Abstract
Chikungunya virus (CHIKV) spread rapidly throughout the Caribbean region in 2014, and the first serologically confirmed case was seen in Grenada in July. This study investigated the outbreak of CHIKV in Grenada to identify the distinguishing clinical manifestations and the symptoms that corresponded the closest with serological test results. Sera were tested by IgM enzyme-linked immunosorbent assay and polymerase chain reaction to distinguish between cases positive or negative for CHIKV. Of 493 cases, 426 (86%) tested positive for CHIKV. The diagnostic decision rule, “Define as CHIKV positive a patient presenting with joint pain and any combination of fever, body pain, or rash,” produced the closest agreement (85%) with the serological test results (Cohen's kappa, k = 0.289, P value < 0.001). When laboratory facilities are not available for diagnostic confirmation, syndromic surveillance using these four symptoms could be useful to define cases during a CHIKV outbreak when CHIKV is the predominant circulating arbovirus.
Arthropod-borne viruses (arboviruses) comprise many of the most important emerging pathogens due to their geographic expansion and their increasing impact on vulnerable populations.1–4 During the last decade, chikungunya virus (CHIKV) has expanded its range across Africa and Asia, and has emerged in Europe.5–12 In December 2013, CHIKV reemerged in the Americas after a presumed absence of more than 200 years.13 The Caribbean outbreak is by the Asian genotype first identified in southeast Asia.14,15 Since then, more than 1.6 million confirmed and suspected cases have been reported across all of the Caribbean islands,16 in multiple countries in Central and South America,16 and in southern Florida in the United States.17
In the Caribbean, CHIKV is transmitted by diurnally feeding urban Aedes aegypti vectors.18,19 CHIKV's rapid spread has been facilitated by immunologically naive populations in the region mixing with huge numbers of travelers arriving by air or by sea from neighboring mainland countries and traveling among the region's islands. CHIKV is now likely to be endemic to the region, and will continue to be a cause of severe febrile disease in communities of the Caribbean and along the Gulf of Mexico. For example, 7 months after the introduction of CHIKV to the Caribbean island of Saint Martin, the seroprevalence rate on the island was estimated to be nearly 17% with 39% of infected persons being asymptomatic indicating the disease can circulate inconspicuously throughout the region.20
Information on the most common clinical manifestations can facilitate syndromic surveillance and could be diagnostically valuable since the capability to make a laboratory confirmation of CHIKV in the region is limited and there are no reliable diagnostic test kits commercially available. In the Caribbean, only Puerto Rico, Cuba, Dominican Republic, Saint Martin, Martinique, Guadeloupe, French Guiana, Aruba, Curacao, Saba, and Suriname have laboratories capable of rapidly implementing diagnostic testing to detect the emergence of a novel virus. All other Caribbean countries have limited diagnostic capacity and samples are sent to the Caribbean Public Health Agency (CARPHA) in Trinidad for analysis. The huge number of samples received at CARPHA means that only a few samples can be tested from each country, and consequently, many countries must rely on syndromic surveillance to manage epidemics.
Anticipating the imminent arrival of CHIKV into Grenada in 2014 and since no local CHIKV laboratory diagnostic facilities existed, a collaborative link was established between St. George's University School of Medicine and the Naval Infectious Diseases Diagnostic Laboratory (NIDDL) at the Naval Medical Research Center in the United States. Subsequently, in Summer 2014, CHIKV transmission was confirmed in Grenada, and an explosive outbreak lasted for 13 weeks from mid-July to mid-October 2014.
On the basis of cases observed during the outbreak, the 3-fold objectives of this study were to:
-
1.
describe the demographic attributes of patients suspected to be CHIKV infected,
-
2.
determine whether the CHIKV and dengue virus (DENV) were cocirculating in Grenada, and
-
3.
identify a set of observed symptoms that could provide the closest agreement with serological test results.
From the time of presentation of the first suspected CHIKV case in Grenada in July, patients with symptoms consistent with either CHIKV or DENV were enrolled in the study from health facilities throughout the country. Cases came from all regions in rough proportion to the overall population with the highest number coming from St. George Parish—the largest urban area.
This investigation was a “rapid public health response” and approval was obtained from the St. George's University Institutional Review Board. All participants were counseled about the aims and risks of the study and were enrolled only if they wished to participate.
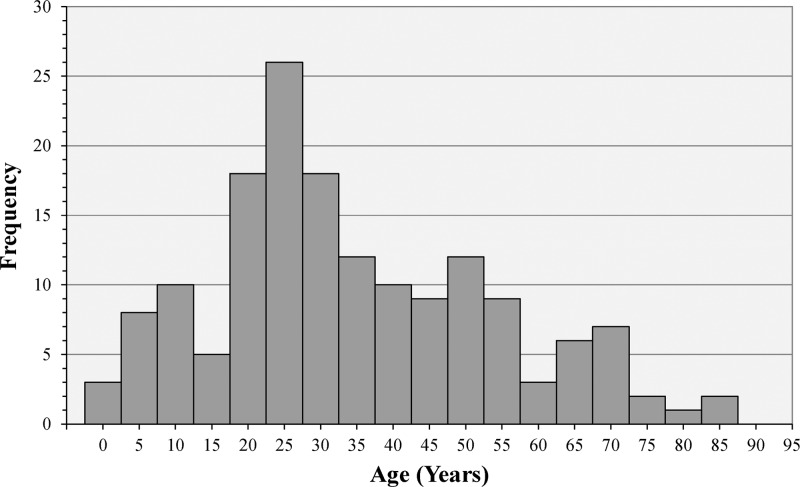
During the 2014 outbreak in Grenada, demographic information, symptom data, and whole blood specimens were collected from 493 suspected cases from the index case on July 9 until October 9, by which time symptomatic patients were no longer presenting at the clinics. The onset coincided with the beginning of the annual rainy season in Grenada. Of the 493 cases enrolled, 325 (66%) were female and 165 were male (34%)—the gender was not recorded for three cases. Although the observed cases were not a representative sample drawn from the general population, the cases spanned the entire range of ages in Grenada from less than 1 year to over 80 years, with a median age of 34.5 years with no significant difference in the age distributions by gender (Figure 1 ). Consequently, the outbreak had the potential to adversely impact all aspects of society from children's school attendance to adults' business and economic activities.
Figure 1.
The distribution of ages for all enrolled cases. Although children are underrepresented among the observed cases relative to the general population, the adult age groups roughly approximate the age distribution of the adult population. There was no significant difference in the age distributions by gender.
Thirty symptoms were clinically observed in the cases enrolled in the study. The top 10 clinical symptoms are shown in Table 1 listed in descending order of frequency observed. The remaining symptoms were observed in less than 20% of the cases, and therefore were deemed nonindicative of the presence of CHIKV.
Table 1.
Top ten clinical symptoms observed during the acute presentation phase of the 2014 CHIKV outbreak in Grenada
| Cases with symptom | ||||
|---|---|---|---|---|
| Female cases (%) | Male cases (%) | All cases (%) | CHIKV ± significance | |
| Joint pain | 90.8 | 88.5 | 90.0 | < 0.001* |
| Fever | 88.3 | 89.1 | 88.6 | 0.002* |
| Body pain | 65.2 | 69.7 | 66.7 | 0.025* |
| Headache | 52.9 | 55.8 | 53.9 | 0.077 |
| Chills | 50.8 | 49.1 | 50.2 | < 0.001* |
| Rash | 48.0 | 32.7 | 42.9 | < 0.001* |
| Enlarged lymph | 29.8 | 26.7 | 28.8 | 0.116 |
| Eye pain | 28.9 | 24.2 | 27.3 | 0.056 |
| Nausea | 27.7 | 25.5 | 26.9 | 0.513 |
| Joint swelling | 28.9 | 17.6 | 25.1 | 0.002* |
CHIKV = chikungunya virus. The top ten clinical symptoms are listed in descending order of frequency observed in the cases enrolled during the acute presentation phase of the 2014 CHIKV outbreak in Grenada. The predominant symptoms observed for both genders are joint pain and fever. Statistical significance (*) indicates evidence of a dependence between a symptom and its observed frequency in CHIKV-positive cases compared with negative cases.
Sera samples were tested at the NIDDL using the indirect CHIKV IgM enzyme-linked immunosorbent assay (ELISA) with a cutoff value for seropositivity > 0.30 mean adjusted net optical density. Since CHIKV was the predominant circulating alphavirus during the outbreak, there were few, if any, other infections that would have been cross-reactive with the ELISA test. In addition, a quantitative multiplex real-time polymerase chain reaction (PCR) for CHIKV and DENV was performed by the NIDDL using an in-house developed and Clinical Laboratory Improvement Amendments–validated assay with a cutoff for positivity < 37 cycle time.21
Of all 493 cases in the study, 426 (86%) tested positive for CHIKV by either IgM or PCR, with 49 testing positive by both tests. By gender, 286 of 325 (88%) female cases and 140 of 165 (85%) male cases tested positive. Only two cases were asymptomatic cases yet tested positive. No cases tested positive for DENV.
Positive CHIKV cases in Grenada were most likely to have joint pain, but unlike the CHIKV outbreak in Trinidad22—the largest neighboring country to Grenada—abdominal pain and sore throat were not associated with CHIKV in Grenada. The clinical presentations of CHIKV in Grenada were more similar to what was seen in Colombia where joint pain, fever, headache, and rash were the most common symptoms.23
Table 1 also shows the results of using likelihood ratio tests for independence between a symptom and its observed frequency in the CHIKV positive and negative cases. Significance was evaluated using the Holm–Bonferroni sequential procedure for multiple comparisons to control for a false discovery rate of 5%. As a result, six symptoms were identified as possibly indicative of CHIKV: joint pain, fever, body pain, chills, rash, and joint swelling.
Joint pain was by far the most statistically significant clinical symptom distinguishing between positive and negative CHIKV cases. Of 441 cases with joint pain, 395 (90%) tested positive for CHIKV, whereas only 31 of 49 (63%) cases without joint pain tested positive. Thus, the proportion of cases who presented with joint pain and tested positive for CHIKV was 42% greater than the proportion of cases without joint pain. Comparing males and female cases with joint pain, no evidence was found (Mantel–Haenszel P value = 0.678) contrary to homogeneity of the two gender groups for the proportion testing positive for CHIKV. However, fever and chills were more distinguishing between CHIKV positive and negative cases in males (P values = 0.023 and 0.035, respectively).
Generalized linear modeling with binary categorical response and explanatory variables was used to test all possible combinations of the observed symptoms ranging from single symptoms to all six possibly indicative symptoms to determine the combination of symptoms that would most closely agree with the serological test results for each gender and for all cases combined. All analyses were performed using IBM® SPSS® Statistics version 22 (Armonk, NY).
Although a study in Mayotte (Indian Ocean area) reported in 2010 that joint pain and fever correctly classified 87% of serologically confirmed CHIKV cases,24 in Grenada, those two symptoms alone were insufficient to reliably classify cases. In fact, no single symptom or combinations of only two symptoms were sufficient as diagnostic indicators.
In the Grenada cases, the decision rule to “Diagnose as CHIKV positive patients who present with joint pain along with any combination of the fever, body pain, or rash,” would have provided 85% agreement with the serological tests. Table 2 shows the agreement between the symptomatic and serological diagnoses by gender and for all cases. Agreement was tested using Cohen's kappa statistic and was adjusted for prevalence. The decision rule applied to both genders with 85% agreement with the serological results for females and 82% agreement for males. No other decision rule would have produced significantly closer agreement with the serological tests. On the basis of the resultant P value for each kappa statistic, agreement between the symptomatic diagnostic decision rule and the serological testing results was unlikely to have occurred by chance for each gender and for all cases combined.
Table 2.
Percentage of agreement between the symptomatic and serological diagnoses by gender and for all cases
| Symptomatic diagnostic decision rule results | |||||
|---|---|---|---|---|---|
| Agreement (%) | Kappa | Kappaadj | Kappamax | Significance | |
| Female cases | 84.9 | 0.244 | 0.699 | 0.923 | 0.014 |
| Male cases | 82.4 | 0.281 | 0.649 | 0.926 | 0.020 |
| All cases | 84.5 | 0.289 | 0.690 | 0.944 | < 0.001 |
CHIKV = chikungunya virus. The symptomatic diagnostic decision rule that most closely agreed with the serological test results was to diagnose as CHIKV positive a patient presenting with joint pain and any combination of fever, body pain, or rash. The percentage of agreement is shown by gender and for all cases combined, along with the kappa statistic for each. Also shown are adjusted kappa statistics accounting for the high prevalence rates of the disease among the observed cases. The maximum achievable kappa values are shown for comparison. All of the kappa statistics are significant, indicating that each agreement is unlikely to have been by chance.
In summary, due to the absence of cocirculating DENV, this study has provided a clear picture of the clinical manifestations of CHIKV unconfounded with DENV. Although the results of this study are not generalizable beyond the cases studied, a diagnostic decision rule such as the one developed in this study can give close agreement with serological tests. Since CHIKV is now presumed to be endemic in the Caribbean region, syndromic surveillance could be used to define cases in similar immunologically naive populations, where laboratory facilities for serological diagnostic confirmation are not available, as was the situation in Grenada during the 2014 CHIKV outbreak.
Footnotes
Authors' addresses: Calum Macpherson, Windward Islands Research and Education Foundation, St George's University, Saint George, Grenada, and Graduate Studies Department, St. George's University, Saint George, Grenada, E-mail: cmacpherson@sgu.edu. Trevor Noël, Microbiology, St. George's University, Saint George, Grenada, and Windward Islands Research and Education Foundation, St. George's University, Saint George, Grenada, E-mail: trevornoel@sgu.edu. Paul Fields, Windward Islands Research and Education Foundation, Saint George, Grenada, E-mail: pjfphd@comcast.net. Donald Jungkind, Microbiology, St. George's University, Saint George, Grenada, E-mail: jungkin2@msn.com. Katherine Yearwood, University Health Services, St. George's University, Saint George, Grenada, E-mail: kyearwood@sgu.edu. Monika Simmons and Susana Widjaja, Naval Medical Research Center, Infectious Diseases Directorate, Silver Spring, MD, E-mails: monika.simmons@med.navy.mil and susana.widjaja@med.navy.mil. George Mitchell and Dolland Noel, Ministry of Health, Government of Grenada, Saint George, Grenada, E-mails: mitgeogw@gmail.com and dnoel@sgu.edu. Satesh Bidaisee, Public Health and Preventive Medicine, St. George's University, Saint George, Grenada, E-mail: sbidaisee@sgu.edu. Todd E. Myers, Naval Infectious Diseases Diagnostic Laboratory, Silver Spring, Naval Medical Research Center, MD, E-mail: todd.e.myers.mil@mail.mil. A. Desiree LaBeaud, Pediatric Infectious Diseases, Stanford University, Stanford, CA, E-mail: dlabeaud@stanford.edu.
References
- 1.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 2.Gubler D. The emergence of epidemic dengue fever and dengue hemorrhagic fever in the Americas: a case of failed public health policy. Rev Panam Salud Publica. 2005;17:221–224. doi: 10.1590/s1020-49892005000400001. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis. 1998;4:442–450. doi: 10.3201/eid0403.980326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 5.Pastorino B, Muyembe-Tamfum JJ, Bessaud M, Tock F, Tolou H, Durand JP, Peyrefitte CN. Epidemic resurgence of chikungunya virus in Democratic Republic of the Congo: identification of a new central African strain. J Med Virol. 2004;74:277–282. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- 6.Hertz JT, Munishi OM, Ooi EE, Howe S, Lim WY, Chow A, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Gubler DJ, Crump JA. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–177. doi: 10.4269/ajtmh.2012.11-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faruque LI, Zaman RU, Alamgir AS, Gurley ES, Haque R, Rahman M, Luby SP. Hospital-based prevalence of malaria and dengue in febrile patients in Bangladesh. Am J Trop Med Hyg. 2012;86:58–64. doi: 10.4269/ajtmh.2012.11-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis. 2008;14:416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini P, Macini P, Finarelli AC, Pol C, Venturelli C, Bellini R, Dottori M. Chikungunya epidemic outbreak in Emilia-Romagna (Italy) during summer 2007. Parassitologia. 2008;50:97–98. [PubMed] [Google Scholar]
- 10.Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L'Ambert G, Cochet A, Prat C, Foulongne V, Ferre JB, Catelinois O, Fusin O. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20:21108. doi: 10.2807/1560-7917.es2015.20.17.21108. [DOI] [PubMed] [Google Scholar]
- 11.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 12.Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 13.Halstead SB. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg Infect Dis. 2015;21:557–561. doi: 10.3201/eid2104.141723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo TH, Her Z, Tan JJL, Lum FM, Lee WWL, Chan YH, Ong RY, Kam YW, Leparc-Goffart I, Gallian P, Rénia L, de Lamballerie X, Ng LFP. Caribbean and La Réunion chikungunya virus isolates differ in their capacity to induce proinflammatory Th1 and NK cell responses and acute joint pathology. J Virol. 2015;89:7955–7969. doi: 10.1128/JVI.00909-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 16.Fischer M, Staples JE. Notes from the field: chikungunya virus spreads in the Americas: Caribbean and South America, 2013–2014. Morb Mortal Wkly Rep. 2014;63:500–501. [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) First Chikungunya Case Acquired in the United States Reported in Florida. 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html Available at. Accessed January 1, 2016.
- 18.Nsoesie EO, Ricketts RP, Brown HE, Fish D, Durham DP, Ndeffo Mbah ML, Christian T, Ahmed S, Marcellin C, Shelly E, Owers K, Wenzel N, Galvani AP, Brownstein JS. Spatial and temporal clustering of chikungunya virus transmission in Dominica. PLoS Negl Trop Dis. 2015;9:e0003977. doi: 10.1371/journal.pntd.0003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega-Rua A, Lourenco-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yebakima A, Gustave J, Girod R, Dusfour I, Leparc-Goffart I, Vanlandingham DL, Huang YJ, Lounibos LP, Mohamed Ali S, Nougairede A, de Lamballerie X, Failloux AB. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9:e0003780. doi: 10.1371/journal.pntd.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gay N, Rousset D, Huc P, Matheus S, Ledrans M, Rosine J, Cassadou S, Noel H. Seroprevalence of Asian lineage chikungunya virus infection on Saint Martin Island, 7 months after the 2013 emergence. Am J Trop Med Hyg. 2015;94:393–396. doi: 10.4269/ajtmh.15-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons M, Myers T, Williams M, Jungkind D, Houng H. Development and validation of a quantitative, one-step, multiplex, real-time RT-PCR assay for the detection of dengue and chikungunya viruses. J Clin Microbiol. 2016;54:1766–1773. doi: 10.1128/JCM.00299-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahadeo N, Mohammed H, Allicock OM, Auguste AJ, Widen SG, Badal K, Pulchan K, Foster JE, Weaver SC, Carrington CV. Molecular characterisation of chikungunya virus infections in Trinidad and comparison of clinical and laboratory features with dengue and other acute febrile cases. PLoS Negl Trop Dis. 2015;9:e0004199. doi: 10.1371/journal.pntd.0004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattar S, Miranda J, Pinzon H, Tique V, Bolanos A, Aponte J, Arrieta G, Gonzalez M, Barrios K, Contreras H, Alvarez J, Aleman A. Outbreak of chikungunya virus in the north Caribbean area of Colombia: clinical presentation and phylogenetic analysis. J Infect Dev Ctries. 2015;9:1126–1132. doi: 10.3855/jidc.6670. [DOI] [PubMed] [Google Scholar]
- 24.Sissoko D, Ezzedine K, Moendandze A, Giry C, Renault P, Malvy D. Field evaluation of clinical features during chikungunya outbreak in Mayotte, 2005–2006. Trop Med Int Health. 2010;15:600–607. doi: 10.1111/j.1365-3156.2010.02485.x. [DOI] [PubMed] [Google Scholar]