Abstract
Objective To identify and characterise non-specific immunological effects after routine childhood vaccines against BCG, measles, diphtheria, pertussis, and tetanus.
Design Systematic review of randomised controlled trials, cohort studies, and case-control studies.
Data sources Embase, PubMed, Cochrane library, and Trip searched between 1947 and January 2014. Publications submitted by a panel of experts in the specialty were also included.
Eligibility criteria for selecting studies All human studies reporting non-specific immunological effects after vaccination with standard childhood immunisations. Studies using recombinant vaccines, no vaccine at all, or reporting only vaccine specific outcomes were excluded. The primary aim was to systematically identify, assemble, and review all available studies and data on the possible non-specific or heterologous immunological effects of BCG; measles; mumps, measles, and rubella (MMR); diphtheria; tetanus; and pertussis vaccines.
Results The initial search yielded 11 168 references; 77 manuscripts met the inclusion criteria for data analysis. In most included studies (48%) BCG was the vaccine intervention. The final time point of outcome measurement was primarily performed (70%) between one and 12 months after vaccination. There was a high risk of bias in the included studies, with no single study rated low risk across all assessment criteria. A total of 143 different immunological variables were reported, which, in conjunction with differences in measurement units and summary statistics, created a high number of combinations thus precluding any meta-analysis. Studies that compared BCG vaccinated with unvaccinated groups showed a trend towards increased IFN-γ production in vitro in the vaccinated groups. Increases were also observed for IFN-γ measured after BCG vaccination in response to in vitro stimulation with microbial antigens from Candida albicans, tetanus toxoid, Staphylococcus aureas, lipopolysaccharide, and hepatitis B. Cohort studies of measles vaccination showed an increase in lymphoproliferation to microbial antigens from tetanus toxoid and C albicans. Increases in immunogenicity to heterologous antigens were noted after diphtheria-tetanus (herpes simplex virus and polio antibody titres) and diphtheria-tetanus-pertussis (pneumococcus serotype 14 and polio neutralising responses) vaccination.
Conclusions The papers reporting non-specific immunological effects had heterogeneous study designs and could not be conventionally meta-analysed, providing a low level of evidence quality. Some studies, such as BCG vaccine studies examining in vitro IFN-γ responses and measles vaccine studies examining lymphoproliferation to microbial antigen stimulation, showed a consistent direction of effect suggestive of non-specific immunological effects. The quality of the evidence, however, does not provide confidence in the nature, magnitude, or timing of non-specific immunological effects after vaccination with BCG, diphtheria, pertussis, tetanus, or measles containing vaccines nor the clinical importance of the findings.
Introduction
Many published reports and commentaries have suggested that several vaccines routinely administered to infants could have heterologous or non-specific effects on mortality, unrelated to the prevention of illness and deaths caused by the specific diseases against which the vaccines were developed.1 2 3 For example, studies have suggested that receipt of both the BCG and measles vaccine are associated with a reduced risk of death (that is, all cause mortality) beyond that expected by a reduction in deaths from measles and tuberculosis, while receipt of diphtheria-tetanus-pertussis (DTP) vaccine might be associated with an increased risk of death, at least among female infants.4 5 6 Nearly all studies that showed these effects were observational or derived from secondary analyses. Consequently, poorly controlled or uncontrolled confounding and various types of selection and information bias have been suggested as alternative justifications for these findings.7 8
The biological plausibility of one or more vaccines having heterologous effects, either detrimental or beneficial, is supported by several studies in animals and observations in humans.9 10 11 12 Indeed it is these heterologous properties that are exploited in specific circumstances in adults in whom BCG has been used for the treatment of bladder cancer and melanoma, while MMR has been used as a treatment for warts.13 14 15 Nevertheless, the biological mechanisms and immune pathways that could underlie and rationalise such effects remain largely unspecified. The WHO Strategic Advisory Group of Experts (SAGE) requested the WHO Secretariat to review the evidence surrounding the possible non-specific/heterologous effects of vaccines included in the routine infant immunisation schedule in 2013.16 The WHO Secretariat working group commissioned this review to determine whether the current evidence is sufficient to warrant further scientific investigation and, if so, to define the path towards obtaining unequivocal evidence on these issues that would support future robust, evidence based adjustments to immunisation policies, if warranted.
The possible implications of any such heterologous effects of vaccines for the formulation or re-formulation of the infant immunisation schedule remain unclear, but it has been suggested that if such effects can be established beyond a reasonable doubt, the infant immunisation schedule might need to be reconfigured.16 Previous reviews, including periodic assessments by the WHO Global Advisory Committee on Vaccine Safety, have indicated that any such effects remain inconclusive and are therefore not a justification for altering the current schedule recommendations.17 At the meeting of SAGE during which the data from this review were presented, no changes to the current policy were recommended.18
We systematically identified, assemble, reviewed, and critically appraised all available human studies with immunological endpoints describing the possible non-specific or heterologous effects of BCG, diphtheria, pertussis, tetanus, and measles containing vaccines.
Methods
Definitions
We defined specific immunological effects as an effect of immunisation on an immunological variable in response to an antigen contained within the initial immunisation. The terms heterologous and non-targeted effects have also been used in the literature to describe non-specific immunological effects. For the purpose of this review we defined non-specific immunological effects as an effect on the immune system as a result of immunisation that modifies the way it subsequently responds to antigens that were not present in the initial immunisation.
Study design and selection criteria
Using a comprehensive search strategy, we identified and critically appraised available evidence (published and unpublished) that addressed possible non-specific effects of vaccines. We included in the review randomised controlled trials, quasi-randomised control trials, clinical trials, cohort studies, case-control studies, case series, and case reports. The vaccines examined included live attenuated vaccines (BCG and measles containing vaccines), inactivated vaccines, and toxoids (all diphtheria and tetanus toxoids, and Bordetella pertussis containing vaccines). Though the target population was infants aged under 5, inclusion of studies was not limited to this age group to ensure all relevant studies were identified. Sex, age at vaccination, and co-administration of vitamin A were examined as possible effect measure modifiers.
Were excluded ecological, animal, and in vitro studies; studies using recombinant vaccines or no vaccine at all; and those studies reporting/generating only immunological endpoints specific to the study vaccine.
Search strategy
Embase.com, which includes all records from Medline, was searched from 1947 to December 2012 (appendix 1). Complementary, less extensive searches of the PubMed library, the Cochrane library, and trip database, were performed to detect any articles missed by the search on Embase.com. In addition, we manually searched the reference lists of all included articles found and all relevant review articles to identify studies not included in the previously described search. Experts in the specialty were asked if they were aware of any unpublished reports of studies possibly meeting the inclusion criteria. Full text of all articles identified were sought, using internet downloads, interlibrary loans, and contacting of authors. Articles in any language were sought. We carried out a further limited search from December 2012 to January 2014 in the PubMed library using the same search terms to provide an update. Experts in the specialty were also asked to review the initial search results and identify any further studies that could be included.
Data extraction
We used specifically created data extraction forms and DistillerSR software to acquire consistent data from studies, such as participants, methods, potential confounders and background data. All relevant data were extracted from articles meeting inclusion criteria and entered on the database. There was no pre-specified effect measure that was of interest but rather all summary estimates of effect were extracted whenever possible.
Selection of eligible Studies
Two independent reviewers examined each full text article, and a database of studies considered eligible for inclusion was created (tables A-F in appendix 2). We included in the review studies identified by both reviewers as being eligible for inclusion and having adequate data for extraction. Authors of studies in which non-specific immunological data were generated but not reported were requested to provide data. When there were discrepancies, the reasons for these were discussed, and a decision about inclusion was reached by consensus. If there was no agreement, a further independent reviewer adjudicated to make a final decision regarding eligibility.
Assessment of risk of bias
At least two independent reviewers assessed each included study according to the Cochrane Library risk of bias tool (tables G-L, appendix 2). When there was a conflict in the assessment, a third independent reviewer adjudicated to make a final decision. The overall risk of bias was calculated by assigning each criteria a score of 0 for high, 1 for unclear, and 2 for low and then averaging the total score across all of the criteria.
Data analysis
We generated descriptive tables summarising information about study design, study quality, and results of all included studies. Data on non-specific immunological effects were extracted from papers, which reported summary statistics in tabular form. When results were presented in figures, we extracted data whenever possible with GetData Graph Digitizer version 2.26.0.20.
We summarised the overall immunological outcomes of the included studies graphically for all available variables to provide a perspective on the effect of vaccination on these variables. We calculated the direction of effect for each variable by creating a ratio of the response in those vaccinated compared with the response in the unvaccinated participants, or alternatively the ratio of the estimated response after vaccination compared with the response before vaccination. The response could be presented as a median, mean, geometric mean, fold-rise, or proportion of seroconversion, depending on the statistics reported in the paper. Participants were not all measured at comparable time points across studies nor were of similar age. We could not formally combine these ratios as they are statistically non-comparable, the timings differ, and many studies did not present estimates of variability. Not all studies reported results of significance tests and for those that did the statistical test reported was not always the appropriate one. Furthermore, because of the multitude of variables tested and small study sizes, there were related multiple testing issues that would have increased the rate of type 1 errors. For these reasons we designed the plots to be descriptive of the overall diversity of responses and point to any general trends that could be occurring in the data without the calculation of any summary statistics or testing specific hypotheses that would have required more consistent data. For papers that reported comparisons at multiple time points for the same children we plotted only the first comparison so that each cohort of children is reported only once per study per variable per type of stimulant.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Results
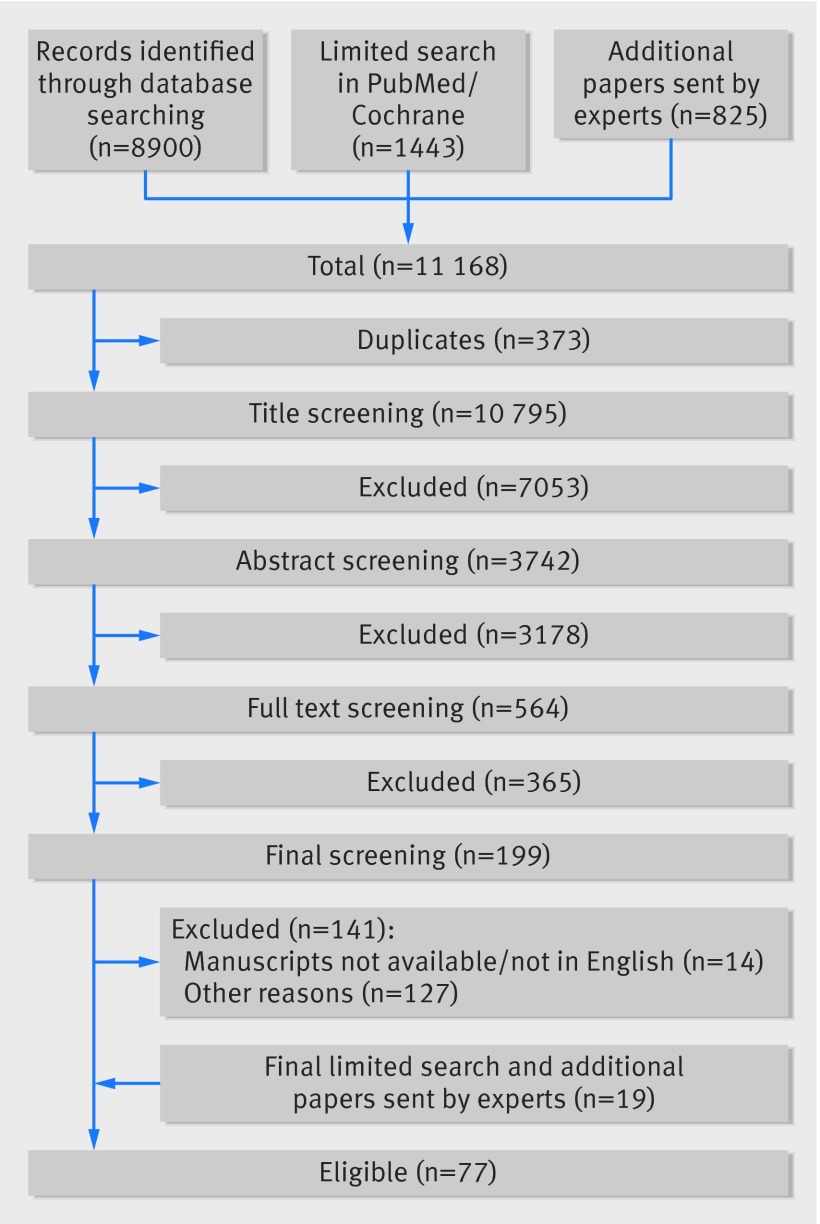
The search yielded 11 168 references, and 77 studies met our eligibility criteria (fig 1). Fourteen of these studies were from submissions made by experts in the specialty. Of the two studies published on the randomised controlled trial by Burl and colleagues, we included additional data from the associated PhD thesis.19 We identified relatively equal proportions of randomised controlled trials, cohort studies, and case-control studies (table 1). There was a wide range (3-2345; mean 206) of total study participants involved across the studies. Most studies (37/77, 48%) used BCG as the study vaccine intervention, while 47/77 (61%) were exclusively conducted in children. In 54/77 (70%) studies, the final time point of outcome measurement was performed between one and 12 months after vaccination. At least one non-specific immunological variable was reported as significant in 29/77 (38%) of the studies (tables 2-5). Because of the heterogeneity, we could not conduct a meta-analysis and for that reason none of the trends, where evident, are statistically sound.
Fig 1 Identification of studies in review of non-specific immunological effects of selected routine childhood immunisations
Table 1.
Overview of studies included in systematic review of non-specific immunological effects of selected routine childhood immunisations
| Study vaccine | No of participants |
|---|---|
| BCG | 37 |
| Measles | 14 |
| MMR | 3 |
| DTP | 7 |
| Pertussis | 1 |
| DT | 4 |
| TT | 11 |
| Other vaccine/s used in study: | |
| Yes | 24 |
| No | 31 |
| Not described/not applicable | 22 |
| Age group: | |
| Neonate | 15 |
| Infant | 18 |
| Children | 14 |
| Adults | 19 |
| Elderly | 0 |
| Combination | 11 |
| Sex of study population: | |
| Male and female | 39 |
| Male | 2 |
| Female | 1 |
| NR | 35 |
| Geographical location: | |
| Africa | 19 |
| Europe | 22 |
| Asia | 8 |
| Americas | 20 |
| Oceania | 4 |
| Combination | 4 |
| Co-administration with vitamin A? | |
| Yes | 3 |
| No/NR | 74 |
| Presence of attribute that could affect response? | |
| Yes | 22 |
| No | 55 |
| Interval between vaccine administration and final outcome measure (months): | |
| <1 | 11 |
| 1-<6 | 29 |
| 6-≤12 | 25 |
| >12 | 10 |
| NR | 2 |
| No of participants: | |
| Mean | 206 |
| Median | 77 |
| Range | 3-2345 |
| Study design: | |
| Randomised controlled trial | 25 |
| Prospective cohort | 23 |
| Prospective case-control | 23 |
| Other | 6 |
NR=not reported.
Table 2.
Summary characteristics of all included BCG studies with significant findings reported
| Author | Vaccine | Design | Participants | Interventions | Outcomes (time of measurement) | NSIE outcomes | Difference in NSIE outcome | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Burl, 200919 | BCG | RCT | Neonates (n=103) | BCG at birth or 4.5 months | Cytokine (IL-10, IL-13, and IFN-γ) responses from whole blood cultures stimulated with PPD, SEB, BCG, and ESAT-6/CFP-10. Blood phenotyping (birth, 4.5, and 9 months) | Cytokine responses from whole blood cultures stimulated with SEB. Blood phenotyping | Increased IL-10 production at 4.5 months in SEB stimulated cultures of vaccinated v unvaccinated (P<0.042) | Low |
| Djuardi, 201020 | BCG | Cohort | Indonesian newborns (n=147) | BCG | Cytokine responses (baseline, 5, 12, and 24 months) | Cytokine responses from whole blood cultures stimulated with PHA, LPS, or media only | Increased IFN-γ production to PHA at 24 months v baseline (P<0.001). Decreased IL-5 to PHA at 5 months v baseline (P<0.05). Decreased TNF-α and IL-10 to LPS at 24 months v baseline (P<0.001) | High |
| Faustman, 201221 | BCG | RCT | Type 1 diabetic adults and healthy controls (n=12) | BCG v placebo | T cells, autoantibodies and C peptide (up to weekly sampling from baseline to week 20 after vaccination) | Autoreactive T cells, insulin autoantibodies (GAD, IA-2A, ZnT8A), C peptide | GAD: two BCG participants had significant (one increase and one decrease) changes from baseline. ZnT8A: decrease in one BCG subject only. (All values after vaccine compared with baseline values with regression model) | Unclear |
| Kleinnijenhuis, 201222 | BCG | Cohort | Adults (n=20) | BCG | Cytokines in response to TB, S aureus and C albicans and phenotype of circulating monocytes. (baseline, 2 weeks, and 3 months after vaccination) | IFN-γ, TNF-α, and IL-1β production from PBMC cultures stimulated with S aureus and C albicans. No of CD14 cells with TLR4 and CD11b surface expression. IL-1β and TNF-α mRNA expression | IFN-γ, TNF-α, and IL-1β increase at 2 weeks and 3 months (except S aureus and IL-1β at 2 weeks). CD14 cells increase at 2 weeks (P<0.05). CD14 TLR4 expression decreases at 2 weeks (P<0.05) and increase at 3 months (P<0.005). CD14 CD11b expression increases at 2 weeks (P<0.05) and 3 months (P<0.01) | High |
| Libraty, 201423 | BCG | Case-control | Infants (n=51) | BCG in first 2 weeks or after first DTP | IFN-γ ELISpot. Flow cytometry (age 8-12 weeks) | IFN-γ ELISpot to TT, polio, HBsAg, and PHA. T cell intracellular cytokine staining for; IFN-γ+/TNF-α+/CD4+/CD4RO+/−FoxP3+TNF-α/CD4+/CD4RO+/- | Between age 8-12 age infants vaccinated with BCG as neonates had increased IFN-γ ELISpot to TT (P=0.046) and IFN-γ+/TNF-α+/CD4+/CD4RO+ T cells (P=0.018) compared with infants who did not receive BCG as neonate | Unclear |
| Marks, 200324 | BCG | Case-control | Children (n=751) | BCG v unvaccinated | Cytokine responses, Clinical components of allergy (age 7-14 years) | IL-4, IL-5, IL-10 and IFN-γ secreted in response to house dust mite stimulation of PBMCs. Total serum IgE | IL-10 lower for BCG recipients (P<0.001) v unvaccinated at age 7-14 years | High |
| Ota, 200225 | BCG | RCT | Newborns (n=151) | BCG at birth, 2, or 4.5 months | Proliferation, cytokine, and vaccine specific antibody responses (birth, 2, and 4.5 months) | PBMC proliferation to HBsAg, TT, and PHA. IL-5, IL-13 and IFN-γ PBMC responses to HBsAg, TT and PHA. Antibody responses to HBsAg, TT, polio, and diphtheria toxin | At age 2 and 4.5 months proliferation, IL-5, IL-13, and IFN-γ responses to TT and HBsAg higher in groups where BCG was given earlier. At age 2 and 4.5 antibody responses to HBsAg higher in group given BCG at birth compared with controls (P=0.03 and P=0.004). At 4.5 months antibody titres to PV1 higher in group receiving BCG at 2 months compared with controls (P=0.002) | Unclear |
| Tastan, 200526 | BCG | RCT | Newborns in Turkey (n=40) | BCG at birth v 2 months | Total lymphocytes and TCR phenotyping (birth and 2 months) | Total lymphocytes, αβ+ T cells, and γδ+ T cells | Increased total and TCR- lymphoctyes (P=0.001) and decreased αβ+ cells (0.01) at 2 months for group vaccinated at birth compared with 2 months | Unclear |
| Vargas, 200427 | BCG | RCT | Asthmatic children in Mexico (n=82) | BCG v placebo | Symptoms questionnaire, leukocyte count, eosinophil count, IgE, esosinophils in nasal mucus, parasites in stools. IL-4 and IFN-γ from PMA and ionomycin stimulated PBMCs (baseline and 12 months after vaccination) | Symptoms questionnaire. Leukocyte count, eosinophil count, IgE, eosinophils in nasal mucus, parasites in stools. IL-4 and IFN-γ from phorbol myristate acetate and ionomycin stimulated PBMCs | Significant change over time in placebo group for IgE (P<0.002), IL-4 (P<0.05), and IFN-γ (P<0.05). Decrease in monocyte (P<0.001) and eosinophil percentage (P<0.05) for BCG and decrease in monocyte (P<0.05), eosinophil (P<0.05) and eosinophil percentage (P<0.001) for placebo from nasal cytology | Unclear |
| Vijaya Lakshmi, 200528 | BCG | Case-control | Children (n=107) | BCG v unvaccinated and active TB patients | Lymphoproliferation and cytokine responses (age 5-7 years) | IL-2 dependent lymphocyte transformation supplemented with supernatant from lymphocyte culture stimulated with control antigens. Supernatant IFN-γ of lymphocyte cultures stimulated with control antigens | Higher stimulation index of lymphocyte transformation for vaccinated v unvaccinated healthy children (P<0.05). Increased IFN-γ in vaccinated children (P<0.01) | High |
| Weir, 200429 | BCG | RCT | Malawi adults (n=633) and UK children (n=424) | BCG v placebo | Cytokine responses to mycobacterial antigens. (baseline and 12 months after vaccination) | Cytokines (TNF-α, IL-10, IL-1β) from whole blood culture supernatants | IL-10 decrease from baseline in Malawi subjects for M scrofulaceum and M vaccae only (P=0.015 and 0.02) | Unclear |
NSIE=non-specific immunological effects, TCR=T cell receptor, PPD=purified protein derivative, SEB=staphylococcal enterotoxin B, GAD=glutamic acid decarboxylase, TT=tetanus toxoid, TB=Mycobacterium tuberculosis, PBMC=peripheral blood mononuclear cells, PHA=phytohaemagglutinin, HBsAg=hepatitis B surface antigen.
Table 3.
Summary characteristics of all included measles and MMR vaccine studies with significant findings reported
| Author | Vaccine | Design | Participants | Interventions | Outcomes (time of measurement) | NSIE outcomes | Difference in NSIE outcome | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Hennino, 200730 | Measles | RCT | Infants in the US with atopic dermatitis (n=12) | Schwartz strain v placebo | Severity of atopic dermatitis (baseline, 1, 3 and 6 months after vaccination), seroconversion, serum levels of CCL18 and E-selectin (baseline and 1 month after vaccination) | Severity of atopic dermatitis, seroconversion, serum levels of CCL18 and E-selectin | CCL18 decrease in 2 individual measles treated participants compared to baseline (P=0.018 and 0.001) | High |
| Hussey, 199631 | Measles | Cohort | Infants in South Africa (n=88) | EZ strain at 6 months or Schwarz strain at 6 and 9 months or Schwarz strain at 9 months | Measles antibody responses. Proliferation, IL-2 receptor, CD4, CD8, β2 microglobulin and neopterin in response to PHA. Lymphocyte subsets. Comparison between males and females (baseline, 2 weeks, and 3 months after vaccination) | Proliferation, IL-2 receptor, CD4, CD8, β2 microglobulin and neopterin in response to PHA. Lymphocyte subsets | Decrease in PHA proliferation in Schwarz strain groups at 3 months after immunisation (P=0.013, 0.002). Decrease in PHA proliferation at 2 months after immunisation in Schwarz strain at 9 months group (P<0.001). Increase in soluble CD8 (P=0.02) and β2 microglobulin (P=0.04) from baseline in Schwarz strain at 9 months group. Difference between all groups for soluble IL-2 receptor (P<0.001), soluble CD4 (P=0.015) and absolute CD8 count (P=0.008) | High |
| Lisse, 199432 | Measles | Case-control | Guinea-Bissau infants (n=78) | Medium or high titre EZ strain at 4 months, or standard titre Schwarz strain at 9 months or control | Total white cells and lymphocyte subsets (presented for each trial and by sex) (age 3-5 years) | Total white cells and lymphocyte subsets (presented for each trial and by sex) | Lower percentage of lymphocytes and CD4:CD8 ratio and higher percentage and total CD8 cells in females receiving high titre EZ strain (P<0.05) | High |
| Ovsyannikova, 200333 | Measles | Cohort | Infants/children in the USA (n=57) | Edmonston strain, first dose in infants vs second dose in children | PHA stimulated cytokine production (IL-2, IL-4, IL-6, IFN-γ and TNF-α), plasma cytokine concentrations, and measles antibody titres (baseline, 2, 5, 10, 15, 20, 30, and 40 days after vaccination) | PHA stimulated cytokine production (IL-2, IL-4, IL-6, IFN-γ and TNF-α), plasma cytokine concentrations | In children decrease in IFN-γ to PHA at day 20. Higher overall median IFN-γ to PHA in children compared with infants. Decrease in median plasma IFN-γ (P=0.0027), TNF-α (P=0.0001), and sIL2-R (P=0.0001) in children compared with infants | High |
| Samb, 199534 | Measles | Case-control | Rural Senegalese children (n=136) | Rabies vaccine after high titre EZ at 5 months v placebo at 5 months and Schwarz at 10 months (n=143) | Immunogenicity to measles, yellow fever, and rabies vaccines. Skin tests. Haematological variables (baseline and 3 months after rabies vaccination; baseline age 36-44 months) | Rabies and yellow fever immune responses. CD3, CD4, and CD8 lymphocyte counts | Females who previously received EZ had higher rabies neutralisation (P=0.012) and ELISA (P=0.03) antibody at 3 months after initial rabies vaccine dose | Unclear |
| Schnorr, 200135 | Measles | RCT | Bangladeshi infants (n= 78) | Standard titre EZ or had Schwarz at 6 and 9 months v 9 months only v unvaccinated (n=78) | Measles antibody titres. DTH skin test. Cell phonotype. Cytokine responses in response to stimulation with PHA (1, 6, and 24 weeks after vaccination) | DTH skin test. Cell phenotype. Cytokine responses in response to stimulation with PHA | Increased anergy to candida in DTH assay (P=0.015 for 6 month group and P=0.04 for 9 month group) Difference in CD71 (P=0.04), and CD30 (P=0.004) expression between groups at baseline. Difference in expression of CD25 (P=0.02), CD69 (P=0.04), CD71 (P=0.04), and CD30 (P=0.006) between groups from week 1 to week 6. Difference in expression of NK (P=0.009) and CD69 (P=0.03) between groups at week 24. Increased IL-2 (P=0.007) at 6 weeks and IL-10 (P=0.04) at 24 weeks between groups | High |
| Pabst, 199736 | MMR | Cohort | Infants (n=124) | MMR | PBMC blast transformation to measles antigen, Vero cell control antigen, TT, and candida antigen. Production of sIL-2r, IFN-γ, IL-4 and IL-10 stimulated by PHA. CD4, CD8 and NK cells (baseline, 14, 22, 30 and 38 days after vaccination) | PBMC blast transformation to Vero cell control antigen, TT and candida antigen. Production of sIL-2r, IFN-γ, IL-4 and IL-10 stimulated by PHA. CD4, CD8, and NK cells | Decrease in PBMC blast transformation to candida antigen (P<0.01) at day 22 v day 0. CD4 decrease at day 22 and 38; CD8 increase at days 14, 30, and 38; NK cell increase at day 14 and 30 (P<0.05). Increased IFN-γ to PHA at day 14 and 38 (P<0.01) | Unclear |
| Rager-Zisman, 200337 | MMR | Cohort | Children (n=38) | MMR | Measles, mumps, and rubella IgG, total IgM, IgG and IgE. White cell count, CD8, CD4 and CD4:CD8. Lymphoproliferative responses to PHA and TT. NK cells and NK specific activity (baseline and 30 days after vaccination) | White cell count, CD8, CD4 and CD4:CD8. Lymphoproliferative responses to PHA and TT. NK cells and NK specific activity | Decrease in total leukocytes (P<0.001), CD4% (P=0.028), and CD8% (P=0.041) from before to after immunisation. Increased proliferative response to TT (P=0.006) from before to after immunisation. Increase in CD56+ cells (P=0.01) from before to after immunisation | Unclear |
NSIE=non-specific immunological effects, PPD=purified protein derivative, TT=tetanus toxoid, PBMC=peripheral blood mononuclear cells, PHA=phytohaemagglutinin, EZ=Edmonston-Zagreb, DTH=delayed type hypersensitivity, NK=natural killer.
Table 4.
Summary characteristics of all included tetanus toxoid vaccine studies with significant findings reported
| Author | Vaccine | Design | Participants | Interventions | Outcomes (time of measurement) | NSIE outcomes | Difference in NSIE outcome | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Armitage, 199338 | TT | Case-control | Elderly and young adults (n=46) | TT based on vaccination history | Immunogenicity, proliferation and blastogenesis (2 weeks post vaccination) | Lymphoproliferation to ConA and PHA | Decrease in ConA and PHA blastogenesis in elderly v young adults (P=0.01 and P=0.001) | High |
| Borut, 198039 | TT | Case-control | Agammaglobulinaemic and healthy children, and adults (n=80) | TT v unvaccinated | Skin testing, lymphocyte proliferation, monocyte chemotaxis, and immunogenicity (single measurement at recruitment) | Monocyte chemotaxis | Higher monocyte chemotaxis in those with positive tetanus toxoid skin test (P<0.01) | High |
| Chollet, 197940 | TT | Case-control | Adults (n=75) | Boosting with TT v no boosting | Lymphocyte proliferation, phenotyping (by electrophoresis), cytotoxicity, and rosettes (baseline, 1, 2, 3, 4, and 8 days after vaccination) | B, T1, and T2 cell phenotypes by electrophoresis. Lymphoproliferation to PHA, PWM, and ConA | Increase in T2 cells at days 2 (P<0.01), 3 (P<0.01), and 8 (P<0.05) and T1 cells at day 2 (P<0.05) and 3 (P<0.01) in vaccinated group | High |
| Gentile, 200641 | TT | Case-control | Adults with and without allergic rhinitis (n=30) | TT | PBMC cytokine responses, skin testing to allergens, and TT immunogenicity (baseline, 3, 7, 14, and 28 days after vaccination) | IFN-γ and IL-13 responses to PHA | PHA and TT induced IFN-γ increased on days 7 and 14 for non-allergic rhinitis v allergic rhinitis group (P<0.05). PHA induced IL-13 was increased on day 7 for non-allergic rhinitis v allergic rhinitis group (P<0.05) | High |
| Mahalingham, 201042 | TT | RCT | Adult females (n=108) | Palm oil v placebo. All participants received TT | Lymphoproliferation, cytokines in culture supernatants, TT immunogenicity, and plasma vitamin E concentration (baseline, 28, and 56 days after recruitment). NB TT given on day 28 | IFN-γ and IL-4 production in response to ConA. L-6 production in response to LPS | Increase in ConA induced IFN-γ and IL-4 at day 56 for both groups (P<0.001). Increase in IL-6 to LPS in the intervention group at day 56 (P<0.001) | Unclear |
NSIE=non-specific immunological effects, ConA=Concanavalin A, PWM=pokeweed mitogen, LPS=lipopolysaccharide, TT=tetanus toxoid, PBMC=peripheral blood mononuclear cells, PHA=phytohaemagglutinin.
Table 5.
Summary characteristics of all included DTP and DT vaccine studies with significant findings reported
| Author | Vaccine | Design | Participants | Interventions | Outcomes (time of measurement) | NSIE outcomes | Difference in NSIE outcome | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Fernande, 201043 | DT | Cohort | Male adults in Canada (n=20) | Single dose of tetanus/diphtheria vaccine | Frequency of B cell subsets. IgG, IgA and tetanus/diphtheria specific antibody secretion. IgA and IgG antibody to polio virus and HSV (baseline, 7, and 14 days after vaccination) | Frequency of B cell subsets. IgA and IgG antibody to polio virus and HSV | Increase in HSV IgG (P≤0.01), IgA (P<0.01), and polio virus IgA (P<0.005) at day 7 v baseline | Unclear |
| Halasa, 200844 | DTP | RCT | Infants (n=50) | DTaP and HBV at birth v HBV only at birth | Adverse reactions. IgG to PT, FHA PRN and Fimbriae 2/3. PRP Hib capsule antigen. Pneumococcal capsule antigens, diphtheria and tetantus toxoids. Neutralisation assays for polio and HBsAg antibodies (baseline, age 6, 7, 17, and 18 months) | Polio neutralisation assays, pneumococcal serotypes 6, 14, 23 and HBsAg antibodies | Higher GMC for pneumococcal serotype 14 in controls at 7 months (P=0.035) and higher microneutralisation titres in controls for poliovirus 1 and 3 at 18 months (P=0.39 and 0.041) | Unclear |
| Heine, 201145 | DT | RCT | Adults (n=32) | Oral vitamin D3 oil/day v equal volume of neutral oil (participants received tetanus/diphtheria toxoid vaccine at 9 weeks) | 25-hydroxyvitamin D. TT specific IgG and IgA. Peripheral B and T lymphocytes. T cell activation to stimulation by SEB. TT specific cytokine profile (IL-2, IL-4, IL-5, IL-10, TNF-α and IFNγ). Adverse events. Leukocyte counts and IgG, IgA and IgM titres (baseline and 7 days after vaccination) | T cell activation to stimulation by Staphylococcus enterotoxin and no antigen. Pre vaccination data not reported. Leukocyte counts and IgG, IgA and IgM titres | Monocyte count decreased in placebo group (P=0.04). | High |
| Rowe, 200046 | DTP | Cohort | Infants in Australia (n=55) | DTaP at 2, 4, and 6 months. Infants also received oral polio and Hib titre vaccines | IL-4, IL-5, IL-6, IL-9, IL-10, IL13, and IFN γ stimulated by TT and PHA. IL-4 and IL-9 mRNA (2, 4, 6, and 12 months of age) | IL-4, IL-5, IL-6, IL-9, IL-10, IL13, and IFN-γ stimulated by PHA | Increase in IL-5 (P=0.01) and IL-13 (P=0.01) at 12 v 6 months. Increase in IL-5 (P=0.05) at 6 v 4 months | High |
| El Yousfi, 200547 | DT | Case-control | Elderly v young adults (n=15) | Single dose DT-polio and Typhim Vi vaccine | Acute phase proteins (CRP, AGP, Fibrinogen, α1-Antitrypsin, Haptoglobulin, Albumin, Transthyretin, Transferrin). White cells counts. Plasma cytokine levels (TNFα, IL-6, IL-10). IL-6 and IL-10 production by LPS stimulated whole blood. IFN-γ production by PHA stimulated whole blood (baseline and 2 days after vaccination) | Acute phase proteins (CRP, AGP, fibrinogen, α1-antitrypsin, haptoglobulin, albumin, transthyretin, transferrin). White cells counts. Plasma cytokine concentrations (TNFα, IL-6, IL-10). IL-6 and IL-10 production by LPS stimulated whole blood. IFN-γ production by PHA stimulated whole blood | Increase from before to after (day 2) vaccine for CRP (P=0.003), AGP (P<0.001), fibrinogen (P=0.004), haptoglobin (P=0.002) and transthyretin (P=0.01). Increase from before to after vaccine in monocytes (P=0.007), lymphocytes (P=0.002), and neutrophils (P=0.04). Eldery group plasma IL-6 and IL-10 increases from baseline (P<0.05). Both groups had increased IFN- γ production to PHA from baseline (P<0.05). Young adults had increased IL-6 production to LPS from baseline (P<0.05) | Unclear |
NSIE=non-specific immunological effects, DT=diptheria-tetanus, TT=tetanus toxin, PT=pertussis toxin, FHA=filamentous hemagglutinin, PRN=pertactin, HSV=herpes simplex virus, HBsAg=hepatitis B virus surface antigen, SEB=staphylococcal enterotoxin B, CRP=C reactive protein, AGP=alpha-1-acid glycoprotein, LPS=lipopolysaccharide, PHA=phytohaemagglutinin.
BCG vaccine studies
Overall 37 studies were identified that measured non-specific immunological effects of BCG vaccination (appendix 2). In 11 of these papers, the results of assays conducted were not reported as they were not the main focus of the paper. Of the included studies, 24 enrolled children aged under 5 years. Twenty papers reported non-specific immunological effects with data reported in tables or graphs, which could be extracted with a digitizer program, and one study supplied raw data. These papers reported 89 different immunological variables. There were 20 types of stimulants used in theses assays, resulting in 167 unique combinations.
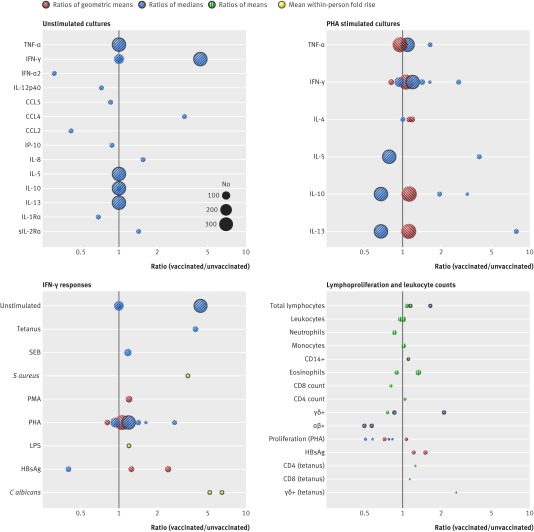
Immunological responses from unstimulated and phytohaemagglutinin (PHA) stimulated cultures were most commonly reported (fig 2; appendix 4 provides detailed tabulated data for the figure). No general patterns according to pro-inflammatory or anti-inflammatory classifications were observable for unstimulated responses when comparisons were between vaccinated and unvaccinated groups, though a distinct increase in IFN-γ was noted within one cohort study reporting cytokine responses in unstimulated cultures after BCG vaccination.20
Fig 2 Non-specific immunological responses to BCG vaccination for unstimulated cultures, PHA stimulated cultures, IFN-γ responses, and lymphoproliferation and leukocyte counts from included BCG studies. Each circle represents responses from one study, calculated as either ratio of responses in those vaccinated compared with those unvaccinated (no black outer circle) or responses before vaccination compared with after vaccination (black outer circles). No estimate of variability is given and pooling of results was not justified because of inconsistency in summary statistics reported and poor reporting of results. Size of circles is proportionate to number of samples analysed. Ratios of geometric means (red circles with left hatching), ratios of medians (blue circles with right hatching), ratios of means (green circles with vertical hatching), and mean fold rise within individual (yellow circles)
Cytokine responses in PHA stimulated cultures showed a trend towards increases in vaccinated compared with unvaccinated groups for all cytokines, although there were studies assessing the pro-inflammatory cytokines IFN-γ and TNF-α that also fell below the null value. In comparisons of cytokine responses in PHA stimulated cultures before and after vaccination within a cohort study, there was a trend towards an increase in pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines.
IFN-γ was the most commonly reported immunological variable. Data for IFN-γ were extracted from 11 papers and one PhD thesis and one study author supplied unpublished raw data from unstimulated assays on request (fig 2). Those studies that made comparisons between vaccinated and unvaccinated groups showed a trend towards an increase in IFN-γ after stimulation. Increases after vaccination were observed in a small cohort study of IFN-γ measured in response to in vitro stimulation with Candida albicans and Staphylococcus aureas.22
T cells and T cell subsets were the most commonly reported leukocyte variable (fig 2). Ex vivo total leukocyte counts had larger cohort sizes and trended around the null value. Counts of subsets for vaccinated compared with unvaccinated groups showed a decrease in neutrophils, CD8, and γδ+ T cells; increase in CD4 T cells and monocytes; and no consistent direction for eosinophils. Cohort studies that compared values before and after vaccination showed an increase in total lymphocytes and CD14+ cells, decrease in αβ+ T cells, and inconsistent effect for γδ+ T cells.22 26 In vitro proliferation assays to tetanus toxoid and hepatitis B surface antigen (HBsAg) all showed increases in the vaccinated compared with unvaccinated group.25 48
Measles vaccine studies
Various different measles vaccine strains and titres were used, with the Edmonston-Zagreb and Schwarz strains most commonly applied (appendix 2, table B). Data available from measles studies reported 23 different immunological variables including B cells, β2 microglobin, CD4, CD4:CD8 ratio, CD8, IFN-γ, IL-10, IL-2, sIL-2Ra, IL-4, IL-6, lymphocytes, lymphoproliferation, malaria parasites, MIP-1β, Neopterin, sCD4, sCD8, T-cell proliferation, TNF-α, and total white blood cell count (WBC). There were six types of stimulants used in these assays (including C albicans, PHA, and tetanus toxoid), resulting in 31 unique combinations. All the papers contained children aged under 5.
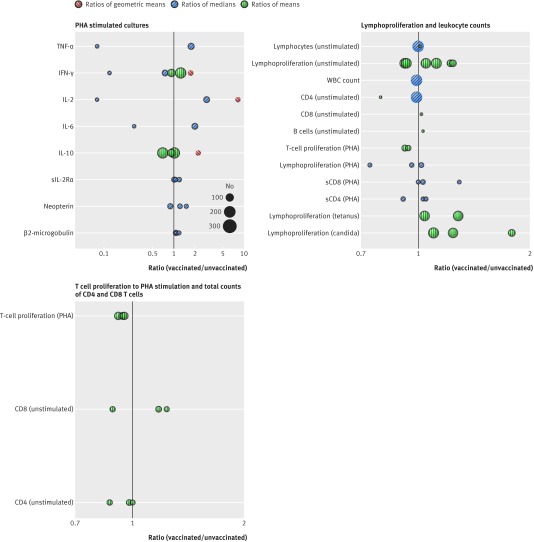
In studies of PHA stimulated assays IFN-γ was the most commonly reported variable followed by IL-10 (fig 3; appendix 4 provides detailed tabulated data for the figure). One study that compared responses between vaccinated groups showed an increase in IFN-γ, IL-2, and IL-10 in the vaccinated compared with the control group.35 The remaining responses to PHA were all from cohort studies and did not show a consistent direction of effect for any of the variables except for sIL-2Ra and β2 microglobulin, which showed small relative increases after vaccination in relatively smaller cohort sizes. Cohort studies in which unstimulated assays were conducted showed decreases in IL-4, MIP-1β, and sIL-2Rα and no consistent direction of response for IFN-γ (fig G in appendix 3).
Fig 3 Non-specific immunological responses to measles and MMR vaccination. PHA stimulated cultures, lymphoproliferation and leukocyte counts, and T cell proliferation to PHA stimulation and total counts of CD4 and CD8 T cells in included MMR vaccine studies. Each circle represents responses from one study, calculated as either ratio of responses in those vaccinated compared with those unvaccinated (no black outer circle) or responses before vaccination compared with after vaccination (black outer circles). No estimate of variability is given and pooling of results was not justified due to inconsistency in summary statistics reported and poor reporting of results. Size of circles is proportionate to number of samples analysed. Ratios of geometric means (red circles with left hatching), ratios of medians (blue circles with right hatching), and ratios of means (green circles with vertical hatching)
Lymphoproliferation was reported in response to tetanus toxoid, Candida species, and PHA stimulation as well as unstimulated assays (fig 3). In cohort studies reporting responses to C albicans and tetanus toxoid, lymphoproliferation was consistently raised after vaccination. Total T cell proliferation was consistently reduced and soluble CD8 responses were consistently increased after PHA stimulation. One study that compared vaccinated with unvaccinated children showed overall values for ex vivo white blood cell counts and in vitro proliferative responses by unstimulated lymphocyte and CD4 T cells that lay close to the null.32
MMR vaccine studies
Overall data were extracted from three papers reporting responses to non-specific stimuli in MMR studies (table C, appendix 2). Two papers conducted studies in children aged under 5, while the third followed up vaccinated infants at mean age of 6.14 years. These papers reported 10 different immunological variables using five types of stimulants, resulting in 13 unique combinations.
CD4, and CD8 responses were the most commonly reported variables in cohort studies of T cell proliferation in response to PHA stimulation (fig 3). T cell proliferation in response to PHA stimulation was consistently reduced. Unstimulated CD4 responses also trended towards a reduction while unstimulated CD8 responses had no consistent direction of effect.
Tetanus vaccine studies
Ten studies reported responses to non-specific stimuli after tetanus vaccination (table D in appendix 2). They reported 21 different immunological variables (primarily lymphocyte proliferation and cytokines) and used 14 types of stimulants, resulting in 36 unique combinations. Only one study (by Borut and colleagues39) involved children aged under 5, who made up only a fraction of the total study cohort.
Two studies reported cytokine production to mitogens after tetanus toxoid vaccination. After PHA stimulation, increases in IFN-γ and IL-13 were noted in participants with non-allergic rhinitis compared with allergic rhinitis.41 One study included women randomised to palm oil or placebo and compared lymphoproliferative responses before and 56 days after vaccine receipt; stimulation with concanavalin A (ConA) resulted in higher IFN-γ and IL-4 titres in both groups, while lipopolysaccharide generated higher IL-6 titres only in women who received palm oil.45 Age seemed to play a role in blastogenesis to ConA and PHA after tetanus toxoid vaccination, with reduced responses in elderly adults compared with young adults.38 One study that examined skin test responses to tetanus toxoid noted that there was increased monocyte chemotaxis in those participants with positive skin test responses.39
DTP, DT, and pertussis vaccine studies
One study explored the effect of vitamin A on cytokine (IFN-γ, TNF-α, IL-10, IL-5, and IL-13) responses in relation to receipt of DTP vaccination and noted no significant differences (fig H in appendix 3).49 This study, however, did report a significant decrease in monocyte count in the group not receiving vitamin A in conjunction with DTP. Interestingly, one DT (diphtheria-tetanus) and one DTP study showed vaccine interference. The DT study showed increased herpes simplex virus and polio antibody titres, while the DTP study showed an increase in antibody to pneumococcus serotype 14 and polio neutralising responses.
We identified 10 studies that contained assays of non-specific immunological responses after immunisation with DTP or DT (table E in appendix 2). Notably, five studies reported to have or were likely to have co-administered a polio vaccine.
One study reported the comparison of a monovalent to trivalent pertussis vaccine in adults (table F in appendix 2).50 In this study culture stimulation indices to tetanus toxoid stimulation were measured but no statistical testing was performed.
Discussion
Principal findings
This is the first systematic review of non-specific immunological effects after human vaccination. Study designs were heterogeneous, and we could not carry out a meta-analysis. Included studies also had a low level of evidence quality. We could not conclude from the current available data that there are any consistent findings to confirm or discard the occurrence of non-specific immunological effects after vaccination with BCG, diphtheria, pertussis, tetanus, or measles containing vaccines. In addition, data from the included papers were not presented in a form that allowed us to assess the effect of sex on non-specific immunological effects. More meaningful conclusions might be drawn if raw data analyses could be conducted with unpublished and published data. If the same summary statistics could be computed for each study then meta-analysis might be possible, though there would still remain large diversity in study design, timing of assessments, and age at vaccination.
Strengths and limitations
Our review showed that a multitude of variables have been used to assess non-specific immunological effects of vaccines over the past six decades. Many of these are reported only once, and it is in these situations that single significant P values need to be interpreted with caution. Stronger evidence for any effect can be found when more than one study has assessed the same variable and when confirmatory results can be found from different studies.
The improvement in technology for testing immunological variables (such as multiplex assays) allows multiple tests to be assessed at one time with one blood sample and greatly increases the chances of false positive results occurring because of chance alone. The standard arbitrary cut point used for significance testing in these situations (P<0.05) means that there is a 5% chance of a false positive result with every P value computed. If a study reports the results of a multiplex assay testing multiple separate variables and each one is tested at the P<0.05 level, then the chances that one of those variables will show a significant difference where none exists is high.
Comparison with other studies
Conceptually, we would expect heterogeneity of responses because the reported variables are singular measures of a complex biological system where a matrix of positive and negative responses by immunological variables can be seen. It is also important to consider potential confounders that could play a role—for example, contamination might explain some changes in unstimulated culture systems. Notably, this review shows some consistent patterns of effect that could be relevant in an immunological context. For example, in most studies that reported IFN-γ responses in people vaccinated with BCG, there was an increase in production of the cytokine in unstimulated and stimulated in vitro conditions, both over time within a cohort and within groups that had received BCG compared with controls. The cytokine profile of PHA stimulated assays from BCG studies also showed a trend towards a pro-inflammatory response in cohort studies. Interestingly, the studies comparing vaccinated versus control groups indicate a more general increase in both pro-inflammatory and anti-inflammatory profiles. This suggests that the effect seen in the cohort studies could be ontogenetic in nature, consistent with in vitro studies of human blood stimulated with toll-like receptor agonists that show an increase in pro-inflammatory and decrease in anti-inflammatory cytokines during infancy.51
Three study cohorts assessed proliferative and IFN-γ responses to HBsAg stimulation of peripheral blood mononuclear cells after BCG vaccination.23 25 In two, IFN-γ showed an increase in the respective variable for the vaccinated compared with the control group.25 Interestingly, responses to bacterial and fungal antigens in both BCG and measles vaccine studies showed increases in the respective variables measured. These findings are consistent with the results of animal studies that have shown acquired cellular resistance as a result of altered responsiveness of monocytes and macrophages.52 This is supported by the trend towards an increase in monocyte responses in BCG vaccinated compared with control groups in this review. Furthermore, both animal and human studies support a role for activation of innate pattern recognition receptors triggering metabolic pathways and epigenetic changes that result in this response.53 22
Conclusions and policy implications
The lack of clear high quality evidence does not confirm or exclude the possibility of non-specific immunological effects after vaccination, which are well described in animal studies and accepted by many immunologists as occurring in humans.54 55 The human data, however, do not provide the necessary evidence to provide any confidence in the nature, quality, quantity, kinetics, or impact of non-specific immunological effects in young children after vaccination nor its translation into explaining morbidity or mortality outcomes.
Measurement of conventional immunological variables (such as antibody titres and cellular responses) provides little mechanistic insight into the relatedness of vaccination to an epidemiological outcome. Technological advances, however, mean that it might now be possible to design studies that can examine this issue systematically. For example, by using systems biology approaches, studies on yellow fever and influenza vaccination have uncovered some key processes that drive vaccine immune responses.56 57 In addition, studies examining disease conditions (including infectious causes) have described the roles specific molecular pathways play in susceptibility.58 Thus systems biology provides an avenue for describing the biological networks that are perturbed by immunisation and how these can mechanistically relate to well defined epidemiological outcomes such as susceptibility to infectious disease. In addition, it has advantages in being able to incorporate complex considerations such as multiple vaccine antigens administered simultaneously. By examining how the molecular expression profiles generated after vaccination relate to those found at the time of a measured epidemiological outcome it would be possible to identify whether heterologous effects exist and, if so, how they exert their effects. Further validation would then be required perhaps in animal models or using in vitro systems.
Design of studies that would aid understanding of heterologous effects of vaccines should take account of four key considerations: identification of the main endpoints (morbidity or mortality) that will be used to assess the importance of any observed immunological effects; strategies to take account of the complexity of multiple antigens given at one time; the order in which vaccines are given in the immunisation schedule; and which biological measurements to use in the assessment. To appropriately examine these considerations it is necessary to undertake pilot mechanistic studies that would use new technological approaches to identify suitable laboratory variables to study in a larger epidemiological study with relevant endpoints. These studies would most readily be carried out in small cohorts of adults or animal based studies in which multiple sampling time points can be acquired, analysed with new high throughput technology such as standardised cellular phenotyping59 and multiplex serological assays coupled with the high dimensional molecular systems biology approaches to define these mechanisms.
Future detailed studies using a systems biology approach to capture the transcriptional, epigenetic, and immunological effects of vaccines could provide data on the timing, duration, quality, and magnitude of such effects. This would entail a rigorous statistical approach to correct for multiple testing. It is particularly important to gain an understanding of whether any such measurable effects are able to influence future inflammatory or innate/acquired immunological responses to exposure with vaccines or infectious agents. If reproducible signals are identified, these could be used in large scale studies with relevant epidemiological endpoints in children to characterise the clinical importance of such vaccine effects.
What is already known on this topic
Observational studies across a limited geographical distribution have suggested the presence of non-specific effects on all cause mortality for BCG, measles, diphtheria, pertussis, and tetanus vaccines
No causal immunological mechanism has yet been elucidated
What this study adds
In some BCG and measles vaccine studies, there are consistent trends or patterns of immunological response after vaccination that are suggestive of non-specific immunological effects
There is no conclusive immunological evidence from previously conducted human studies to support the presence of clinically relevant, geographically generalisable, non-specific immunological effects after vaccination with BCG, diphtheria, pertussis, tetanus, or measles containing vaccines
Web Extra.
Extra material supplied by the author
Appendix 1: Search terms
Appendix 2: Supplementary tables A-L and references
Appendix 3: Supplementary figures with tabulated data
Appendix 4: Detailed tabulated data for figs 2 and 3
We acknowledge the support provided by WHO, Department of Immunization Vaccines and Biologicals, and all the members of the WHO SAGE working group on non-specific effects of vaccines. We also thank Henry Ebron and Peter O’Blenis for their support in using DistillerSR software.
Contributors: RK performed screening, selected articles for inclusions, assessed risk of bias, analysed data, and wrote the report. MV extracted and analysed the data. FMcQ performed screening, selected articles for inclusion, and assessed risk of bias. CS, DO’C, KdN, RR, OO, and UU performed screening and extracted data. AJP revised the protocol, performed screening, and adjudicated on conflicts on article inclusion and risk of bias assessment. All authors approved this version for publication. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. RK and AJP are guarantors.
Funding: This work was supported by WHO. The work was commissioned as an independent evidence review by WHO. WHO staff provided advice about the topic area throughout the project and implemented the search of bibliographic databases.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that AJP has previously conducted vaccine studies on behalf of Oxford University that were sponsored by manufacturers of vaccines but does not receive any personal payments from them. The University of Oxford has received unrestricted educational grants from vaccine manufacturers.
Ethical approval: Not required.
Data sharing: No additional data available. A copy of the review protocol is available from the corresponding author.
Transparency: The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat Immunol 2014;15:895-9. 10.1038/ni.2961 pmid:25232810. [DOI] [PubMed] [Google Scholar]
- 2.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013;34:431-9. 10.1016/j.it.2013.04.004 pmid:23680130. [DOI] [PubMed] [Google Scholar]
- 3.Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2012;2:e000707 10.1136/bmjopen-2011-000707 pmid:22619263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaby P, Ravn H, Roth A, et al. Early diphtheria-tetanus-pertussis vaccination associated with higher female mortality and no difference in male mortality in a cohort of low birthweight children: an observational study within a randomised trial. Arch Dis Child 2012;97:685-91. 10.1136/archdischild-2011-300646 pmid:22331681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaugelade J, Pinchinat S, Guiella G, Elguero E, Simondon F. Non-specific effects of vaccination on child survival: prospective cohort study in Burkina Faso. BMJ 2004;329:1309 10.1136/bmj.38261.496366.82 pmid:15550402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan A, Srivastava R, Dwivedi P, Ng N, Byass P, Pandav CS. Non-specific sex-differential effect of DTP vaccination may partially explain the excess girl child mortality in Ballabgarh, India. Trop Med Int Health 2013;18:1329-37. 10.1111/tmi.12192 pmid:24103109. [DOI] [PubMed] [Google Scholar]
- 7.Pollard AJ. Non-specific effects of vaccines: RCTs, not observational studies, are needed. Arch Dis Child 2012;97:677-8. 10.1136/archdischild-2012-301873 pmid:22753770. [DOI] [PubMed] [Google Scholar]
- 8.Farrington CP, Firth MJ, Moulton LH, Ravn H, Andersen PK, Evans S. Working Group on Non-specific Effects of Vaccines. Epidemiological studies of the non-specific effects of vaccines: II--methodological issues in the design and analysis of cohort studies. Trop Med Int Health 2009;14:977-85. 10.1111/j.1365-3156.2009.02302.x pmid:19531116. [DOI] [PubMed] [Google Scholar]
- 9.Rehermann B, Shin E-C. Private aspects of heterologous immunity. J Exp Med 2005;201:667-70. 10.1084/jem.20050220 pmid:15753200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh RM, Fujinami RS. Pathogenic epitopes, heterologous immunity and vaccine design. Nat Rev Microbiol 2007;5:555-63. 10.1038/nrmicro1709 pmid:17558423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sørup S, Benn CS, Stensballe LG, Aaby P, Ravn H. Measles-mumps-rubella vaccination and respiratory syncytial virus-associated hospital contact. Vaccine 2015;33:237-45. 10.1016/j.vaccine.2014.07.110 pmid:25446818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaby P, Garly M-L, Nielsen J, et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: Observations from vaccination trials in Guinea-Bissau. Pediatr Infect Dis J 2007;26:247-52. http://www.ncbi.nlm.nih.gov/pubmed/17484223. 10.1097/01.inf.0000256735.05098.01 pmid:17484223. [DOI] [PubMed] [Google Scholar]
- 13.Yokomizo A, Kanimoto Y, Okamura T, et al. Randomized Controlled Study of the Efficacy, Safety and Quality of Life of Low-Dose Bacillus Calmette-Guerin Instillation Therapy for Non-Muscle-Invasive Bladder Cancer. J Urol 2015;195:41-6, 10.1016/j.juro.2015.08.075 [DOI] [PubMed] [Google Scholar]
- 14.Stewart JH 4th, , Levine EA. Role of bacillus Calmette-Guérin in the treatment of advanced melanoma. Expert Rev Anticancer Ther 2011;11:1671-6. 10.1586/era.11.163 pmid:22050015. [DOI] [PubMed] [Google Scholar]
- 15.Nofal A, Nofal E, Yosef A, Nofal H. Treatment of recalcitrant warts with intralesional measles, mumps, and rubella vaccine: a promising approach. Int J Dermatol 2015;54:667-71. 10.1111/ijd.12480 pmid:25070525. [DOI] [PubMed] [Google Scholar]
- 16.WHO. SAGE Working Group on non-specific effects of vaccines (March-June 2013). http://www.who.int/immunization/sage/sage_wg_non_specific_effects_vaccines_march2013/en/
- 17.WHO. Weekly Epidemiological Record 2008;83,32:285-292. http://www.who.int/wer/2008/wer8332/en/.
- 18.WHO. Weekly Epidemiological Record 2014;89,21:221-236. http://www.who.int/wer/2014/wer8921/en/.
- 19.Burl S. The Role of Regulatory T cells in Early Life Immunity to BCG: Influence of Exposure to Environmental Mycobacteria.Open University, 2009. [Google Scholar]
- 20.Djuardi Y, Sartono E, Wibowo H, Supali T, Yazdanbakhsh M. A longitudinal study of BCG vaccination in early childhood: the development of innate and adaptive immune responses. PLoS One 2010;5:e14066 10.1371/journal.pone.0014066 pmid:21124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustman DL, Wang L, Okubo Y, et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS One 2012;7:e41756 10.1371/journal.pone.0041756 pmid:22905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109:17537-42. 10.1073/pnas.1202870109 pmid:22988082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libraty DH, Zhang L, Woda M, et al. Neonatal BCG vaccination is associated with enhanced T-helper 1 immune responses to heterologous infant vaccines. Trials Vaccinol 2014;3:1-5. 10.1016/j.trivac.2013.11.004 pmid:24611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks GB, Ng K, Zhou J, et al. The effect of neonatal BCG vaccination on atopy and asthma at age 7 to 14 years: an historical cohort study in a community with a very low prevalence of tuberculosis infection and a high prevalence of atopic disease. J Allergy Clin Immunol 2003;111:541-9. 10.1067/mai.2003.171 pmid:12642835. [DOI] [PubMed] [Google Scholar]
- 25.Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J Immunol 2002;168:919-25. 10.4049/jimmunol.168.2.919 pmid:11777990. [DOI] [PubMed] [Google Scholar]
- 26.Taştan Y, Arvas A, Demir G, Alikaşifoğlu M, Gür E, Kiray E. Influence of Bacillus Calmette-Guèrin vaccination at birth and 2 months old age on the peripheral blood T-cell subpopulations [gamma/delta and alpha-beta T cell]. Pediatr Allergy Immunol 2005;16:624-9. 10.1111/j.1399-3038.2005.00329.x pmid:16343082. [DOI] [PubMed] [Google Scholar]
- 27.Vargas MH, Bernal-Alcántara DA, Vaca MA, Franco-Marina F, Lascurain R. Effect of BCG vaccination in asthmatic schoolchildren. Pediatr Allergy Immunol 2004;15:415-20. 10.1111/j.1399-3038.2004.00198.x pmid:15482516. [DOI] [PubMed] [Google Scholar]
- 28.Vijaya Lakshmi V, Kumar S, Surekha Rani H, Suman LG, Murthy KJ. Tuberculin specific T cell responses in BCG vaccinated children. Indian Pediatr 2005;42:36-40.pmid:15695856. [PubMed] [Google Scholar]
- 29.Weir RE, Black GF, Dockrell HM, et al. Mycobacterial purified protein derivatives stimulate innate immunity: Malawians show enhanced tumor necrosis factor alpha, interleukin-1beta (IL-1beta), and IL-10 responses compared to those of adolescents in the United Kingdom. Infect Immun 2004;72:1807-11. 10.1128/IAI.72.3.1807-1811.2004 pmid:14977992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennino A, Cornu C, Rozieres A, et al. Influence of measles vaccination on the progression of atopic dermatitis in infants. Pediatr Allergy Immunol 2007;18:385-90. 10.1111/j.1399-3038.2007.00537.x pmid:17617807. [DOI] [PubMed] [Google Scholar]
- 31.Hussey GD, Goddard EA, Hughes J, et al. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune response in infants. J Infect Dis 1996;173:1320-6. 10.1093/infdis/173.6.1320 pmid:8648203. [DOI] [PubMed] [Google Scholar]
- 32.Lisse IM, Aaby P, Knudsen K, Whittle H, Andersen H. Long term impact of high titer Edmonston-Zagreb measles vaccine on T lymphocyte subsets. Pediatr Infect Dis J 1994;13:109-12. 10.1097/00006454-199402000-00006 pmid:8190534. [DOI] [PubMed] [Google Scholar]
- 33.Ovsyannikova IG, Reid KC, Jacobson RM, Oberg AL, Klee GG, Poland GA. Cytokine production patterns and antibody response to measles vaccine. Vaccine 2003;21:3946-53. 10.1016/S0264-410X(03)00272-X pmid:12922130. [DOI] [PubMed] [Google Scholar]
- 34.Samb B, Whittle H, Aaby P, et al. No evidence of long-term immunosuppression after high-titer Edmonstron-Zagreb measles vaccination in Senegal. J Infect Dis 1995;171:506-8. 10.1093/infdis/171.2.506 pmid:7844403. [DOI] [PubMed] [Google Scholar]
- 35.Schnorr JJ, Cutts FT, Wheeler JG, et al. Immune modulation after measles vaccination of 6-9 months old Bangladeshi infants. Vaccine 2001;19:1503-10. 10.1016/S0264-410X(00)00349-2 pmid:11163674. [DOI] [PubMed] [Google Scholar]
- 36.Pabst HF, Spady DW, Carson MM, Stelfox HT, Beeler JA, Krezolek MP. Kinetics of immunologic responses after primary MMR vaccination. Vaccine 1997;15:10-4. 10.1016/S0264-410X(96)00124-7 pmid:9041660. [DOI] [PubMed] [Google Scholar]
- 37.Rager-Zisman B, Bazarsky E, Skibin A, et al. The effect of measles-mumps-rubella (MMR) immunization on the immune responses of previously immunized primary school children. Vaccine 2003;21:2580-8. 10.1016/S0264-410X(03)00053-7 pmid:12744894. [DOI] [PubMed] [Google Scholar]
- 38.Armitage KB, Duffy EG, Mincek MA, et al. Transient normalization of lymphocyte blastogenic and specific antibody responses following boosting of healthy elderly subjects with tetanus toxoid. J Gerontol 1993;48:M19-25. 10.1093/geronj/48.1.M19 pmid:7678105. [DOI] [PubMed] [Google Scholar]
- 39.Borut TC, Ank BJ, Gard SE, Stiehm ER. Tetanus toxoid skin test in children: correlation with in vitro lymphocyte stimulation and monocyte chemotaxis. J Pediatr 1980;97:567-73. 10.1016/S0022-3476(80)80010-2 pmid:7420219. [DOI] [PubMed] [Google Scholar]
- 40.Chollet P, Chassagne J, Philippe P, et al. Peripheral lymphocytes changes in the anamnestic response to tetanus toxoid challenge. Clin Exp Immunol 1979;37:152-61.pmid:487652. [PMC free article] [PubMed] [Google Scholar]
- 41.Gentile D, Trecki J, Patel A, Fausnight T, Angelini B, Skoner D. Effect of tetanus immunization on t-helper cytokine production in adults with and without allergic rhinitis. Allergy Asthma Proc 2006;27:197-201. 10.2500/aap.2006.27.2857 pmid:16913261. [DOI] [PubMed] [Google Scholar]
- 42.Mahalingam D, Radhakrishnan AK, Amom Z, Ibrahim N, Nesaretnam K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur J Clin Nutr 2011;65:63-9. 10.1038/ejcn.2010.184 pmid:20859299. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes JR, Wasserman S, Snider DP. Stimulation of anti-polio and anti-HSV IgA pre-plasma cell response in blood following parenteral immunization with tetanus-diphtheria vaccine. Vaccine 2010;28:1493-8. 10.1016/j.vaccine.2009.11.057 pmid:20003921. [DOI] [PubMed] [Google Scholar]
- 44.Halasa NB, O’Shea A, Shi JR, LaFleur BJ, Edwards KM. Poor immune responses to a birth dose of diphtheria, tetanus, and acellular pertussis vaccine. J Pediatr 2008;153:327-32. 10.1016/j.jpeds.2008.03.011 pmid:18534242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heine G, Drozdenko G, Lahl A, et al. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur J Clin Nutr 2011;65:329-34. 10.1038/ejcn.2010.276 pmid:21224870. [DOI] [PubMed] [Google Scholar]
- 46.Rowe J, Macaubas C, Monger TM, et al. Antigen-specific responses to diphtheria-tetanus-acellular pertussis vaccine in human infants are initially Th2 polarized. Infect Immun 2000;68:3873-7. 10.1128/IAI.68.7.3873-3877.2000 pmid:10858197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Yousfi M, Mercier S, Breuillé D, et al. The inflammatory response to vaccination is altered in the elderly. Mech Ageing Dev 2005;126:874-81. 10.1016/j.mad.2005.03.008 pmid:15876450. [DOI] [PubMed] [Google Scholar]
- 48.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guérin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol 1998;161:1045-54.pmid:9670986. [PubMed] [Google Scholar]
- 49.Jorgensen MJ, Fisker AB, Sartono E, et al. The effect of at-birth vitamin A supplementation on differential leucocyte counts and in vitro cytokine production: an immunological study nested within a randomised trial in Guinea-Bissau. Br J Nutr 2013;109:467-77. 10.1017/S0007114512001304. pmid:23168172. [DOI] [PubMed] [Google Scholar]
- 50.Di Tommaso A, Bartalini M, Peppoloni S, Podda A, Rappuoli R, De Magistris MT. Acellular pertussis vaccines containing genetically detoxified pertussis toxin induce long-lasting humoral and cellular responses in adults. Vaccine 1997;15:1218-24. 10.1016/S0264-410X(97)00023-6 pmid:9286047. [DOI] [PubMed] [Google Scholar]
- 51.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012;37:771-83. 10.1016/j.immuni.2012.10.014 pmid:23159225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacKaness GB. The Immunological Basis of Acquired Cellular Resistance. J Exp Med 1964;120:105-20. 10.1084/jem.120.1.105 pmid:14194388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014;345:1251086 10.1126/science.1251086 pmid:25258085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol 2005;95:571-8. 10.1016/S1081-1206(10)61021-6 pmid:16400898. [DOI] [PubMed] [Google Scholar]
- 55.Parra M, Liu X, Derrick SC, et al. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PLoS One 2013;8:e66115 10.1371/journal.pone.0066115 pmid:23861742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakaya HI, Wrammert J, Lee EK, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 2011;12:786-95. 10.1038/ni.2067 pmid:21743478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaucher D, Therrien R, Kettaf N, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 2008;205:3119-31. 10.1084/jem.20082292 pmid:19047440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mejias A, Ramilo O. Transcriptional profiling in infectious diseases: ready for prime time?J Infect 2014;68(Suppl 1):S94-9. 10.1016/j.jinf.2013.09.018 pmid:24139187. [DOI] [PubMed] [Google Scholar]
- 59.Kalina T, Flores-Montero J, Lecrevisse Q, et al. Quality assessment program for EuroFlow protocols: summary results of four-year (2010-2013) quality assurance rounds. Cytometry A 2015;87:145-56. 10.1002/cyto.a.22581 pmid:25345353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Search terms
Appendix 2: Supplementary tables A-L and references
Appendix 3: Supplementary figures with tabulated data
Appendix 4: Detailed tabulated data for figs 2 and 3