Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is induced by emotions or exercise in patients without organic heart disease and may be polymorphic or bidirectional in nature. The prognosis of CPVT is not good, and therefore prevention of sudden death is of utmost importance. Genetic variants of CPVT include RyR2, CASQ2, CALM2, TRD, and possibly KCNJ2 and ANK2 gene mutations. Hypotheses that suggest the causes of CPVT include weakened binding of FKBP12.6 and RyR2, a store overload-induced Ca2+ release (SOICR), unzipping of intramolecular domain interactions in RyR2, and molecular and functional abnormalities caused by mutations in the CASQ2 gene. The incidence of an RyR2 anomaly in CPVTs is about 35–79%, whereas anomalies in the CASQ2 gene account for 3–5% CPVTs. The ping-pong theory, suggesting that reciprocating delayed after depolarization induces bigeminy of the right and left bundle branches, may explain the pathogenesis of bidirectional ventricular tachycardia. Flecainide, carvedilol, left sympathetic nerve denervation, and catheter ablation of the PVC may serve as new therapeutic strategies for CPVT while gene-therapy may be applied to some types of CPVT in the future. Although, not all sudden cardiac deaths in CPVT patients are currently preventable, new medical and interventional therapies may improve CPVT prognosis.
Keywords: Catecholaminergic polymorphic ventricular tachycardia (CPVT), Ryanodine (RyR2), Calsequestrine (CASQ2), Delayed after depolarization, Left cardiac sympathetic denervation
1. Introduction
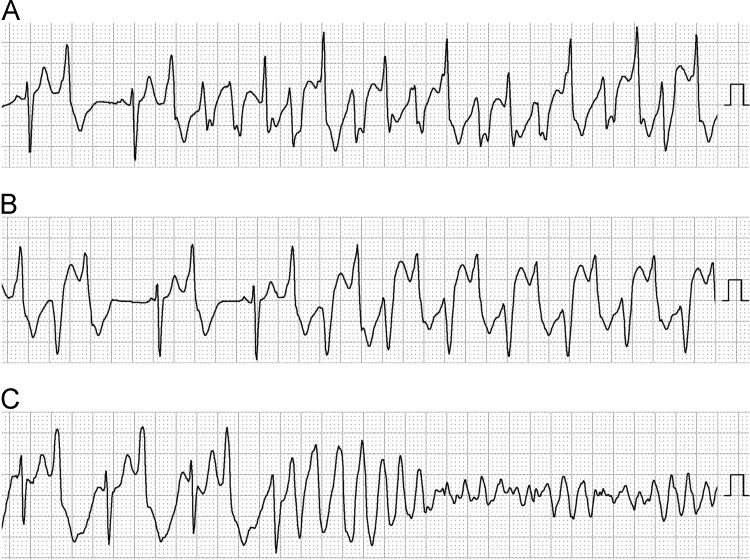
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is induced by emotional stress or exercise in patients without organic heart disease and may be polymorphic or bidirectional (Fig. 1) [1], [2], [3]. This ventricular arrhythmia sometimes degenerates into rapid polymorphic ventricular tachycardia and ventricular fibrillation (Fig. 1) and may lead to syncope or sudden death. The incidence of CPVT is reported to be as high as 1:10,000, but its real prevalence is unclear.
Fig. 1.
Typical features of ventricular tachycardia in a patient with CPVT. (A) Polymorphic ventricular tachycardia. (B) Bidirectional ventricular tachycardia. (C) Rapid polymorphic ventricular tachycardia deteriorating into ventricular fibrillation. These electrocardiograms were recorded by Holter monitoring in the CM3 lead in the same patient.
2. Clinical manifestations and prognosis
The first clinical manifestations of CPVT are syncope or aborted sudden cardiac death during exercise or emotional stress and appear during the first or second decade of life [1], [2], [3]. CPVT differs from seizures, in that almost all syncopal events are associated with physical activity or emotional stress and do not occur during a resting state.
The prognosis of CPVT is very poor. About 40% patients die within 10 years of diagnosis [3]. Although prognosis in recent times could be better than previous reports, sudden death and severe brain damage are still reported in CPVT patients.
3. Diagnosis of CPVT
CPVT patients usually have a normal resting ECG, or just a lower heart rate than is normal for their age [3]. During exercise in these patients, monomorphic premature ventricular contractions (PVCs) increase, then polymorphic, or bidirectional PVC bigeminy appear, followed by bidirectional or polymorphic VT. Exercise induced supraventricular arrhythmias (atrial fibrillation, premature atrial contraction, and atrial tachycardia) are also common in the patients with CPVT [4]. The diagnostic criteria of CPVT are as follows [5]:
-
1.
CPVT is diagnosed in the presence of a structurally normal heart, normal ECG, and unexplained exercise or catecholamine-induced bidirectional VT, polymorphic ventricular premature beats or VT in individuals <40 years of age.
-
2.
CPVT is diagnosed in patients (index case or family member) who have a pathogenic mutation.
-
3.
CPVT is diagnosed in family members of a CPVT index case with a normal heart who manifests exercise-induced PVCs or bidirectional/polymorphic VT.
-
4.
CPVT can be diagnosed in the presence of a structurally normal heart and coronary arteries, normal ECG, and unexplained exercise or catecholamine-induced bidirectional VT, polymorphic ventricular premature beats or VT in individuals >40 years of age.
4. Mechanism of CPVT
The major pathogenic mechanism of CPVT is thought to involve the malfunction of RyR2. RyR2 is a large tetrameric protein expressed on the sarcoplasmic reticulum (SR) membrane. RyR2 is anchored to calsequestrin (CASQ2) by satellite proteins such as calmodulin (CaM), FKBP12.6, (calstabin2), protein kinase A (PKA), phosphatase 1 (PP1), and phosphatase 2A (PP2A) bound to the cytoplasmic region and junction, and triadin (TRD) bound to the luminal side [6].
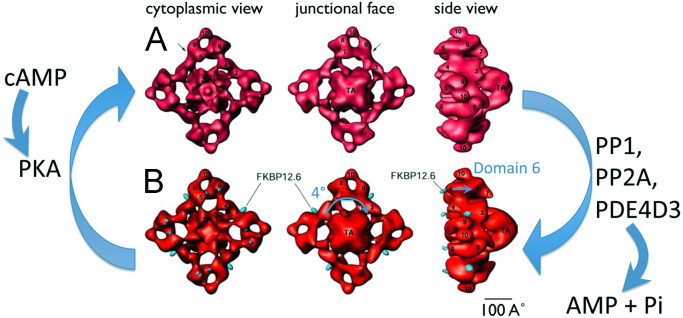
A three-dimensional reconstruction of RyR2 bound to FKBP12.6 is shown in Fig. 2B. When RyR2 is bound to FKBP12.6, it forms a stable structure with closed pores, the domain 6 of RyR2 was found to protrude into the luminal side, when observed from the junctional face after the transmembrane assembly (TA) was rotated counterclockwise by about 4° [7]. When unbound to FKBP12.6, RyR2 assumes an open state (Fig. 2A) [7].
Fig. 2.
Surface representations of RyR2 3D reconstructions with and without bound FKBP12.6. [7]. (A) High activity state of RyR2. A 3D map of RyR2, obtained by in vitro assembly of purified RyR2 incubated with FKBP12.6 alone. (B) Low activity state of RyR2. A 3D map of RyR2, obtained by incubating RyR2 with FKBP12.6 and an excess of FK506. FKBP12.6 is denoted by the blue dots. The major difference in these structures is observed in domain 6, which extends in the vertical direction (shown by the blue arrow), and the transmembrane assembly is rotated about 4° (shown by the blue arrow in the lower center panel). FKBP12.6: calstabin2, protein kinase A: PKA, phosphatase 1: PP1, phosphatase 2A: PP2A, phosphodiesterase 4D3: PDE 4D3, TA: transmembrane assembly.
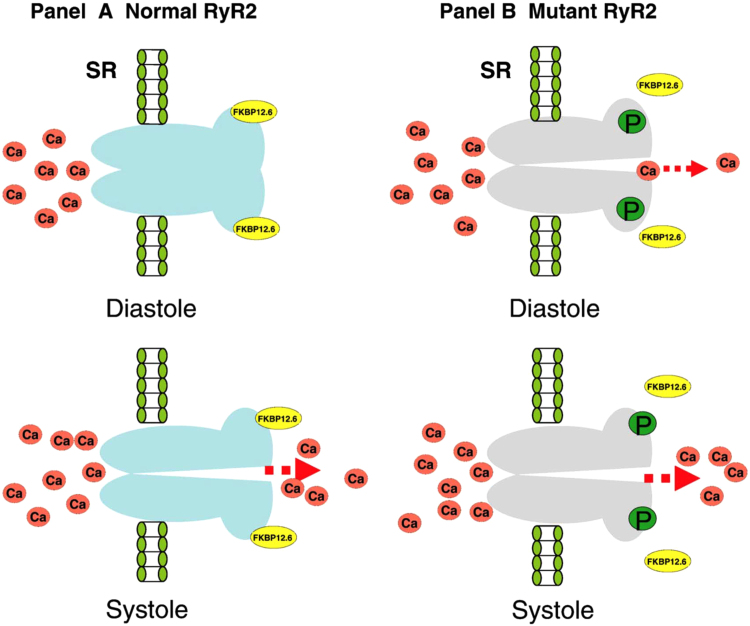
Several pathogenic hypotheses have been reported regarding the causes of CPVT [8]. The first theory suggests the dissociation of FKBP12.6 from RyR2. The normal RyR2 channel is stabilized by FKBP12.6 and closes during diastole. With mutant RyR2, the binding affinity with FKBP12.6 is weakened, and phosphorylation of RyR2 by protein kinase A (PKA) results in dissociation of FKBP12.6 from RyR2, resulting in open channels which may leak Ca2+ during diastole (Fig. 3).
Fig. 3.
FKBP12.6 dissociation from mutant RyR2 in the pathogenesis of CPVT [8]. FKBP12.6 acts as a stabilizer that preserves the closed RyR2 channel during diastole. Weakened binding affinity with FKBP12.6 may lead to a Ca2+ leak during diastole.
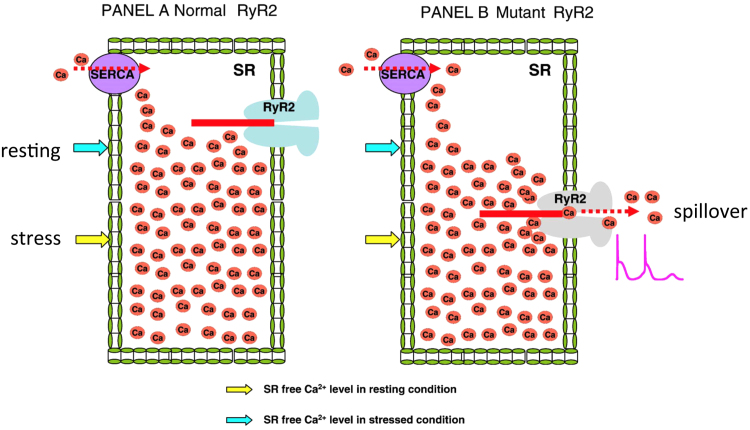
The second hypothesis is a store overload-induced Ca2+ release (SOICR) theory [8]. With normal RyR2, the resting and stress levels of free Ca2+ are below the SOICR level. However, with mutant RyR2, the SOICR threshold drops below the level of free Ca2+ in the SR. This may cause a spillover of Ca2+ from the SR (Fig. 4).
Fig. 4.
The store overload-induced Ca2+ release (SOICR) hypothesis [8]. With normal RyR2, the resting and stress levels of free calcium are below the SOICR threshold (panel A). If the SOICR threshold falls below the level of free SR calcium as with mutant RyR2, a leak of Ca2+ will occur and generate a delayed after-depolarization.
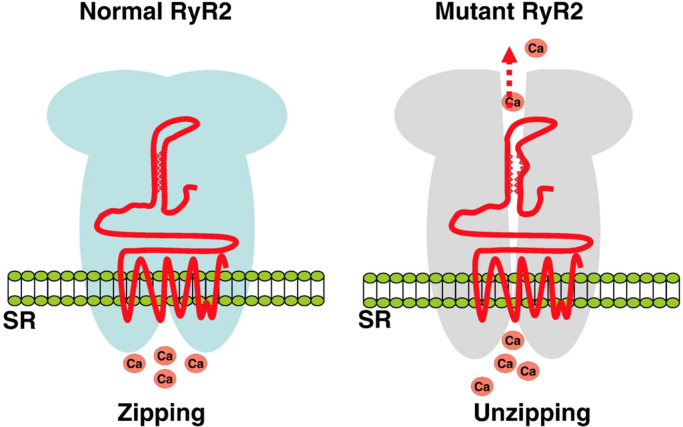
The third hypothesis considers defective intramolecular domain interaction [8]. RyR2 is stabilized by a tight zipping of the intramolecular structure. If a mutation interferes with this zipping structure, the intramolecular domain interaction is weakened, causing an unzipping of the interdomain structure and leads leaking of Ca2+ from the SR (Fig. 5).
Fig. 5.
Defective intramolecular domain interactions in RyR2 mutations [8]. The N terminal domain and the central domain of RyR2 interact with a tight “zipping” that serves to stabilize the channel (left panel). A mutation in either domain weakens this interaction (unzipping), which results in leaking of Ca2+ from RyR2 (right panel).
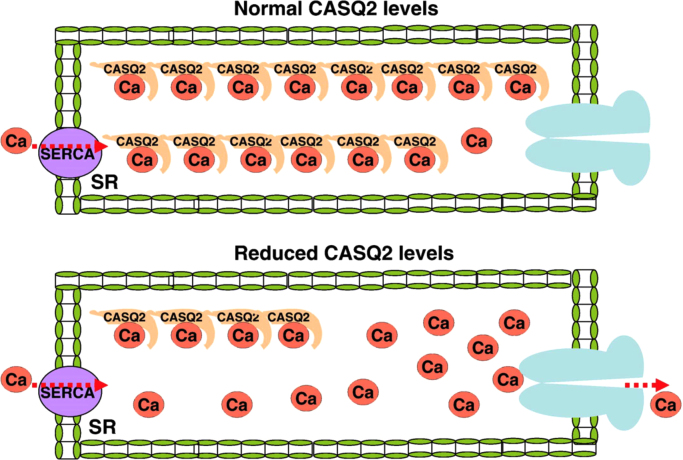
The fourth hypothesis suggests that the molecular and functional abnormalities are related to mutations in the CASQ2 gene [8]. CASQ2 is a Ca2+ storage protein inside the SR. The functional storage capacity of CASQ2 or its reduced levels, may lead to increased levels of free Ca2+ inside the SR, leading to a Ca2+ leak during diastole (Fig. 6). It is also known that CASQ2 stabilizes binding of RyR2 with TRD and the junction.
Fig. 6.
Molecular and functional abnormalities related to mutations in the CASQ2 gene [8]. Storage of Ca2+ in the SR largely depends on the level and function of CASQ2 (upper panel). Decreased levels or function of CASQ2 results in increase in the free SR Ca2+ that may result in a Ca2+ leak from RyR2 during diastole (lower panel).
This Ca2+ overload activates the forward mode of the Na+/Ca+ exchanger (NCX), increases the transient inward current (Iti), and induces ventricular arrhythmias due to delayed after depolarizations (DADs).
5. Subtypes of CPVT
Several subtypes of CPVT have been reported (Table 1). The most common type of CPVT is caused by an anomaly in the RyR2 gene (CPVT1) [9], [10]. This accounts for more than 50% of CPVT cases. In our CPVT cohort, about 79% of the CPVT cases were related to an anomaly in the RyR2 gene. The inheritance of CPVT1 is autonomic dominant, and sudden death was observed in about 10% of these patients. There were no sex differences noted in this CPVT.
Table 1.
Subtypes of CPVT.
| Subtypes |
Juvenile type |
Adult type | ||||||
|---|---|---|---|---|---|---|---|---|
| CPVT1 | CPVT2 | CPVT3 | CPVT4 | CPVT5 |
CPVT related diseases |
|||
| ATS | LQT4 | |||||||
| Incidence (%) | 50–60 | 1 | ≪1 | ≪1 | ≪1 | ≪1 | ≪1 | ≈30 |
| Inheritance | AD | AR | AR | AD | Sporadic | AD | AD | Sporadic |
| Onset of symptoms | 10 years | 7 years | 10 years | 4 years | 2, 26 years | 14, 9, 17 years | ? | >20 years (40 years) |
| Sex | M:F=1:1 | M:F=1:1 | M:F=1:1 | M:F=1:1 | M=3 | F>M? | ? | F≫M |
| Chromosome locus | 1q43 | 1p13.1 | 7p22–p14 | 14q32.11 | 6q22.31 | 17q24.3 | 4q25-26 | |
| Gene | RyR2 | CASQ2 | ? | CALM1 | TRD | KCNJ2 | ANK2 | RyR2≈30% |
| Protein | CaM | Kir2.1α | Ankyrin-B | |||||
| Sudden death (%) | ≈10 | ≈42 | ≈75 | ≈18 | ≈25 | ? | ? | 0 |
The second most common type of CPVT is caused by a CASQ2 gene anomaly (CPVT2) [11], [12]. The inheritance of CPVT2 is autosomal recessive, and the rate of sudden death is higher than that observed in CPVT1. However, autosomal dominant mutations of CASQ2 are also reported [13], [14], [15].
CPVT3 was reported in a family with showing a 7p22-p14 chromosome anomaly, but the gene responsible has not been identified yet [16]. Recently, calmodulin (CALM) [17] and triadin (TRD) [18] anomalies have been found to responsible for CPVT4 and CPVT5, respectively.
CALM is a protein that involves the calcium dependent ICa inactivation of the L-type Ca channel. Further, CALM also stabilizes the RyR2 channel. Thus, a mutation in CALM may easily cause Ca2+ overload. TRD is a protein that connects CASQ to RyR2, and stabilizes the RyR2 channel. A mutation in TRD may also result in a diastolic leak of Ca2+ and Ca2+ overload in the myocytes.
KCNJ2 encodes the cardiac inward rectifier K channel. A mutation in KCNJ2 causes the Andersen–Tawil syndrome (LQT7), and is also reported in patients with exercise induced bi-directional VT [19]. Whether or not this type of mutation should be included as a subtype of CPVT is a matter of controversy. Mutations in the ANK2 gene are well known as a cause of LQT4. Recently, a patient with an ANK2 mutation was reported to have bi-directional VT [20]. This may be another disease related to CPVT.
A type of adult CPVT has also been reported [21], [22]. In this disease, the patients are predominantly female, with CPVT onset at the age of around 40 years, and no sudden death is reported. We believe that this may not be a specific type of CPVT, but rather a mild form of the disease.
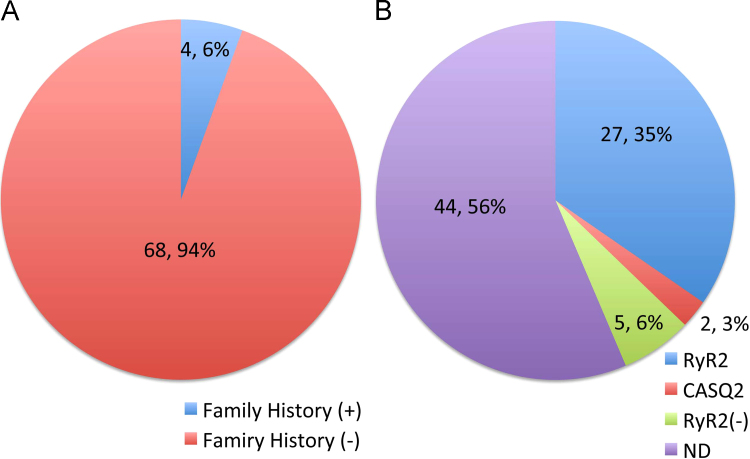
In the Japanese CPVT registry, 78 patients (M:F=26:52, age=11.2±8.2 years) were enrolled. In this registry, only 6% of the cases were familial cases whereas 94% of the cases were sporadic (Fig. 7A). In this cohort, 56% of the patients had not undergone genetic testing. However, of the 46% patients who underwent genetic testing, 79% of the patients had an RyR2 gene anomaly, 6% had a CASQ2 gene anomaly, and in 15% of the patients the specific causative gene anomaly was unknown (Fig. 7B). The estimated RyR2 genotype percentage is reported to range from 35% [23] up to 65% [24], [25], and the CASQ2 genotyped patients are estimated to account for approximately 3–5% [25].
Fig. 7.
Family history and gene anomalies in the Japanese registry. (A) Family history in the registry. (B) Gene mutations ND; gene testing was not performed.
The proportions of familial cases reported in other studies were 21.3% [26] and 30% [21]. The lower percentage of familial cases observed in our cohort may be because half of the registered cases are over 15 years old, at which time only information of familial history was taken without exercise or genetic testing. This may result in an apparently lower percentage of familial cases. Kawamura et al. have reported that RyR2 positive CPVT cases are more likely to have clinically diagnosed CPVT-affected family members with bidirectional VT, and sinus bradycardia [26].
6. The mechanism of bidirectional VT
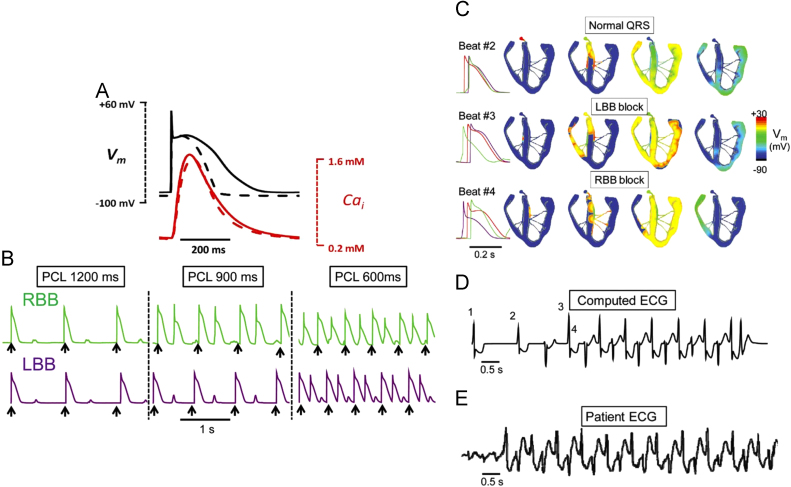
Bidirectional VT is the most characteristic feature of CPVT. In the His–Purkinje system, DAD induced bigeminy may differ depending on whether they are induced by the right bundle branch or the left bundle branch. The right bundle branch (RBB) caused a DAD induced bigeminy at a pacing rate of 900 ms (Fig. 8B), whereas the left bundle branch (LBB) induced a bigeminy at a pacing rate of 600 ms (Fig. 8B) [27]. In these situations, the sinus rate exceeded the threshold of the RBB-DAD induced bigeminy rate, and the beat after the sinus beat may have been induced from the RBB, resulting in a LBB block (LBBB) type PVC. The coupling interval of the normal sinus beat to the LBBB type PVC exceeded the threshold of the LBB-DAD induced bigeminy, and the next beat arose from LBB, resulting in a RBB block (RBBB) type PVC. When the coupling interval of the LBBB type PVC and RBBB type PVC exceeded the threshold of the RBBDAD induced bigeminy, the next beat arose from the RBB followed by a beat from the LBB, one after the other (Fig. 8C) [27]. This computer simulation suggests a mechanism for the bidirectional VT.
Fig. 8.
A possible mechanism for bidirectional ventricular tachycardia: ping-pong in the His–Purkinje system [27]. (A) Comparison of simulated rabbit ventricular (dashed line) and Purkinje (solid line) action potentials (APs) and Cai transients during pacing at 600 ms. (B) Rate dependence of delayed after depolarizations (DADs) and bigeminy in Purkinje cell AP models. For the green trace, the rate threshold for DAD-induced bigeminy was 67 bpm (pacing cycle length [PCL] 900 ms), such that pacing (black arrows) at both 900 and 600 ms induced bigeminy. For the purple trace, the bigeminy rate threshold was 100 bpm (PCL 600 ms), such that pacing at 600 ms, but not 900 ms, induced bigeminy. LBB: left bundle branch; RBB: right bundle branch. (C) Voltage snapshots depicting the activation sequence at BVT onset Beat #2 is the last paced beat, with normal activation. Beat #3 is the first beat of BVT, due to a DAD-triggered action potential (AP) arising in the right bundle branch (RBB), resulting in QRS with a left bundle branch (LBB) block pattern. Beat #4 is the second beat of BVT, due to a DAD-triggered AP arising in the LBB and results in a QRS with RBB block pattern. Traces on the right show the timing of APs recorded from the His bundle (red), RBB (green), and LBB (purple). (D) Computed ECG from the simulation in A, showing BVT. (E) ECG recorded in a patient during BVT.
7. Therapy for the CPVT
7.1. β Blockers
The long acting β blocker, nadolol, is preferred for prophylactic treatment of CPVT. Propranolol is also an effective medication. However, β blockers cannot completely suppress the arrhythmic events in CPVT patients [28].
Carvedilol is reported to inhibit the SOICR in an HEK 293 cell culture model. Among various β blockers, only carvedilol inhibits RyR2 activity [29]. Thus, carvedilol may be an effective β blocker for CPVT, but its β blocking effect may be weak in comparison to the other β blockers. Therefore, the efficacy of carvedilol needs to be further investigated.
7.2. Verapamil
Verapamil has also shown beneficial effects in some CPVT patients [30], [31]. However, the long-term efficacy of verapamil is still controversial.
7.3. Flecainide
Flecainide is an effective medication for CPVT [32], [33], [34]. Flecainide treatment shows improvement of ventricular arrhythmias in 74% of the genotype positive CPVT cases [32], and in 92% of the genotype negative CPVT cases [34]. Flecainide is thought to function by direct suppression of the RyR2 receptor. Among the Class I anti-arrhythmic medications, only flecainide and propafenone inhibit RyR2 activity [35]. However, recent report denies the direct suppression of RyR2 by flecainide [36]. That may suggest another mechanism of flecainide, such as inhibition of NCX.
7.4. Left cardiac sympathetic denervation
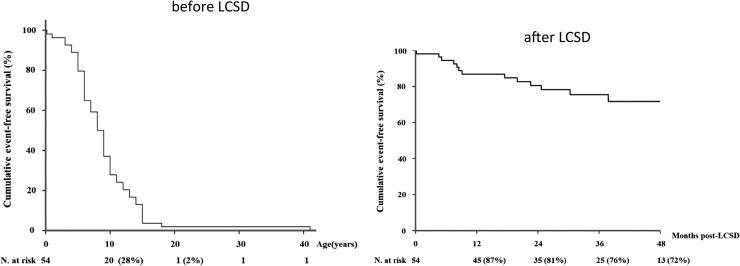
Left cardiac sympathetic denervation is reported to be a useful therapeutic method for suppressing ventricular arrhythmias in CPVT patients [37], [38]. In patients with uncontrollable ventricular arrhythmias, left cardiac sympathetic denervation is highly useful in controlling ventricular tachyarrhythmias (Fig. 9). The rate of complications involving Horner syndrome is very low if denervation is performed in the lower half of the T1 sympathetic ganglion through the T4 ganglion [38].
Fig. 9.
Kaplan–Meier curve of cumulative survival to a first major cardiac event before and after left cardiac sympathetic denervation (LCSD) in symptomatic patients with CPVT [38]. In 63 patients with CPVT, the cumulated event free survival significantly improved after LCSD.
7.5. ICD
Implantation of an ICD should be considered in patients in the absence of controlled optimal therapy [39]. However, implantation of an ICD in children still has a number of technical problems [40]. Moreover, inappropriate or painful shocks may increase the risk of further ventricular arrhythmias, and electrical storms that may result in lethal events.
7.6. Catheter ablation
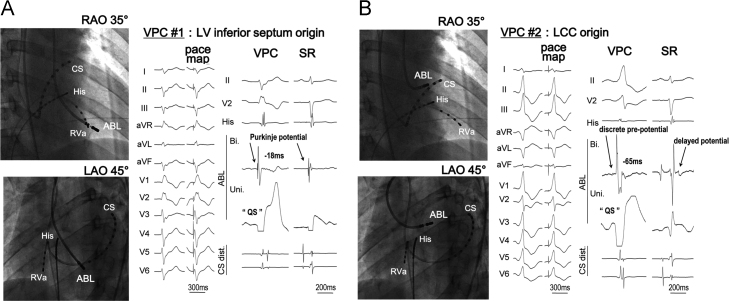
Pulmonary vein isolation is reported to be effective in some CPVT patients with atrial fibrillation [41]. Purkinje cells are reported to be more arrhythmogenic than ventricular myocytes in a mutant knockout mouse model of CPVT [42]. The onset of CPVT may be initiated from Purkinje cells. Successful catheter ablation has been reported at the site of Purkinje potentials or discrete pre-potentials (Fig. 10) [43].
Fig. 10.
Pace mapping of PVC in a patient with CPVT [43]. (A) A perfect pace map of the second beat of the CPVT was obtained on the left ventricular septum. Purkinje potential at that point was recorded during the PVC and sinus rhythm. (B) A perfect pace map of the first beat of a PVC was obtained at the left coronary cusp. A discrete pre-potential was recorded during the PVC, and a delayed potential was recorded during sinus rhythm.
7.7. Gene therapy
The homozygous R33Q knock-in mouse has a dysfunctional CASQ2, which may cause CPVT. In this mouse model, isoproterenol induced DADs, which were markedly reduced after 12 months following infection with an adenoviral vector (serotype 9), that carried the normal CASQ2 gene [44]. This report suggested the possible use of gene therapy for some types of CPVT in the future.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgment
This work was supported by Health Science Research grant from the Ministry of Health, Labour and Welfare of Japan for Clinical Research on Measures for Intractable Diseases (2016-032).
References
- 1.Coumel P., Fidelle J., Lucet V. Catecholamine-induced severe ventricular arrhythmias with Adams–Stokes syndrome in children: report of four cases. Br Heart J. 1978;40(Suppl.):S28–S37. [Google Scholar]
- 2.Leenhardt A., Lucet V., Denjoy I. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 3.Sumitomo N., Harada K., Nagashima M. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumitomo N., Sakurada H., Taniguchi K. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1551–1554. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 5.Priori S.G., Wilde A.A., Horie M. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. J Arrhythm. 2014;30:29–47. doi: 10.1093/europace/eut272. [DOI] [PubMed] [Google Scholar]
- 6.Yano M., Yamamoto T., Kobayashi S. Role of ryanodine receptor as a Ca2(+) regulatory center in normal and failing hearts. J Cardiol. 2009;53:1–7. doi: 10.1016/j.jjcc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Sharma M.R., Jeyakumar L.H., Fleischer S. Three-dimensional visualization of FKBP12.6 binding to an open conformation of cardiac ryanodine receptor. Biophys J. 2006;90:164–172. doi: 10.1529/biophysj.105.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu N., Rizzi N., Boveri L. Ryanodine receptor and calsequestrin in arrhythmogenesis: what we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol. 2009;46:149–159. doi: 10.1016/j.yjmcc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Priori S.G., Napolitano C., Tiso N. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 10.Laitinen P.J., Brown K.M., Piippo K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 11.Lahat H., Eldar M., Levy-Nissenbaum E. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome. Circulation. 2001;103:2822–2827. doi: 10.1161/01.cir.103.23.2822. [DOI] [PubMed] [Google Scholar]
- 12.Lahat H., Pras E., Olender T. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Fuente S., Van Langen I.M., Postma A.V. A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin Electrophysiol. 2008;31:916–919. doi: 10.1111/j.1540-8159.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- 14.Postma A.V., Denjoy I., Hoorntje T.M. Absence of calsequestrin2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–e26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 15.Roux-Buisson N., Egea G., Denjoy I. Germline and somatic mosaicism for a mutation of the ryanodine receptor type2 gene: implication for genetic counseling and patient caring. Europace. 2011;13:130–132. doi: 10.1093/europace/euq331. [DOI] [PubMed] [Google Scholar]
- 16.Bhuiyan Z.A., Hamdan M.A., Shamsi E.T. A novel early onset lethal form of catecholaminergic polymorphic ventricular tachycardia maps to chromosome. J Cardiovasc Electrophysiol. 2007;18:1060–1066. doi: 10.1111/j.1540-8167.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- 17.Nyegaard M., Overgaard M.T., Sondergaard M.T. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–712. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux-Buisson N., Cacheux M., Fourest-Lieuvin A. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega A.L., Tester D.J., Ackerman M.J. Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2009;2:540–547. doi: 10.1161/CIRCEP.109.872309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohler P.J., Splawski I., Napolitano C. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sy R.W., Gollob M.H., Klein G.J. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2011;8:864–871. doi: 10.1016/j.hrthm.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 22.Sumitomo N. Are there juvenile and adult types in patients with catecholaminergic polymorphic ventricular tachycardia? Heart Rhythm. 2011;8:872–873. doi: 10.1016/j.hrthm.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Bai R., Napolitano C., Bloise R. Yield of genetic screening in inherited cardiac channelopathies: how to prioritize access to genetic testing. Circ Arrhythm Electrophysiol. 2009;2:6–15. doi: 10.1161/CIRCEP.108.782888. [DOI] [PubMed] [Google Scholar]
- 24.Priori S.G., Napolitano C., Memmi M. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 25.Ackerman M.J., Priori S.G., Willems S. HRS/HER a expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura M., Ohno S., Naiki N. Genetic background of catecholaminergic polymorphic ventricular tachycardia in Japan. Circ J. 2013;77:1705–1713. doi: 10.1253/circj.cj-12-1460. [DOI] [PubMed] [Google Scholar]
- 27.Baher A.A., Uy M., Xie F. Bidirectional ventricular tachycardia: ping pong in the His–Purkinje system. Heart Rhythm. 2011;8:599–605. doi: 10.1016/j.hrthm.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi M., Denjoy I., Extramiana F. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q., Xiao J., Jiang D. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosso R., Kalman J.M., Rogowski O. Calcium channel blockers and beta- blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007;4:1149–1154. doi: 10.1016/j.hrthm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Swan H., Laitinen P., Kontula K. Calcium channel antagonism reduces exercise-induced ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia patients with RyR2 mutations. J Cardiovasc Electrophysiol. 2005;16:162–166. doi: 10.1046/j.1540-8167.2005.40516.x. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H., Chopra N., Laver D. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Werf C., Kannankeril P.J., Sacher F. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe H., van der Werf C., Roses-Noguer F. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2013;10:542–547. doi: 10.1016/j.hrthm.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang H.S., Hasdemir C., Laver D. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bannister M.L., Thomas N.L., Sikkel M.B. The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circ Res. 2015;116:1324–1335. doi: 10.1161/CIRCRESAHA.116.305347. [DOI] [PubMed] [Google Scholar]
- 37.Wilde A.A., Bhuiyan Z.A., Crotti L. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 38.De Ferrari G.M., Dusi V., Spazzolini C. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation. 2015;131:2185–2193. doi: 10.1161/CIRCULATIONAHA.115.015731. [DOI] [PubMed] [Google Scholar]
- 39.van der Werf C., Zwinderman A.H., Wilde A.A. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace. 2012;14:175–183. doi: 10.1093/europace/eur277. [DOI] [PubMed] [Google Scholar]
- 40.Sumitomo N. Device therapy in children and patients with congenital heart disease. J Arrhythmia. 2014;30:428–432. [Google Scholar]
- 41.Sumitomo N., Nakamura T., Fukuhara J. Clinical effectiveness of pulmonary vein isolation for arrhythmic events in a patient with catecholaminergic polymorphic ventricular tachycardia. Heart Vessels. 2010;25:448–452. doi: 10.1007/s00380-009-1214-6. [DOI] [PubMed] [Google Scholar]
- 42.Kang G., Giovannone S.F., Liu N. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res. 2010;107:512–519. doi: 10.1161/CIRCRESAHA.110.221481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneshiro T., Naruse Y., Nogami A. Successful catheter ablation of bidirectional ventricular premature contractions triggering ventricular fibrillation in catecholaminergic polymorphic ventricular tachycardia with RyR2 mutation. Circ Arrhythm Electrophysiol. 2012;5:e14–e17. doi: 10.1161/CIRCEP.111.966549. [DOI] [PubMed] [Google Scholar]
- 44.Denegri M., Bongianino R., Lodola F. Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation. 2014;129:2673–2681. doi: 10.1161/CIRCULATIONAHA.113.006901. [DOI] [PubMed] [Google Scholar]