Abstract
Maternal microchimerism, which occurs naturally during gestation in hemochorial placental mammals upon transplacental migration of maternal cells into the fetus, is suggested to significantly influence the fetal immune system. In our previous publication, we explored the sensitivity of quantitative polymerase chain reaction and flow cytometry to detect cellular microchimerism. With that purpose, we created mixed cells suspensions in vitro containing reciprocal frequencies of wild type cells and cells positive for enhanced green fluorescent protein or CD45.1+, respectively. Here, we now introduce the H-2 complex, which defines the major histocompatibility complex in mice and is homologous to HLA in human, as an additional target to detect maternal microchimerism among fetal haploidentical cells. We envision that this advanced approach to detect maternal microchimeric cells by flow cytometry facilitates the pursuit of phenotypic, gene expression and functional analysis of microchimeric cells in future studies.
Keywords: fetal immune development, flow cytometry, maternal microchimerism, mouse model
The presence of a small population of cells in a genetically different individual is referred to as microchimerism (MC). In humans, mice, and other eutherian mammals, MC occurs naturally during gestation, by cell trafficking from the fetus to the mother (fetal MC) and from the mother to the fetus (maternal MC).1,2 Despite the low frequencies of maternal and fetal MC cells, experimental evidence suggests that these forms of MC significantly influence the host's immune responses.3 For example, fetal MC cells may affect the course of disease in women suffering from multiple sclerosis4 or rheumatoid arthritis.5-7 In turn, maternal MC plays a role in the generation of regulatory T (Treg) cells,1,8,9 tolerance to allografts,8,10,11 and even in susceptibility to asthma12 in the offspring.
A major technical challenge faced in research endeavors aiming to understand the functional role of MC in various settings is the difficulty to distinctly identify chimeric cells due to their generally extremely low frequencies. Mouse models allow to overcome these limitations, since transgenic or congenic markers can be employed for MC identification. To date, quantitative polymerase chain reaction (qPCR)2,13 and flow cytometry9,11 targeting such transgenic or congenic markers enable to effectively identify MC cells in different settings. However, a thorough validation of the sensitivity of these approaches was long elusive.
In our recent publication, we explored the sensitivity of 2 methods, qPCR and flow cytometry, to detect cellular MC.14 We generated MC cell suspensions in vitro by combining murine wild-type (wt) and genetically modified cells expressing either the enhanced green fluorescent protein (eGFP) or the congenic leukocyte cell surface receptor CD45.1 in various ratios. The lowest detection limit of MC cells by flow cytometry was 0.05% for eGFP and 0.2% for CD45.1, and the detection limit of eGFP by qPCR was 0.2%. The sensitivity rates in relation to the transgenes and tissues in which the techniques were applied and related sensitivity limitations are thoroughly discussed in our14 and others publications.15 While both PCR and flow cytometry proved to reliably detect low numbers of chimeric cells with high sensitivity, our observations suggested the presence of potentially false positive cells among the MC population. This could result from intrinsic limitations of the flow cytometry technique such as cell auto-fluorescence, unspecific antibody binding or high spectral overlap in the fluorescent signal emission, as discussed in our previous publication.14
In the present work we aimed to address these technical limitations by adding a second independent marker to phenotypically identify naturally occurring maternal MC haematopoietic cells among haploidentical fetal cells in mice.
Similar to our previous approach, we used the model of CD45.2+ C57Bl/6 females mated to CD45.1+ BALB/c males.14 Here, we took advantage of the natural polymorphism between mouse inbred strains with regard to their H-2D gene, one of the subclasses comprised in the major histocompatibility complex (MHC) class I. This is H-2Db in the females of the C57Bl/6 strain and H-2Dd in Balb/c males. Figure 1 illustrates the mating combination we used, including the parental and fetal genotypes.
Figure 1.
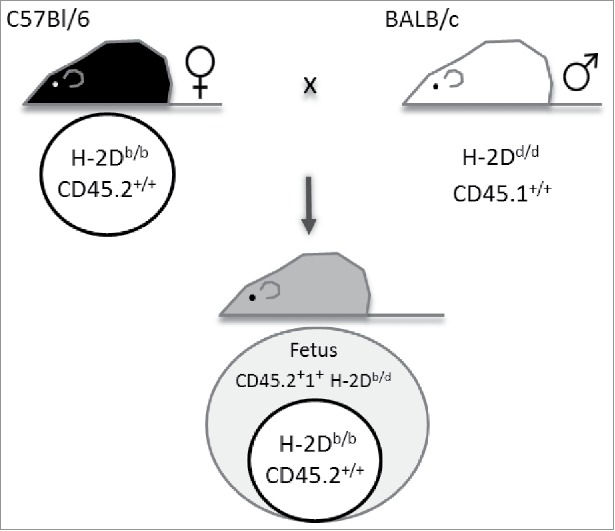
Mating combination to detect maternal cells in fetal organs. Wt C57Bl/6 females expressing CD45.2 and major histocompatibility complex (MHC) class I haplotype H-2Db were mated to MHC I H-2Dd-expressing BALB/c males congenic for CD45 (CD45.1). This mating combination allows the identification of maternal cells positive for CD45.2 and H-2Db among fetal cells expressing CD45.2+1+ H-2Db/d.
Maternal cells were harvested from uterus-draining lymph nodes or bone marrow (BM) on gestational day (GD) 18.5, fetal cells were obtained from thymus and BM of the respective offspring. Paternal cells were harvested from BM of adult male mice and served as an aid for the gating strategies in the subsequent flow cytometric analyses. Cells were stained according to our standard protocol14 and frequencies were acquired using a BD LSRFortessa analyzer. Gate thresholds were set following the ‘fluorescence minus one’ (FMO) controls for each fluorescent dye one by one. Figure 2 depicts the gating strategy used to identify maternal cells among fetal leukocytes. This gating strategy allows the identification of maternal CD45.2+H-2Db (Fig. 2A), paternal CD45.1+H-2Dd (Fig. 2B), fetal CD45.2+1+H-2Db/d (Fig. 2C) haematopoietic cells as well as maternal CD45.2+H-2Db MC cells among the haploidentical fetal CD45.2+1+H-2Db/d cells (Fig. 2C). To note, while the H-2 complex is expressed in virtually all leukocytes obtained from adult organisms (Fig. 3A and B), a gradient from H-2Db/d high expression over dim expression to negativity was observed in leukocytes obtained from fetal BM (Fig. 3C). Thus, the high frequency of H-2Db/d negative fetal cells may further enhance the scrutiny of detecting maternal H-2Db single positive cells among fetal BM-derived cells.
Figure 2.
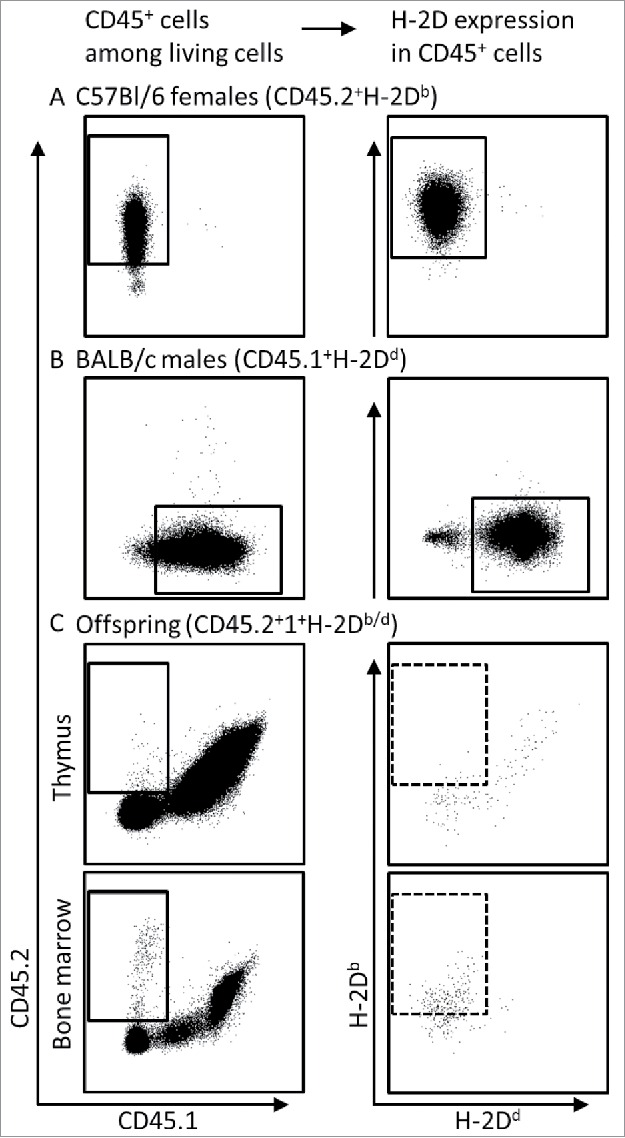
Approach used for detection of maternal MC cells among fetal cells by flow cytometry. Living cells were detected in lymph nodes from C57Bl/6 females (A), bone marrow of BALB/c males (B) and in fetal thymus and bone marrow in offspring of BALB/c-mated C57Bl/6 females (C), based on their 7AAD negativity (not shown) and gated for CD45 positivity (left panel). Within the respective CD45+ gates (solid squares), the frequency of cells expressing H-2Db or H-2Dd was detected (right panel). Maternal MC was identified based on the frequency of CD45.2+H-2Db cells among CD45.2+1+H-2Db/d fetal cells (stippled squares in C, right panel).
Figure 3.
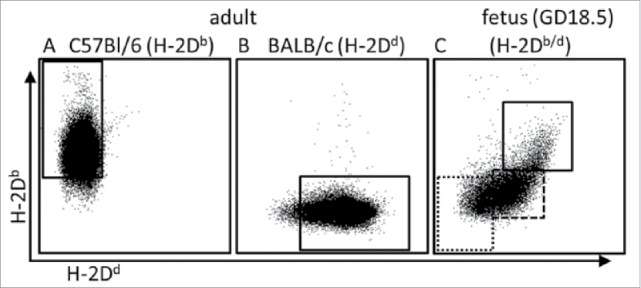
H-2D expression on adult and fetal bone marrow cells. Representative examples for frequencies of respective H-2D-positive cells among maternal (H-2Db, marked by the solid gate in (A), paternal (H-2Dd, marked by the solid gate in (B) and fetal (H-2Db/d, marked by the solid gate in (C) CD45+ BM-derived leukocytes. Fetal cells were obtained from offspring according to the mating combination shown in Figure 1. Note that a gradient ranging from H-2Db/d high expression (solid square in (C) to H-2Db/d dim expression (marked by stippled gate) and H-2Db/d negativity (marked by dotted gate) was observed among the fetal leukocytes.
As shown in Table 1, the use of H-2D as a second marker for detecting MC cells improved the specificity of the detection as it permitted to exclude false positive CD45.2+ cells among the maternal MC population. Interestingly, the purity of maternal cells among CD45.2+ cells was higher in cells derived from fetal BM compared to thymus-derived cells (Fig. 2C; Table 1). The reasons for this organ dependent cell differences are unknown. We hypothesize that they could result from the different tissue processing methods applied in solid and non-solid tissues. Here, the thymus, as a solid tissue, was processed mechanically to prepare the single cell suspensions. This constitutes a challenge for cell homeostasis which could enhance the unspecific antibody binding, when compared to non-solid BM isolated simply by flushing the bone cavity. Further, differences in the phenotype and consequently in the gene expression of the maternal MC cells localized in each organ could also account for the differences in detection efficiencies. Indeed, we were able to identify different populations of haematopoietic cells among CD45.2+H-2Db MC cells, including stem cells and terminally differentiated immune cells (data not shown), suggesting that MC cell phenotype does not influence the detection significantly in the present set up. Similarly, we could demonstrate that virtually all the haematopoietic CD45+ cells in the maternal samples express H-2D (Fig. 2A; Table 1), suggesting that these 2 markers can be efficiently used to detect maternal cells among haploidentical fetal cells. In contrast, we envision these markers not to be efficient to detect fetal MC cells among maternal cells, as fetal CD45 and H-2D gene expression follows a maturation pattern (Fig. 3C), which could signify that more immature CD45 and H-2D negative fetal cells could not be distinguished among maternal cells, leading to false negative results.
Table 1.
Frequencies of CD45.2+ and CD45.2+H-2Db in 106 living cells per organ
| Cells organ (n) | CD45.2+ in 106 living cells (mean ± SEM) | CD45.2+ H-2Db in 106 living cells (mean ± SEM) |
|---|---|---|
| fetal thymus (4) | 216 ± 76 | 27 ± 12 |
| fetal bone marrow (4) | 9102 ± 2250 | 5192 ± 1046 |
| maternal lymph node (1) | 984572 | 983321 |
Fetal BM not only revealed a more precise identification of maternal MC but also showed higher frequencies of MC cells when compared to fetal thymus (Fig 2C; Table 1). This observation is in line with earlier reports, showing that the major haematopoietic organs in prenatal immune development, liver and BM, are concomitantly prominent sites for MC cell localization during fetal life.2,16 These different frequencies could be explained by an enhanced migration or proliferation of maternal MC cells in the fetal BM, compared to fetal thymus. However, differences in blood flow could be also a potential explanation for the differences in MC cell frequencies between tissues, as no perfusion could be conducted in these fetal organs and therefore the maternal MC cells could be both tissue resident and blood cells. These hypotheses shall be tested in future endeavors employing this experimental set up to analyze for example migration or proliferation markers by flow cytometry or PCR after cell sorting to enrich MC cells.
In summary, we here introduce H-2D gene expression as an additional marker that significantly improves the specificity of MC cell detection by minimizing the false positive results. We could demonstrate that this technique is suitable to reliably detect and quantify low frequencies of maternal MC cells among fetal haploidentical cells. H-2D could also be used to further verify the maternal origin of microchimeric cells by qPCR after cell sorting, as H-2Dd can be specifically detected among other H-2D subclasses.13 In our experimental setting, a positive H-2Dd signal among the sorted H-2Db MC cells could only derive from contaminating fetal H-2Db/d cells. However, this approach would be technically challenging to verify the purity of MC cells by PCR determination of the CD45 haplotype. CD45.2 and CD45.1 genes differ in only 12 nucleotides,17 which makes it very difficult to distinguish them by genomic PCR. Previously, maternal MC had also been studied in mice by differentiating MC and fetal cells by transgenic markers, such as eGFP,13,18 luciferase19 or neoR,20 upon adoptive transfer of labeled, transgenic leukocytes into the mother,21 by using antigen-specific T cell mouse models,9,22 or adoptively transferring blastocysts from normal matings into LacZ+ transgene-tagged mothers.2,23 All of these approaches can be considered as interventions that may have immunogenic properties. In contrast, this experimental setup takes advantage of the natural genomic variation between mouse strains to detect MC cells, minimizing the interventions during pregnancy and prenatal life which could also play a role on microchimerism. Here, the use of an allogeneic mating combination results in fetuses that are semiallogeneic to the mother, which resembles human pregnancies. This is of note since maternal MC has been shown to differ between syngeneic, allogenic and outbred offspring.16,24 MC is higher in syngeneic fetuses, indicating that the capacity of microchimeric cells to migrate into or reside in fetal tissue is dependent on inherited antigen differences. Further, opposed to experimental setups using litter comprised of wt, heterozygote and homozygote mutant mice,13,18-20 in the set-up we developed, all fetuses are genetically identical. Hence, no genotyping of the offspring is required. This saves a considerable amount of resources and allows experimenting with freshly isolated MC cells, which may be critical to study MC cell function. This—along with the great potential of flow cytometry to identify various markers on a single cell basis and to separate cells according to their phenotype - provides significant progress in the phenotypic, gene expression and functional analysis of maternal MC cells, i.e. upon cell sorting.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008; 322(5907):1562-5; PMID:19056990; http://dx.doi.org/ 10.1126/science.1164511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marleau AM, Greenwood JD, Wei Q, Singh B, Croy BA. Chimerism of murine fetal bone marrow by maternal cells occurs in late gestation and persists into adulthood. Lab Invest 2003; 83(5):673-81; PMID:12746477; http://dx.doi.org/ 10.1097/01.LAB.0000067500.85003.32 [DOI] [PubMed] [Google Scholar]
- 3. Seppanen E, Fisk NM, Khosrotehrani K. Pregnancy-acquired fetal progenitor cells. J Reprod Immunol 2013; 97(1):27-35; PMID:23432869; http://dx.doi.org/ 10.1016/j.jri.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 4. Bloch EM, Reed WF, Lee TH, Montalvo L, Shiboski S, Custer B, Barcellos L. Male microchimerism in peripheral blood leukocytes from women with multiple sclerosis. Chimerism 2011; 2(1):6-10; PMID:21547029; http://dx.doi.org/ 10.4161/chim.15151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Østensen M, Villiger PM, Förger F. Interaction of pregnancy and autoimmune rheumatic disease. Autoimmun Rev 2012; 11(6-7):A437-46; PMID:22154710; http://dx.doi.org/ 10.1016/j.autrev.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 6. Yan Z, Aydelotte T, Gadi VK, Guthrie KA, Nelson JL. Acquisition of the rheumatoid arthritis HLA shared epitope through microchimerism. Arthritis Rheum 2011; 63(3):640-4; PMID:21360493; http://dx.doi.org/ 10.1002/art.30160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 2013; 19(5):548-56; PMID:23652115; http://dx.doi.org/ 10.1038/nm.3160 [DOI] [PubMed] [Google Scholar]
- 8. Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, et al. . Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol 2007; 179(10):6749-61; PMID:17982065; http://dx.doi.org/ 10.4049/jimmunol.179.10.6749 [DOI] [PubMed] [Google Scholar]
- 9. Leveque L, Hodgson S, Peyton S, Koyama M, Macdonald KP, Khosrotehrani K. Selective organ specific inflammation in offspring harbouring microchimerism from strongly alloreactive mothers. J Autoimmun 2014; 50:51-8; PMID:24268809; http://dx.doi.org/ 10.1016/j.jaut.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 10. Dutta P, Burlingham WJ. Correlation between post transplant maternal microchimerism and tolerance across MHC barriers in mice. Chimerism 2011; 2(3):78-83; PMID:22163065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nijagal A, Wegorzewska M, Jarvis E, Le T, Tang Q, MacKenzie TC. Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice. J Clin Invest 2011; 121(2):582-92; PMID:21245575; http://dx.doi.org/ 10.1172/JCI44907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson EE, Myers RA, Du G, Aydelotte TM, Tisler CJ, Stern DA, Evans MD, Graves PE, Jackson DJ, Martinez FD, et al. . Maternal microchimerism protects against the development of asthma. J Allergy Clin Immunol 2013; 132(1):39-44; PMID:23434286; http://dx.doi.org/ 10.1016/j.jaci.2012.12.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, Burlingham WJ. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 2009; 114(17):3578-87; PMID:19700665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thiele K, Holzmann C, Solano ME, Zahner G, Arck PC. Comparative sensitivity analyses of quantitative polymerase chain reaction and flow cytometry in detecting cellular microchimerism in murine tissues. J Immunol Methods 2014; PMID:24657636; http://dx.doi.org/ 10.1016/j.jim.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 15. Fujiki Y, Johnson KL, Peter I, Tighiouart H, Bianchi DW. Fetal cells in the pregnant mouse are diverse and express a variety of progenitor and differentiated cell markers. Biol Reprod 2009; 81(1):26-32; PMID:19279322; http://dx.doi.org/ 10.1095/biolreprod.108.074468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vernochet C, Caucheteux SM, Gendron MC, Wantyghem J, Kanellopoulos-Langevin C. Affinity-dependent alterations of mouse B cell development by noninherited maternal antigen. Biol Reprod 2005; 72(2):460-9; PMID:15469995; http://dx.doi.org/ 10.1095/biolreprod.104.035048 [DOI] [PubMed] [Google Scholar]
- 17. Zebedee SL, Barritt DC, Raschke WC. Comparison of mouse Ly5a and Ly5b leucocyte common antigen alleles. Dev Immunol 1991; 1(4):243-54; PMID:1822988; http://dx.doi.org/ 10.1155/1991/52686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, Shimamura M. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology 2000; 101(4):570-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Su EC, Johnson KL, Tighiouart H, Bianchi DW. Murine maternal cell microchimerism: analysis using real-time PCR and in vivo imaging. Biol Reprod 2008; 78(5):883-7; PMID:18256332; http://dx.doi.org/ 10.1095/biolreprod.107.063305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan J, Land S. Influence of maternal-fetal histocompatibility and MHC zygosity on maternal microchimerism. J Immunol 2005; 174(11):7123-8; PMID:15905555 [DOI] [PubMed] [Google Scholar]
- 21. Wienecke J, Hebel K, Hegel KJ, Pierau M, Brune T, Reinhold D, Pethe A, Brunner-Weinzierl MC. Pro-inflammatory effector Th cells transmigrate through anti-inflammatory environments into the murine fetus. Placenta 2012; 33(1):39-46; PMID:22093381; http://dx.doi.org/ 10.1016/j.placenta.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 22. Roy E, Leduc M, Guegan S, Rachdi L, Kluger N, Scharfmann R, Aractingi S, Khosrotehrani K. Specific maternal microchimeric T cells targeting fetal antigens in β cells predispose to auto-immune diabetes in the child. J Autoimmun 2011; 36(3-4):253-62; PMID:21414756; http://dx.doi.org/ 10.1016/j.jaut.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 23. Piotrowski P, Croy BA. Maternal cells are widely distributed in murine fetuses in utero. Biol Reprod 1996; 54(5):1103-10; PMID:8722632; http://dx.doi.org/ 10.1095/biolreprod54.5.1103 [DOI] [PubMed] [Google Scholar]
- 24. Vernochet C, Caucheteux SM, Kanellopoulos-Langevin C. Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta 2007; 28(7):639-49; PMID:17116327; http://dx.doi.org/ 10.1016/j.placenta.2006.10.006 [DOI] [PubMed] [Google Scholar]


