Abstract
Chronic musculoskeletal pain, by its very nature, will be associated with negative emotions and psychological distress. There are individual differences in personality, coping skills, behavioral adaptation, and social support that dramatically alter the psychological outcomes of patients with chronic pain. Patients that have an aspect of central pain amplification associated with mechanical or inflammatory pain and patients with fibromyalgia (FM) are likely to exhibit higher levels of psychological distress and illness behaviors. This manuscript will discuss several different constructs for the association between chronic pain, central pain amplification, and psychological distress. The first key question addresses mechanisms shared in common between chronic pain and mood disorders, including the individual factors that influence psychological comorbidity. Second, how pain affects mood and vice versa. Finally, the utility of cognitive behavioral approaches to the management of chronic pain symptoms will be discussed.
Keywords: Fibromyalgia, Pain, Depression, Psychological
COGNITIVE AND EMOTIONAL ASPECTS OF CHRONIC PAIN
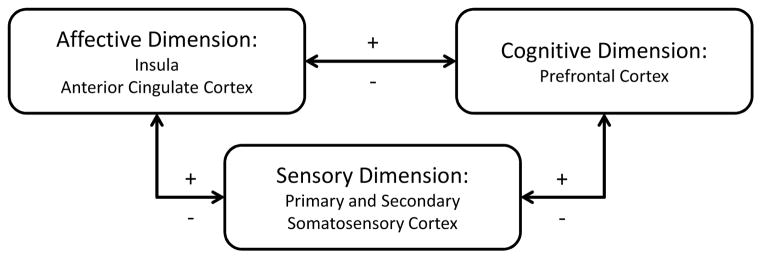
Chronic pain is a complex sensory and emotional experience that varies widely between people depending on the context and meaning of the pain and the psychological state of the person [1]. Cognitive and emotional factors have a surprisingly important influence of pain perception and these relationships lie in the connectivity of brain regions controlling pain perception, attention or expectation, and emotional states (Figure 1). Imaging studies have confirmed that activity of afferent and descending pain pathways are altered by attentional state, positive and negative emotions among many other factors unrelated to the pain stimulus itself. The physiology of central pain amplification at the level of the brain takes into account these important connections. There are now numerous studies that demonstrate that patients with chronic pain have alterations in brain regions involved in cognitive and emotional modulation of pain [1]. This complex interplay may explain why patients with long-term chronic pain develop anxiety and depression, but also why those with cognitive distortion and psychological distress are at increased risk for chronic pain and central amplification of pain.
Figure 1.
Brain regions involved in the dimensions of pain. There are feedback loops involving dimensions of pain. Pain has negative effects on emotions and cognitive functions but negative of positive emotion or cognitive states can directly modulate pain perception. The cortical regions consistently showing changes in patients with chronic pain include the insula, anterior cingulate cortex, and prefrontal cortex [1].
FROM PSYCHOGENIC RHEUMATISM TO SOMATIC SYMPTOM DISORDER
There is a long medical literature regarding the interplay between musculoskeletal pain and psychological distress, though only a few of these will be discussed. A starting point is the description of “psychogenic rheumatism” where one of the earliest case series was recorded in 1946 by Nobel laureate Philip S. Hench and Edward W. Boland as they describe the characteristics of US Army veterans returning from war [2]. Psychogenic rheumatism was one of the more common diagnoses, affecting approximately 20% of patients in specialized rheumatic disease centers. According to their report, “Psychogenic rheumatism – the musculo-skeletal expression of functional disorders, tension states, or psychoneurosis – is one of the commonest causes of generalized or localized aches and pains in muscles and/or joints in either civilian or military life.” They identify that this condition may occur either alone, or as an overlay to rheumatoid arthritis or fibrositis, their term for soft tissue rheumatic disorders such as bursitis or tendonitis. They went on to describe that primary fibrositis “puts its victims at the mercy of changes in external environment: thus weather, heat, cold, humidity, rest, exercise, etc. characteristically influences most of them for better or for worse.” On the other hand, psychogenic rheumatism “puts its victims at the mercy of changes in the internal environment: thus their symptoms may vary with mood or psyche, pleasure, excitement, mental distraction, worry, or fatigue.” The description of psychogenic rheumatism included an attitude that was tense, anxious, defensive, and antagonistic. The chief symptoms were described as burning, tightness, weakness, numbness, tingling, queer or tired sensations that were often continuous day and night. They also describe severe fatigue causing disability, worsening of symptoms during and after exercise, and a “touch me not” reaction to examination. Psychotherapy was the preferred treatment approach for these patients. It is likely that patients collectively referred in the present day as fibromyalgia have components of both fibrositis and psychogenic rheumatism.
Arthur J. Barsky and Jonathan F. Borus describe “functional somatic syndromes”, which include fibromyalgia, characterized by higher levels of symptoms, suffering, and disability than by consistently demonstrable tissue abnormality [3]. These authors point out that the symptoms common to the functional somatic syndromes include fatigue, weakness, sleep difficulties, headaches, muscle aches and joint pain, problems with memory, attention, and concentration, nausea and other gastrointestinal symptoms, anxiety, depression, irritability, palpitations and racing heart, shortness of breath, dizziness or light-headedness, sore throat and dry mouth are highly prevalent in the population in general. Furthermore, as is true with fibromyalgia, patients often meet the criteria for other syndromes in part because of the overlap in diagnostic criteria. In their analysis, these authors implicate “four psychosocial factors that propel symptom amplification including the belief that one has a serious disease, the expectation that one’s condition is likely to worsen, the sick role including the effects of litigation and compensation, and the alarming portrayal of the condition as catastrophic and disabling. [3]” The biological process of selecting sensations believed to have pathological significance for conscious attention lies in the realm of cognitions around these sensation and, certainly, pain is a symptom often selected for significance. There is also often an influence of negative memory of past symptoms and expectations of future symptoms that may play a role in the cognitive amplification processes.
More recently, Frederick Wolfe has been a proponent of the term “polysymptomatic distress” that incorporates multifocal musculoskeletal pain and neuropsychological symptoms of fatigue, unrefreshing sleep, dyscognition, and other functional somatic syndromes into a quantitative scale that allows for a flexible and continuous application in patients with any rheumatic disease and in the general population. It is clear from his work that patients with a variety of rheumatic diseases and individuals in the general population can have a variable degree of polysymptomatic distress that influences clinical outcomes [4].
The American Psychiatric Association in the 5th Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) has replaced the previous category of somatoform disorders with “Somatic Symptom Disorder (SSD)” [5]. This diagnosis is characterized by “distressing somatic symptoms plus abnormal thoughts, feelings, and behaviors in response to these symptoms”. Importantly, the previous requirement that symptoms had to be medically unexplained is removed and psychological symptoms surrounding the somatic symptoms have been added. These include excessive thoughts, feelings, or behaviors related to somatic symptoms or associated health concerns as manifested by rumination and/or high level of anxiety about health or symptoms and/or excessive time and energy devoted to symptoms or health concerns.
It is likely that there are genetic factors that play a role in the vulnerability of patients to developing SSD. Patients with fibromyalgia and other forms of central pain amplification are at risk for other psychiatric disorders, especially depression, post-traumatic stress disorder and other anxiety disorders, and bipolar disorder [6]. In addition, there are exposures that alter pain-related emotions and cognitions that include socioeconomic deprivation, traumatic events, and chronic stress [7]. Finally, the sociocultural environment and health care system that require a diagnosis to legitimize and treat symptoms may contribute to maladaptive illness behaviors.
In concept, patients with chronic musculoskeletal pain of any etiology may experience, to a greater or lesser degree, excessive emotional, cognitive, and behavioral responses to chronic pain. Centrally amplified pain, as occurs in fibromyalgia, may be more likely than nociceptive pain to be associated with multiple somatic symptoms and associated distress. It is suggested that clinicians identify patients with pain-associated SSD and address these issues using cognitive behavioral management strategies. Thus, a patient with fibromyalgia or rheumatoid arthritis or systemic lupus erythematosus may also have SSD that could be managed as part of a comprehensive approach to treatment.
SHARED GENETIC VULNERABILITY TO PAIN AND PSYCHOLOGICAL SYMPTOMS
Many of the pathways identified as important for chronic widespread pain and fibromyalgia sit within pathways also important for mood. For example, two major neurotransmitter pathways have been repeatedly associated with musculoskeletal pain [8]. The first is the adrenergic pathway, in which COMT, the gene encoding the enzyme catechol O-methyltransferase that is responsible for the catabolism of catechol neurotransmitters such as epinephrine, norepinephrine, and dopamine, is the most frequently associated with chronic musculoskeletal pain conditions. Most studies of COMT report an increased risk of chronic pain associated with a Val159Met (rs4680) that encodes a protein with lower enzymatic activity [8]. More extensive studies have expanded the functional locus to three major haplotypes that modify expression and activity of the enzyme thus conferring low and high risk phenotypes for acute pain sensitivity as well as risk for developing chronic pain [9]. Additional genetic variation in the β2-adrenergic receptor gene (ARDRB2; rs1042713 and rs1042714) has been associated with an increased risk of FM and chronic widespread pain. Haplotype variants that regulate β2-adrenergic receptor expression and internalization are associated with differences in susceptibility to chronic pain [10]. The second pathway associated with chronic pain syndromes is the serotonin pathway. Specific genes include the 5-hydroxytryptamin receptor 2A (HTR2A) and 5HT transporter (SLC6A4) [11] [12] [13]. A 44-base pair insertion/deletion polymorphism in the promoter region of SLC6A4 is most frequently associated with risk of chronic pain conditions, including FM [8].
Both of these genetic pathways are also associated with several “endophenotypes” or intermediate measurable phenotypes that are present in patients with FM. These include autonomic dysregulation, altered pain processing and modulation, sleep dysfunction, and anxiety in the case of the adrenergic pathway [8]. Personality and affective traits such as somatic awareness, depression and anxiety have been associated with genetic variation in the serotonin pathway and are associated with risk for chronic pain [8].
NEUROBIOLOGY OF SOMATOSENSORY AMPLIFICATION
Somatosensory amplification refers to the tendency to experience a wide range of bodily sensations as intrusive, intense, noxious, and disruptive. This is associated with “heightened attentional focus on bodily sensations, the tendency to select out certain weak and infrequent sensations, and the disposition to react to these sensations with affect and cognitions that intensify them and make them more alarming and distressing” [14]. Presently, the ability to image the central nervous system has allowed investigators to determine the pathways involved in the affective and cognitive circuits associated with the relationships between afferent processing and interpretation of symptoms [15]. It should be noted that both limbic and cortical regions receive input from pain transmission neurons of the spinothalamic tract.
Functional neuroimaging has contributed much to the understanding of fibromyalgia. Patient’s symptoms of pain can objectively be demonstrated as changes in pain transmission and representation confirming that patients are accurately describing their experiences. One of the first studies to address this issue demonstrated that greater regional cerebral blood flow receiving pain pathway input was related to the patient’s report of pain rather than to the stimulus intensity [16]. Other neuroimaging techniques have emerged and provided information in fibromyalgia and other chronic pain states. Using proton magnetic resonance spectroscopy (1H-MRS), patients with fibromyalgia were found to have low levels of N-acetyl-aspartate, a metabolite believe to be a marker of neuronal density and viability where low levels may indicate loss of neural function and activity, in the hippocampus [17]. Also using 1H-MRS, it was found that the main excitatory neurotransmitter, glutamate/glutamine, was elevated in the posterior insular cortex and a decrease in the signal was correlated with improvements in pain [18] [19]. Other brain regions of fibromyalgia patients, including the amygdala, posterior cingulate, and ventral lateral prefrontal cortex, had elevated levels of glutamate/glutamine suggesting a possible role for this neurotransmitter in pain and perhaps other symptoms of FM [17].
Focusing on the psychological aspects of chronic pain, the hippocampal formation and its connectivity to the anterior cingulate cortex (ACC) and posterior insula has been linked to negative expectancy. Negative attentional bias, which is the enhanced sensitivity and detection of aversive or upleasant stimuli, and pain catastrophizing involve the ACC, amygdala, and lateral prefrontal cortex [15]. It has been proposed that the dorsal ACC and anterior middle cingulate cortex are positioned to integrate negative affect, pain, and cognitive control [20].
SOCIAL AND PSYCHOLOGICAL FACTORS
Studies of social and psychological risk factors are more abundant for patients with chronic widespread pain and fibromyalgia than for nociceptive pain. However when studied, similar risk factors are associated with poor outcome in other forms of chronic musculoskeletal pain.
Life stress and socioeconomic factors
In many longitudinal epidemiologic studies, chronic pain and other somatic symptoms can be predicted by childhood abuse and traumas, low educational attainment, social isolation, depression and anxiety [21]. In a population-based study to determine psychosocial factors that predicted new-onset chronic widespread pain, investigators identified a random sample of subjects from socio-demographically disparate backgrounds then identified more than 3,000 who did not have pain at baseline and more than 300 that had new widespread musculoskeletal pain at follow-up [7]. The strongest predictors were premorbid somatic symptoms, illness behaviors, and sleep problems. In another community-based study, perceived physical and emotional trauma as precipitating factors for FM were associated with health-care seeking rather than pain severity [22].
Lower socioeconomic status predicts greater symptom severity and functional impairment in patients with FM even controlling for levels of pain, depression, and anxiety [23]. The biopsychosocial model of pain posits that pain experience and its impact of the individual is a function of interacting combinations of nociceptive input, psychological processes including beliefs, coping repertoire and mood, and environmental contingencies that would include family, community, and cultural rules or expectations [24]. All of these factors are likely to play a key role on the clinical expression and health impact of FM.
Personality, cognitive, and psychological factors
It has been suggested that patients with FM may have a specific personality profile characterized, for example, by high levels of neuroticism. However, many studies include state variables, such as catastrophizing, self-esteem, motivation or coping strategies, with personality traits. It is sometimes unclear whether a personality feature is a state or a trait, for example catastrophizing may be seen as a personality feature, a cognitive error, or a coping strategy [25]. A recent study using a standard five-factor personality scale (neuroticism, extraversion, openness to experience, agreeableness, conscientiousness) showed that FM patients were not different from patients with other rheumatic pain conditions or other chronic illnesses [26]. Furthermore, the scores fell within the normal range for the general population. However, a cluster of FM patients was identified with a personality profile (high neuroticism, lower extraversion) that reflects a proneness to experience emotional distress, a difficulty for positive emotion, and a tendency to ineffective use of emotional regulation processes rather exhibiting rumination and maladaptive behaviors. The patients in this cluster also exhibited more psychosocial problems [26].
Another way of stratifying patients is the psychological and behavioral response to chronic pain. Patients with FM have been characterized and divided into groups that predict outcome based on psychological characteristics [27]. Patients classified as “dysfunctional” exhibit the highest pain intensity, interference, distress, and lowest control and activity levels. The “adaptive coper” groups report the lowest levels of pain and interference, as well as the highest activity levels. The “interpersonally distressed” patients report high levels of affective distress and more negative spouse responses to pain.
It is likely that there are important relationships between psychological and physiological pathways in patients with FM. This is certainly not surprising since regulation of domains characterized as psychological and physiological, utilize common mediators. For example, a recent study of patients with FM performed a cluster analysis based on pain characteristics and cognitive, affective, and behavioral responses to pain and stress [28]. The study demonstrated that psychophysiological responses of blood pressure, heart rate, and skin conductance were associated with specific types of psychological coping and psychiatric diagnoses [29].
CHRONIC PAIN AND MOOD DISORDERS
On average, between 30 and 60% of patients with chronic pain have comorbid depression [30]. Furthermore, the prevalence of a lifetime history of major depression or other mood disorder is even higher. These data are confounded by 50% prevalence of pain in patients whose primary diagnosis is depression. Pain complaints are typically amplified in patients with depression. These observations lead to the concept that there is a bidirectional relationship between the presence and severity of pain and depression. Indeed, a large longitudinal study of primary care patients with persistent pain of the back, hip or knee found that change in pain was a strong predictor of depression severity and vice versa [31]. Chronic pain is also strongly associated with posttraumatic stress disorder and there is a negative association with pain, psychological status, quality of life and disability independent from depression [32].
FM is strongly associated with major depressive disorder, bipolar disorder, anxiety disorders including panic disorder, post-traumatic stress disorder, social phobia and obsessive compulsive disorder, and substance abuse disorder [6, 33]. In a study of 108 individuals with and 228 individuals without FM, the odds ratio for patients with FM having bipolar disorder was 153 (95% CI, 26–902, P<0.001), any anxiety disorder 6.7 (95% CI, 2.3–20, P<0.001), substance use disorder 3.3 (95% CI, 1.1–10, P=0.040, and major depressive disorder 2.7 (95% CI, 1.2–6.0 P=0.13) [6].
SUICIDE RISK IN PATIENTS WITH CHRONIC PAIN
Chronic pain has long been considered a risk factor for suicide and a recent study reported that this risk is due, at least in part, to depression and substance use disorders [34]. A 15-year prospective cohort study of Danish patients found that there was a standardized mortality rate of 10.5 (95% confidence intervals 5.5–20.7) for death by suicide in women with fibromyalgia [35]. Of interest, patients who died by suicide did not have a pre-existing psychiatric diagnosis at the time their fibromyalgia was diagnosed. Another large study assessing mortality in FM from the United States reported that FM patients had an Odds Ratio of 3.31 (95% confidence intervals 2.15–5.11) for death by suicide compared to the general population [36]. A large study of veterans found that risk of suicide differs with the type and severity of pain [37].
Risk factors for suicide mortality in patients with chronic pain include the pain characteristics, psychiatric comorbidity, other psychological factors, substance abuse, and ready access to analgesics [34]. Back pain and FM, but not neuropathic pain, is associated with an elevated suicide risk. Among other psychological factors, a Canadian study identified pain-related helplessness as an important predictor of suicidal behavior [38]. Although the absolute risk of suicide is very low, these data emphasize the importance of screening pain patients for substance abuse, psychiatric comorbidity, and suicidal ideation.
COGNITIVE BEHAVIORAL THERAPY FOR CHRONIC PAIN
Cognitive behavioral therapy (CBT) has been part of treatment for chronic pain for decades and the majority of research supports the effectiveness of the intervention. CBT is a form of psychotherapy in which the therapist and patient work together to identify and solve problems.
It is problem-focused and action oriented. CBT includes interventions that are based on the basic premise that chronic pain is maintained by cognitive and behavioral factors, and that psychological treatment leads to changes in these factors through training in specific techniques [39]. These would include cognitive re-structuring and behavioral training, such as relaxation and social skills training. The underlying theory is based on the cognitive model in which the way a person thinks about a situation influences emotional and behavioral reactions. Perceptions are often distorted and dysfunctional when individuals are distressed. CBT helps people identify distressing thoughts and evaluate them realistically to change distorted thinking. When distress decreases, patients are better able to solve related problems and initiate behavioral changes. Therapists use a questioning process to help patients evaluate and respond to their thoughts and beliefs. They also help patients engage in the evaluation process. A recent Cochrane review of CBT in FM selected 23 studies that included more than 2,000 patients [40]. The quality of the evidence was low and the effect sizes were small. However, CBT was shown to provide benefit for reducing pain, negative mood, and disability [40].
Mindfulness-based stress reduction is a cognitive therapy that helps individuals to self-manage and reframe worrisome and intrusive thoughts by mindfulness meditation. This technique was shown to reduce perceived stress, sleep disturbance, and symptom severity though there was no improvement in pain or physical functioning in a randomized controlled clinical trial in FM [41]. A systematic review and meta-analysis of mindfulness-based programs in FM found that this might be a useful approach, but that the quality of the evidence was low [42]. A recent comparison of CBT for pain, mindful awareness and acceptance treatment, and education in patients with RA found that mindfulness produced the broadest improvements in daily pain and stress reactivity [43]. Operant behavioral treatment focuses on the modification of pain behavior by increasing activity levels, reducing healthcare-seeking behavior, and also on reducing pain-reinforcing behaviors in significant relationships [39]. A recent Cochrane review of 21 studies that employed various forms of psychotherapy for patients with somatoform disorders concluded that only CBT has been adequately studied and that this form of therapy reduced somatic symptoms with a small effect size [44]. Although based on approaches used for depression, CBT for pain typically focuses on symptom relief and increased physical functioning [30]. There is evidence that CBT for insomnia improves sleep parameters, but also improves pain in patients with knee osteoarthritis [45].
These interventions are typically delivered in group settings, but versions of CBT can also be delivered by computer interface or by telephone. A recent study compared an internet-delivered individualized CBT for chronic pain patients with comorbid depression and anxiety compared to a moderated on-line discussion forum. CBT resulted in reduced depression and anxiety as well as reduction in pain catastrophizing [46]. Mindfulness-based approaches can also be delivered over the internet. For example, a recent study for a computerized mindfulness-based cognitive therapy “Mindfulness in Action” was compared to a pain management psychoeducation program. Both groups showed equivalent and significant improvements on pain interference, pain acceptance, and catastrophizing. Participants in the MIA group were better able to manage emotions and stress [47].
SUMMARY
Chronic pain and psychological distress frequently coexist, but it is not generally fruitful to determine causative relationships. More important is to identify psychological comorbidities in patients with chronic pain and develop a management plan taking these into account. Incorporating CBT and related treatment approaches may improve outcomes with long-term benefits on the ways patients think about and cope with their pain.
Practice Points. Somatic symptom disorder (SSD) in chronic pain.
Patients with chronic pain of any cause may have SSD
SSD is defined by distressing somatic symptoms plus abnormal thoughts, feelings, and behaviors in response to these symptoms
Examples include rumination and/or high level of anxiety about health or symptoms and/or excessive time and energy devoted to symptoms or health concerns
Footnotes
Conflicts of Interest: None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews Neuroscience. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hench P, Boland E. The management of chronic arthritis and other rheumatic diseases among soldiers of the United States army. Ann Rheum Dis. 1946;5:106–114. doi: 10.1136/ard.5.4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barsky AJ, Borus JF. Functional somatic syndromes. Annals of internal medicine. 1999;130:910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Brahler E, Hinz A, et al. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis care & research. 2013;65:777–785. doi: 10.1002/acr.21931. [DOI] [PubMed] [Google Scholar]
- 5.Association AP. Diagnostic and Statistical Manual of Mental Disorders - DSM-5. Washington, D.C: American Psychiatric Publishing; 2013. [Google Scholar]
- 6.Arnold LM, Hudson JI, Keck PE, et al. Comorbidity of fibromyalgia and psychiatric disorders. The Journal of clinical psychiatry. 2006;67:1219–1225. doi: 10.4088/jcp.v67n0807. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Silman AJ, Ray D, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford) 2007;46:666–671. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 8.Diatchenko L, Fillingim RB, Smith SB, et al. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nature reviews Rheumatology. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 10.Hocking LJ, Smith BH, Jones GT, et al. Genetic variation in the beta2-adrenergic receptor but not catecholamine-O-methyltransferase predisposes to chronic pain: results from the 1958 British Birth Cohort Study. Pain. 2010;149:143–151. doi: 10.1016/j.pain.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Bondy B, Spaeth M, Offenbaecher M, et al. The T102C polymorphism of the 5-HT2A-receptor gene in fibromyalgia. Neurobiology of Disease. 1999;6:433–439. doi: 10.1006/nbdi.1999.0262. [DOI] [PubMed] [Google Scholar]
- 12.Offenbaecher M, Bondy B, de Jonge S, et al. Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheum. 1999;42:2482–2488. doi: 10.1002/1529-0131(199911)42:11<2482::AID-ANR27>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Nicholl BI, Holliday KL, Macfarlane GJ, et al. Association of HTR2A polymorphisms with chronic widespread pain and the extent of musculoskeletal pain: results from two population-based cohorts. Arthritis and rheumatism. 2011;63:810–818. doi: 10.1002/art.30185. [DOI] [PubMed] [Google Scholar]
- 14.Barsky AJ, Goodson JD, Lane RS, et al. The amplification of somatic symptoms. Psychosomatic medicine. 1988;50:510–519. doi: 10.1097/00006842-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. The Journal of neuropsychiatry and clinical neurosciences. 2015;27:e40–50. doi: 10.1176/appi.neuropsych.13070170. [DOI] [PubMed] [Google Scholar]
- 16.Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 17.Napadow V, Harris RE. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of ‘centralized’ pain? Arthritis research & therapy. 2014;16:425. doi: 10.1186/s13075-014-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harte SE, Clauw DJ, Napadow V, et al. Pressure Pain Sensitivity and Insular Combined Glutamate and Glutamine (Glx) Are Associated with Subsequent Clinical Response to Sham But Not Traditional Acupuncture in Patients Who Have Chronic Pain. Medical acupuncture. 2013;25:154–160. doi: 10.1089/acu.2013.0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholl BI, Macfarlane GJ, Davies KA, et al. Premorbid psychosocial factors are associated with poor health-related quality of life in subjects with new onset of chronic widespread pain - results from the EPIFUND study. Pain. 2009;141:119–126. doi: 10.1016/j.pain.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaron LA, Bradley LA, Alarcon GS, et al. Perceived physical and emotional trauma as precipitating events in fibromyalgia. Associations with health care seeking and disability status but not pain severity. Arthritis Rheum. 1997;40:453–460. doi: 10.1002/art.1780400311. [DOI] [PubMed] [Google Scholar]
- 23.Fitzcharles MA, Rampakakis E, Ste-Marie PA, et al. The association of socioeconomic status and symptom severity in persons with fibromyalgia. J Rheumatol. 2014;41:1398–1404. doi: 10.3899/jrheum.131515. [DOI] [PubMed] [Google Scholar]
- 24.Blyth FM, Macfarlane GJ, Nicholas MK. The contribution of psychosocial factors to the development of chronic pain: the key to better outcomes for patients? Pain. 2007;129:8–11. doi: 10.1016/j.pain.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Hassett AL, Cone JD, Patella SJ, et al. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis and rheumatism. 2000;43:2493–2500. doi: 10.1002/1529-0131(200011)43:11<2493::AID-ANR17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Torres X, Bailles E, Valdes M, et al. Personality does not distinguish people with fibromyalgia but identifies subgroups of patients. General hospital psychiatry. 2013;35:640–648. doi: 10.1016/j.genhosppsych.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Thieme K, Turk DC, Flor H. Responder criteria for operant and cognitive-behavioral treatment of fibromyalgia syndrome. Arthritis Rheum. 2007;57:830–836. doi: 10.1002/art.22778. [DOI] [PubMed] [Google Scholar]
- 28.Thieme K, Turk DC, Gracely RH, et al. The relationship among psychological and psychophysiological characteristics of fibromyalgia patients. J Pain. 2015;16:186–196. doi: 10.1016/j.jpain.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Thieme K, Rose U, Pinkpank T, et al. Psychophysiological responses in patients with fibromyalgia syndrome. J Psychosom Res. 2006;61:671–679. doi: 10.1016/j.jpsychores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Current psychiatry reports. 2013;15:421. doi: 10.1007/s11920-013-0421-0. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12:964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. Journal of behavioral medicine. 2015 doi: 10.1007/s10865-015-9628-3. [DOI] [PubMed] [Google Scholar]
- 33.Bradley LA. Psychiatric comorbidity in fibromyalgia. Current pain and headache reports. 2005;9:79–86. doi: 10.1007/s11916-005-0042-3. [DOI] [PubMed] [Google Scholar]
- 34.Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Current pain and headache reports. 2014;18:436. doi: 10.1007/s11916-014-0436-1. [DOI] [PubMed] [Google Scholar]
- 35.Dreyer L, Kendall S, Danneskiold-Samsoe B, et al. Mortality in a cohort of Danish patients with fibromyalgia: increased frequency of suicide. Arthritis and rheumatism. 2010;62:3101–3108. doi: 10.1002/art.27623. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe F, Hassett AL, Walitt B, et al. Mortality in fibromyalgia: a study of 8,186 patients over thirty-five years. Arthritis care & research. 2011;63:94–101. doi: 10.1002/acr.20301. [DOI] [PubMed] [Google Scholar]
- 37.Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA psychiatry. 2013;70:692–697. doi: 10.1001/jamapsychiatry.2013.908. [DOI] [PubMed] [Google Scholar]
- 38.Racine M, Choiniere M, Nielson WR. Predictors of suicidal ideation in chronic pain patients: an exploratory study. The Clinical journal of pain. 2014;30:371–378. doi: 10.1097/AJP.0b013e31829e9d4d. [DOI] [PubMed] [Google Scholar]
- 39.Bernardy K, Fuber N, Kollner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 40.Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. The Cochrane database of systematic reviews. 2013;9:CD009796. doi: 10.1002/14651858.CD009796.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cash E, Salmon P, Weissbecker I, et al. Mindfulness Meditation Alleviates Fibromyalgia Symptoms in Women: Results of a Randomized Clinical Trial. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2014 doi: 10.1007/s12160-014-9665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500–510. doi: 10.1016/j.jpsychores.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Davis MC, Zautra AJ, Wolf LD, et al. Mindfulness and cognitive-behavioral interventions for chronic pain: differential effects on daily pain reactivity and stress reactivity. Journal of consulting and clinical psychology. 2015;83:24–35. doi: 10.1037/a0038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. The Cochrane database of systematic reviews. 2014;11:CD011142. doi: 10.1002/14651858.CD011142.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavior therapy for insomnia in knee osteoarthritis: A double-blind, randomized, active placebo controlled clinical trial. Arthritis & rheumatology (Hoboken, NJ) 2015 doi: 10.1002/art.39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buhrman M, Syk M, Burvall O, et al. Individualized Guided Internet-delivered Cognitive Behaviour Therapy for Chronic Pain Patients with Comorbid Depression and Anxiety: A Randomized Controlled Trial. The Clinical journal of pain. 2014 doi: 10.1097/AJP.0000000000000176. [DOI] [PubMed]
- 47.Dowd H, Hogan MJ, McGuire BE, et al. Comparison of an Online Mindfulness-based Cognitive Therapy Intervention with Online Pain Management Psychoeducation: A Randomized Controlled Study. The Clinical journal of pain. 2015 doi: 10.1097/AJP.0000000000000201. [DOI] [PubMed] [Google Scholar]