol. 2015;13:651. doi: 10.1038/nrmicro3523. [DOI] [PubMed] [Google Scholar]
- 314.Servick K. Science. 2015;348:850. doi: 10.1126/science.348.6237.850. [DOI] [PubMed] [Google Scholar]
- 315.Fisher JF, Meroueh SO, Mobashery S. Chem Rev. 2005;105:395. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 316.Pulcini C, Bush K, Craig WA, Frimodt-Moller N, Grayson ML, Mouton JW, Turnidge J, Harbarth S, Gyssens IC. Clin Infect Dis. 2012;54:268. doi: 10.1093/cid/cir838. [DOI] [PubMed] [Google Scholar]
- 317.Theuretzbacher U, Van Bambeke F, Cantón R, Giske CG, Mouton JW, Nation RL, Paul M, Turnidge JD, Kahlmeter G. J Antimicrob Chemother. 2015;70:2177. doi: 10.1093/jac/dkv157. [DOI] [PubMed] [Google Scholar]