Abstract
Objectives
Chronic hypertension is the most critical risk factor for cardiovascular disease, heart failure, and stroke.
Approach and Results
Here we show that wild-type mice infused with Angiotensin II develop hypertension, cardiac hypertrophy, perivascular fibrosis and endothelial dysfunction with enhanced Stromal interaction molecule 1 (STIM1) expression in heart and vessels. All these pathologies were significantly blunted in mice lacking STIM1 specifically in smooth muscle (Stim1SMC−/−). Mechanistically, STIM1 up-regulation during Angiotensin II-induced hypertension was associated with enhanced endoplasmic reticulum (ER) stress and smooth muscle STIM1 was required for ER stress-induced vascular dysfunction through TGF-β and NADPH oxidase-dependent pathways. Accordingly, knockout mice for the ER stress pro-apoptotic transcriptional factor, CHOP (CHOP−/−) were resistant to hypertension-induced cardiovascular pathologies. Wild-type mice infused with Angiotensin II, but not Stim1SMC−/− or CHOP−/− mice showed elevated vascular NADPH oxidase activity and reduced phosphorylated endothelial Nitric Oxide Synthase (eNOS), cGMP and nitrite levels.
Conclusion
Thus, smooth muscle STIM1 plays a crucial role in the development of hypertension and associated cardiovascular pathologies and represents a promising target for cardiovascular therapy.
Keywords: Stromal interaction molecule 1 (STIM1), Endothelial Nitric Oxide Synthase (eNOS), Nicotinamide adenine dinucleotide phosphate (NADPH), vascular reactivity, cardiac hypertrophy, hypertension, ER stress
INTRODUCTION
Hypertension is a major risk factor for cardiovascular complications including heart failure and stroke in animal models and patients1, 2. Vascular reactivity is of paramount importance in regulating local blood flow and ensuring constant tissue perfusion. We and others reported that hypertension impairs vascular function through reduced endothelial Nitric Oxide (eNOS) activity and enhanced activation of molecular pathways of stress, including endoplasmic reticulum (ER) stress and oxidative stress3–7. It is well established that increased intracellular calcium (Ca2+) concentration is a key second messenger involved in cardiovascular homeostasis in terms of flow-induced dilation, and vascular smooth muscle cell (SMC) and cardiomyocyte contractility8–10. Adequate physiological functions of both endothelial cells and SMC require accurate intracellular Ca2+ regulation11–13. In particular, the ubiquitous store-operated Ca2+ entry (SOCE) pathway has been shown to regulate many cell functions14–17. In vascular disease states, SOCE is functional in endothelial cells, VSMCs, and cardiomyocytes, and plays an essential role in the regulation of proliferation, migration, hypertrophy and apoptosis16–25. STIM1 is an endoplasmic reticulum (ER) Ca2+ sensor which plays a critical role in the activation of Orai1 channels at the plasma membrane that mediate SOCE15, 18, 26–29. Physiologically, SOCE is activated upon receptor-mediated depletion of inositol-1,4,5-trisphopshate (IP3)-sensitive ER Ca2+ stores. SOCE contributes to intracellular Ca2+ refilling of the ER and also provides Ca2+ micro-domains crucial for downstream signaling to the nucleus30. In smooth muscle, we showed that STIM1 proteins are also required for the activation of another channel contributed by heteromultimeric of Orai1 and Orai3, which is activated by store-independent means involving intracellular actions of the inflammatory lipid second messenger, leukotriene C4 (LTC4)31–34. Our recent studies determined that the deletion of STIM1 specifically in SMC of mice reduces vascular contractile response to sympathetic stimulation with no effect on endothelium-dependent relaxation23. Others and we showed that increased STIM1 expression is critical for the development of vascular and cardiac remodeling in animal models24, 35–37. The STIM1 expression is also enhanced in vessels of hypertensive rats compared to normotensive rats and is associated with elevated spontaneous tone and force generation38. Collectively, these studies suggest that up-regulation of smooth muscle STIM1 could play a crucial role in vascular dysfunction and cardiac hypertrophy during hypertension. Thus, in the present study we sought to determine the role and mechanisms of smooth muscle STIM1 in hypertension and the associated vascular dysfunction and cardiac hypertrophy using various tissue-specific knockout mice strains.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
One of the most obvious observations from this in vivo study is the reduced body weight of Stim1SMC−/− mice, which is in agreement with previous studies3, 39. Indeed, regardless of whether mice were infused with saline or with Angiotensin II (Ang II), body weight was significantly reduced in Stim1SMC−/− compared to WT, Stim1SMC−/+, and CHOP−/− mice (Figure 1A). Importantly, in the Ang II-infused mice, hypertension was significantly delayed in Stim1SMC−/− and CHOP−/− compared to WT and Stim1SMC−/+ mice (Figure 1B)
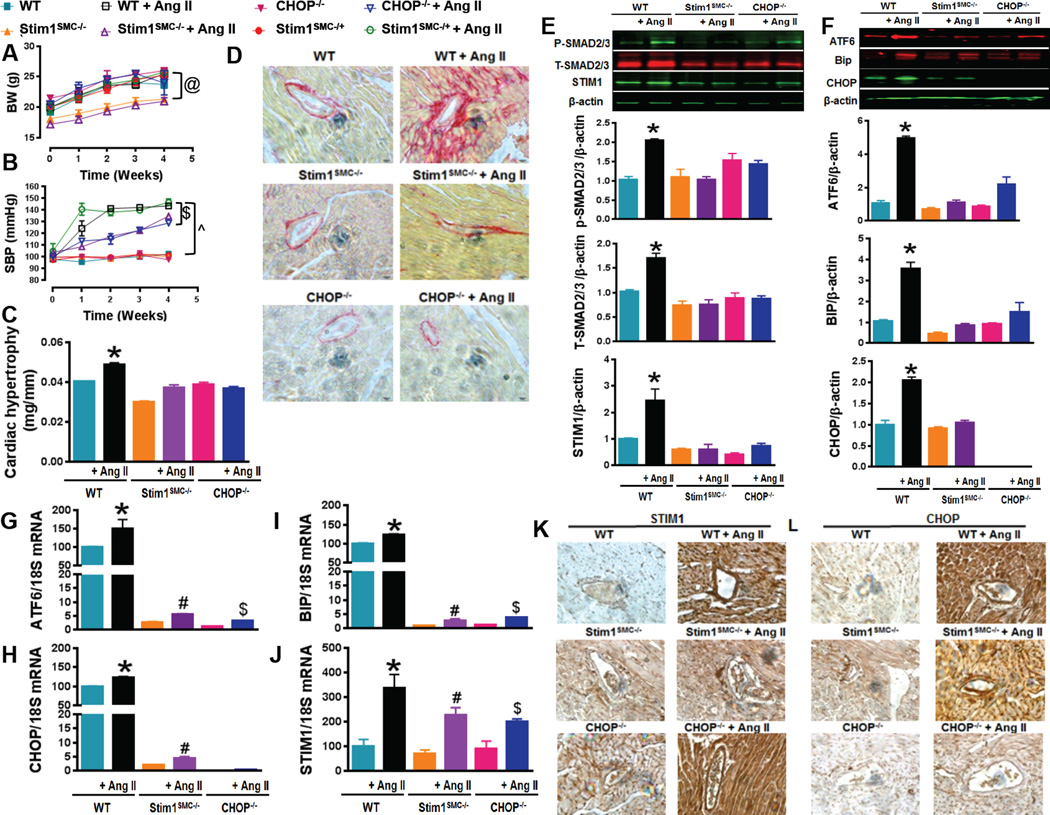
Figure 1. STIM1 and CHOP mediate hypertension-induced cardiac damage and fibrosis.
Body weight (A, n=6) and Systolic blood pressure measured by tail cuff machine (B, n=6) in WT, heterozygous (Stim1SMC−/+) and homozygous Stim1 knockout specifically in SMC (Stim1SMC−/−), and CHOP knockout (CHOP−/−) mice infused with or without Ang II. Cardiac hypertrophy determined by evaluating heart weight/tibia length ratios (C, n=6), Cardiac fibrosis stained with the collagen-specific Sirius-red (D, n=3), Western blot and quantification showing phosphorylated and total SMAD 2/3, STIM1 (E, n=3) and ER stress markers (ATF6, BIP CHOP) (F, n=3), mRNA levels of ER stress marker (ATF6 CHOP and BIP) (G, H, I, n=3) and STIM1 (J, n=3) and, Immunohistochemistry showing STIM1 (K, n=3) and CHOP (L, n=3) in heart from WT, Stim1SMC−/− and CHOP−/− mice infused with saline or Ang II. Two-way repeated measured ANOVA followed by Tukey's Post-Hoc test were applied for figures (A, B). One-way ANOVA followed by Bonferroni Post-Hoc test were applied for figures (G, H, I, J, E, F). @p<0.05 between STIMSMC−/−, STIMSMC−/− + AngII VS WT, WT + Ang II, STIMSMC−/+, STIMSMC−/+ + Ang II, CHOP−/−, CHOP−/− + Ang II. $p<0.05 between STIMSMC−/− + AngII, CHOP−/− + Ang II vs. STIMSMC−/+ + Ang II, WT + Ang II. ^p<0.05 between WT, STIMSMC−/−, CHOP−/− vs WT + Ang II, STIMSMC−/− + AngII, CHOP−/− + Ang II. *p<0.05 between WT + Ang II vs WT, STIMSMC−/−, STIMSMC−/− + AngII, CHOP−/−, CHOP−/− + Ang II. #p<0.05 between STIMSMC−/− vs STIMSMC−/− + AngII. &p<0.05 between CHOP−/− vs CHOP−/− + Ang II.
STIM1 and CCAAT-enhancer-binding protein homologous protein (CHOP) deletion inhibit hypertension-mediated cardiac hypertrophy and fibrosis
Cardiac hypertrophy was increased in WT, and Stim1SMC−/+ subjected to Ang II infusion while Stim1SMC−/− and CHOP−/− mice infused with Ang II were protected against cardiac hypertrophy (Figure 1C). Histological examination using collagen-specific Sirius-red staining on heart slices clearly demonstrated that chronic Ang II infusion induces perivascular fibrosis in WT group but not in Stim1SMC−/− and CHOP−/− group (Figure 1D). Similarly, Western blot analysis on heart tissues showed that total and phosphorylated Smad2/3, STIM1, and the ER stress markers Bip, ATF6 and CHOP protein expression were increased in the WT group infused with Ang II but not in Stim1SMC−/− and CHOP−/− groups (Figure 1E, F). We used RT-PCR on heart tissue samples to demonstrate that upregulation of these proteins in the WT group infused with Ang II occur at the mRNA level (Figure 1G–J). Immunohistochemistry on heart sections showed an increase in STIM1 both in WT and CHOP−/− groups infused with Ang II but not in hearts from Stim1SMC−/− mice infused with Ang II (Figure 1K, Supp. Figure 9A). However, CHOP protein levels were only increased in WT mice infused with Ang II (Figure 1L, Supp. Figure 9B). Together, these data suggest that STIM1 is acting upstream of CHOP and ER stress.
Effect of STIM1 and CHOP deletion on vascular reactivity in hypertension
The constriction of mesenteric resistance artery (MRA) in response to phenylephrine (PE) was significantly reduced in STIM1SMC−/− compared to WT, Stim1SMC−/+, and CHOP−/− mice (Figure 2A). However, this reduction in contraction in Stim1SMC−/− was not altered in Ang II-infused hypertensive mice (Figure 2D). The contraction to a thromboxaneA2 agonist (U-46619) was similar in all groups (Figure 2B). However, in hypertension thromboxaneA2 receptor-induced contraction was enhanced but to a lesser extent in STIM1SMC−/− and CHOP−/− groups infused with Ang II (Figure 2E). The endothelium-dependent relaxation of MRA was identical in all control saline-infused groups (Figure 2C). However, in mice infused with Ang II the endothelium-dependent relaxation in response to acetylcholine (ACh) was impaired in WT and STIM1SMC−/+ groups (Figure 2F). Interestingly, the endothelium-dependent relaxation was only partially inhibited (~50%) in Stim1SMC−/− infused with Ang II and was comparable to control levels in CHOP−/− mice infused with Ang II (Figure 2F).
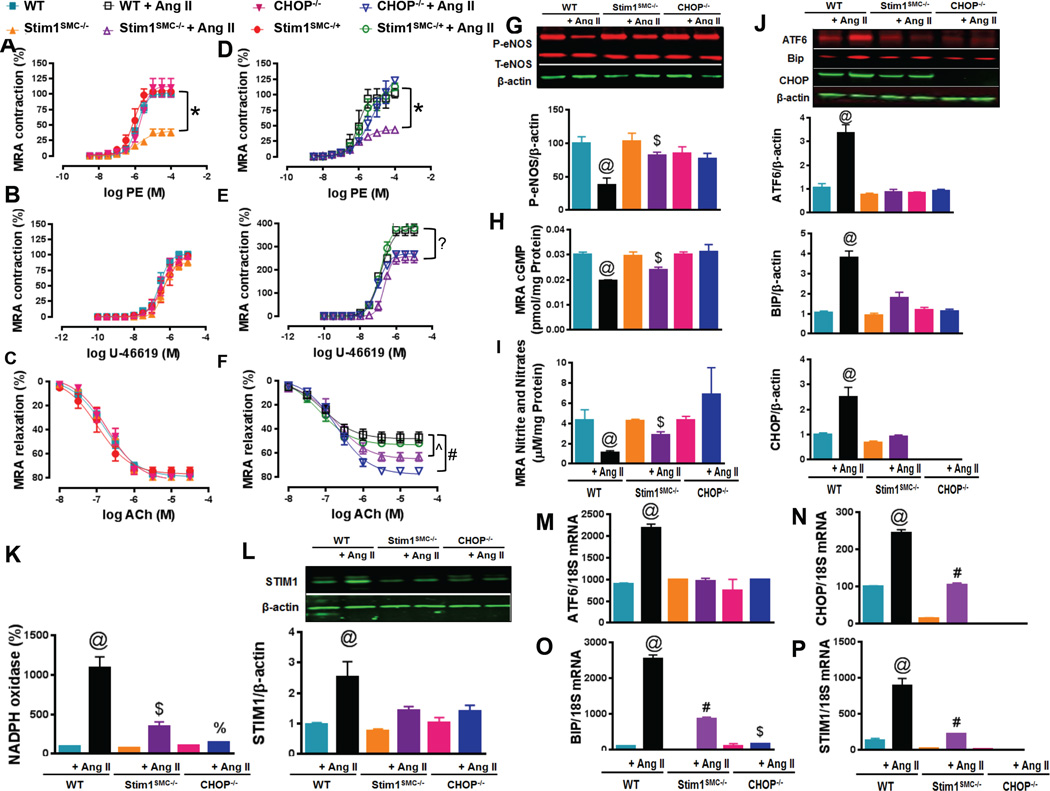
Figure 2. STIM1 and CHOP regulate hypertension-induced vascular damage.
Wire Myograph vascular reactivity showing vessel contraction response to phenylephrine (A, D, n=5–6) and ThromboxaneA2 analogue U-46619 (B, E, n=5), and endothelial-dependent relaxation in response to acetylcholine (C, F, n=5) in mesenteric resistance arteries (MRA) from WT, heterozygous (Stim1SMC−/+) and homozygous Stim1 knockout specifically in SMC (Stim1SMC/−), and CHOP knockout (CHOP−/−) mice infused with saline or Ang II. Western blot showing phosphorylated and total eNOS (G, n=3), Elisa showing cGMP levels using a sandwich enzymelinked immunosorbent assay (H, n=3) and nitrites/nitrate levels using the Griess reaction (I, n=3), protein levels and quantification of ER stress markers (ATF6, BIP and CHOP) (J, n=3), NADPH oxidase activity using lucigenin chemiluminescence (K, n=5), Western blot showing STIM1 (L, n=3), mRNA of ER stress markers (ATF6, BIP and CHOP) (M, N, O, n=3) and STIM1 (P, n=3) in mesenteric resistance arteries from WT, Stim1SMC−/− and CHOP−/− mice infused with or without Ang II. Two-way repeated measured ANOVA followed by Tukey's Post-Hoc test were applied for figures (A, B, C, D, E, F). One-way ANOVA followed by Bonferroni Post-Hoc test were applied for figures (K, L, H, I, J, M, N, O, P). *p<0.05 between STIMSMC−/− vs. WT, STIMSMC−/+, and CHOP−/−. &p<0.05 between STIMSMC−/− + AngII vs WT + Ang II, STIMSMC−/+ + Ang II, CHOP−/− + Ang II. ?p<0.05 between STIMSMC−/− + AngII, CHOP−/− + Ang II VS WT + Ang II, STIMSMC−/+ + Ang II. ^p<0.05 between WT + Ang II, STIMSMC−/+ + Ang II vs. STIMSMC−/− + AngII. #p<0.05 between WT + Ang II, STIMSMC−/+ + Ang II Vs. STIMSMC−/− + AngII vs CHOP−/− + Ang II. @p<0.05 between WT + Ang II vs WT, STIMSMC−/−, STIMSMC−/− + AngII, CHOP−/−, CHOP−/− + Ang II. $p<0.05 between STIMSMC−/− vs STIMSMC−/− + AngII. %p<0.05 between CHOP−/− vs. CHOP−/− + Ang II.
Western blots demonstrated a more pronounced reduction in eNOS phosphorylation in WT infused with Ang II compared to Stim1SMC−/− infused with Ang II, with the CHOP−/− mice infused with Ang II showing normal levels of eNOS phosphorylation (Figure 2G). Similarly, the cGMP and nitrite/nitrate levels (markers for NO signaling) were less reduced in STIM1SMC−/− infused with Ang II by comparison to WT mice infused with Ang II (Figure 2 H, I). However, CHOP−/− mice infused with Ang II had essentially normal levels of cGMP and nitrite/nitrate (Figure 2H, I). Consistent with eNOS, cGMP and nitrite/nitrate data above, NADPH oxidase activity (Figure 2K), p47phox expression (Supp. Figure 5A), mRNA levels of NOX isoforms (Nox2 and 4) (Supp. Figure 6) and 8-OHD (Supp. Figure 7A) were significantly elevated in WT mice infused with Ang II compared to STIM1SMC−/− mice and CHOP−/− mice infused with Ang II, with NADPH oxidase activity in CHOP−/− mice infused with Ang II showing levels comparable to those of control saline-infused mice (Figure 2K). Interestingly, we noticed that Nox2 mRNA expression level was significantly reduced after STIM1 deletion in SMC (Supp. Figure 6B).
The results obtained above with the MRA were confirmed in conductance arteries (thoracic aortas). Indeed, contractility in response to PE (Supp. Figure 1A, B) and thromboxaneA2 analogue (Supp. Figure 1C, D), the relaxation to ACh (Supp. Figure 1E, F), eNOS levels (Supp. Figure 1G), cGMP and nitrite/nitrate (Supp. Figure 1H, I), and NADPH oxidase activity (Supp. Figure 1J) p47phox expression (Supp. Figure 5B) and 8-OHD (Supp. Figure 7B) in thoracic aorta were similar to the results observed in MRA.
To study the involvment of eNOS coupling in vascular reactivity we measured the expression of eNOS T495 phosphorylation and the phosphorylated and total PKCα/β in MRA and thoracic aorta (Supp. Figure 5). Our data indicates that there was no difference in the expression of eNOS T495 (Supp. Figure 5B) among groups, however, the ratio P-PKCα/β/T-PKCα/β was significantly increased in WT mice infused with Ang II compared to STIM1SMC−/− mice and CHOP−/− mice infused with Ang II (Supp. Figure 5A, B).
To confirm our data, we measured in heart, MRA, and aorta tissues the NADPH oxidase activity in the presence and absence of L-NAME and apocynin (Supp. Figure 8). Our data showed that the increased NADPH oxidase in the WT group infused with Ang II was significantly reduced after treatment with L-NAME and Apocynin (Supp. Figure 8).
STIM1 and CHOP deletion inhibits vascular ER stress in hypertension
Since STIM1 is an ER protein of major importance in the maintenance of ER Ca2+ homeostasis and since specific lack of STIM1 in SMC protects against disruption of vascular and cardiac function during hypertension, we reasoned that STIM1 upregulation that occurs in hypertension might be mediating negative cardiovascular effects through exacerbation of ER stress. Indeed, we found that the expression of ER stress marker proteins Bip and ATF6 were significantly enhanced in MRA from WT mice infused with Ang II compared to all other groups (Figure 2J). STIM1 protein expression in MRA was greatly augmented in WT mice infused with Ang II compared to control saline-infused mice (Figure 2L). STIM1 protein expression was also significantly increased in Stim1SMC−/− mice and CHOP−/− mice infused with Ang II compared to control saline-infused mice, but this increase was less pronounced in these two groups of mice compared to WT mice infused with Ang II (Figure 2L). The increase in STIM1 expression observed in MRA from Stim1SMC−/− mice most likely reflects contributions from endothelial cells and possibly adventitial fibroblasts23.
We also demonstrated that changes in protein expression observed above occur at the mRNA levels. Indeed, all ER stress markers Bip, ATF6 and CHOP mRNA levels, as well as STIM1 mRNA levels, were increased after Ang II infusion and these increases were significantly decreased in Stim1SMC−/− and blunted in CHOP−/− mice infused with Ang II (Figure 2M–P).
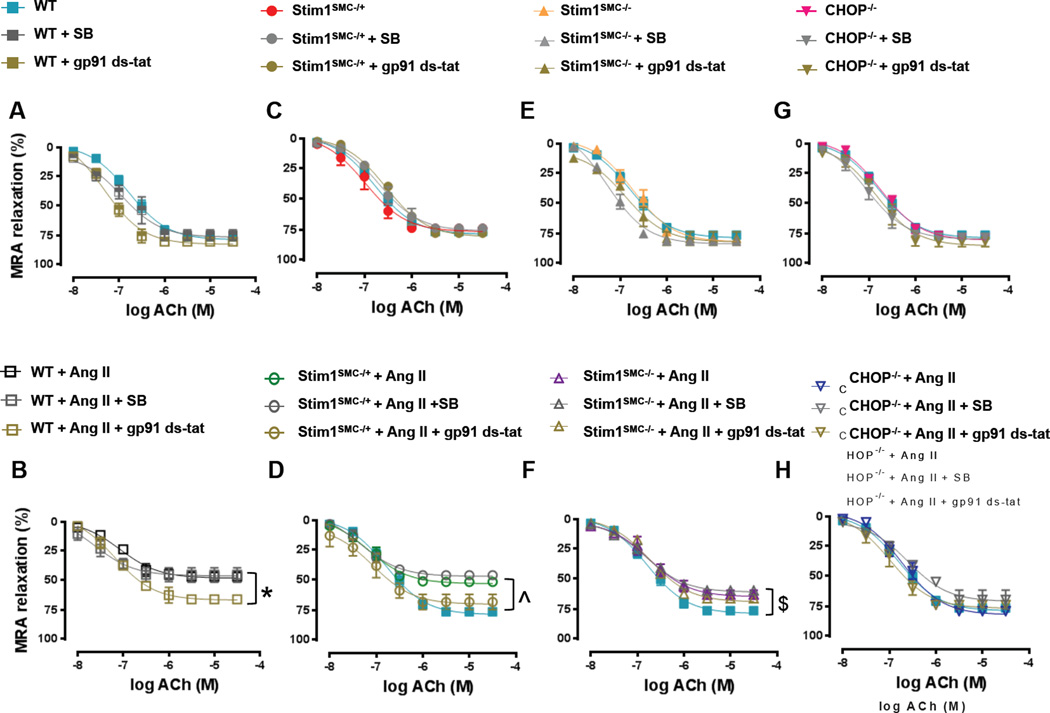
Effect of STIM1 and CHOP deletion on vascular TGF-β and reactive oxygen species in hypertension
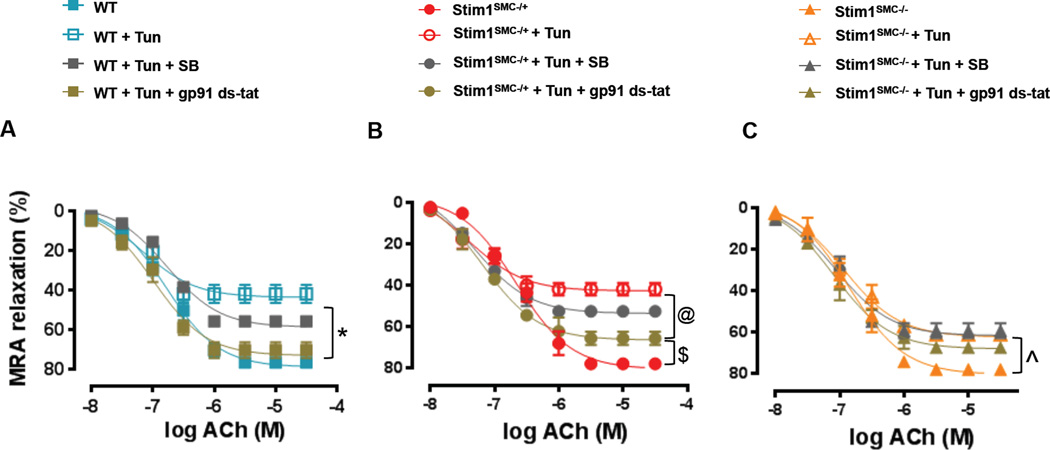
ER stress is an important determinant in the initiation of oxidative stress and TGF-β signaling. Our previous studies showed that induction of ER stress in mice causes endothelial dysfunction and inhibits vascular reactivity through TGF-β and reactive oxygen species-dependent mechanisms3. Therefore, we sought to determine the contribution of oxidative stress and TGF-β signaling in mediating vascular dysfunction downstream of STIM1 and ER stress using WT, Stim1SMC−/+, Stim1SMC−/− and CHOP−/− mice. We incubated MRA ex-vivo with the TGF-β inhibitor, SB431542 or the NADPH oxidase inhibitor, gp-91-stat. In the control groups of WT, Stim1SMC−/−, Stim1SMC−/+, and CHOP−/− saline-infused mice, the inhibition of TGF-β and NADPH oxidase had no effect on the endothelium-dependent relaxation of MRA (Figure 3A, C, E, G). Similar results were obtained when thoracic aortas were used instead of MRA (Supp. Figure 2A, C, E, G). Interestingly, when mice were infused with Ang II the NADPH oxidase inhibitor gp-91-stat improved endothelium-dependent relaxation of MRA from WT and Stim1SMC−/+ mice (Figure 3B, D) but had no effect in thoracic aorta (Supp. Figure 2B, D). Reciprocally, the inhibition of TGF-β improved endothelium-dependent relaxation in thoracic aorta of WT and Stim1SMC−/+ infused with Ang II (Supp. Figure 2B, D) but had no effect in MRA (Figure 3B, D). Consistent with a role for SMC STIM1 in promoting vascular dysfunction through ER stress-dependent mechanisms, endothelium-dependent relaxation of MRA and thoracic aorta was only partially inhibited in homozygous Stim1SMC−/− mice and was preserved in CHOP−/− mice infused with Ang II. Furthermore, in both Stim1SMC−/− and CHOP−/− mice the TGF-β inhibitor and the NADPH oxidase inhibitor were without effect (Figure 3 F, H; Supp. Figure 2F, H).
Figure 3. Effect of inhibition of NADPH oxidase and TGF-β signaling on Vascular reactivity in STIM1 and CHOP knockout mice.
Wire Myograph vascular reactivity showing endothelial-dependent relaxation in response to acetylcholine before and after incubation with TGF-β inhibitors (SB431542) and NADPH oxidase inhibitor (gp91 ds-tat) in mesenteric resistance arteries from: - WT mice infused with saline or Ang II (A, B, n=5) - Heterozygous Stim1 knockout specifically in SMC (Stim1SMC−/+) mice infused with saline or Ang II (C, D, n=5) - Homozygous Stim1 knockout specifically in SMC (Stim1SMC−/−) mice infused with saline or Ang II (E, F, n=5) - CHOP knockout (CHOP−/−) mice infused with saline or Ang II (G, H, n=5) Two-way repeated measured ANOVA followed by Tukey's Post-Hoc test were applied for figures (A, B, C, D, E, F, G H). *p<0.05 between WT + Ang II + gp91 ds-tat vs WT + Ang II, WT + Ang II + SB. ^p<0.05 between WT, STIMSMC−/+ + Ang II + gp91 ds-tat vs STIMSMC−/+ + Ang II, STIMSMC−/+ + Ang II + SB. $p<0.05 between WT vs. STIMSMC−/− + Ang II + gp91 ds-tat, STIMSMC−/− + Ang II, STIMSMC−/− + Ang II + SB.
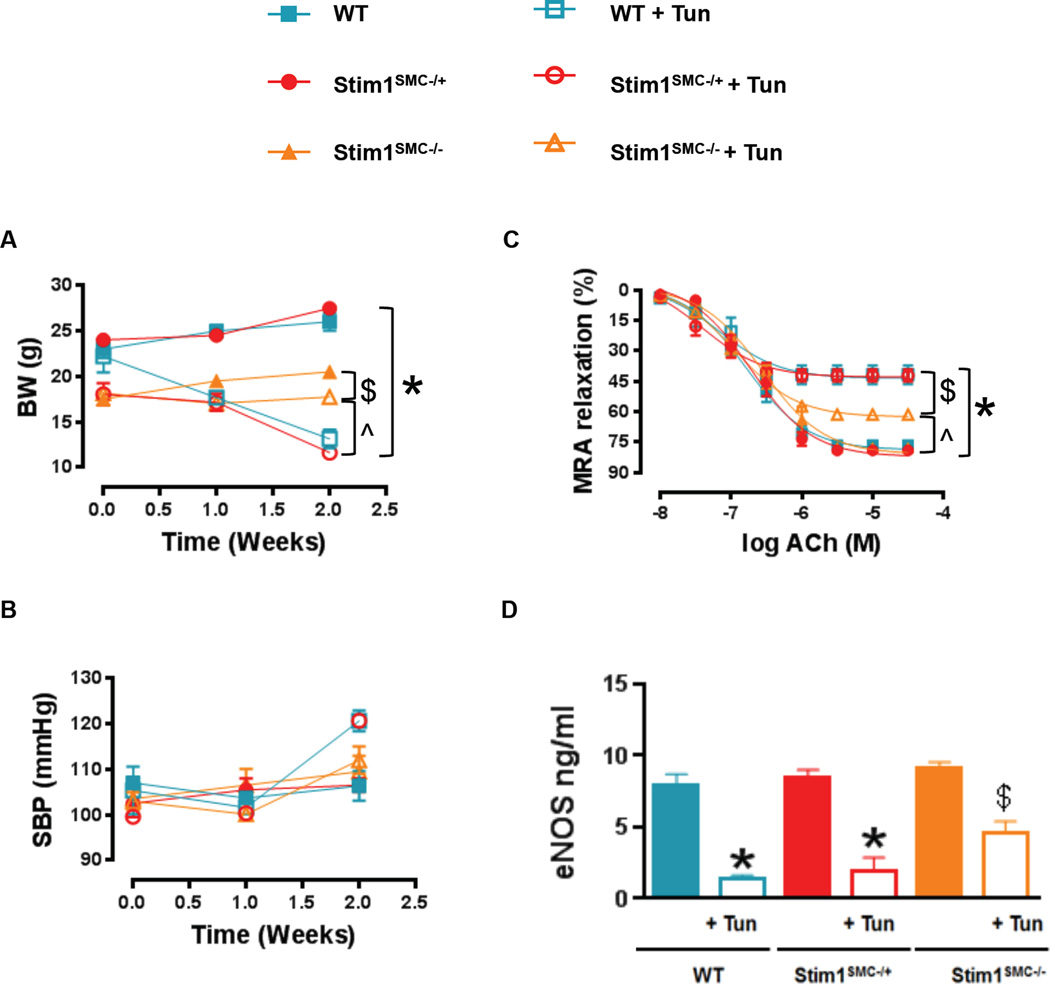
Relationship between ER stress and STIM1
To directly establish the relationship between ER stress and STIM1, we injected WT, Stim1SMC−/+ and Stim1SMC−/− with the ER stress inducer Tunicamycin followed by measurements of vascular reactivity after two weeks. Tunicamycin significantly reduced the body weight in WT and heterozygous Stim1SMC−/+ mice but had a noticeably smaller effect on the body weight of homozygous Stim1SMC−/− mice (Figure 4A); please note that as shown earlier untreated Stim1SMC−/− mice have reduced body weight compared to WT and Stim1SMC−/+ mice. Tunicamycin treatment had marginal effects on blood pressure that was essentially normal in all groups of mice (Figure 4B). Interestingly, endothelium-dependent relaxation in both MRA (Figure 4C) and thoracic aorta (Supp. Figure 3A) was significantly impaired in WT and heterozygous Stim1SMC−/+ mice injected with Tunicamycin while it was protected by approximately 50% in homozygous Stim1SMC−/− mice infused with Tunicamycin. Consistently, infusion with Tunicamycin caused a more pronounced reduction in total levels of eNOS in MRA (Figure 4D) and thoracic aorta (Supp. Figure 3B) from WT and heterozygous Stim1SMC−/+ mice compared to homozygous Stim1SMC−/− mice.
Figure 4. Effect of tunicamycin-induced ER stress on vascular reactivity in STIM1 knockout mice.
Body weight (A, n=5) and systolic blood pressure measured by tail cuff machine (B, n=5), Wire Myograph vascular reactivity showing endothelial-dependent relaxation in response to acetylcholine (C, n=5) and eNOS levels determined by an ELISA Kit (D, n=3) in MRA from WT, heterozygous (Stim1SMC−/+) and homozygous Stim1 knockout specifically in SMC (Stim1SMC−/−) mice treated with saline or Tunicamycin. Two-way repeated measured ANOVA followed by Tukey's Post-Hoc test were applied for figures (A, B, C). One-way ANOVA followed by Bonferroni Post-Hoc test were applied for figures (D). $p<0.05 between STIMSMC−/+ + Tunica vs. WT, STIMSMC−/−, STIMSMC−/+. *p<0.05 between STIMSMC−/+ + Tunica, WT + Tunica vs. WT, STIMSMC−/−, STIMSMC−/+. &p<0.05 between STIMSMC−/− vs. STIMSMC−/− + Tunica. ^p<0.05 between STIMSMC−/−, STIMSMC−/− + Tunica vs. STIMSMC−/+ + Tunica, WT + Tunica. %p<0.05 between STIMSMC−/− + Tunica vs. STIMSMC−/+ + Tunica and WT + Tunica.
Ex vivo experiments using the NADPH oxidase and TGF-β inhibitors as described above were performed to determine the contributions of oxidative stress and TGF-β signaling during ER stress induction in WT, Stim1SMC−/+ and Stim1SMC−/− mice. Consistent with the results from Figure 3 and Supp. Figure 2, the NADPH oxidase inhibitor gp-91-stat greatly improved endothelium-dependent relaxation of MRA from WT and Stim1SMC−/+ mice treated with Tunicamycin (Figure 5A, B), compared to the effect observed in thoracic aorta (Supp. Figure 4A, B). Reciprocally, the inhibition of TGF-β signaling greatly improved endothelium-dependent relaxation in thoracic aorta of WT and Stim1SMC−/+ treated with Tunicamycin compared with the effect in MRA (Figure 5A, B; Supp. Figure 4A, B). Consistent with results obtained with Ang II infusion, endothelium-dependent relaxation in MRA and aortas from homozygous Stim1SMC−/− mice was only partially inhibited by Tunicamycin treatment and additional treatment with the NADPH oxidase inhibitor or the TGF-β inhibitor was with no effect (Figure 5C; Supp. Figure 4C). The main finding in this study is summarized in figure supplementary 10.
Figure 5. Effect of inhibition of NADPH oxidase and TGF-β signaling on Vascular reactivity in tunicamycin-treated STIM1 knockout mice.
Wire Myograph vascular reactivity showing endothelial-dependent relaxation in response to acetylcholine before and after incubation with TGF-β inhibitor (SB431542) and NADPH oxidase inhibitor (gp91 ds-tat) in mesenteric resistance arteries from: - WT mice treated with saline or Tunicamycin (A, n=5) - Heterozygous Stim1 knockout specifically in SMC (Stim1SMC−/+) mice treated with saline or Tunicamycin (B, n=5) - Homozygous Stim1 knockout specifically in SMC (Stim1SMC−/−) mice treated with saline or Tunicamycin (C, n=5) Two-way repeated measured ANOVA followed by Tukey's Post-Hoc test were applied for figures (A, B, C). *p<0.05 between WT, WT + Tunica + gp91 ds-tat vs Sham + Tunica, WT + Tunica + SB. $p<0.05 between STIMSMC−/+ vs STIMSMC−/+ + Tunica + gp91 ds-tat. @p<0.05 between STIMSMC−/+ + Tunica + gp91 ds-tat vs STIMSMC−/+ + Tunica, STIMSMC−/+ + Tunica + SB. $p<0.05 between STIMSMC−/− vs STIMSMC−/− + Tunica, STIMSMC−/− + Tunica + gp91 ds-tat, STIMSMC−/− + Ang II, STIMSMC−/− + Tunica + SB.
DISCUSSION
The present study illustrates a novel mechanism connecting STIM1 to ER stress in mediating cardiac hypertrophy and vascular dysfunction in hypertension. The role of STIM1 in controlling several physiological processes such as endothelial and smooth muscle functions has been well established32, 33, 36, 40, 41. Others and we have previously shown that STIM1 is a master regulator of cardiovascular function; STIM1 protein expression is upregulated in vascular smooth muscle during vascular remodeling that is associated with phenotypic switching of smooth muscle from contractile to proliferative phenotypes12, 40–43. The prevention of STIM1 upregulation using in vivo delivery of siRNA into vessels of living rats inhibits vascular remodeling and neointimal hyperplasia35–37. While STIM1 expression is increased in vessels from hypertensive rats38, the role of STIM1 in hypertension and associated cardiac and vascular dysfunction remained unknown. Our data demonstrate a significant up-regulation of STIM1 expression in heart and arteries during hypertension-induced cardiac damage and vascular dysfunction. We used mice with targeted gene deletion of STIM1 specifically in smooth muscle and infused them with Ang II to demonstrate that lack of STIM1 in smooth muscle prevents hypertension and hypertension-induced cardiac hypertrophy and vascular dysfunction through abrogation of ER stress.
It is well established that hypertension in animals and patients triggers cardiac hypertrophy and cardiac fibrosis44, 45. We show that cardiac hypertrophy and fibrosis induced by Ang II were associated with enhanced STIM1 expression. Interestingly, STIM1 deletion in SMC reduced Ang II–induced cardiac hypertrophy and fibrosis suggesting that STIM1 in SMC is an important contributor to the development of cardiac damage in hypertension. Our data are supported by studies from our group and others showing increased STIM1 expression in neointimal hyperplasia and cardiac hypertrophy35–37, 46, 47. Additionally, our data indicate that ER stress markers were increased in the heart from WT mice infused with Ang II, which is in agreement with previous publications3, 48. STIM1 is a key regulator of Ca2+ homeostasis by supporting communications between the ER and plasma membrane. Therefore, STIM1 up-regulation could lead to ER stress and either directly or indirectly dictates the development of cardiovascular complications under hypertensive conditions. Thus, we determined that ER stress markers were reduced in the heart from Stim1SMC−/− and CHOP−/− mice infused with Ang II. Also, we observed that CHOP−/− mice infused with Ang II were protected against the development of hypertension, cardiac fibrosis, and hypertrophy. Our data showed that STIM1 expression was increased in the heart from CHOP−/− mice infused with Ang II, suggesting that STIM1 is upstream of the ER stress marker (CHOP) but likely they are part of a positive loop.
It is well established that hypertension is associated with impaired vascular reactivity in animal models and patients5, 49, but the mechanisms involved in vascular dysfunction during hypertension are not fully understood. Here we report that vasoconstriction in response to PE is significantly reduced in Stim1SMC−/− compared to WT consistent with previous studies23, 39. This effect was independent of hypertension since vasoconstriction in response to PE in Stim1SMC−/− mice infused with Ang II is also reduced. The vasoconstriction in response to PE in CHOP−/− mice with or without Ang II was similar to WT mice. Under control conditions (i.e. in the absence of Ang II infusion), the vasoconstriction in response to the thromboxaneA2 analog U46619 was identical in all groups. However, in mice infused with Ang II, the U46619-induced contraction was augmented in WT and heterozygous Stim1SMC−/+ and to a lesser extent in Stim1SMC−/− and CHOP−/− mice. The difference in the vasoconstriction response between PE and U46619 could be explained by the fact that thromboxaneA2 receptor signaling is not coupled to STIM1 while α1-adrenoceptor signaling is.
Endothelium-dependent relaxation of arteries depends on the activation of eNOS and cGMP signaling50. The deletion of STIM1 and CHOP did not alter the endothelium-dependent relaxation when compared to WT. However, after infusion of Ang II, the endothelium-dependent relaxation and eNOS phosphorylation were impaired in WT and Stim1SMC−/+ but protected in Stim1SMC−/− and CHOP−/− mice infused with Ang II. These data indicate that in hypertension, enhanced smooth muscle STIM1, and ER stress impair vascular endothelium-dependent relaxation. Several experimental and clinical evidence have linked the enhanced production of reactive oxygen species (ROS) to hypertension51, 52. We showed an increase in NADPH oxidase activity in mice infused with Ang II and this increase was blunted in Stim1SMC−/− and CHOP−/− infused with Ang II. We previously reported that TGF-β, an important cytokine produced by smooth muscle, plays a major role in the impairment of endothelium-dependent relaxation in conductance arteries while NADPH oxidase signaling is involved in resistance arteries3. Consistent with our previous studies, we found that in WT mice infused with Ang II, the inhibition of NADPH oxidase improves endothelium-dependent relaxation in resistance arteries while TGF-β inhibition improves endothelium-dependent relaxation in the thoracic aorta. Interestingly, in Stim1SMC−/− infused with Ang II, the inhibition of NADPH oxidase and TGF-β pathway did not improve further the endothelium-dependent relaxation, suggesting that STIM1 and NADPH oxidase/TGF-β pathways are not additive and likely mediating their effects through the same pathway. To determine the relationship between STIM1 and ER stress independently of hypertension, we treated mice with the ER stress inducer, Tunicamycin. In agreement with our previous studies3, 4, Tunicamycin did not enhance arterial blood pressure while impairing vascular endothelium-dependent relaxation in WT and heterozygous Stim1SMC−/+ mice. However, in homozygous Stim1SMC−/−, endothelium-dependent relaxation was protected after injection of Tunicamycin. These results suggest a circular effect between STIM1 and ER stress. Future molecular studies are needed to determine the interaction between STIM1 and ER stress and the interplay; how STIM1 regulates ER stress and how ER stress regulates STIM1.
The mechanism by which SMC-specific deletion of STIM1 protects the endothelium-dependent relaxation in hypertension is still unknown. It is likely that SMC STIM1 regulates factors released by SMC that interact with endothelial cells and therefore eNOS activity. Future studies are needed to determine the link between SMC STIM1 and the endothelium-dependent relaxation.
The important implication from these data is that direct induction of ER stress independently of hypertension impairs vascular endothelium-dependent relaxation, and specific deletion of STIM1 in smooth muscle overcomes ER stress-induced vascular dysfunction. Our study provides new insights into the in vivo contribution and molecular mechanisms of smooth muscle STIM1 in hypertension-induced cardiac damage and vascular dysfunction. Therefore specific targeting of smooth muscle STIM1 has the potential to overcome hypertension-induced cardiovascular complications.
LIMITATION PARAGRAPH
Previous studies by Giachini et al. showed that normotensive and spontaneously hypertensive female rats have reduced Store-operated Ca2+ entry compared to normotensive and hypertensive males, respectively and that was due to reduced expression of Orai1 and STIM1 in females53. The same authors showed that when spontaneously hypertensive females were ovariectomized, aortas from these female rats showed increased contraction and enhanced Orai1 expression, with no changes in STIM1 expression, suggesting that female sex hormones may down-regulate Orai1-mediated Ca2+ entry, thus contributing to vascular protection in females. In fact, studies by Flourakis et al showed that the expression of Orai1 in prostate cancer is controlled by androgen54 while our previous studies showed that estrogen regulates the expression of the Orai3 isoform in breast cancer55, 56. Taken together, these data suggest that potentially a smaller ratio of Orai1 to Orai3 in females compared to males might contribute to vascular protection observed in females. Since STIM1 regulates the function of all three Orai isoforms, it is likely that our current findings have major relevance to both females and males. Clearly, additional studies comparing males and females side by side, similar to those of Giachini et al.,53 would be required to conclusively address this issue.
Supplementary Material
Highlights.
We have found that protein expression of the Ca2+ sensor stromal interaction molecule 1 (STIM1) is enhanced in heart and vessels of hypertensive mice.
We show that abrogating STIM1 expression specifically in smooth muscle protects against hypertension and associated cardiovascular dysfunction
SMC STIM1 deletion protects the cardiovascular system likely through the modulation of the ER stress.
Acknowledgments
N/A
SOURCES OF FUNDING
This work was supported by the National Institutes of Health (HL095566; PI: Dr. Matrougui), (HL097111 and HL123364; PI: Dr. Trebak), AHA grant 14GRNT18880008 (PI: Dr. Trebak) and T32 HL007121 to Aboud Cardiovascular Research Center, University of Iowa (Dr. kassan; PI: Dr. F. Aboud)
Abbreviation
- ER stress
Endoplasmic reticulum stress
- MRA
Mesenteric Resistance Arteries
- STIM1
Stromal interaction molecule 1
- CHOP
CCAAT-enhancer-binding protein homologous protein
- NADPH
Nicotinamide adenine dinucleotide phosphate
- SOCE
Ubiquitous store-operated Ca2+ entry
- eNOS
Endothelial Nitric Oxide Synthase
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Leong XF, Ng CY, Jaarin K. Animal models in cardiovascular research: Hypertension and atherosclerosis. Biomed Res Int. 2015;2015:528757. doi: 10.1155/2015/528757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efrati S, Zaidenstein R, Dishy V, Beberashvili I, Sharist M, Averbukh Z, Golik A, Weissgarten J. Ace inhibitors and survival of hemodialysis patients. American journal of kidney diseases. 2002;40:1023–1029. doi: 10.1053/ajkd.2002.36340. [DOI] [PubMed] [Google Scholar]
- 3.Kassan M, Galán M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galán M, Kassan M, Kadowitz PJ, Trebak M, Belmadani S, Matrougui K. Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2014;1843:1063–1075. doi: 10.1016/j.bbamcr.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: Insights and directions. Current hypertension reports. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitler KM, Matsumoto T, Webb RC. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. American Journal of Physiology-Heart and Circulatory Physiology. 2013;305:H344–H353. doi: 10.1152/ajpheart.00952.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasty AH, Harrison DG. Endoplasmic reticulum stress and hypertension—a new paradigm? The Journal of clinical investigation. 2012;122:3859. doi: 10.1172/JCI65173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matchkov VV, Kudryavtseva O, Aalkjaer C. Intracellular ca2+ signalling and phenotype of vascular smooth muscle cells. Basic & clinical pharmacology & toxicology. 2012;110:42–48. doi: 10.1111/j.1742-7843.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 9.Bito V, Sipido KR, Macquaide N. Assessing ca2+-removal pathways in cardiac myocytes. Cold Spring Harbor Protocols. 2015;2015 doi: 10.1101/pdb.prot076992. pdb. prot076992. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Ngai CY, Huang Y, Ko WH, Wu M, He GW, Garland CJ, Dora KA, Yao X. Depletion of intracellular ca2+ stores enhances flow-induced vascular dilatation in rat small mesenteric artery. British journal of pharmacology. 2006;147:506–515. doi: 10.1038/sj.bjp.0706639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflügers Archiv-European Journal of Physiology. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trebak M. Stim/orai signalling complexes in vascular smooth muscle. The Journal of physiology. 2012;590:4201–4208. doi: 10.1113/jphysiol.2012.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflügers Archiv-European Journal of Physiology. 2008;457:405–415. doi: 10.1007/s00424-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh AB, Putney JW. Store-operated calcium channels. Physiological reviews. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ruhle B, Trebak M. Emerging roles for native orai ca2+ channels in cardiovascular disease. Curr Top Membr. 2013;71:209–235. doi: 10.1016/B978-0-12-407870-3.00009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trebak M, Zhang W, Ruhle B, Henkel MM, Gonzalez-Cobos JC, Motiani RK, Stolwijk JA, Newton RL, Zhang X. What role for store-operated ca(2)(+) entry in muscle? Microcirculation. 2013;20:330–336. doi: 10.1111/micc.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putney JW. A model for receptor-regulated calcium entry. Cell calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Venkatachalam K, van Rossum DB, Patterson RL, Ma H-T, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nature Cell Biology. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 20.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA. Orai1 and stim1 regulate normal and hypertrophic growth in cardiomyocytes. Journal of molecular and cellular cardiology. 2010;48:1329–1334. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and orai1 mediate crac currents and store-operated calcium entry important for endothelial cell proliferation. Circulation research. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, Gonzalez-Cobos JC, Zhang W, Matrougui K, Vincent PA, Trebak M. Stim1 controls endothelial barrier function independently of orai1 and ca2+ entry. Science signaling. 2013;6:ra18. doi: 10.1126/scisignal.2003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassan M, Zhang W, Aissa KA, Stolwijk J, Trebak M, Matrougui K. Differential role for stromal interacting molecule 1 in the regulation of vascular function. Pflügers Archiv-European Journal of Physiology. 2014;467:1195–1202. doi: 10.1007/s00424-014-1556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouille A, Dupuis M, Hadri L, Jeong D, Muhlstedt S, Schmitt J, Braun A, Benard L, Saliba Y, Laggerbauer B, Nieswandt B, Lacampagne A, Hajjar RJ, Lompre AM, Engelhardt S. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. Stim1/orai1-mediated soce: Current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol. 2013;305:H446–H458. doi: 10.1152/ajpheart.00104.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the crac channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 27.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. Stim1, an essential and conserved component of store-operated ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors stim1 and stim2 in t cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. Stim is a ca2+ sensor essential for ca2+-store-depletion-triggered ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh AB, Muallem S. Ca(2+) signalling and gene regulation. Cell Calcium. 2011;49:279. doi: 10.1016/j.ceca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. Store-independent orai1/3 channels activated by intracrine leukotriene c4: Role in neointimal hyperplasia. Circ Res. 2013;112:1013–1025. doi: 10.1161/CIRCRESAHA.111.300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, González-Cobos JC, Schindl R, Muik M, Ruhle B, Motiani RK, Bisaillon JM, Zhang W, Fahrner M, Barroso M. Mechanisms of stim1 activation of store-independent leukotriene c4-regulated ca2+ channels. Molecular and cellular biology. 2013;33:3715–3723. doi: 10.1128/MCB.00554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Zhang W, González-Cobos JC, Jardin I, Romanin C, Matrougui K, Trebak M. Complex role of stim1 in the activation of store-independent orai1/3 channels. The Journal of general physiology. 2014;143:345–359. doi: 10.1085/jgp.201311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the orai1 message. Sci Signal. 2015;8:ra74. doi: 10.1126/scisignal.aaa8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompre AM, Hulot JS. Rna interference targeting stim1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther. 2009;17:455–462. doi: 10.1038/mt.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M. Orai1-mediated icrac is essential for neointima formation after vascular injury. Circulation research. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo RW, Yang LX, Li MQ, Pan XH, Liu B, Deng YL. Stim1- and orai1-mediated store-operated calcium entry is critical for angiotensin ii-induced vascular smooth muscle cell proliferation. Cardiovasc Res. 2012;93:360–370. doi: 10.1093/cvr/cvr307. [DOI] [PubMed] [Google Scholar]
- 38.Giachini FR, Chiao C-W, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC. Increased activation of stromal interaction molecule-1/orai-1 in aorta from hypertensive rats a novel insight into vascular dysfunction. Hypertension. 2009;53:409–416. doi: 10.1161/HYPERTENSIONAHA.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mancarella S, Potireddy S, Wang Y, Gao H, Gandhirajan RK, Autieri M, Scalia R, Cheng Z, Wang H, Madesh M. Targeted stim deletion impairs calcium homeostasis, nfat activation, and growth of smooth muscle. The FASEB Journal. 2013;27:893–906. doi: 10.1096/fj.12-215293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for stim1-and orai1-dependent store-operated calcium influx through icrac in vascular smooth muscle cells: Role in proliferation and migration. The FASEB Journal. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Essential role for stim1/orai1-mediated calcium influx in pdgf-induced smooth muscle migration. American Journal of Physiology-Cell Physiology. 2010;298:C993–C1005. doi: 10.1152/ajpcell.00325.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lompre AM, Benard L, Saliba Y, Aubart F, Fauconnier J, Hulot JS. Stim1 and orai in cardiac hypertrophy and vascular proliferative diseases. Front Biosci (Schol Ed) 2013;5:766–773. doi: 10.2741/s406. [DOI] [PubMed] [Google Scholar]
- 44.Katholi RE, Couri DM. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. International journal of hypertension. 2011;2011 doi: 10.4061/2011/495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuspidi C, Ciulla M, Zanchetti A. Hypertensive myocardial fibrosis. Nephrology Dialysis Transplantation. 2006;21:20–23. doi: 10.1093/ndt/gfi237. [DOI] [PubMed] [Google Scholar]
- 46.Hulot J-S, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouillé A, Dupuis M. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. Stim1-dependent store-operated ca 2+ entry is required for pathological cardiac hypertrophy. Journal of molecular and cellular cardiology. 2012;52:136–147. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isodono K, Takahashi T, Imoto H, Nakanishi N, Ogata T, Asada S, Adachi A, Ueyama T, Oh H, Matsubara H. Parm-1 is an endoplasmic reticulum molecule involved in endoplasmic reticulum stress-induced apoptosis in rat cardiac myocytes. PLoS One. 2010;5:e9746. doi: 10.1371/journal.pone.0009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puddu P, Puddu GM, Zaca F, Muscari A. Endothelial dysfunction in hypertension. Acta cardiologica. 2000;55:221–232. doi: 10.2143/AC.55.4.2005744. [DOI] [PubMed] [Google Scholar]
- 50.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization beyond nitric oxide and cyclic gmp. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 51.Kassan M, Montero M, Sevilla M. Chronic treatment with pravastatin prevents early cardiovascular changes in spontaneously hypertensive rats. British journal of pharmacology. 2009;158:541–547. doi: 10.1111/j.1476-5381.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by cd4+ cd25+ natural regulatory t cells improves microvascular endothelial function through inhibition of nadph oxidase activity in hypertensive mice. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giachini FR, Lima VV, Filgueira FP, Dorrance AM, Carvalho MHC, Fortes ZB, Webb RC, Tostes RC. Stim1/orai1 contributes to sex differences in vascular responses to calcium in spontaneously hypertensive rats. Clinical Science. 2012;122:215–226. doi: 10.1042/CS20110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flourakis M, Lehen'kyi V, Beck B, Raphael M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell death & disease. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by orai3 selective requirement of orai3 versus orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. Journal of Biological Chemistry. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor α-regulated ca2+ channel that promotes tumorigenesis. The FASEB Journal. 2013;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.