Abstract
Microsomal prostaglandin E synthase-1 (mPGES-1) is an inducible enzyme that specifically catalyzes the conversion of prostaglandin (PG)H2 to PGE2. We showed that mPGES-1 null mice had a significantly reduced incidence and severity of collagen-induced arthritis (CIA) compared to wild-type (WT) mice associated with a marked reduction in antibodies to type II collagen. In the present study, we further elucidated the role of mPGES-1 in the humoral immune response. Basal levels of serum IgM and IgG were significantly reduced in mPGES-1 null mice. Compared with WT mice, mPGES-1 null mice exhibited a significant reduction of hapten-specific serum antibodies in response to immunization with the T-cell dependent antigen DNP-KLH. Immunization with the T-cell independent type-1 antigen TNP-LPS or the T-cell independent type-2 antigen DNP-Ficoll revealed minimal differences between strains. Germinal center formation in the spleens of mPGES-1 null and WT mice were similar after immunization with DNP-KLH. To determine if the effect of mPGES-1 and PGE2 was localized to hematopoietic or non-hematopoietic cells, we generated bone marrow chimeras. We demonstrated that mPGES-1 deficiency in non-hematopoietic cells was the critical factor for reduced T-cell dependent antibody production. We conclude that mPGES-1 and PGE2-dependent phenotypic changes of non-hematopoietic/mesenchymal stromal cells play a key role in T-cell dependent humoral immune responses in vivo. These findings may have relevance to the pathogenesis of rheumatoid arthritis and other autoimmune inflammatory diseases associated with autoantibody formation.
Keywords: autoantibodies, lipid mediators, prostaglandin
Introduction
Prostaglandin (PG) E2 is a ubiquitous mediator of many physiologic and pathologic functions whose production is regulated by expression and activity of its biosynthetic enzymes, most notably microsomal prostaglandin E synthase-1 (mPGES1) (1). PGE2 exerts its actions via four different G protein-coupled receptor subtypes, EP 1–4, by triggering their respective downstream signaling cascades (2). PGE2 is the most prominent PG in chronic inflammatory disorders including rheumatoid arthritis (3), influencing both innate and acquired immunity. In general, PGE2 suppresses the functions of neutrophils and macrophages while exerting stimulatory effects on stromal and vascular endothelial cells under stimulated conditions. Additionally, PGE2 has important modulatory effects on lymphocytes that are highly dependent on its concentration and on the cytokine/growth factor milieu (4–6). PGE2 has been shown to alter the balance of Th1/Th2 subsets of T cells and differentially modulate the production of cytokines from these subsets (7, 8). Recent studies have also shown that PGE2 facilitates expansion of the Th17 subset of T helper cells via its specific receptor subtypes (9, 10).
mPGES-1 is a specific, highly inducible, biosynthetic enzyme for PGE2 that acts downstream of COX (11, 12). mPGES-1 null mice have been generated and provide insight into the role of mPGES-1 in a number of different disease states (13–18). Studies using mPGES-1 null mice have demonstrated that this enzyme is a key mediator of many physiological and pathophysiological events including inflammation, pain, stress, angiogenesis, fever, bone metabolism, tumorigenesis, atherosclerosis and reproduction (19–27). We recently reported that resistance to bovine type II collagen (CII) induced arthritis in mPGES-1 deficient mice is associated with a failure to develop a CII-specific antibodies, suggesting an important role of mPGES-1 and its derived PGE2 in the development of acquired immune response (28). However, the mechanisms underlying the impaired humoral immune response under mPGES-1 deficiency remain to be fully understood.
Humoral immunity is a critical event for autoimmune disease as well as in normal host defenses. The humoral immune responses can be divided into T-cell dependent (thymus dependent: TD) and T-cell independent (thymus independent: TI) responses, according to their requirement of T-cells for generation of antibodies. In TI immunity, the antibody response occurs directly after B-cell activation followed by binding of antigens to toll-like receptors or the B-cell receptor (29). Based on their ability to stimulate an immune-deficient strain of mice (CBA/N) to produce antibodies, TI antigens are further divided into two types, TI type 1 (TI-1) and type-2 (TI-2) (30–32). In contrast to TI responses, TD responses require T-cell-mediated B-cell activation and maturation, including MHC-restricted presentation of antigen to T-cells by antigen presenting cells and T-cell/B-cell interaction that occur in secondary lymphoid organs. A series of complex events such as CD40-CD40L interaction and B-cell receptor signaling are essential for B-cell activation and differentiation into antibody-producing cells in TD humoral immune responses (33, 34). These events are supported in vivo by formation of germinal centers and interactions with mesenchymal stromal cells (35, 36).
To characterize the functional significance of mPGES-1 during antibody response under exposure to antigens in vivo, we assessed humoral responses to TD, TI-1 and TI-2 antigens in mPGES-1 null mice. We report here that genetic deletion of mPGES-1 results in a dramatic reduction of antibody production in response to a TD antigen. At the same time, mPGES-1 deletion has a minimal impact in TI responses. By utilizing a radiation bone marrow (BM) transplantation chimera strategy, we demonstrate clearly that the reduction of TD antibody production seen in mPGES-1 null mice is a result of deficient mPGES-1 expression in non-hematopoietic/mesenchymal rather than in hematopoietic cells. These results indicate a significant role for mPGES-1 and its derived PGE2 in B-cell maturation and antibody production in vivo, specifically for T-cell-dependent humoral immunity. Our findings provide novel insights relevant to the therapeutic potential for pharmacologic inhibition of mPGES-1 in autoimmune inflammatory diseases by targeting TD immune responses.
Materials and Methods
Mice
mPGES-1 heterozygous(Het) male and female mice on a DBA1 lac/J background were provided from Pfizer (13). mPGES-1 Het mice were mated to generate mPGES-1 null, Het and littermate wild-type (WT) mice, and genotypes were identified by PCR, as previously reported (28). Mice were housed in microisolator cages in a specific pathogen-free (SPF) barrier facility, and all experiments were performed under the IACUC guidelines as set forth by the University of Kentucky. Male and female mice used in this study were 8–10 weeks old.
Measurement of total IgG and IgM in serum
Serum samples were collected as previously described (37). The levels of total IgG and IgM in serum were assessed by ELISA according to the manufacturer’s protocol (Bethyl Laboratories). Briefly, 96 well plates were coated with goat anti-mouse IgG or goat anti-mouse-IgM. After blocking, plates were incubated overnight at 4 °C with diluted serum samples. After washing, horse radish peroxidase (HRP)-conjugated antibody was added for 1 hr at room temperature. After further washing, the color was developed with TMB, terminated by 2M H2SO4, and then measured at 450 nm using a plate reader (BioRad).
Immunization protocols for T-cell dependent and T-cell independent humoral immune responses
To analyze T-cell dependent humoral immune responses, mice were intraperitoneally immunized with 100 μg of a dinitrophenyl-keyhole limpet hemocyanin (DNP-KLH) in complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis H37 RA and are boosted with DNP-KLH in incomplete Freund’s adjuvant (IFA) at 21 days after the first immunization. To examine T-cell independent type1 (TI-1) and type 2 (TI-2) humoral responses, mice are intraperitoneally immunized with 50 μg of trinitrophenyl-lipopolysaccharide (TNP-LPS: E. coli. O111:B4: a TI-1 antigen) or 250 μg of DNP-Ficoll (a TI-2 antigen) in saline.
Measurement of DNP- and TNP-specific antibodies
Serum samples were collected as previously described (37). The levels of anti-DNP and anti-TNP antibodies in serum were assessed by ELISA. Briefly, 96 well plates were coated with 10 μg/ml of a DNP-BSA (Biosearch Technologies) or a TNP-BSA (Biosearch Technologies) overnight at 4°C. After blocking with 50 mM TBS (pH 8.0) containing 1% BSA, serum samples were added and incubated overnight at 4°C. After washing, HRP-conjugated antibody (Bethyl Laboratories) was added for 1 hr at room temperature. After further washing, the color was developed with tetramethyl benzidine (TMB), terminated by 2M H2SO4, and then measured at 450 nm using a plate reader (BioRad). Titer of the pooled standard from a WT mouse was defined as 1000 units/ml.
Western blot analysis
Splenocytes were lysed in Tris-buffered saline (TBS) containing 0.1 % sodium dodecyl sulfate (SDS). After determined protein content by BCA protein assay reagent with bovine serum albumin as standard, cell lysates adjusted to equal equivalents of protein (100 μg) were applied to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for electrophoresis and then the proteins were electroblotted onto PVDF membrane. After blocking the membranes in 10 mM TBS containing 0.1 % Tween-20 (TBS-T) containing 5 % skim milk, the membranes were probed with the respective antibodies [1:500 for COX-1 (Cayman Chemicals; #160109), COX-2 (Cayman Chemicals; #160106), mPGES-1 (Cayman Chemicals; #160140), mPGES-2 (Cayman Chemicals; #160145) and hPGDS (Cayman Chemicals; #10004348); 1:1000 for HPRT (Santa Cruz; sc-20975)] in TBS-T. After washing the membranes with TBS-T, the membranes were incubated with HRP-conjugated secondary antibody (Jackson ImmunoResearch; 1:10,000 dilution in TBS-T containing 5% skim milk) for overnight at 4°C. After further washing with TBS-T, protein bands were visualized with an ECL Western blot analysis system using a Chemidoc Apparatus (BioRad).
Measurement of prostaglandins and cytokines
Spleen was isolated 10 days after immunization with DNP-KLH in CFA. Single cell suspensions were prepared in complete RPMI-1640. Splenocytes (1.5 × 106/0.3 ml) were cultured in 12 well plates for 1, 2, 4 and 6 days with complete RPMI-1640 in the absence or presence of 10 μg/ml of DNP-KLH. The levels of PGE2 and PGD2 in culture medium were measured by ELISA (Cayman Chemicals) according to the manufacturer’s protocol. The concentration of IL-4, IL-2, IL-17 and IFNγ in culture medium was measured by an ELISA with using capture and detection antibody sets (BD Biosciences).
Proliferative response of splenocytes
Proliferation of splenocytes was assessed by 5-bromo-2′-deoxyuridine (BrdU) cell proliferation assay kit (Roche Diagnostics) according to the manufacturer’s protocol. Briefly, spleen was isolated at 10 day after immunization with DNP-KLH in CFA. Splenocytes (1.25 × 105 cells / 0.1 ml) were cultured in flat bottom 96 well plates with or without 10 μg/ml of DNP-KLH for 1, 2, 3, 4 and 5 days, and then further incubated with BrdU for 18 hrs. Proliferative activity was estimated from the nuclear incorporation of BrdU as measured by ELISA.
Real-Time RT-PCR
Total RNA was isolated from the spleen as well as superficial, axillary, and inguinal lymph nodes of the wild type and mPGES-1 knockout mice using TRIzol reagent (Ambion) following the manufacturer instructions. Total RNA was quantitatively converted into single stranded cDNA by using High Capacity cDNA Archive Kit (Applied Biosystems). The particular genes were detected using the respective TaqMan Gene Expression Assays (Applied Biosystems) on the 7300 Real-Time RT-PCR system from the same manufacturer by relative quantitation employing G3PDH as the reference gene.
Germinal center staining
Spleens from immunized mice were embedded in OCT compound, snap-frozen and stored at −80°C. Cryostat sections (6 μm) were fixed in acetone and stained with peanut agglutinin (PNA: Vector Laboratories), antibody to B-cells, B220/CD45R (eBioscience) and IgD (Southern Biotechnology), follicular dendritic cells, FDC; CR1/CD35 (BD Pharmingen) and T-cells, Thy1.2/CD90.2 (BD Pharmingen) followed by incubation with alkaline phosphatase (AP)-streptavidin and HRP-conjugated secondary antibody. Color development for bound AP and HRP was performed using a Blue AP Substrate Kit (Vector Laboratories) and 3,3′-Diaminobenzidine (DAB) with an Enhance Orange Buffer Solution (KPL Inc.).
Bone marrow transplantation (BMT)
Recipient female mice (9–10 weeks age) were irradiated twice (450 rads each dose, 4 hours apart). Donor male mice (9–10 weeks of age) were euthanatized and bone marrow was harvested from the femurs and tibias. Bone marrow cells (10 × 106 cells/100 μl) in 2% FBS RPMI 1640 were injected into the tail vein of recipient mice. The mice were allowed to recover for 5 weeks and then used for experiments. To prevent mice from infection after bone marrow transplantation (BMT), recipient mice were treated with antibiotic water including 160 μg/ml sulfamethoxazole and 35 μg/ml trimethoprim (Sulfatrim pediatric suspension; Alpharma USPD Inc) for a week before irradiation and for 4 weeks after. Complete blood counts were performed by the Hemavet 950FS System (Drew Scientific Inc). The number of leukocytes and erythrocytes in peripheral blood of BM chimeras was within the normal range.
Efficiency of bone marrow transplant monitored by FISH
In order to evaluate the efficacy of BMT, BM cells were cultured overnight in complete RPMI medium. Cells were further incubated for an additional 40 min in the presence of 0.1 ug/ml colcemid (Invitrogen/Gibco, Carlsbad, CA). Red blood cells were disrupted in 0.075 M KCl hypotonic solution, and then fixed in methanol/acetic acid solution (3:1 ratio). For each sample, a drop of this solution was placed on a microscope slide and allowed to dry at r.t., then dehydrated with ethanol. Slides were denatured at 65 °C in denaturing solution and then quenched in 70% ice-cold ethanol and dehydrated again in ethanol. Cells were then hybridized to Cy3-labeled mouse Y-chromosome paint in hybridization solution (Cambio), under cover slips, overnight, at 37 °C. Slides were then washed in stringent conditions (50% formamide and 0.5 X SSC) at 45 °C for 5 min. Slides were then washed in 1X SSC at 45 °C for 5 min (2 times). After incubating in detergent solution (4 X SSC, 0.05% Tween-20) for 4 minutes at 45 °C (3 times), slides were mounted with DAPI counterstain and examined using the Axio Observer D1 microscope connected to the AxioCam MRm digital camera (Carl Zeiss).
Statistical analysis
Data are expressed as the means ± SEM. Statistical analysis was performed with the Sigmastat 3.5 software (Systat Software). The comparison of more than two groups was analyzed with one-way ANOVA by Turkey’s multiple comparison test. For comparison of two groups, Student’s t-test was performed. P <0.05 was considered statistically significant.
Results
Effect of mPGES-1 deficiency on basal levels of immunoglobulin in vivo and antibody production in vitro
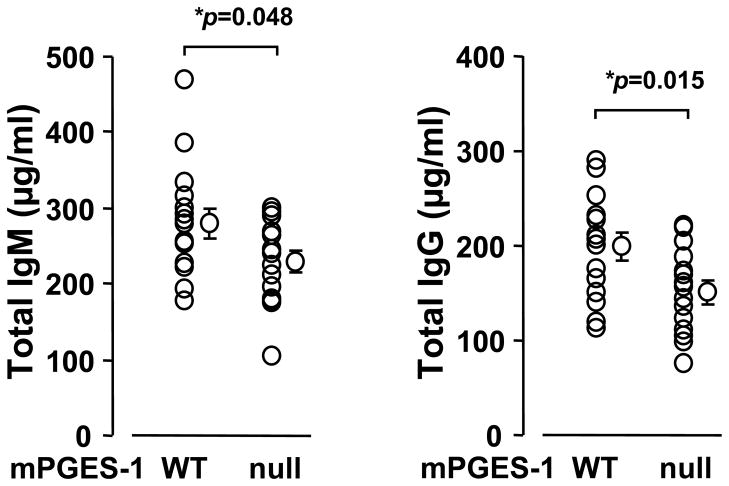
We examined whether genetic deletion of mPGES-1 affected basal antibody production by comparing IgG and IgM levels in serum from peripheral blood of mPGES-1 null and WT mice exposed to the same diet and environment. As shown in Figure 1, serum immunoglobulin levels of unimmunized mPGES-1 null mice exhibited significantly lower levels of both total IgM and IgG in serum compared to WT mice, although the levels may vary considerably between individual mice. This result indicates the importance of mPGES-1 in immunoglobulin generation under the basal condition in vivo.
Fig. 1. Effect of mPGES-1 gene deletion on the basal levels of total IgM and total IgG in vivo.
Basal levels of total IgM and IgG in serum of mPGES-1 null (n=15) and WT (n=15) mice were measured by an ELISA. Data are expressed as the mean ± SEM. * indicates significance at P<0.05 in mPGES-1 null versus WT mice.
Antibody response to a T-cell dependent antigen in mPGES-1 null mice
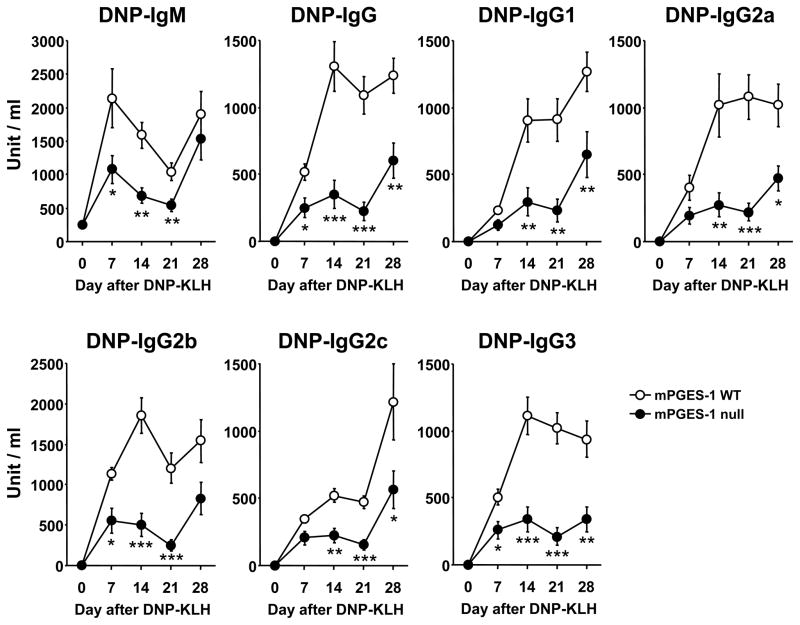
We next examined primary and secondary hapten-specific antibody production in mPGES-1 null and WT mice immunized with soluble hapten-protein TD antigen, DNP-KLH in CFA. As shown in Figure 2, the levels of hapten-specific IgM were significantly lower in mPGES-1 null mice compared to WT mice, on days 7, 14 and 21 after a primary immunization, but not after a secondary immunization. Hapten-specific IgG production was also significantly lower in mPGES-1 null mice, even after a secondary immunization. In addition, the levels of hapten-specific IgG subclasses IgG1, IgG2a, IgG2b, IgG2c and IgG3 were all significantly lower in mPGES-1 null mice. These data demonstrate the enhancing property of mPGES-1 in the TD humoral immune response.
Fig. 2. Humoral immune responses to a T-cell dependent (TD) antigen in mPGES-1 null mice.
mPGES-1 WT (n=5) and null (n=8) mice were injected i.p. with T-cell dependent (TD) antigen DNP-KLH in CFA on day 0 and bled on the indicated days. All mice were challenged with antigen in IFA on day 21. Titers of DNP-specific IgM, IgG and IgG subclass in serum were measured by ELISA. Data are expressed as the mean ± SEM in arbitrary units. *, ** and *** indicate significance at P<0.05, P<0.01 and P<0.001, respectively. Pooled serum collected from WT mice at day 28 was used for the standard curve. The titer of a pooled standard was defined as 1000 units/ml.
Antibody response to T-cell independent type 1 and T-cell independent type 2 antigens in mPGES-1 null mice
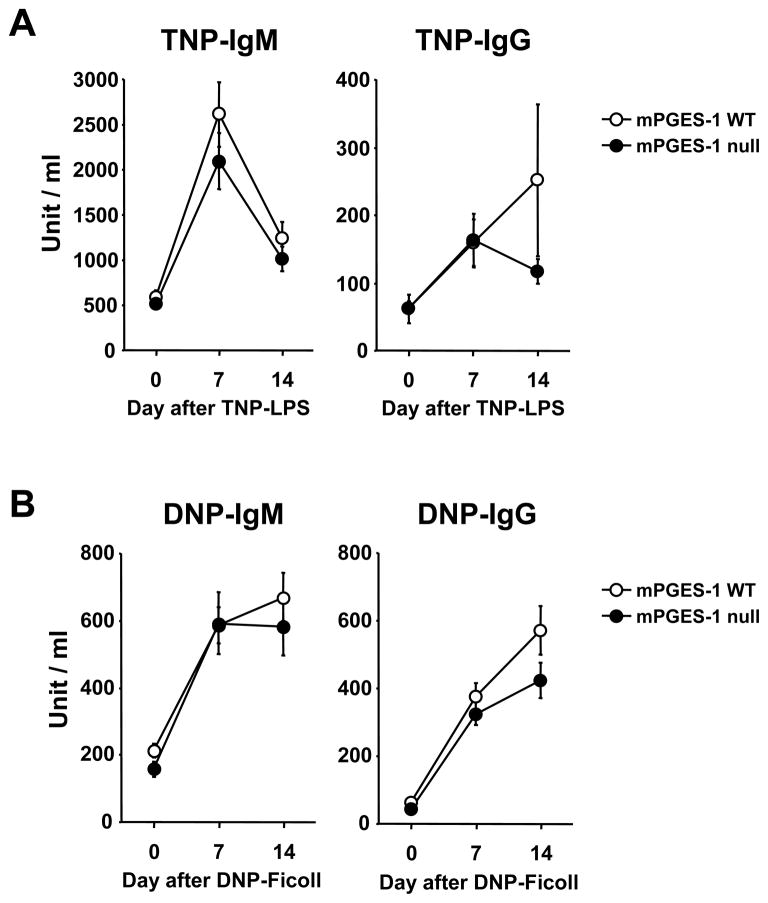
We also determined the effect of mPGES-1 genetic deletion on TI humoral responses including TI-1 and TI-2. As shown in Figure 3, no significant changes in hapten-specific IgM or IgG were identified after immunization with either TNP-LPS (TI-1) or DNP-Ficoll (TI-2). IgG subclasses were also not different between mPGES-1 WT and null mice immunized with TNP-LPS (data not shown). Statistically lower levels of IgG2a and IgG2c were observed in mPGES-1 null when compared with WT mice responses after immunization with DNP-Ficoll (data not shown). These data indicate that mPGES-1 is not a critical factor for the TI-1 humoral immune response and that there was only a minor effect on the TI-2 humoral immune response.
Fig. 3. Humoral immune responses to a T-cell independent type1 (TI-1) and a type2 (TI-2) antigen in mPGES-1 null mice.
(A) mPGES-1 WT (n=8) and null (n=8) mice were injected i.p. with T-cell independent type1 (TI-1) antigen TNP-LPS on day 0 and bled on the indicated days. Titers of TNP-specific IgM and IgG in serum were measured by ELISA. (B) mPGES-1 WT (n=11) and null (n=8) mice were injected i.p. with T-cell independent type2 (TI-2) antigen DNP-Ficoll on day 0 and bled on the indicated days. Titers of DNP-specific IgM and IgG in serum were measured by ELISA. Data are expressed as the mean ± SEM in arbitrary units. Pooled serum collected from WT mice at day 14 was used for the standard curve. The titer of the pooled standard was defined as 1000 units/ml.
Expression of COX, PGES and PGDS in response to a T-cell dependent antigen in splenocytes
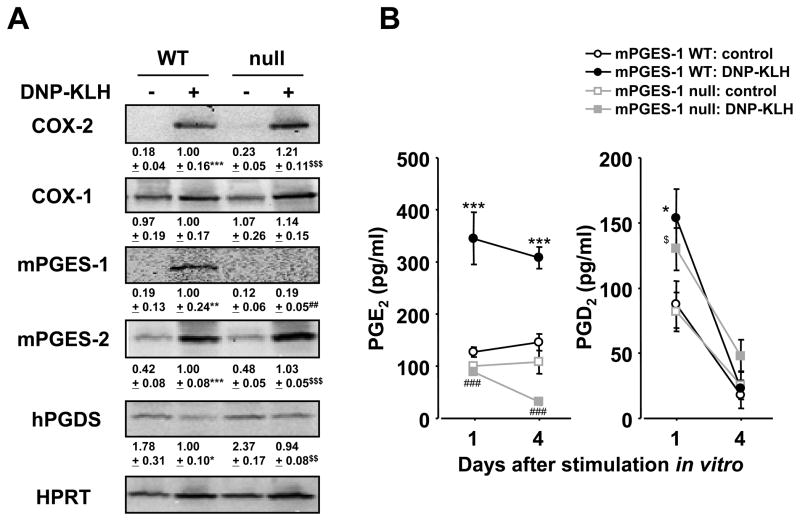
Since we observed marked reduction of TD humoral response in mPGES-1 null mice, we examined the protein expression of PG biosynthetic enzymes in WT and mPGES-1 null splenocytes isolated from mice immunized with DNP-KLH in CFA, followed by DNP-KLH stimulation in vitro (Figure 4A). COX-2 protein was not expressed basally, but significantly increased to a similar degree in both WT and null cells after DNP-KLH stimulation in vitro. COX-1 protein was present constitutively in both WT and null cells and its expression was not changed after stimulation with DNP-KLH. WT cells did not basally express mPGES-1 protein, but upregulated expression in the presence of DNP-KLH. As expected, mPGES-1 null cells did not express mPGES-1 protein, either with or without stimulation. Interestingly, mPGES-2, an isozyme of PGE synthase, had a low level of basal expression in WT and mPGES-1 null cells and was significantly upregulated in response to treatment with DNP-KLH. Hematopoietic PGD synthase (hPGDS) protein, on the other hand, was present constitutively in both WT and null cells and its expression did not change after stimulation with DNP-KLH. These data demonstrate that genetic deletion of mPGES-1 had no measurable effect on the expression of COXs and other PG terminal synthases in splenocytes and that those enzymes responded similarly to DNP-KLH stimulation in WT and mPGES-1 null cells.
Fig. 4. Production of prostaglandins and expression of prostaglandin synthetic enzymes in cultured splenocytes with mPGES-1 deficiency.
Splenocytes were isolated from mPGES-1 WT and null mice at day 10 post immunization with DNP-KLH in CFA. (A) Protein expression of COX-1, COX-2, mPGES-1, hPGDS and HPRT in splenocytes cultured with or without DNP-KLH (10 μg/ml) for day 4 was determined by Western blotting. Results are representative examples from 4 mice. Levels of protein expression were normalized against HPRT; data are shown as the fold induction relative to WT DNP-KLH (assigned the value “1”) and are the mean ± SEM from 4 mice. Single, double and triple characters indicate significance at P<0.05, P<0.01 and P<0.001, respectively. *, WT control versus WT DNP-KLH; #, WT DNP-KLH versus null DNP-KLH; $, null control versus null DNP-KLH. (B) Cells were cultured with or without DNP-KLH (10 μg/ml) for indicated days. The levels of PGE2 and PGD2 in culture medium were measured by ELISA. Data are expressed as mean ± SEM (n=7). Single, double and triple characters indicate significance at P<0.05, P<0.01 and P<0.001, respectively. *, WT control versus WT DNP-KLH; #, WT DNP-KLH versus null DNP-KLH; $, null control versus null DNP-KLH.
Production of PG and T-cell cytokines in response to a T-cell dependent antigen in splenocytes
Since both PG and cytokines are known to be important mediators of humoral immunity, we probed their basal and DNP-KLH-stimulated production in WT and mPGES-1 null animals. As expected, the PGE2 level in the spleens of mPGES-1 null mice was significantly lower than that of WT mice at baseline and after immunization with TD (days 14 and 28) or TI (day 14) (Table 1). In cultured splenocytes isolated from mice immunized by DNP-KLH, DNP-KLH stimulation in vitro resulted in a significant increase in PGE2 production by WT, but not mPGES-1 null cells despite upregulated expression of mPGES-2 (Figure 4B). On the other hand, WT and mPGES-1 deficient splenocytes displayed similar baseline levels of PGD2 that increased significantly after DNP-KLH stimulation, but no significant differences were seen between groups. Taken together, these data indicate that mPGES-1 is the synthase that mediates the production of PGE2 in spleen at baseline and after stimulation.
Table 1.
PGE2 production in spleen of mPGES-1 null and WT mice
| Immunization | PGE2 (ng/mg protein)
|
|
|---|---|---|
| WT | nul | |
| DNP-KLH (day 14) | 5.14 ± 1.45 | 0.34 ± 0.08** |
| DNP-KLH (day 28) | 9.13 ± 1.94 | 0.61 ± 0.08*** |
| TNP-LPS (day 14) | 6.72 ± 0.70 | 0.60 ± 0.08*** |
| DNP-Ficoll (day 14) | 8.18 ± 0.27 | 0.74 ± 0.07*** |
mPGES-1 WT and null mice were injected i.p. with T-cell dependent (TD) antigen DNP-KLH in CFA, T-cell independent type1 (TI-1) antigen TNP-LPS or T-cell independent type2 (TI-2) antigen DNP-Ficoll on day 0. Spleen was isolated at the indicated time point and the levels of PGE2 were determined by ELISA. Data are expressed as the mean + SEM (n=4–11).
indicate statistical significance at P<0.01 and P<0.001, versus WT mice.
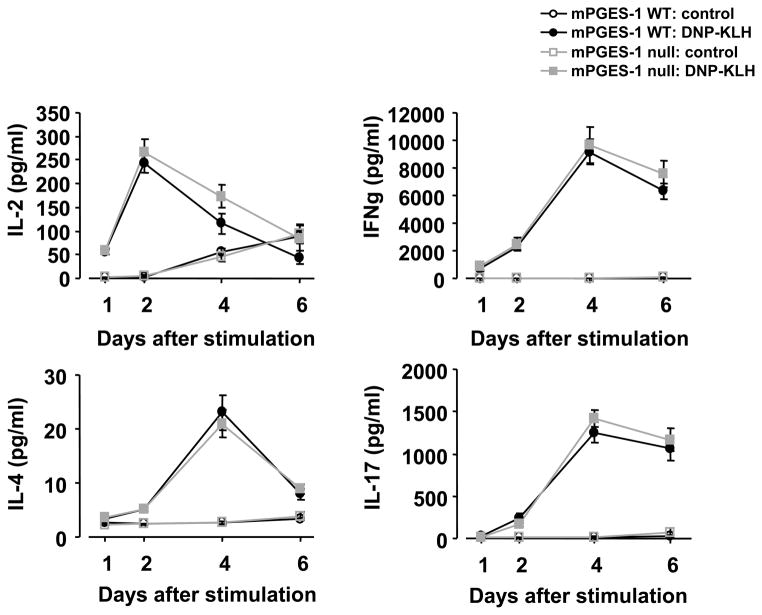
To determine whether mPGES-1 null mice display a perturbed profile of T-cell cytokines, we measured interferon-γ (IFNγ), interleukin (IL)-2, IL-4 and IL-17 in cultured splenocytes isolated from mice immunized by DNP-KLH, followed by stimulation by DNP-KLH in vitro. The levels of each cytokine were increased in mPGES-1 null splenocytes by DNP-KLH although the respective levels were not different from those of WT splenocytes (Figure 5). In addition, both WT and mPGES-1 null cells exhibited similar proliferative response in exposure to DNP-KLH [unstimulated WT splenocytes: 0.15 ± 0.06 (n=4), DNP-KLH stimulated WT splenocytes: 1.31 ± 0.09 (n=4), unstimulated mPGES-1 null splenocytes: 0.19 ± 0.08 (n=4), DNP-KLH stimulated mPGES-1 null splenocytes: 1.00 ± 0.24 (n=4) (optical density at 450 to 655 nm: 5 days post stimulation in vitro)].
Fig. 5. Effect of mPGES-1 genetic deletion on Th1, Th2 and Th17 cytokines in splenocytes in response to a TD antigen.
Splenocytes were isolated from mPGES-1 WT (n=13) and null (n=11) mice at day 10 post immunization with DNP-KLH in CFA. The cells were cultured for 1, 2, 4 and 6 days in the presence or absence of DNP-KLH (10 μg/ml) in vitro. The concentration of IL-2, IFNγ, IL-4 and IL-17 in culture medium was measured by ELISA. Data are expressed as the mean ± SEM.
Germinal center formation in response to a T-cell dependent antigen in mPGES-1 null mice
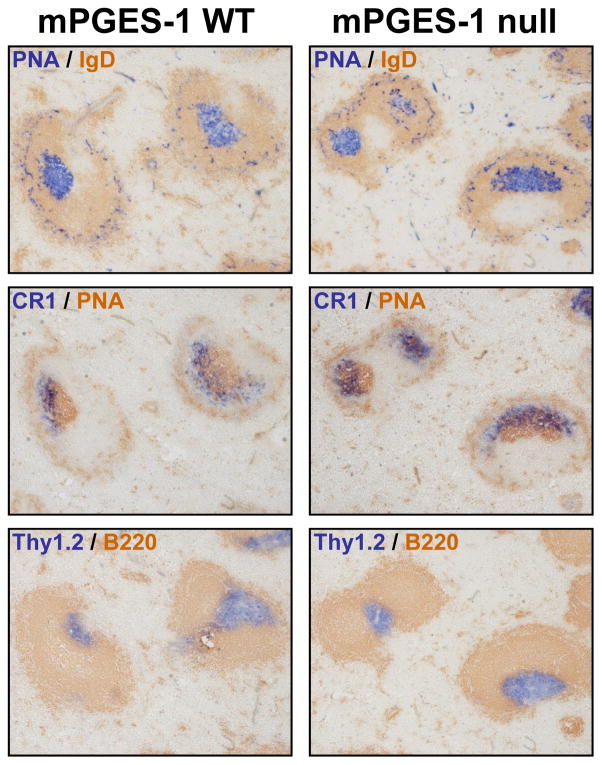
In normal TD responses, germinal centers (GC) are formed, and GC B-cells can be identified by their reactivity with PNA and the lack of surface IgD several days after an immunization (38–40). In contrast, TI response do not generate detectable GC (41). We probed the GC formation in the WT and mPGES-1 KO mice by immunohistochemistry (Figure 6). mPGES-1 null mice demonstrated the appearance of PNA and CR1 (follicular dendritic cell marker) characteristic of normal GC structures and were phenotypically similar to WT mice in response to TD antigen. In addition, there was no difference in the numbers of CD11c+CR1+ cells in mPGES-1 null and WT mice in our study [WT: 9.7 ± 0.7 % (n=4) vs. null: 8.6 ± 1.8 % (n=3)]. The appearance of Thy1.2 positive T-cell and B220 positive B-cell regions were also normal in mPGES-1 null and WT spleens. In addition, there was no difference in the frequency of GC formation between mPGES-1 null and WT mice (Table 2). These results indicate that the formation of GC in response to TD antigen is not impaired in mPGES-1 null mice.
Fig. 6. Germinal center formation in spleen post immunization with a TD antigen in mPGES-1 WT and null mice.
mPGES-1 WT and null mice were injected i.p. with TD antigen DNP-KLH in CFA on day 0 and challenged with antigen in IFA on day 21. On day 28, spleen cryosections were stained with both PNA and anti-IgD (top panel), with both anti-CR1 and PNA (middle panel), or with both Thy1.2 and B220 (bottom panel). Representative results from 4 mice are shown. Magnification: x10.
Table 2.
Germinal-center formation in mPGES-1 null and WT mice
| Immunization | Frequency (%)
|
|
|---|---|---|
| WT | null | |
| Non- Immunization | 3.0 ± 1.4 | 3.7 ±2.4 |
| DNP-KLH (day 28) | 43.5 ± 1.8 | 40.8 ± 2.1 |
mPGES-1 WT and null mice were injected i.p. with T-cell dependent (TD) antigen DNP-KLH in CFA on day 0 and challenged with antigen in IFA on day 21. Spleens from mice of mPGES-1 null and WT mice on day 28 were assessed for frequency (%) of PNA positive germinal centers to IgD positive regions. Values represent the mean percentage ± SEM (n=3–4). Differences between mPGES-1 null mice and WT littermates were not significant (Student’s t-test)
Efficiency of BMT in mPGES-1 null mice
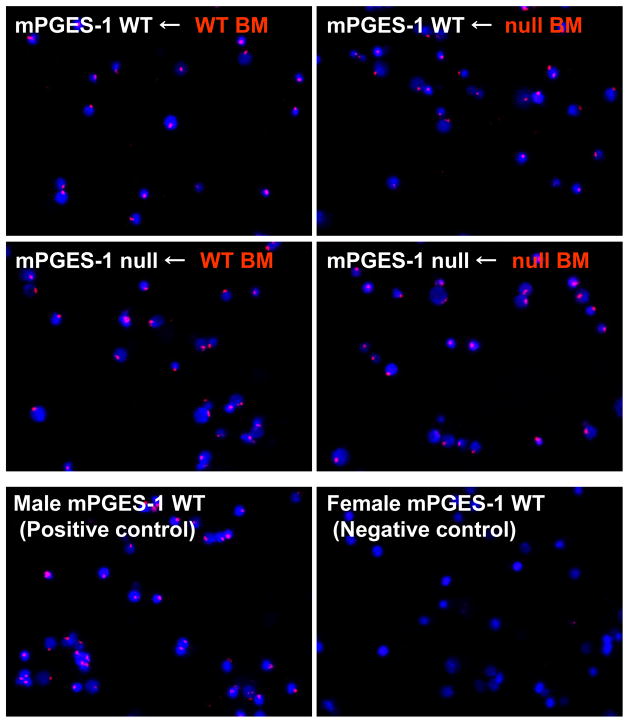
The role of non-hematopoietic/mesenchymal versus hematopoietic cell mPGES-1 deficiency in TD response was examined using a radiation bone marrow (BM) transplantation chimera strategy. Chimeric mice were generated using a protocol where irradiated female recipient WT and mPGES-1 null mice were reconstituted with BM cells from male donor WT or mPGES-1 null mice. This protocol enabled us to monitor the efficiency of BM transplantation by detecting donor-derived Y-chromosome positive population in BM cells of chimeras. As shown in Figure 7, Y-chromosomes (red) in the most BM cells isolated from chimeras were stained along with DAPI nucleus staining (blue), while a negative control was not. Quantitative analysis revealed that approximately 95% of BM cells in each group of chimeras were Cy3-labeled Y chromosome probe positive (Table 3). There was no significant difference in the percentage of cells with Cy3-labeled Y chromosome probe positive among the chimera groups, which was almost identical to a male mouse used as a positive control (95.9 ± 0.6 %). These data indicate complete transplantation and reconstruction of BM in our study, but also demonstrate no impact of mPGES-1 deficiency in reconstruction of BM after transplantation.
Fig. 7. Efficiency of bone marrow transplant monitored by FISH.
Bone marrow (BM) chimeras were generated by reconstituting lethally irradiated recipient female mice with 1 × 107 BM cells from donor male mice. At the end point of experiment (on day 14 post immunization with DNP-KLH in CFA), bone marrow (BM) cells were harvested to check the efficacy of chimerism by FISH with Cy3 labeled Y chromosome paint (Y chromosome: red) and DAPI (nuclei: blue) staining. mPGES-1 WT Male and female mice without bone marrow transplantation were also used as a positive and negative control for the staining, respectively. Results are representative data from 6–7 mice in each group of BM chimeras and 4 mice in positive and negative control.
Table 3.
Efficiency of bone marrow transplant monitored by FISH
| Chimeras | Frequency (%) |
|---|---|
| WT ← WT BM | 95.4 ± 0.8 |
| WT ← null BM | 95.5 ± 0.8 |
| null ← WT BM | 94.8 ± 1.1 |
| null ← null BM | 93.8 ± 1.9 |
| WT male (positive control) | 95.9 ± 0.6 |
| WT Female (negative control) | 2.5 ± 0.9 |
Efficiency of bone marrow transplant were assessed for frequency (%) of Y chromosome paint positive cells to DAPI positive cells by FISH. Data are expressed as mean ± SEM from 6–7 mice in each group of BM chimeras and 4 mice in positive and negative control. Data were analyzed by 3 observers.
Antibody response to a T-cell dependent antigen in bone marrow chimeras of mPGES-1
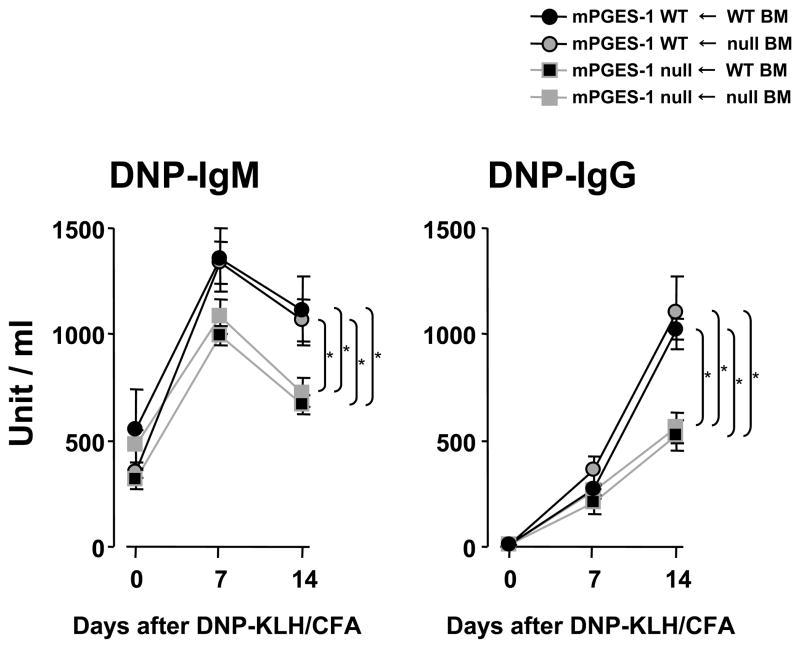
Chimeras of WT mice with mPGES-1 null BM showed a marked increase of hapten-specific antibody production in response to immunization with a TD antigen that was similar to the response in WT mice transplanted with WT BM. However, chimeras of mPGES-1 null mice with WT BM exhibited deficient hapten-specific antibody production after immunization with the TD antigen similar to mPGES-1 null mice transplanted with mPGES-1 null BM. These data indicated that mPGES-1 deficiency in mesenchymal cells rather than in BM-derived hematopoietic cells led to the reduction of the humoral immune response against DNP-KLH (Figure 8).
Fig. 8. Detection of DNP-specific IgG and IgM in bone marrow chimeras.
Mixed chimeras were generated by reconstituting lethally irradiated female recipient mice with 1 × 107 bone marrow (BM) cells from donor male mice. At 5 weeks after BM transplantation, chimeric mice were immunized with DNP-KLH in CFA (i.p.) on days 0 and serum was collected at the indicated time points. DNP-specific IgG and IgM were assayed by ELISA. Data are expressed as mean ± SEM (n=6–7). The titer of the pooled standard was defined as 1000 units/ml. * indicates significance at P<0.05.
Mesenchymal stromal cell cytokines in response to a T-cell dependent antigen in splenocytes
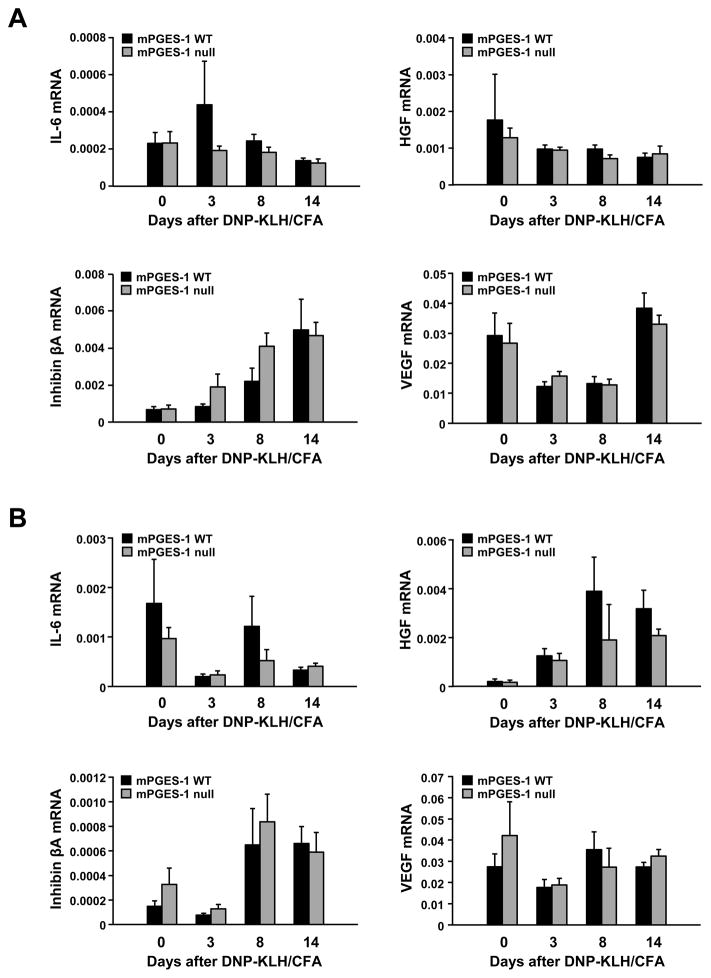
We examined expression of cytokines whose expression is known to be influenced by PGE2 and that support B-cell maturation in spleen (Figure 9A) and lymph nodes (Figure 9B) at day 0 and at days 3, 8, and 14 after immunization with DNP-KLH. There were no statistical differences between WT and mPGES-1 null mice in expression of mRNA for IL-6, HGF, inhibin βA, or VEGF in either spleen or lymph node over the time course examined.
Fig. 9. Effect of mPGES-1 genetic deletion on mesenchymal stromal cell cytokines mRNA expression in response to a TD antigen.
Spleen (A) or lymph nodes (B) were harvested from unimmunized (day 0) or at days 3, 8, or 14 following immunization with DNP-KLH in CFA. mRNA expression for IL-6, HGF, inhibin βA, and VEGF were determined by TaqMan real time PCR. Data are expressed relative to GAPDH and represent the mean ± SEM (n=6–8).
Discussion
The present study using a mouse model of mPGES-1 deficiency demonstrates a significant role for mPGES-1 in TD, but not TI humoral immune responses. Genetic deletion of mPGES-1 results in marked reduction in hapten-specific antibody production induced by a TD antigen. Further, we demonstrate for the first time that mPGES-1 expression in non-hematopoietic/mesenchymal cells rather than in BM-derived hematopoietic cells is required to promote the TD humoral immune response whereas mPGES-1 is not required for normal antibody production in directly stimulated B-lymphocytes.
We demonstrated for the first time that mPGES-1 genetic deletion resulted in the significantly lower basal levels of both IgG and IgM in serum from peripheral blood. These mice were ages 8–10 weeks and the relatively modest differences could be attributed to delayed maturation. Also possible is that the differences would be greater in mice with fully adult IgG levels. To date, there is no report showing the impact of other relevant enzymes within the PGE2 biosynthetic pathway including COX and cPLA2 in the basal levels of IgG and IgM in vivo. We previously reported that in vitro stimulation with LPS of splenocytes from mPGES- null mice compared with WT mice resulted in very similar levels of total IgM and IgG secretion, despite the fact that PGE2 production was abolished in mPGES-1 null mice (28). These data demonstrate that in the setting of mPGES-1 and consequently PGE2 deficiency, B-cells are not intrinsically deficient in their ability to synthesize and secrete antibodies.
The findings reported here are strongly supportive of our previous report which showed a significant reduction in serum anti-CII antibody production in mPGES-1 null mice associated with reduced arthritis in the CIA model (28). It has also been shown that pharmacological inhibition of COX-2 by non-steroidal anti-inflammatory drugs (NSAIDs) including non-specific and/or COX-2 selective inhibitors reduces antigen-specific antibody production in mouse in vivo models of TD response using a purified tetanus anatoxin or an ovalbumin emulsified in CFA (42, 43). Similarly, a Mycobacterium butyricum-induced adjuvant arthritis in a rat model showed a significant reduction in anti-Mycobacterium antibodies after administration of selective COX-2 inhibitors (44). Furthermore, studies using COX-2 null mice have demonstrated reduced antigen specific IgG responses to a vaccine, human papillomavirus type 16 virus-like particles (HPV 16 VLPs), or CII in CFA (45, 46). These previous findings indicate a vital role for COX-2 in promoting a TD humoral immune response in vivo. Taken together with the present study, these evidences implicate COX-2/mPGES-1/PGE2 upregulation as critical in the development of humoral immune response to TD antigens.
We show that mPGES-1 deficiency does not change the expression of COX and other PG synthases in splenocytes. The deletion of mPGES-1 abolished production of PGE2, but not PGD2. These results indicate that the synthetic machinery for other PGs remains intact in absence of mPGES-1. Shunting phenomenon toward other PGs in some types of mPGES-1 null cells has been reported (15, 17, 18, 47), however, there was no shunting toward PGD2 production in splenocytes in the experimental conditions employed in the present experiments.
We have also shown that mPGES-1, but not mPGES-2, is the primary enzyme for increases in PGE2 production in splenocytes. This preferential requirement of mPGES-1 correlates with similar findings in different types of cells such as macrophages (14, 17), embryo fibroblasts (15, 16) and dendritic cells (47) in response to a various inflammatory stimuli. A previous study reported that PGE2 production in spleen under basal conditions and after LPS-injection was reduced in an mPGES-1 gene dose-dependent manner (18). On the other hand, a study using mPGES-2 null mice showed that loss of mPGES-2 does not result in a measurable decrease in PGE2 levels in any tissues or cell types examined from healthy mice (48). The mPGES-2 enzyme is capable of in vitro synthesis of PGE2 from the COX metabolite PGH2 (49). However, our study further emphasized the uncertain role of mPGES-2 for PGE2 production in vivo.
It has been known that the many types of cells including both hematopoietic cells and mesenchymal cells express mPGES-1 and have ability to produce PGE2. For example, in immune cells of hematopoietic origin, mPGES-1 is required for PGE2 production in murine BM-derived dendritic cells in vitro, though mPGES-1 null dendritic cells exhibit normal maturation and migration in a FITC sensitization model in vivo (47). As described above, mPGES-1 expressing macrophages are also a major source of PGE2 after exposure to inflammatory stimuli (14, 17). Activated human T-cells have ability to induce COX-2 in vitro (50). Activated human B cells can also express COX-2 and produce PGE2 (51, 52). These studies also suggested an autocrine effect of COX-2 derived PGs from B-cells on antibody production in vitro. However, it has not been yet elucidated whether those hematopoietic immune cells and/or surrounding mesenchymal cells/tissues of lymphoid organ are critical for humoral immune response in an in vivo setting.
The present study using mPGES-1 BM chimeras demonstrates that reduction of TD humoral response in mPGES-1 null mice is associated with the absence of non-hematopoietic/mesenchymal mPGES-1 rather than hematopoietic mPGES-1 in vivo. These findings suggest that mPGES-1 expression and its derived PGE2 in mesenchymal cells/tissues is required to promote optimal antibody responses. Although a role for non-hematopoietic cells at the site of immunization cannot be excluded as contributing to the altered acquired immune response, the presence of normally proliferating hapten-specific T-cells and T-cell cytokines not different between WT and mPGES-1 null mice suggest a mechanism involving secondary lymphoid organs. We did not see deficient germinal center formation, but it is known that interactions between germinal center lymphocytes and mesenchymal stromal cells play a key role in B-cell maturation to plasma cells. Furthermore, mesenchymal stromal cells play a key role in immunologic memory in the bone marrow (53).
In secondary lymphoid organs, there are a number of stromal subsets that include fibroblastic reticular cells, follicular dendritic cells (FDC), red pulp fibroblasts, lymph node medullary fibroblasts, and lymphatic and vascular endothelial cells. Follicular dendritic cells (FDC) are of mesenchymal origin, while lymphoid and myeloid dendritic cells are of hematopoietic origin. Fang et. al. (54) reported an absolute requirement for CR1 expressed on FDC in a TD immune response in vivo using BM chimeras generated by null and WT BM reconstitution of lethally irradiated host mice (55). In the present study, we showed that mPGES-1 null mice exhibit immunoreactivity to CR1 antibody in GC similar to WT mice in response to a TD antigen. There was no difference in the numbers of CD11c+CR1+ cells in mPGES-1 null and WT mice in our study. Interestingly, it has been reported that FDC isolated from lymph follicles of human tonsils produces high levels of PGE2 in vitro (56). In addition, it was shown that human FDC express COX-2 and mPGES-1 that are induced after stimulation with pro-inflammatory cytokines, while prostacyclin synthase is constitutively expressed (57). In vitro, PGE2 and PGI2 inhibit T cell proliferation and protect against activation-induced cell death. Deficiency of mPGES-1 in FDC, which could lead to alteration of the PG production profile, cannot be excluded as contributing to our results showing reduced TD immune response in BM chimeras with mesenchymal mPGES-1 deficiency.
Costimulatory signals via engagement of CD40 on T-cell and CD40 ligand on B-cells have significant effects on TD immune responses. Studies using CD40 null mice (58) and CD40 ligand null mice (59) have demonstrated that both molecules are essential for antigen-specific antibody production in response to TD antigens, but are also required for basal immunoglobulin generation. Importantly, antigen-specific antibody response against TI antigens is induced in these null mice. The blockade of CD40/CD40 ligand interaction using anti-CD40 ligand also reduces both primary and secondary TD humoral immune responses to TD antigens without altering the TI-2 response (60). In addition, the injection of anti-CD40 ligand reduce the development of arthritis along with the reduction of serum antibody titer against CII (61), as reported in studies using mPGES-1 null (28) and COX-2 null mice (46). An in vitro study has also shown that IgM and IgG production in human B-cells by stimulation with CD40 ligand plus anti-IgM antibody are attenuated by NSAIDs in vitro, although the pharmacological inhibition of COX results in blocking not only PGE2 but also other PGs. A mechanism involving CD40/CD40 ligand cannot be excluded as contributing to our findings.
Mice with a genetic deletion of mPGES-1 display a reduction of antigen-specific IgM as well as IgG, suggesting that the absence of mPGES-1 may not affect class switch in TD response by DNP-KLH. This observation is not in agreement with a previous study that COX-2 null mice exhibit 4-fold increase in IgM, but reduction in IgG, post vaccination with HPV 16 VLP. It is possible for there to be differential effects on antibody production in vivo related to the specific antigen or adjuvant. Inconsistent results for IgM generation in TD responses have been recognized in several studies. The blockade of CD40/CD40 ligand interaction using anti-CD40 ligand abolished both IgM and IgG antibody response to the TD antigens sheep red blood cells and KLH (60), while CD40 null mice (58) and CD40 ligand null mice (59) display reduction of IgG but not IgM in response to hapten-conjugated KLH.
We evaluated a limited number of proteins known to be both influenced by PGE2 and be contributors to B cell development or survival. IL-6 and HGF influence the development of B cells as evidenced by acquisition of a plasma cell phenotype and an increase in IgG secretion (35). HGF, through its receptor c-met expressed on B-cells, also mediates cell-cell interactions between stromal cells and B-cells to promote the survival of mature B cells (62). Of note, in several cell types and systems, PGE2 induces production of HGF (63–65). In this study, we showed a non-significant reduction in both IL-6 and HGF in lymph node. Activin A, a homodimer of inhibin βA, negatively regulates B cell lymphopoiesis (66). Although the differences in IL-6, HGF and inhibin βA expression were not significant, it remains possible that phenotypic changes in at least some types of mesenchymal stromal cells may contribute to altered TD humoral immunity in mPGES-1 deficient mice.
mPGES-1 is an attractive target for drug development, since inhibition would specifically abolish the up-regulated PGE2 production associated with inflammation. Drug development programs have been hampered by species differences between rodents and humans (67). However, studies in a guinea pig model of arthritis suggest that these agents may be effective anti-inflammatory analgesic compounds (68). The present study provides potentially important information affecting the therapeutic potential for pharmacologic inhibition of mPGES-1 that will need to be evaluated in pre-clinical or early clinical studies.
Acknowledgments
We would like to thank Dr. Lihua Yang for her technical and secretarial assistance. We would also like to thank Drs. Alan Daugherty, Victoria L. King, Haruhito A. Uchida, Debra Rateri and Deborah Howatt (University of Kentucky) for their assistance regarding BM transplantation. We also thank Dr. John Zhang (Medical University of South Carolina) for his suggestions on FISH staining protocol. We would also like to thank Dr. James W. Thomas (Vanderbilt University) for his review of the B-cell biology.
Grant support: This work was supported by the NIH/NIAMS R01 AR049010, the NIH EY14060 and a travel award from the Japanese Society of Clinical Pharmacology and Therapeutics.
Footnotes
Disclosures: None
References
- 1.Legler DF, Bruckner M, Uetz-von Allmen E, Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol. 2010;42:198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 3.Crofford LJ. Prostaglandin biology. Gastroenterol Clin North Am. 2001;30:863–876. doi: 10.1016/s0889-8553(05)70217-x. [DOI] [PubMed] [Google Scholar]
- 4.Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2:603–630. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 5.Sakata D, Yao C, Narumiya S. Emerging roles of prostanoids in T cell-mediated immunity. IUBMB Life. 2010;62:591–596. doi: 10.1002/iub.356. [DOI] [PubMed] [Google Scholar]
- 6.Kalinski P. Regulation of immune responses by prostaglandin e2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 8.Gold KN, Weyand CM, Goronzy JJ. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;37:925–933. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- 9.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 10.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 13.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Shunting of prostanoid biosynthesis in microsomal prostaglandin E synthase-1 null embryo fibroblasts: regulatory effects on inducible nitric oxide synthase expression and nitrite synthesis. Faseb J. 2006;20:2387–2389. doi: 10.1096/fj.06-6366fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator-activated receptor gamma: regulation by prostaglandin E2 via the phosphatidylinositol 3-kinase and Akt pathway. J Biol Chem. 2007;282:5356–5366. doi: 10.1074/jbc.M610153200. [DOI] [PubMed] [Google Scholar]
- 17.Trebino CE, Eskra JD, Wachtmann TS, Perez JR, Carty TJ, Audoly LP. Redirection of eicosanoid metabolism in mPGES-1-deficient macrophages. J Biol Chem. 2005;280:16579–16585. doi: 10.1074/jbc.M412075200. [DOI] [PubMed] [Google Scholar]
- 18.Boulet L, Ouellet M, Bateman KP, Ethier D, Percival MD, Riendeau D, Mancini JA, Methot N. Deletion of microsomal prostaglandin E2 (PGE2) synthase-1 reduces inducible and basal PGE2 production and alters the gastric prostanoid profile. J Biol Chem. 2004;279:23229–23237. doi: 10.1074/jbc.M400443200. [DOI] [PubMed] [Google Scholar]
- 19.Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- 20.Mabuchi T, Kojima H, Abe T, Takagi K, Sakurai M, Ohmiya Y, Uematsu S, Akira S, Watanabe K, Ito S. Membrane-associated prostaglandin E synthase-1 is required for neuropathic pain. Neuroreport. 2004;15:1395–1398. doi: 10.1097/01.wnr.0000129372.89000.31. [DOI] [PubMed] [Google Scholar]
- 21.Kamei D, Yamakawa K, Takegoshi Y, Mikami-Nakanishi M, Nakatani Y, Oh-Ishi S, Yasui H, Azuma Y, Hirasawa N, Ohuchi K, Kawaguchi H, Ishikawa Y, Ishii T, Uematsu S, Akira S, Murakami M, Kudo I. Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin e synthase-1. J Biol Chem. 2004;279:33684–33695. doi: 10.1074/jbc.M400199200. [DOI] [PubMed] [Google Scholar]
- 22.Saha S, Engstrom L, Mackerlova L, Jakobsson PJ, Blomqvist A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1100–1107. doi: 10.1152/ajpregu.00872.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kubota K, Kubota T, Kamei D, Murakami M, Kudo I, Aso T, Morita I. Change in prostaglandin E synthases (PGESs) in microsomal PGES-1 knockout mice in a preterm delivery model. J Endocrinol. 2005;187:339–345. doi: 10.1677/joe.1.06169. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Zukas AM, Hui Y, Ricciotti E, Pure E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc Natl Acad Sci U S A. 2006;103:14507–14512. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J Immunol. 2006;177:1879–1885. doi: 10.4049/jimmunol.177.3.1879. [DOI] [PubMed] [Google Scholar]
- 26.Elander L, Engstrom L, Ruud J, Mackerlova L, Jakobsson PJ, Engblom D, Nilsberth C, Blomqvist A. Inducible prostaglandin E2 synthesis interacts in a temporally supplementary sequence with constitutive prostaglandin-synthesizing enzymes in creating the hypothalamic-pituitary-adrenal axis response to immune challenge. J Neurosci. 2009;29:1404–1413. doi: 10.1523/JNEUROSCI.5247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda-Matsuo Y, Ota A, Fukada T, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:11790–11795. doi: 10.1073/pnas.0604400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima F, Kapoor M, Yang L, Fleishaker EL, Ward MR, Monrad SU, Kottangada PC, Pace CQ, Clark JA, Woodward JG, Crofford LJ. Defective generation of a humoral immune response is associated with a reduced incidence and severity of collagen-induced arthritis in microsomal prostaglandin E synthase-1 null mice. J Immunol. 2008;180:8361–8368. doi: 10.4049/jimmunol.180.12.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kindred B. Nude mice in immunology. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- 30.Slack J, Der-Balian GP, Nahm M, Davie JM. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980;151:853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mond JJ, Scher I, Mosier DE, Baese M, Paul WE. T-independent responses in B cell-defective CBA/N mice to Brucella abortus and to trinitrophenyl (TNP) conjugates of Brucella abortus. Eur J Immunol. 1978;8:459–463. doi: 10.1002/eji.1830080703. [DOI] [PubMed] [Google Scholar]
- 32.Press JL. The CBA/N defect defines two classes of T cell-dependent antigens. J Immunol. 1981;126:1234–1240. [PubMed] [Google Scholar]
- 33.Banchereau J, Rousset F. Human B lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol. 1992;52:125–262. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- 34.Galibert L, Burdin N, de Saint-Vis B, Garrone P, Van Kooten C, Banchereau J, Rousset F. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J Exp Med. 1996;183:77–85. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skibinski G, Skibinska A, James K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology. 2001;102:506–514. doi: 10.1046/j.1365-2567.2001.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skibinski G, Skibinska A, Stewart GD, James K. Enhancement of terminal B lymphocyte differentiation in vitro by fibroblast-like stromal cells from human spleen. Eur J Immunol. 1998;28:3940–3948. doi: 10.1002/(SICI)1521-4141(199812)28:12<3940::AID-IMMU3940>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 38.Rose ML, Birbeck MS, Wallis VJ, Forrester JA, Davies AJ. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980;284:364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- 39.Bhan AK, Nadler LM, Stashenko P, McCluskey RT, Schlossman SF. Stages of B cell differentiation in human lymphoid tissue. J Exp Med. 1981;154:737–749. doi: 10.1084/jem.154.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardie DL, Johnson GD, Khan M, MacLennan IC. Quantitative analysis of molecules which distinguish functional compartments within germinal centers. Eur J Immunol. 1993;23:997–1004. doi: 10.1002/eji.1830230502. [DOI] [PubMed] [Google Scholar]
- 41.Szomolanyi-Tsuda E, Welsh RM. T-cell-independent antiviral antibody responses. Curr Opin Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 42.Yamaki K, Uchida H, Harada Y, Yanagisawa R, Takano H, Hayashi H, Mori Y, Yoshino S. Effect of the nonsteroidal anti-inflammatory drug indomethacin on Th1 and Th2 immune responses in mice. J Pharm Sci. 2003;92:1723–1729. doi: 10.1002/jps.10380. [DOI] [PubMed] [Google Scholar]
- 43.Lupu AR, Cremer L, Durbaca S, Calugaru A, Herold A, Kerek F, Szegli G, Radu DL. COX-2 inhibitors can down-regulate in vivo antibody response against T-dependent antigens. Roum Arch Microbiol Immunol. 2006;65:59–65. [PubMed] [Google Scholar]
- 44.Turull A, Queralt J. Selective cyclooxygenase-2 (COX-2) inhibitors reduce anti-Mycobacterium antibodies in adjuvant arthritic rats. Immunopharmacology. 2000;46:71–77. doi: 10.1016/s0162-3109(99)00159-9. [DOI] [PubMed] [Google Scholar]
- 45.Ryan EP, Malboeuf CM, Bernard M, Rose RC, Phipps RP. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol. 2006;177:7811–7819. doi: 10.4049/jimmunol.177.11.7811. [DOI] [PubMed] [Google Scholar]
- 46.Myers LK, Kang AH, Postlethwaite AE, Rosloniec EF, Morham SG, Shlopov BV, Goorha S, Ballou LR. The genetic ablation of cyclooxygenase 2 prevents the development of autoimmune arthritis. Arthritis Rheum. 2000;43:2687–2693. doi: 10.1002/1529-0131(200012)43:12<2687::AID-ANR8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Monrad SU, Kojima F, Kapoor M, Kuan EL, Sarkar S, Randolph GJ, Crofford LJ. Genetic deletion of mPGES-1 abolishes PGE2 production in murine dendritic cells and alters the cytokine profile, but does not affect maturation or migration. Prostaglandins Leukot Essent Fatty Acids. 2011;84:113–121. doi: 10.1016/j.plefa.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jania LA, Chandrasekharan S, Backlund MG, Foley NA, Snouwaert J, Wang IM, Clark P, Audoly LP, Koller BH. Microsomal prostaglandin E synthase-2 is not essential for in vivo prostaglandin E(2) biosynthesis. Prostaglandins Other Lipid Mediat. 2008 doi: 10.1016/j.prostaglandins.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murakami M, Nakashima K, Kamei D, Masuda S, Ishikawa Y, Ishii T, Ohmiya Y, Watanabe K, Kudo I. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem. 2003;278:37937–37947. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 50.Pablos JL, Santiago B, Carreira PE, Galindo M, Gomez-Reino JJ. Cyclooxygenase-1 and -2 are expressed by human T cells. Clin Exp Immunol. 1999;115:86–90. doi: 10.1046/j.1365-2249.1999.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 52.Bernard MP, Phipps RP. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin Immunol. 2007;125:138–148. doi: 10.1016/j.clim.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 54.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 55.Humphrey JH, Grennan D, Sundaram V. The origin of follicular dendritic cells in the mouse and the mechanism of trapping of immune complexes on them. Eur J Immunol. 1984;14:859–864. doi: 10.1002/eji.1830140916. [DOI] [PubMed] [Google Scholar]
- 56.Heinen E, Cormann N, Braun M, Kinet-Denoel C, Vanderschelden J, Simar LJ. Isolation of follicular dendritic cells from human tonsils and adenoids. VI. Analysis of prostaglandin secretion. Ann Inst Pasteur Immunol. 1986;137D:369–382. [PubMed] [Google Scholar]
- 57.Lee IY, Cho W, Kim J, Park CS, Choe J. Human follicular dendritic cells interact with T cells via expression and regulation of cyclooxygenases and prostaglandin E and I synthases. J Immunol. 2008;180:1390–1397. doi: 10.4049/jimmunol.180.3.1390. [DOI] [PubMed] [Google Scholar]
- 58.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 59.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foy TM, Shepherd DM, Durie FH, Aruffo A, Ledbetter JA, Noelle RJ. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Durie FH, Fava RA, Foy TM, Aruffo A, Ledbetter JA, Noelle RJ. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 62.Gordin M, Tesio M, Cohen S, Gore Y, Lantner F, Leng L, Bucala R, Shachar I. c-Met and its ligand hepatocyte growth factor/scatter factor regulate mature B cell survival in a pathway induced by CD74. J Immunol. 2010;185:2020–2031. doi: 10.4049/jimmunol.0902566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohnishi T, Suwa M, Oyama T, Arakaki N, Torii M, Daikuhara Y. Prostaglandin E2 predominantly induces production of hepatocyte growth factor/scatter factor in human dental pulp in acute inflammation. J Dent Res. 2000;79:748–755. doi: 10.1177/00220345000790020801. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L, Himi T, Murota S. Induction of hepatocyte growth factor (HGF) in rat microglial cells by prostaglandin E(2) J Neurosci Res. 2000;62:389–395. doi: 10.1002/1097-4547(20001101)62:3<389::AID-JNR9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 65.Plantier L, Marchand-Adam S, Marchal-Somme J, Leseche G, Fournier M, Dehoux M, Aubier M, Crestani B. Defect of hepatocyte growth factor production by fibroblasts in human pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol. 2005;288:L641–647. doi: 10.1152/ajplung.00249.2004. [DOI] [PubMed] [Google Scholar]
- 66.Shoham T, Parameswaran R, Shav-Tal Y, Barda-Saad M, Zipori D. The mesenchymal stroma negatively regulates B cell lymphopoiesis through the expression of activin A. Ann N Y Acad Sci. 2003;996:245–260. doi: 10.1111/j.1749-6632.2003.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 67.Pawelzik SC, Uda NR, Spahiu L, Jegerschold C, Stenberg P, Hebert H, Morgenstern R, Jakobsson PJ. Identification of key residues determining species differences in inhibitor binding of microsomal prostaglandin E synthase-1. J Biol Chem. 2010;285:29254–29261. doi: 10.1074/jbc.M110.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu D, Rowland SE, Clark P, Giroux A, Cote B, Guiral S, Salem M, Ducharme Y, Friesen RW, Methot N, Mancini J, Audoly L, Riendeau D. MF63 [2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)-isophthalonitrile], a selective microsomal prostaglandin E synthase-1 inhibitor, relieves pyresis and pain in preclinical models of inflammation. J Pharmacol Exp Ther. 2008;326:754–763. doi: 10.1124/jpet.108.138776. [DOI] [PubMed] [Google Scholar]