Abstract
It has become increasingly clear that the immune system of viviparous mammals is much more in the business of acquiring tolerance to non-self antigens, than it is in rejecting cells that express them (for a recent review, highlighting the role of Treg cells, see ref.1). It is also clear that both self-tolerance, and acquired tolerance to non-self is a dynamic process, with a natural ebb and flow. As has been often said of an effective team defense in sports, tolerance will “bend but does not break.” How microchimerism, defined as the presence of extremely rare [1/104–1/106] cells of a genetically different individual, can induce either new immunogenetic pressures that push self-tolerance to the breaking point, or alternatively, provide relief from pre-existing immunogenetic risk, preventing development of autoimmune disease, remains a mystery. Indeed, the inability to directly correlate DNA-level microchimerism detected in blood samples by qPCR, with naturally occurring regulation to minor H and MHC alloantigens expressed by the rare cells themselves, has been frustrating to researchers in this field.2 [Haynes, W.J. et al, this issue] However, recent developments in the areas of transplantation and reproductive immunology offer clues to how the effects of microchimerism can be amplified, and how a disproportionate immune impact might occur from a very limited cell source.
Keywords: microchimerism, exosomes, tolerance, anergy
Transplant Immunobiology-Pathways of allorecognition
The major barriers to kidney transplantation success are products of the major histocompatibility complex (MHC) genes. Foreign MHC class I and II molecules displayed by an allograft, in the context of co-stimulation, can drive a large subset of T cells [as much as 10%] to divide, most likely due to cross-reactivity with endogenous T naïve and memory cells specific for viral or bacterial peptide-plus-self MHC. This is called the “direct pathway” of allorecognition (Fig. 1, far left), because it requires direct interaction of host T cells with allograft donor antigen presenting cells (APC). Of interest, the direct pathway may also be quite self peptide-specific as well as allo-MHC-specific as predicted nearly 40 y ago and recently proven.3,4 The direct pathway is commonly assayed using the in vitro mixed lymphocyte culture (MLC) test, which was the means by which MHC class II antigens were originally discovered.5
Figure 1.
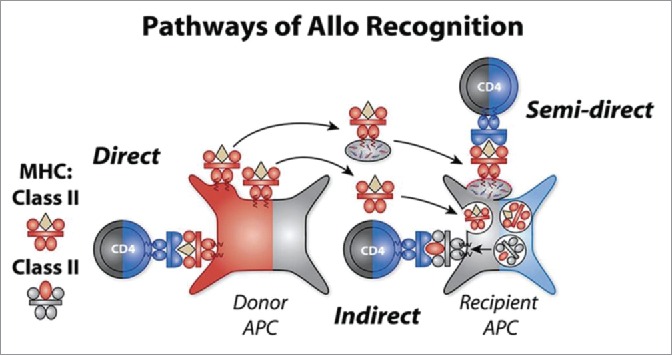
Three major pathways of allorecognition. The three pathways of allorecognition (i.e. direct, indirect, and semi-direct) are shown in this figure. In the context of pregnancy, the role of donor cells in the fetus is played by maternal cells expressing non-inherited maternal antigen or NIMA (red). Direct pathway implies the recognition of intact allo-MHC molecules and indirect pathway implies the recognition of allopeptide-self MHC II complexes derived by normal Ag processing. In the semi-direct pathway, allogeneic MHC molecules (NIMA) are acquired via exosomes, which contain microRNA with the capacity to reprogram the APC. IMA—inherited maternal atigens– are shown in gray, NIMA in red, and IPA—inherited paternal antigens– in blue.
On the other hand, the standard way that antigens are processed and presented to host T cells by host APC also applies to allografts of cells, tissues and organs; therefore a second form of alloreactivity, directed toward allo-peptides derived from the foreign MHC, was implied and eventually discovered.6,7 This came to be known as indirect pathway alloreactivity (Fig. 1, center),. Using the trans-vivo DTH assay to measure indirect alloreactivity, we have reported that a characteristic feature of the tolerant organ transplant recipient is the regulation of this pathway by allopeptide-specific T regulatory cells producing TGF β1, IL-10 or IL-35.8-11 A similar phenomenon, under the name of “cross-presentation” is seen in the case of tolerance to grafts mismatched for minor H antigens only, where the sharing of all MHC class I and II proteins by donor and recipient allows minor H antigens to be “seen” by peptide-specific regulatory T cells of both CD4, as well as CD8 lineage.2,12
The existence of 2 such very different pathways, direct vs. indirect, of allo-recognition in MHC mismatched transplants posed a major problem for transplant immunologists. Once the indirect pathway was described,6,7 and the helper function of the indirect pathway CD4 T cells toward direct pathway CD8 T cells was discovered, it dawned on researchers in the field that there was no plausible mechanism to account for the finding of synergy between indirect/CD4 and direct/CD8 T cells, since each pathway appeared to require a completely different [host vs. donor] antigen presenting cell (APC). Enter the semi-direct pathway.13 By means of either trogocytosis [literally pulling off a membrane fragment from a recently encountered cell], or, via exocytosis and subsequent exosome - fusion with host APC, the host cell may become “cross-dressed,” displaying the MHC/peptide complexes of another cell (Fig. 1, right). If that cell source happens to be allogeneic, one can detect these acquired membrane complexes easily using microscopy and fluorescent-labeled anti-MHC alloantibody. Any host APC –B cell, monocyte, plasmacytoid and myeloid DC (mDC)– can retain the acquired alloantigen on the cell surface in the short term and thus prime direct pathway T cells.14 Given the continuous recirculation of MHC between the intracellular compartments where they are loaded and the cell surface, it would seem that such a mechanism would be quickly diluted amid the “noise” of normal p/MHC trafficking, and as the intact MHC alloantigens are rapidly degraded. However, the mDC is uniquely capable of retaining and concentrating the acquired alloantigen as an intact peptide-MHC complex on the cell surface for a prolonged time period in vivo. When myeloid DC have been decorated in this manner with tiny bits of plasma membrane from an allogeneic cell source –tissue endothelial, epithelial, or leukocyte – direct pathway CD4+ or CD8+, as well as indirect pathway CD4+ T cells could now encounter their target antigen on the same [host] APC. This process, termed “semi-direct” allorecognition, was first described by the Lechler lab13 over10 years ago; its important role in transplant rejection was recently convincingly demonstrated in a heart transplant model by the Pettigrew lab.15 In the scenario illustrated in Figure 1, the semi- direct pathway is shown as a CD4 T cell recognizing acquired MHC class II. This cell could then receive further help froman indirect pathway Th cell recognizing a fragment/peptide from the donor MHC presented in the context of autologous MHCII. If the semi-direct pathway happens to involve a CD8+ T cell recognizing acquired donor MHC class I antigen, then the indirect pathway must provide help to direct pathway CD8+ CTL in order for the host to mount an effective cytotoxic T cell and transplant rejection response.15 In the case of maternal-fetal microchimerism, close encounters of both Th/CD4 and CD8 T effector cells with each one's cognate antigen would seldom occur, given a very low frequency donor APC. However, if that rare cell releases exosomes that are captured by multiple autologous mDCs, the same T cell would gain access, via semi-direct presentation, to help from indirect pathway T helper cells clustered at the same (host) APC.
Two other important aspects of the model shown in Figure 1 should be noted. First, if the captured exosome expresses allo MHC-II, as illustrated here, there is a potential not only for alloreactivity, but also for peptide-specific alloreactivity, i.e. presentation of novel peptides to a CD4 host T cell an allo-restricted manner. This potential will be exploited by the host DC only if the acquired allo MHC-II can recycle from the cell surface to acidic phago-lysosomes and back, something which remains to be formally proven. The transfer of pre-formed complexes of pMHC-II and pMHC-I has however been demonstrated to effectively stimulate peptide-specific T cell clones by antigen acquisition in vitro.13 Second, the model portrays nucleic acids, for ex. microRNAs, as being enclosed in the exosomes. Such microRNAs or miRs are commonly found in plasma exosomes and microvesicles, where they may transport either pro- or anti-inflammatory information to other cells.16 This aspect of exosomes is the ‘wild card’ that not only can spread antigenic targets throughout the body, but also, instructions that result in up- or down regulation of cellular products. Thus, depending on the context of exosome release, the antigen-acquiring ‘cross-dressed’ mDC might become either pro- or anti-inflammatory, with implications for disease risk in the host. How exosome generation occurs in a tolerant host is unknown, but probably involve encounters of host T cells with rare donor-derived DC. If these encounters occur in a “non- inflammatory” environment, a patient with a “metastable” form of tolerance might generate exosomes with miRNA that will cause increased PDL-1 expression in host DC, leading to Th anergy. Innate immunity in the areas of T-alloDC interaction may alter the miRNA content of exosomes released toward a suppression of PDL-1 in the exosome –“cross-dressed” host cells, causing restoration of the Th –CTL axis.
Besides allo-transplantation, a primary role for the “cross-dressing” phenomenon in anti-viral immunity has been demonstrated in a non-transplant setting. By enabling epithelial cells to transfer viral-peptide/MHC complexes to mDC that then traffic to lymph nodes, T cells of the exact anti-viral specificity needed are generated for export to the site of infection.17,18 Three strands of evidence, from 1) human kidney transplantation,19 2) mouse heart transplantation,20 and 3) human lung transplantation,21 all in the context of microchimerism, suggest not only a simple explanation for non-inherited maternal antigen (NIMA) tolerance,22 but also for the phenomenon of foreign HLA-associated risk/susceptibility, based on a hitherto missing link between the immune system of the host, and tiny numbers of foreign cells.23-25
Semi-direct pathway as a factor promoting tolerance
We were ignorant of the cross-dressing/exosome acquisition phenomenon in 1995, when we published the first paper to claim a direct function of microchimerism in a case of kidney transplantation tolerance.19 In this study, we analyzed the direct pathway response of T cells specific for non-inherited maternal HLA-B and DR antigens in a male patient who had stopped all immunosuppressive drug treatment 2 y after receiving a kidney transplant from his mother. We found that he was a microchimera, with a signal for maternal DNA turning up in his peripheral blood and skin. The estimate of maternal cell frequency was between 1/104 –1/105 at both sites, using the then-standard PCR/Southern blot, and a nested PCR technique. The patient manifested donor-specific unresponsiveness in MLC 7day culture with maternal stimulator cells, but his T cells could be “revived” from their anergic state by secondary culture with maternal stimulator cells in the presence of rIL-2. To determine the source of anergy, fresh leukocytes harvested from the patient were added to the secondary culture either as whole cell preparations, or after removal of maternal HLA-Bw6+ cells (patient was homozygous for HLA-Bw4) using immunomagnetic beads. The fresh whole cell preparations caused a dose-dependent inhibition of the patient's direct pathway cytotoxic T lymphocyte (CTL) response; however, the removal of HLA-Bw6+ cells abolished the inhibitory effect. Add back of the immunomagnetic beads containing the NIMA+ cells restored the donor-specific inhibitory effect. We interpreted these results as being consistent with a direct effect of rare maternal cells.19
However, the estimated number of maternal cells in the patient's peripheral blood mononuclear cells (PBMC) did not correspond to the magnitude of the inhibitory effect seen—for ex., significant inhibition of the direct pathway CTL response was seen at an estimated dose of a single maternal cell. At the time we did not suspect that antigen acquisition by host mDCs could play a role; however, we now know that up to 20% of host mDC acquire donor HLA class I and class II antigens during transplant tolerance. This meant that although only a single maternal cell was added to cultures of 2 × 104 CTL, perhaps 20 additional highly specialized host APCs coated with a patchwork of maternal allo-antigens were also present. Since a single mDC is known to be capable of contacting hundreds of T cells, it follows that the inhibitory effects we had attributed to the maternal cells themselves were in fact most likely accomplished by surrogates “cross-dressed” with mother's cell membranes.
Semi-direct pathway and “split” tolerance due to microchimerism
In 2003 we showed that one could replicate the maternal tolerance effect seen in humans22 in a [BDF1 female x B6 male] F1 backcross mouse model originally described by Zhang and Miller,26 using heart instead of skin allografts.27 Offspring were typed for H-2d and those with H-2bxb homozygosity were further analyzed for tolerance to a heart allograft from a DBA/2 donor expressing the non-inherited maternal antigens [NIMA] of the H-2d [MHC] haplotype, along with DBA/2 background minor H antigens. Approximately 47% of the male offspring were tolerant, while the other 53% rejected the heart transplant.27
There were 2 patterns of alloreactivity associated with these 2 different responses to heart allografts from DBA/2 mice. The first was a strong indirect pathway alloreactivity causing rapid DBA/2 heart allograft rejection. This response was found in the 50–60% of male “NIMAd” –exposed offspring that were H-2b homozygous, and was associated with a low level of peripheral MMc [few or no organs containing rare H-2Dd+ maternal cells by quantitative PCR assay], with MMc largely confined to bone marrow c-kit+ stem cells, and no MMc penetration in CD11b or CD11c cell lineages.20 Membrane alloantigen acquisition by host class II-positive cells in spleen and peripheral blood occurs rarely in such mice prior to transplant; however, during DBA/2 allograft rejection, between d 9–12 post –tx, was there a transient pulse of alloantigen acquisition detected in peripheral blood and lymphoid tissue.20,28,29 The second pattern of alloreactivity was one associated with much higher levels of peripheral MMc [2–4 different MMc+ organs] plus the bone marrow. Not only were MMc+ CD11c+ DC present, but the indirect pathway of alloreactivity was silenced due to dominant suppression by Treg cells prior to transplant. In such mice [40–50% of “NIMAd” –exposed mice], pre-transplant regulation toward maternal BDF1 antigens predicted allo-tolerance to a subsequent DBA/2 heart transplant.
Recently, we have used indirect pathway and direct pathway T cell clones to explore the immune status of NIMA-exposed rejectors vs. tolerant offspring. TEa indirect pathway T cells recognize an allopeptide derived from the Eα chain of maternal I-Ed molecules, in the context of IAb, host MHC-II. Using CFSE dilution to follow T cell replication, we recently found that TEa cells proliferate strongly in non-tolerant offspring, but undergo abortive activation and anergy in tolerant mice (Bracamonte-Baran et al., “Membrane alloantigen acquisition by dendritic cells links microchimerism and split tolerance” in preparation). The latter have widespread tissue distribution of maternal microchimerism (MMc), and show maternal cell membrane antigen acquisition, as measured by dim H-2Kd and IAd expression on the surface of class II+ recipient (H-2b homozygous) APC.20,28 On the other hand, 4C Tg T cells, direct pathway T cells that recognize intact IAd antigens gave an opposite result—i.e., they only proliferated in vivo in mice that have acquired membrane I-Ad. While this result indicates that the maternal semi-direct pathway is functional for antigen recognition, it suggests that tolerance to NIMA in mice is ‘split’ (Bracamonte-Baran et al., in preparation), i.e. it features a functional semi-direct, but a non-functional indirect pathway, as was suggested previously.30 This result parallels the data in human PBMC, which show perfectly normal CD4 and CD8 “direct pathway” response to NIMAs in healthy normal subjects using the MLC test,31,32 but a markedly regulated response to NIMAs using the indirect pathway, tvDTH assay.33
The consequence of maintaining a strong semi-direct pathway even when tolerance has been established on the indirect pathway is that direct pathway T cells are sustained long term during tolerance; they are only lost when indirect pathway regulation fails and loss of tolerance results in transplant rejection.34,35 So we can say that in the pre-transplant setting, the successful “mini”-allograft of microchimerism induces a type of “split tolerance”—characterized by a strong semi-direct pathway even while the indirect pathway is being silenced. Interestingly, membrane allo-antigen acquisition in the mouse NIMAd transplant model increased fold10- from pre-transplant levels in mice that became tolerant of a DBA/2 heart.20,36 This may account for the shift from a non-anergic, to an anergic direct/semi-direct pathway in the tolerant host, as in the patient tolerant of a maternal kidney graft, discussed above.
Semi-direct pathway and the challenges to self-tolerance posed by lung transplantation
As stated above, the semi-direct pathway implies that the myeloid DC is capable of retaining and perhaps even concentrating the acquired alloantigens as an intact peptide-MHC complexes on the cell surface for a prolonged time period in vivo. But can a chronically acquired MHC antigen be utilized by the host to recognize new peptide antigens, i.e in an allo-MHC context? Conventional wisdom would say no, since such a response would be considered a form of direct pathway alloreactivity. But consider the strange case of lung transplant patient L86. This case is highlighted in a recent study of HLA-DR15 and the peptide selectivity of Th17 responses to the α1 chain of collagen type V.21 Patient L86 himself was 7 yrs out from transplantation, relatively free of clinical complications, when he developed a strong response to collagen V, typical of those lung and heart allograft recipients who go on to develop fibro-obliterative narrowing of airways and blood vessels. Like many of these patients, including those who had developed collagen V-reactivity in the course of lung or heart disease prior to transplant, his response was found to be α1(V)-specific. Unlike them, he was free of any evidence of bronchiolitis obliterans syndrome (BOS), that afflicts a significant proportion of lung transplant recipients who develop this response.37 His genetic background was HLA-DR1, 11 and his donor was DR1, 15. Through peptide binding studies it was determined that while there was much overlap between patterns of α1(V) peptide binding between DR1 and DR15, there were 2 peptides of α1(V) that exclusively bound to either DR1 (p629) or DR15 (p1049). [Notably, HLA-DR1501 gave by far the highest peptide-binding scores out of the 6 different MHC class II molecules tested, and was a significant risk factor in development of collagen V autoimmunity in the post-transplant period]. This distinction in peptide specificity was confirmed in studies of collagen V-immunized HLA-DR 1 and HLA-DR15 transgenic mice: the DR1 Tg responded only to p629, and not to p1049; the DR15 Tg mouse only to p1049 and not to p629. Remarkably, PBMC from L86 obtained 7 y post-lung transplant responded equally well to BOTH peptides.21
Since peptide p1049 could not be presented by HLA-DR1, the shared allele, or by DR11, based on studies in collagen V-reactive DR11+ patients, it could only mean the HLA-DR15 was somehow present in pt L86s PBMC, and capable of binding the peptide. This unusual finding of donor (allo-)DR-restricted, response to a self antigen, has recently been confirmed in a DR 4,11 patient PP26 who had received a DR15 homozygous lung transplant 1.5 y previously, and who responded very well not only to collagen V, but also to DR15-binding peptide p1049 [Jankowska-Gan & Burlingham, unpublished]. We are currently investigating a) whether this Th17 response occurs via HLA-DR15 antigen acquisition by host mDCs, b) how soon after lung transplant an allo-DR-restricted response to collagen V can occur, and c) whether such a pathway can account for the observed increased risk of obliterative bronchiolitis in transplant pts receiving a DR15+ lung, and for the increased protection afforded by receipt of a lung from a HLA-DR7+ donor.38
Conclusions
In conclusion, I would like to suggest that in normal healthy subjects with maternal or fetal-derived Mc, as well as in lung transplant patients, the acquisition of class II antigens from either the Mc or graft endothelium source greatly amplifies the tolerogenic, or alternatively, immunogenic signals of the transplant, giving rise to substantial impacts of rare cells on allotolerance, or alternatively, disease susceptibility. Based on the mounting evidence that direct [and by implication, also semi-direct] alloreactive T cells can be quite peptide-specific,39 I propose that a semi-direct pathway alloreactivity of CD4 T cells in lung transplant patients such as L86, described here, is responsible for the observed donor-HLA restricted peptide response to collagen V. This would account for the relatively high risk of post–transplant severe BOS development in DR15+ donor lungs, the protection from BOS enjoyed by recipients of DR7-positive donor lungs, since DR7 has very different peptide-binding characteristics from that of DR15 (encoded by the DRB1*1501 allele),40,41 as well as the many observations in the field of autoimmunity, of protective and susceptibility effects of fetal or maternal microchimerism.23,25,42,43
Finally, the semi-direct pathway, fed by exosomes emanating from tissue-resident cells, is the more likely source of profound immune impacts of rare allogeneic cells. The preferred location of maternal and fetal microchimerism, as well as organ-transplant-derived microchimerism, lies in heart, liver, brain, lungs, and bone marrow rather than in the central lymphoid tissues.28,44-46 What better way for these cells to signal their presence to the host than to hijack the preferred system of transporting viral and bacterial peptide/MHC from the peripheral tissue to the lymphoid areas?
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jiang TT, Chaturvedi V, Ertelt JM, Kinder JM, Clark DR, Valent AM, Xin L, Way SS. Regulatory T cells: New keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol 2014; 192(11):4949-56; PMID:24837152; http://dx.doi.org/ 10.4049/jimmunol.1400498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dierselhuis MP, Jankowska-Gan E, Blokland E, Pool J, Burlingham WJ, van Halteren AG, Goulmy E. HY immune tolerance is common in women without male offspring. PLoS One 2014; 9(3):e91274; PMID:24646895; http://dx.doi.org/ 10.1371/journal.pone.0091274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Orsogna LJ, Roelen DL, van der Meer-Prins EM, van der Pol P, Franke-van Dijk ME, Eikmans M, Anholts J, Rossjohn J, McCluskey J, et al.. Tissue specificity of cross-reactive allogeneic responses by EBV EBNA3A-specific memory T cells. Transplantation 2011; 91(5):494-500; PMID:21242884; http://dx.doi.org/ 10.1097/TP.0b013e318207944c [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P, Bevan MJ. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol 1977; 29(1):1-5; PMID:300293; http://dx.doi.org/ 10.1016/0008-8749(77)90269-6 [DOI] [PubMed] [Google Scholar]
- 5.Bach FH, Voynow NK. One-way stimulation in mixed leukocyte culture. Science 1966; 153:545-7; PMID:5938778; http://dx.doi.org/ 10.1126/science.153.3735.545 [DOI] [PubMed] [Google Scholar]
- 6.Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med 1992; 175:305-8; PMID:1730925; http://dx.doi.org/ 10.1084/jem.175.1.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechler RI, Lombardi G, Batchelor JR, Reinsmoen N, Bach FH. The molecular basis of alloreactivity. Immunol Today 1990; 11:83-8; PMID:2186745; http://dx.doi.org/ 10.1016/0167-5699(90)90033-6 [DOI] [PubMed] [Google Scholar]
- 8.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest 2000; 106(1):145-55; PMID:10880058; http://dx.doi.org/ 10.1172/JCI9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes LD, Jankowska-Gan E, Sheka A, Keller MR, Hernandez-Fuentes MP, Lechler RI, Seyfert-Margolis V, Turka LA, Newell KA, Burlingham WJ. Donor-specific indirect pathway analysis reveals a B-cell-independent signature which reflects outcomes in kidney transplant recipients. Am J Transplant. 2012 Mar; 12(3):640-8; PMID:2215123617339499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q, Jankowska-Gan E, Schultz J, Roenneburg DA, Haynes LD, Kusaka S, Sollinger HW, Knechtle SJ, VanBuskirk AM, Torrealba JR, et al.. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol 2007; 178(6):3983-95; PMID:17339499; http://dx.doi.org/ 10.4049/jimmunol.178.6.3983 [DOI] [PubMed] [Google Scholar]
- 11.Olson BM, Sullivan JA, Burlingham WJ. Interleukin 35: A key mediator of suppression and the propagation of infectious tolerance. Front Immunol 2013; 4:315; PMID:24151492; http://dx.doi.org/ 10.3389/fimmu.2013.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, Goulmy E, Burlingham WJ. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med 2004; 199(7):1017-23; PMID:15067036; http://dx.doi.org/ 10.1084/jem.20031012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol 2004; 173(8):4828-37; PMID:15470023; http://dx.doi.org/ 10.4049/jimmunol.173.8.4828 [DOI] [PubMed] [Google Scholar]
- 14.Tocco G, et al.. Presentation of allogeneic MHC on cross-dressed recipient APCs vs. Passenger leukocytes after skin transplantation. Am J Transplant 2014; 14(S3):19(#586 [Google Scholar]
- 15.Sivaganesh S, Harper SJ, Conlon TM, Callaghan CJ, Saeb-Parsy K, Negus MC, Motallebzadeh R, Bolton EM, Bradley JA, Pettigrew GJ. Copresentation of intact and processed MHC alloantigen by recipient dendritic cells enables delivery of linked help to alloreactive CD8 T cells by indirect-pathway CD4 T cells. J Immunol 2013; 190(11):5829-38; PMID:23630361; http://dx.doi.org/ 10.4049/jimmunol.1300458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei L, Wang M, Qu X, Mah A, Xiong X, Harris AG, Phillips LK, Martinez OM, Krams SM. Differential expression of microRNAs during allograft rejection. Am J Transplant 2012; 12(5):1113-23; PMID:22300508; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth LA, Hervouet C, Hayday T, Becker PD, Ellis R, Lechler RI, Lombardi G, Klavinskis LS. Acquisition of MHC:peptide complexes by dendritic cells contributes to the generation of antiviral CD8+ T cell immunity in vivo. J Immunol 2012; 189(5):2274-82; PMID:22821960; http://dx.doi.org/ 10.4049/jimmunol.1200664 [DOI] [PubMed] [Google Scholar]
- 18.Smyth LA, Afzali B, Tsang J, Lombardi G, Lechler RI. Intercellular transfer of MHC and immunological molecules: molecular mechanisms and biological significance. Am J Transplant 2007; 7(6):1442-9; PMID:17511673; http://dx.doi.org/ 10.1111/j.1600-6143.2007.01816.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burlingham WJ, Grailer AP, Fechner JH Jr, Kusaka S, Trucco M, Kocova M, Belzer FO, Sollinger HW. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation 1995; 59:1147-55; PMID:7732562; http://dx.doi.org/ 10.1097/00007890-199504270-00013 [DOI] [PubMed] [Google Scholar]
- 20.Dutta P, Burlingham WJ. Correlation between post transplant maternal microchimerism and tolerance across MHC barriers in mice. Chimerism 2011; 2(3):78-83; PMID:22163065; http://dx.doi.org/ 10.4161/chim.18083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller MR, Haynes LD, Jankowska-Gan E, Sullivan JA, Agashe VV, Burlingham SR, Burlingham WJ. Epitope analysis of the collagen type V-specific T cell response in lung transplantation reveals an HLA-DRB1*15 bias in both recipient and donor. PLoS One 2013; 8(11):e79601; PMID:24265781; http://dx.doi.org/ 10.1371/journal.pone.0079601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, et al.. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors [see comments]. N Engl J Med 1998; 339(23):1657-64; PMID:9834302 [DOI] [PubMed] [Google Scholar]
- 23.ten Wolde S, Breedveld FC, de Vries RR, D'Amaro J, Rubenstein P, Schreuder GM, Claas FH, van Rood JJ. Influence of non-inherited maternal HLA antigens on occurrence of rheumatoid arthritis. Lancet 1993; 341(8839):200-2; PMID:8093497; http://dx.doi.org/ 10.1016/0140-6736(93)90065-O [DOI] [PubMed] [Google Scholar]
- 24.Guthrie KA, Gammill HS, Madeleine MM, Dugowson CE, Nelson JL. Parity and HLA alleles in risk of rheumatoid arthritis. Chimerism 2011; 2(1):11-15; PMID:21547030; http://dx.doi.org/ 10.4161/chim.15424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Z, Aydelotte T, Gadi VK, Guthrie KA, Nelson JL. Acquisition of the rheumatoid arthritis HLA shared epitope through microchimerism. Arthritis Rheum 2011; 63(3):640-4; PMID:21360493; http://dx.doi.org/ 10.1002/art.30160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Miller RG. The correlation of prolonged survival of maternal skin grafts with the presence of naturally transferred maternal T cells. Transplantation 1993; 56:918-921; PMID:8212217; http://dx.doi.org/ 10.1097/00007890-199310000-00027 [DOI] [PubMed] [Google Scholar]
- 27.Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, Tam RC, Illigens BM, Anosova N, Benichou G, et al.. Tolerance to noninherited maternal MHC antigens in mice. J Immunol 2003; 171(10):5554-61; PMID:14607963; http://dx.doi.org/ 10.4049/jimmunol.171.10.5554 [DOI] [PubMed] [Google Scholar]
- 28.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, Burlingham WJ. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 2009; 114(17):3578-87; PMID:19700665; http://dx.doi.org/ 10.1182/blood-2009-03-213561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutta P, Burlingham W. Stem cell microchimerism and tolerance to non-inherited maternal antigens. Chimerism 2010; 1(1):2-10; PMID:21132055; http://dx.doi.org/ 10.4161/chim.1.1.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama Y, Caucheteux SM, Vernochet C, Iwamoto Y, Tanaka K, Kanellopoulos-Langevin C, Benichou G. Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model. J Immunol 2011; 186(3):1442-9; PMID:21178009; http://dx.doi.org/ 10.4049/jimmunol.1003023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadley GA, Phelan D, Duffy BF, Mohanakumar T. Lack of T-cell tolerance of noninherited maternal HLA antigens in normal humans. Hum Immunol 1990; 28:373-381; PMID:2391252; http://dx.doi.org/ 10.1016/0198-8859(90)90032-K [DOI] [PubMed] [Google Scholar]
- 32.Roelen DL, van Bree FP, van Beelen E, van Rood JJ, Claas FH. No evidence of an influence of the non-inherited maternal HLA antigens on the alloreactive T cell repertoire in healthy individuals. Transplantation 1995; 59:1728-33; PMID:7541578; http://dx.doi.org/ 10.1097/00007890-199506270-00015 [DOI] [PubMed] [Google Scholar]
- 33.Jankowska-Gan E, Sheka A, Sollinger HW, Pirsch JD, Hofmann RM, Haynes LD, Armbrust MJ, Mezrich JD, Burlingham WJ. Pretransplant immune regulation predicts allograft outcome: bidirectional regulation correlates with excellent renal transplant function in living-related donor-recipient pairs. Transplantation 2012; 93(3):283-90; PMID:22186938; http://dx.doi.org/ 10.1097/TP.0b013e31823e46a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusaka S, Grailer AP, Fechner JH Jr, Jankowska-Gan E, Oberley T, Sollinger HW, Burlingham WJ. Clonotype analysis of human alloreactive T cells: a novel approach to studying peripheral tolerance in transplant recipients. J of Immunology 2000; 164:2240-7; PMID:10657680; http://dx.doi.org/ 10.4049/jimmunol.164.4.2240 [DOI] [PubMed] [Google Scholar]
- 35.Burlingham WJ, Jankowska-Gan E, VanBuskirk A, Orosz CG, Lee JH, Kusaka S. Loss of tolerance to a maternal kidney transplant is selective for HLA class II: Evidence from trans-vivo DTH and alloantibody analysis. Human Immunology 2000; 61:1395-402; PMID:11163098; http://dx.doi.org/ 10.1016/S0198-8859(00)00217-2 [DOI] [PubMed] [Google Scholar]
- 36.Dutta P, Dart M, Roenneburg DA, Torrealba JR, Burlingham WJ. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant 2011; 11(6):1296-301; PMID:21449933; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al.. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 2007; 117(11):3498-506; PMID:17965778; http://dx.doi.org/ 10.1172/JCI28031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haynes LD, et al.. Early Chronic Rejection In Lung transplant recipients is significantly increased in patients with a HLA-DR15+ donor. Am J Transplant 2014; 14(S3):196; http://dx.doi.org/ 10.1111/ajt.12883 [DOI] [Google Scholar]
- 39.D'Orsogna LJ, van Besouw NM, van der Meer-Prins EM, van der Pol P, Franke-van Dijk M, Zoet YM, van der Slik A, Weimar W, van Kooten C, Mulder A, et al.. Vaccine-induced allo-HLA-reactive memory T cells in a kidney transplantation candidate. Transplantation 2011; 91(6):645-51; PMID:21283063; http://dx.doi.org/ 10.1097/TP.0b013e318208c071 [DOI] [PubMed] [Google Scholar]
- 40.Phelps RG, Jones V, Turner AN, Rees AJ. Properties of HLA class II molecules divergently associated with Goodpasture's disease. Int Immunol 2000; 12(8):1135-43; PMID:10917888; http://dx.doi.org/ 10.1093/intimm/12.8.1135 [DOI] [PubMed] [Google Scholar]
- 41.Freed BM, Schuyler RP, Aubrey MT. Association of the HLA-DRB1 epitope LA(67, 74) with rheumatoid arthritis and citrullinated vimentin binding. Arthritis Rheum 2011; 63(12):3733-9; PMID:22094856; http://dx.doi.org/ 10.1002/art.30636 [DOI] [PubMed] [Google Scholar]
- 42.Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest 1999; 104(1):41-7; PMID:10393697; http://dx.doi.org/ 10.1172/JCI6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, Bean MA, Ober C, Bianchi DW. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma [see comments]. Lancet 1998; 351(9102):559-62; PMID:9492775 [DOI] [PubMed] [Google Scholar]
- 44.Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki PI, Murase N, Kendall RS, Kocova M, Rudert WA, et al.. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher's disease. N Engl J Med 1993; 328:745-9; PMID:8437594; http://dx.doi.org/ 10.1056/NEJM199303183281101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens AM, Hermes HM, Rutledge JC, Buyon JP, Nelson JL. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet 2003; 362(9396):1617-23; PMID:14630442; http://dx.doi.org/ 10.1016/S0140-6736(03)14795-2 [DOI] [PubMed] [Google Scholar]
- 46.Dutta P, Dart ML, Schumacher SM, Burlingham WJ. Fetal microchimerism persists at high levels in c-kit+ stem cells in sensitized mothers. Chimerism 2010; 1(2):51-5:in press; PMID:21327047; http://dx.doi.org/ 10.4161/chim.1.2.14295 [DOI] [PMC free article] [PubMed] [Google Scholar]


