Abstract
Solutions of 5‐N‐arylaminothiazoles containing pyridyl groups exhibited clear halochromism and halofluorism upon addition of Brønsted and Lewis acids. The addition of triflic acid to solutions of 5‐N‐arylaminothiazoles in Et2O induced bathochromic shifts of the absorption and emission bands. DFT calculations suggested that the spectral changes arise from the protonation of the pyridyl group of the thiazoles in Et2O. Single‐crystal X‐ray diffraction analysis of a thiazole and its protonated form revealed the change of the conformation around the thiazole ring. The emission of white light was accomplished from a single fluorescent dye by adjusting the ratio of dye and B(C6F5)3, whereby the International Commission on Illumination coordinates showed a linear change from blue to orange.
Keywords: 5-aminothiazoles, absorption, fluorescent dyes, Lewis acids and bases, white-light emission
Acid–base interactions1 are fundamental chemical phenomena that can control the visible colors of organic molecules through the absorption and emission of light. Halochromic and halofluoric2 properties have a wide range of applications in chemosensors and organic electronic devices.3 Nitrogen‐containing functional groups such as pyridyl,4 pyrazinyl,5 pyrimidyl,6 alkylamino,7 and indolizyl moieties8 in chromophores can be protonated, and the resulting species usually exhibit pH‐dependent bathochromic shifts of their absorption and emission spectra. In addition to the halofluorism induced by Brønsted acids, that triggered by Lewis acids is also of great interest. Structural modifications around Lewis acidic centers with organic functional groups may provide Lewis acids with a wide range of properties that should allow the fine‐tuning of Lewis acid–base interactions. In fact, interactions between structurally modified fluorescent organoboranes9 and Lewis bases such as pyridine,10 or the fluoride anion,11 have resulted in a variety of interesting luminescence properties. Interactions between fluorescent Lewis bases and non‐fluorescent organoboranes12, 13 are also expected to display multicolor emissions, and the combination of such emissions may provide access to white light.14, 15
We have previously reported that the fluorescence properties of 5‐N‐arylaminothiazole 1 (Scheme 1) can be controlled by the substituents, and that the presence of electron‐donating groups on the nitrogen atom at the 5‐position bathochromically shifts the emission.16 5‐Aminothiazole 1 contains monocyclic homo‐ and heteroaromatic groups, whereby their conformation is intricately correlated to both solvent and temperature.
Scheme 1.
5‐N‐Arylaminothiazoles.
Considering the presence of basic nitrogen and sulfur atoms in 1, the addition of acids is expected to trigger changes in its photophysical properties.17 However, only minimal changes were observed after the addition of a large excess of triflic acid (TfOH). Therefore, we have designed and synthesized a series of 5‐N‐arylaminothiazoles (2–6) with 4‐pyridyl groups at the 2‐position, which act as strong Lewis basic sites, and investigated the acid‐induced changes of their photophysical properties. The introduction of various substituents at the para position of the diphenylamino groups should provide a means to tune the absorption and emission spectra. Herein, we report the structures as well as the Brønsted‐ and Lewis‐acid‐induced changes of the photophysical properties of 2‐(4‐pyridyl)‐5‐N‐arylaminothiazoles. The judicious choice of the combination of thiazole and Lewis acid allows the fine‐tuning of the emission color, and the emission of white light can, thus, be accomplished from a single fluorescent dye and a Lewis acid.
To demonstrate the sensitivity of the absorption and emission properties of thiazole 2 toward Brønsted acids, we initially measured the corresponding spectra upon addition of TfOH in increments of 0.25 equivalents [Eq. (1)], (Figure 1). Upon addition of TfOH to 2, a decrease in the intensity of its maximum absorption at 382 nm was observed, concomitant with the emergence of a new absorption band at 474 nm. Owing to the protonation of the nitrogen atom in the 4‐pyridyl group of 2, the color of the solution changed from colorless to pale orange, and a clear isosbestic point18 was observed at 412 nm (Figure 1 a). These results suggest that two absorbing species are present in solution, that is, 2 and a 1:1 complex19 of 2 and TfOH ([H2][OTf]).

Figure 1.
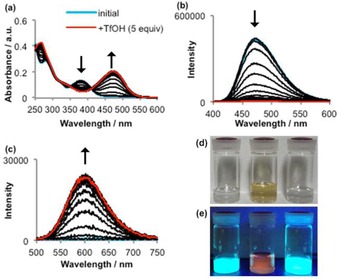
a) UV/Vis absorption spectroscopic titration of thiazole 2 with TfOH (in Et2O, c=10−5 m). b, c) Fluorescence spectroscopic titration of 2 with TfOH (in Et2O, c=10−5 m; λ ex=365 and 470 nm). d, e) Photographic images of Et2O solutions of 2 (left), of 2 after addition of TfOH (middle), and of 2 after consecutive addition of TfOH and Et3N (right). d) Under irradiation from a fluorescent lamp. e) Under irradiation from a UV lamp (λ ex=365 nm).
The fluorescence spectrum of 2 also changed upon addition of TfOH. Initially, we observed light‐blue fluorescence (λ em=472 nm) when 2 was excited at 382 nm in Et2O (Figure 1 b). The fluorescence intensity at 472 nm decreased upon increasing the number of added equivalents of TfOH, and the fluorescence was completely quenched after addition of 5 equivalents of TfOH. Conversely, orange fluorescence at 601 nm could be observed upon addition of TfOH to 2, although the intensity of the emission was weaker than that of pure 2 (Figure 1 c). The protonation of 2 induced bathochromic shifts of the absorption (Δλ abs=92 nm) and emission (Δλ em=129 nm). Upon subsequent addition of Et3N in increments of 0.25 equivalents to the solution of [H2][OTf], the absorption and emission properties reverted to those observed for 2 (Figures 1 d and 1 e), which clearly demonstrates the reversibility of the protonation of 2.
The photophysical properties of 2 and 7 are summarized in Table 1.
Table 1.
Photophysical properties of 2 and 7 in Et2O and CH3CN.
| Solvent | Thiazole | λ ex [nm] | λ em [nm] | τ [ns] | χ 2 | Φ F [a] | K r [b] (×108) | K nr [b] (×108) |
|---|---|---|---|---|---|---|---|---|
| Et2O | 2 | 382 | 479 | 5.85[c] | 1.04 | 0.64 | 1.09 | 0.62 |
| 7 | 475 | 624 | 1.35[d] | 1.10 | 0.08 | 0.59 | 6.81 | |
| CH3CN | 2 | 383 | 523 | 4.81[e] | 1.14 | 0.37 | 0.78 | 1.30 |
| 7 | 474 | 731 | n.d.[f] | 0.004 | n.d.[f] | n.d.[f] |
[a] Absolute fluorescence quantum yield. [b] K r=Φ F/τ, K nr=(1−Φ F/τ).20 [c] Excited at 365 nm. [d] Excited at 470 nm. [f] n.d.=no data.
The fluorescence lifetime of 7 is shorter than that of 2, and 7 displays orange fluorescence with a fluorescence quantum yield (Φ F) of 0.08. The absorption spectra did not reveal any solvent effects, whereas the fluorescence emission was bathochromically shifted to 731 nm in more polar solvents (e.g. acetonitrile) under a concomitant decrease of the emission intensity (see Figure S1 in the Supporting Information).
The structures and absorption spectra of 2 and 7 in Et2O were also investigated computationally, using DFT calculations at the B3LYP/6‐31+G(d,p) and CAM‐B3LYP/6‐31+G(d,p) levels of theory (see the Supporting Information for details). Similar to the experimentally obtained spectra for 2 and 7, a bathochromic shift of the absorption maxima was observed. The calculation revealed that the protonation of 2 should stabilize both the HOMO and LUMO of 7. However, the LUMO should be stabilized more, which would reduce the HOMO–LUMO gap.
The molecular structures of 2 and 7 were determined by using single‐crystal X‐ray diffraction analysis (Figure 2). The extent of the π conjugation can be determined by the dihedral angle (details can be found in the Supporting Information) between the thiazole plane and the tethered π‐electron units, that is, the 4‐pyridyl or 4‐pyridinium groups at the 2‐position (A‐2), the phenyl plane at the 4‐position (A‐4), and the N planes21 at the 5‐position (A‐5). Notably, the dihedral angles A‐4 and A‐5 in 7 are higher than those in 2 (7: 27° and 72°; 2: 1° and 66°), whereas the dihedral angle A‐2 in 7 is smaller than that in 2 (7: 4°; 2: 11°). Moreover, the 4‐pyridinium group is almost co‐planar with the thiazole ring.
Figure 2.
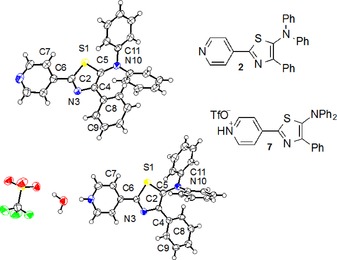
Molecular structures for 2 (top) and 7 (bottom) with thermal displacement parameters set at 50 % probability. Selected dihedral angles (°) and bond lengths (Å): 2: (top): S1−C2−C6−C7 10.7(0), N3−C4−C8−C9 8.1(4), S1−C5−N10−C11 51.8(3), C2−C6 1.482(6), C4−C8 1.484(6), C5−N10 1.396(5), N10−C11 1.428(5); 7 (bottom): S1−C2−C6−C7 6.0(7), N3−C4−C8−C9 24.5(7), S1−C5−N10−C11 60.5(6), C2−C6 1.463(8), C4−C8 1.473(7), C5−N10 1.403(7), N10−C11 1.402(7).
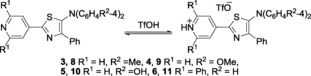
To induce further bathochromic shifts in the absorption and emission spectra, we introduced electron‐donating substituents at the para position of the aryl groups of the nitrogen atom at the 5‐position (3–5) (Table 2, entries 1–3). As expected, the addition of TfOH to Et2O solutions of 3–5 showed redshifts upon the formation of 1:1 complexes (Table 2, Figures S2–S10), and the longest absorption wavelengths shifted by approximately 100 nm, from 398±10 to 500±12 nm. Similar to the formation of 7, the equilibrium constants22 for the reactions between 3–5 and TfOH were larger than that between 2 and TfOH [K a=(8.9±2.4)×104]. However, the emission of 8–10 was quenched differently to that of 7. To avoid emission quenching, phenyl groups were introduced at the 3,5‐positions of the 4‐pyridyl group (entry 4).23 Thus, thiazole 6 displayed visually confirmed fluorescence at 609 nm, but the presence of phenyl groups at the 3,5‐positions of the 4‐pyridyl group in 6 inhibited the protonation of the pyridyl group of 6 with TfOH. Accordingly, the equilibrium constant between 6 and TfOH is by approximately two orders of magnitude smaller than that of the reaction between 2 and TfOH (Figure S10).24
Table 2.
Photophysical properties of thiazoles 3–6 and 8–11.[a]
| Entry | Thiazole | λ abs [nm] | λ em [nm][b] | IP [nm][c] | Thiazole | λ abs [nm] | λ em [nm][b] | K a [m −1] |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 390 | 494 | 421 | 8 | 488 | – | (5.9±1.3)×105 |
| 2 | 4 | 404 | 518 | 434 | 9 | 500 | – | (3.2±0.78)×104 |
| 3 | 5 | 408 | 533 | 443 | 10 | 512 | – | (1.1±0.27)×105 |
| 4 | 6 | 388 | 476 | 420 | 11 | 488 | 609 | (4.5±0.21)×102 |
[a] In Et2O, c=10−5 m. [b] Excited at the wavelength of the maximum absorption. [c] IP=isosbestic point.
Subsequently, we examined the sensitivity of 5‐N‐arylaminothiazoles 2–6 toward Lewis acids. For that purpose, BCl3, AlCl3, and GaCl3 were added to CH2Cl2 solutions of 2 (Table 3, Figures S14–S23). New absorption and emission features were observed at 474–482 nm and 604–607 nm, respectively. Although comparable changes were observed upon addition of BCl3 and AlCl3, the equilibrium constant for the reaction between 2 and BCl3 was, by a factor of 36±11, greater than that for the reaction between 2 and AlCl3 (entries 1 and 2). In contrast, the addition of GaCl3 induced a different effect (entry 3). Up to the addition of 0.5 equivalents of GaCl3, the spectrum of the solution of 2 changed in a manner similar to those that were treated with BCl3 and AlCl3. However, after further addition of GaCl3, the wavelengths of the absorption and emission bands shifted to 526 and 635 nm, respectively, indicative of the formation of more than two absorbing/emitting species in solution.25
Table 3.
Photophysical properties of Lewis acid adducts of 2.[a]
| Entry | Complex | λ abs [nm] | λ em [nm] | IP [nm] | K a [m −1] |
|---|---|---|---|---|---|
| 1 | 2⋅BCl3 | 482 | 605 | 418 | (1.4±0.26)×106 |
| 2 | 2⋅AlCl3 | 476 | 607 | 416 | (3.9±0.49)×104 |
| 3 | 2⋅GaCl3 | 474[b] | 604[b] | 414[b] | |
| 526[c] | 635[c] | 488[c] |
[a] In CH2Cl2, c=10−5 m. [b] 0.25–0.5 equiv of GaCl3. [c] 0.75–3 equiv of GaCl3.
Furthermore, we examined the influence of the Lewis acid B(C6F5)3 12 in toluene (Table 4, Figures 3 a and 3 b). For that purpose, B(C6F5)3 was added to toluene solutions of 2 and 6, which resulted in changes to their photophysical properties, similar to those observed after treatment with other acids (vide supra). The addition of B(C6F5)3 induced a bathochromic shift of the absorption wavelengths of 2 and 6 by 95 and 108 nm, respectively (entries 1 vs. 2 and 3 vs. 4, respectively). As a result, the emission color of 2 and 6 changed from blue to orange, and fluorescence was observed at 576 and 608 nm, respectively. The changes in the emission color of the solution of 2 was measured upon addition of increasing amounts of B(C6F5)3 by using a UV lamp (λ ex=365 nm) (Figure 3 c).
Table 4.
Photophysical properties of 2 and 6 upon the addition of B(C6F5)3.[a]
| Entry | Species | λ ex [nm] | λ em [nm] | Φ F [b] |
|---|---|---|---|---|
| 1 | 2 | 330 | 479 | 0.65 |
| 387 | 479 | 0.78 | ||
| 2 | 2⋅B(C6F5)3 | 345 | 575 | 0.55 |
| 482 | 576 | 0.46 | ||
| 3 | 6 | 330 | 482 | 0.84 |
| 394 | 483 | 0.69 | ||
| 4 | 6⋅B(C6F5)3 | 359 | 607 | 0.38 |
| 502 | 608 | 0.37 |
[a] In toluene, c=10−5 m. [b] Excited at λ ex.
Figure 3.
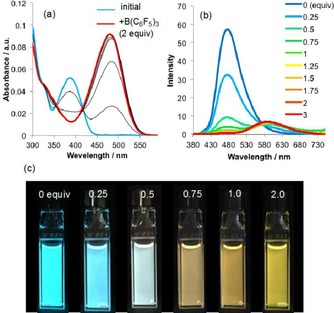
a) UV/Vis absorption spectroscopic titration of thiazole 2 with B(C6F5)3 (in toluene, c=10−5 m). b) Emission spectroscopic titration of thiazole 2 and B(C6F5)3 (in toluene, c=10−5 m, λ ex=387 and 482 nm). c) Photographic images of solutions of 2 that contain different concentrations of B(C6F5)3 in toluene under UV irradiation (λ ex=365 nm).
Interestingly, white emission was achieved upon addition of 0.5 equivalents of B(C6F5)3 (Figure S30). This color is most likely the result of an overlap from the emission bands of the blue fluorescence of 2 and the emission bands of 2⋅B(C6F5)3, although further studies are necessary to disclose the detail of the mechanism of the excitation of 2⋅B(C6F5)3. The emission of multiple colors from a single fluorescent dye26 in one flask may, thus, lead to an emission that approximates white light. However, the 1:1 complex 2⋅B(C6F5)3 is readily formed, and the appropriate ratio between 2 and 2⋅B(C6F5)3 that leads to the emission of highly pure white light could not be realized, as evaluated by the International Commission on Illumination (CIE) coordinates (Figure S30, Table S2).
We then focused on the steric effects of the phenyl groups around the pyridyl group in 6, in the hope of being able to fine‐tune the basicity of 6. In fact, the smaller equilibrium constant between 6 and B(C6F5)3 allowed a fine‐tuning of the emission color by varying the ratio of 6 and 6⋅B(C6F5)3, resulting in the emission of purely white light (Figure 4). To evaluate the obtained colors, their CIE coordinates were calculated from the fluorescence spectra of solutions containing 6 and varying amounts of B(C6F5)3 (Figures 4 and S31, Table S3). Under excitation at 365 nm and continuous addition of B(C6F5)3, the blue fluorescence of 6 [CIE: 0.15, 0.32; 0 equiv of B(C6F5)3] changed to white [CIE: 0.33, 0.35; 4.5 equiv of B(C6F5)3] and eventually to orange [CIE: 0.58, 0.39; 100 equiv of B(C6F5)3] in a linear manner. Most white emissions are observed from RGB or complementary colors (e.g. blue and yellow), usually obtained from the combination of multiple fluorescent dyes.14, 15 In contrast, the present white‐light emission is achieved by adding a non‐fluorescent Lewis acid to a 5‐aminothiazole as a single‐component fluorescent dye7, 26 with a broad emission band (400–700 nm).
Figure 4.

Photographs of a solution of 6 and B(C6F5)3 in toluene under a UV lamp (excitation at 365 nm) (left); CIE of a solution of 6 with 0–100 equiv of B(C6F5)3 (right).
In conclusion, the absorption and emission spectra of 5‐aminothiazoles containing 4‐pyridyl groups exhibited bathochromic shifts upon addition of TfOH, and the resulting protonated 5‐aminothiazoles displayed orange fluorescence, albeit at the expense of lower quantum yields. The addition of Et3N to solutions of these protonated 5‐aminothiazoles and TfOH resulted in the complete reversion of the absorption and emission spectra. 5‐N‐Arylaminothiazoles with electron‐donating groups on the nitrogen atom at the 5‐position displayed more pronounced bathochromic shifts of their absorption and emission spectra, as well as lower equilibrium constants. The addition of Lewis acids such as BCl3 and AlCl3 to the 5‐N‐arylaminothiazoles also changed their halochromic and halofluoric properties in a fashion similar to that observed for the addition of Brønsted acids, whereas the addition of GaCl3 caused changes in the spectra that were consistent with the formation of several absorbing/emitting species. Notably, the use of B(C6F5)3 as a Lewis acid enabled the fine‐tuning of the chromaticity of the emission spectra of the 5‐aminothiazoles. Our system thus provides a new concept for the generation of white‐light emission, that is, the combination of Lewis acids and fluorescent Lewis bases affords single fluorescent dyes, for example, by mixing 5‐aminothiazole 6 and B(C6F5)3 in a 1:2.5 ratio. Further studies on the applications of 5‐N‐arylaminothiazoles as fluorescent dyes are currently in progress in our laboratory.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This work was supported by the following grants‐in‐aid: Scientific Research on Innovative Areas (MEXT), “Stimuli‐Responsive Chemical Species for the Creation of Functional Molecules” [no. 2408], 15H00933 (T.M.) and 24109013 (N.T.), as well as ACT‐C from the Japan Science and Technology Agency (JST).
K. Yamaguchi, T. Murai, J.-D. Guo, T. Sasamori, N. Tokitoh, ChemistryOpen 2016, 5, 434.
References
- 1. Bhatta R. S., Iyer P. P., Dhinojwala A., Tsige M., Modern Phys. Lett. B 2014, 28, 1430014. [Google Scholar]
- 2. Qian G., Qi J., Davey J. A., Wright J. S., Wang Z. Y., Chem. Mater. 2012, 24, 2364. [Google Scholar]
- 3. Jäkle F., Chem. Rev. 2010, 110, 3985; [DOI] [PubMed] [Google Scholar]; Saeed M. A., Le H. T. M., Miljanić O. Š., Acc. Chem. Res. 2014, 47, 2074; [DOI] [PubMed] [Google Scholar]; Li X.-C., Wang C.-Y., Wan Y., Lai W.-Y., Zhao L., Yin M.-F., Huang W., Chem. Commun. 2016, 52, 2748; [DOI] [PubMed] [Google Scholar]; Feng Q., Li Y., Wang L., Li C., Wang J., Liu Y., Li K., Hou H., Chem. Commun. 2016, 52, 3123. [DOI] [PubMed] [Google Scholar]
- 4. Matsui K., Segawa Y., Itami K., Org. Lett. 2012, 14, 1888; [DOI] [PubMed] [Google Scholar]; Hinderer F., Bunz U. H. F., Chem. Eur. J. 2013, 19, 8490; [DOI] [PubMed] [Google Scholar]; Kobayashi N., Kamei Y., Shichibu Y., Konishi K., J. Am. Chem. Soc. 2013, 135, 16078; [DOI] [PubMed] [Google Scholar]; Li W., Yang P.-P., Wang L., Wang H., J. Mater. Chem. C 2015, 3, 3783. [Google Scholar]
- 5. Cai K., Yan Q., Zhao D., Chem. Sci. 2012, 3, 3175; [Google Scholar]; Bindewald E., Lorenz R., Hübner O., Brox D., Herten D.-P., Kaifera E., Himmel H.-J., Dalton Trans. 2015, 44, 3467; [DOI] [PubMed] [Google Scholar]; Fernandez-Cestau J., Bertrand B., Blaya M., Jones G. A., Penfold T. J., Bochmann M., Chem. Commun. 2015, 51, 16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Achelle S., Barsella A., Baudequin C., Caro B., Guen F. R., J. Org. Chem. 2012, 77, 4087; [DOI] [PubMed] [Google Scholar]; Achelle S., Guen F. R., Tetrahedron Lett. 2013, 54, 4491. [Google Scholar]
- 7. Huynh H. V., He X., Baumgartner T., Chem. Commun. 2013, 49, 4899. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y., Garcia-Amorós J., Captain B., Raymo F. M., J. Mater. Chem. C 2016, 4, 2744. [Google Scholar]
- 9. Wakamiya A., Yamaguchi S., Bull. Chem. Soc. Jpn. 2015, 88, 1357; [Google Scholar]; Osumi S., Saito S., Dou C., Matsuo K., Kume K., Yoshikawa H., Awaga K., Yamaguchi S., Chem. Sci. 2016, 7, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sundararaman A., Venkatasubbaiah K., Victor M., Zakharov L. N., Rheingold A. L., Jäkle F., J. Am. Chem. Soc. 2006, 128, 16554; [DOI] [PubMed] [Google Scholar]; Saito S., Matsuo K., Yamaguchi S., J. Am. Chem. Soc. 2012, 134, 9130; [DOI] [PubMed] [Google Scholar]; Matsuo K., Saito S., Yamaguchi S., J. Am. Chem. Soc. 2014, 136, 12580. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Y.-H., Pan H., Fu G.-L., Lin J.-M., Zhao C.-H., Tetrahedron Lett. 2011, 52, 3832; [Google Scholar]; Jun E. J., Xu Z., Lee M., Yoon J., Tetrahedron Lett. 2013, 54, 2755; [Google Scholar]; Poon C.-T., Lam W. H., Wong H.-L., Yam V. W.-W., Chem. Eur. J. 2015, 21, 2182. [DOI] [PubMed] [Google Scholar]
- 12. Welch G. C., Coffin R., Peet J., Bazan G. C., J. Am. Chem. Soc. 2009, 131, 10802; [DOI] [PubMed] [Google Scholar]; Zalar P., Henson Z. B., Welch G. C., Bazan G. C., Nguyen T.-Q., Angew. Chem. Int. Ed. 2012, 51, 7495; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 7613. [Google Scholar]
- 13. Wuttke S., Dietl C., Hinterholzinger F. M., Hintz H., Langhals H., Bein T., Chem. Commun. 2014, 50, 3599. [DOI] [PubMed] [Google Scholar]
- 14.For a selected review, see: Wang J., Zhang F., Zhang J., Tang W., Tang A., Peng H., Xu Z., Teng F., Wang Y., J. Photochem. Photobiol. C 2013, 17, 69. [Google Scholar]
- 15.For examples on white-light emission from blended systems, see: Wang H., Liang Y., Feng L., Xie H., Zhang J., Cheng X., Lu H., Feng S., Dyes Pigm. 2013, 99, 284; [Google Scholar]; Ozawa A., Shimizu A., Nishiyabu R., Kubo Y., Chem. Commun. 2015, 51, 118; [DOI] [PubMed] [Google Scholar]; Malakar P., Modak D., Prasad E., Chem. Commun. 2016, 52, 4309. [DOI] [PubMed] [Google Scholar]
- 16. Murai T., Hori F., Maruyama T., Org. Lett. 2011, 13, 1718; [DOI] [PubMed] [Google Scholar]; Murai T., Yamaguchi K., Hori F., Maruyama T., J. Org. Chem. 2014, 79, 4930; [DOI] [PubMed] [Google Scholar]; Yamaguchi K., Murai T., Hasegawa S., Miwa Y., Kutsumizu S., Maruyama T., Sasamori T., Tokitoh N., J. Org. Chem. 2015, 80, 10742. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X., Jing S.-Y., Huang S.-Y., Zhou X.-W., Bai J.-M., Zhao B.-X., Sens. Actuators B 2015, 206, 663. [Google Scholar]
- 18. Tavker S., Kumar P., J. Geophys. Res. 1997, 102, 30017; [Google Scholar]; Sanjeev R., Jagannadham V., Vrath R. V., Chem. N. Z. 2012, 133. [Google Scholar]
- 19. Niikura K., Anslyn E. V., J. Chem. Soc. Perkin Trans. 2 1999, 2769. [Google Scholar]
- 20. Lakowicz J. R. in Principles of Fluorescence Spectroscopy, 3rd ed., Springer, New York, 2010, p. 9. [Google Scholar]
- 21.The N-plane is defined by the three ipso-carbon atoms on the nitrogen atom.
- 22.S. Akine, TitrationFit, program for analyses of host-guest complexations, Kanazawa University, Kanazawa, Japan, 2013.
- 23. Leblond J., Gao H., Petitjean A., Leroux J.-C., J. Am. Chem. Soc. 2010, 132, 8544. [DOI] [PubMed] [Google Scholar]
- 24.The addition of HCl to 2 also induced spectral band shifts, but no changes were observed upon addition of CH3COOH or CF3COOH (Table S1 and Figures S11–13).
- 25.The coordination of sulfur and nitrogen atoms of benzthiazoles to GaCl3 has been reported, see: Roberts M. F., Jenekhe S. A., Cameron A., McMillan M., Perlstein J., Chem. Mater. 1994, 6, 658. [Google Scholar]
- 26.For examples on white-light emission from single fluorescent dyes, see: Yang Q.-Y., Lehn J.-M., Angew. Chem. Int. Ed. 2014, 53, 4572; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 4660; [Google Scholar]; Findlay N. J., Bruckbauer J., Inigo A. R., Breig B., Arumugam S., Wallis D. J., Martin R. W., Skabara P. J., Adv. Mater. 2014, 26, 7290; [DOI] [PMC free article] [PubMed] [Google Scholar]; Benelhadj K., Muzuzu W., Massue J., Retailleau P., Charaf-Eddin A., Laurent A. D., Jacquemin D., Ulrich G., Ziessel R., Chem. Eur. J. 2014, 20, 12843; [DOI] [PubMed] [Google Scholar]; Maity A., Ali F., Agarwalla H., Anothumakkool B., Das A., Chem. Commun. 2015, 51, 2130; [DOI] [PubMed] [Google Scholar]; Xie Z., Chen C., S. X., Li J., Zhang Y., Liu S., Xu J., Chi Z., Angew. Chem. Int. Ed. 2015, 54, 7181; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 7287; [Google Scholar]; Zhang Z., Wu Y.-S., Tang K.-C., Chen C.-L., Ho J.-W., Su J., Tian H., Chou P.-T., J. Am. Chem. Soc. 2015, 137, 8509; [DOI] [PubMed] [Google Scholar]; Xu B., Mu Y., Mao Z., Xie Z., Wu H., Zhang Y., Jin C., Chi Z., Liu S., Xu J., Wu Y.-C., Lu P.-Y., Lien A., Bryce M. R., Chem. Sci. 2016, 7, 2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


