Abstract
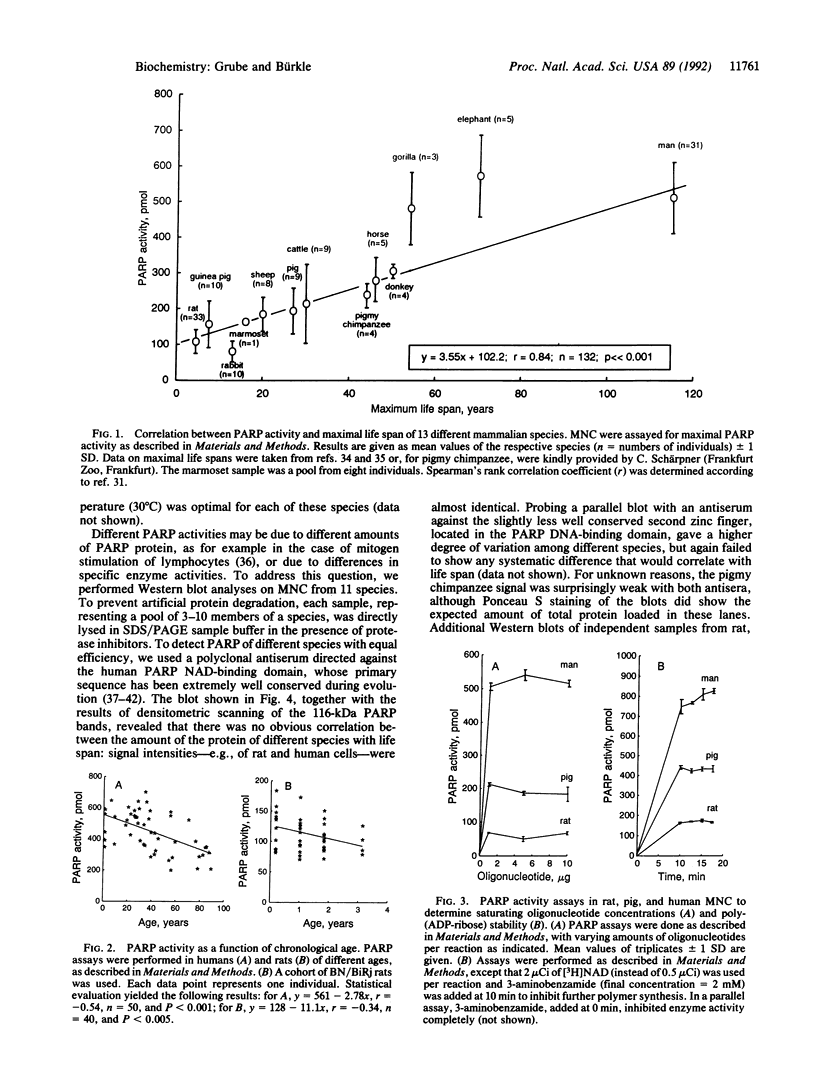
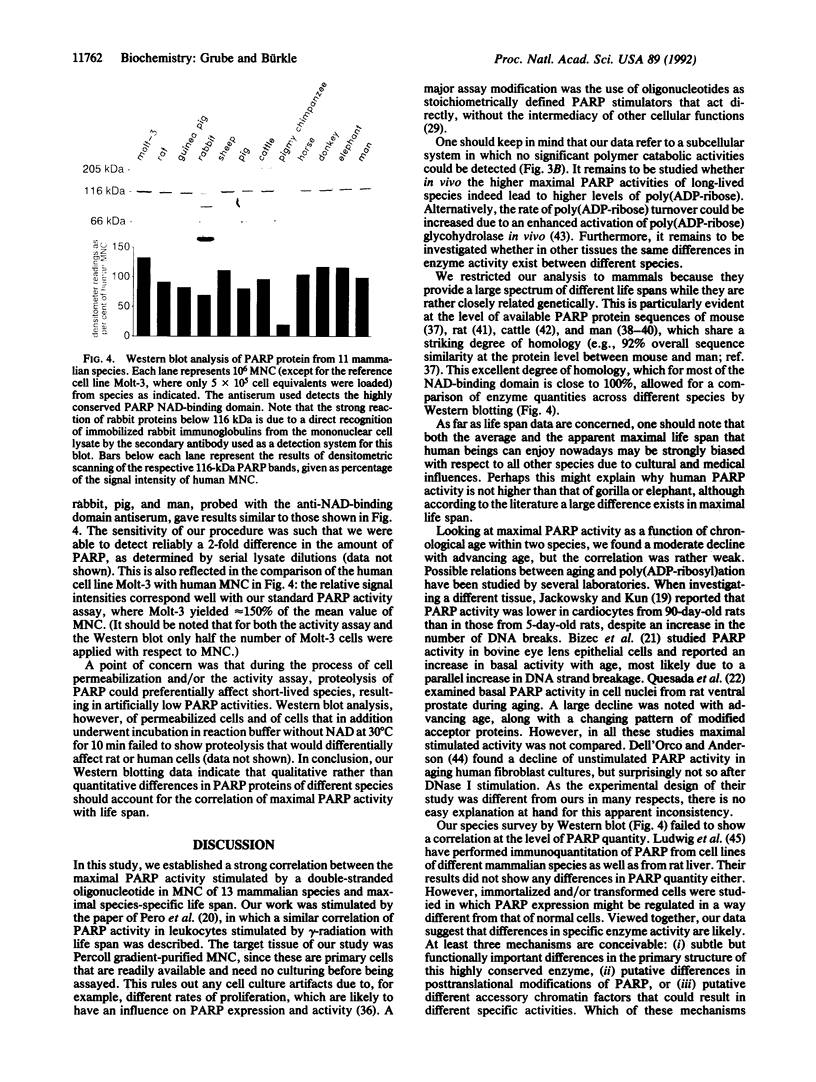
Poly(ADP-ribosyl)ation is a eukaryotic posttranslational modification of proteins that is strongly induced by the presence of DNA strand breaks and plays a role in DNA repair and the recovery of cells from DNA damage. We compared poly(ADP-ribose) polymerase (PARP; EC 2.4.2.30) activities in Percoll gradient-purified, permeabilized mononuclear leukocytes from mammalian species of different maximal life span. Saturating concentrations of a double-stranded octameric oligonucleotide were applied to provide a direct and maximal stimulation of PARP. Our results on 132 individuals from 13 different species yield a strong positive correlation between PARP activity and life span (r = 0.84; P << 0.001), with human cells displaying approximately 5 times the activity of rat cells. Intraspecies comparisons with both rat and human cells from donors of all age groups revealed some decline of PARP activity with advancing age, but it was only weakly correlated. No significant polymer degradation was detectable under our assay conditions, ruling out any interference by poly(ADP-ribose) glycohydrolase activity. By Western blot analysis of mononuclear leukocytes from 11 species, using a crossreactive antiserum directed against the extremely well-conserved NAD-binding domain, no correlation between the amount of PARP protein and the species' life spans was found, suggesting a greater specific enzyme activity in longer-lived species. We propose that a higher poly(ADP-ribosyl)ation capacity in cells from long-lived species might contribute to the efficient maintenance of genome integrity and stability over their longer life span.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Gonzalez R., Althaus F. R. Poly(ADP-ribose) catabolism in mammalian cells exposed to DNA-damaging agents. Mutat Res. 1989 Sep;218(2):67–74. doi: 10.1016/0921-8777(89)90012-8. [DOI] [PubMed] [Google Scholar]
- Bizec J. C., Klethi J., Mandel P. Regulation of protein adenosine diphosphate ribosylation in bovine lens during aging. Ophthalmic Res. 1989;21(3):175–183. doi: 10.1159/000266804. [DOI] [PubMed] [Google Scholar]
- Borek C., Morgan W. F., Ong A., Cleaver J. E. Inhibition of malignant transformation in vitro by inhibitors of poly(ADP-ribose) synthesis. Proc Natl Acad Sci U S A. 1984 Jan;81(1):243–247. doi: 10.1073/pnas.81.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. Relation between carcinogenesis, chromatin structure and poly(ADP-ribosylation) (review). Anticancer Res. 1991 Mar-Apr;11(2):489–527. [PubMed] [Google Scholar]
- Bürkle A., Heilbronn R., zur Hausen H. Potentiation of carcinogen-induced methotrexate resistance and dihydrofolate reductase gene amplification by inhibitors of poly(adenosine diphosphate-ribose) polymerase. Cancer Res. 1990 Sep 15;50(18):5756–5760. [PubMed] [Google Scholar]
- Bürkle A., Meyer T., Hilz H., zur Hausen H. Enhancement of N-methyl-N'-nitro-N-nitrosoguanidine-induced DNA amplification in a Simian virus 40-transformed Chinese hamster cell line by 3-aminobenzamide. Cancer Res. 1987 Jul 15;47(14):3632–3636. [PubMed] [Google Scholar]
- Cherney B. W., McBride O. W., Chen D. F., Alkhatib H., Bhatia K., Hensley P., Smulson M. E. cDNA sequence, protein structure, and chromosomal location of the human gene for poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8370–8374. doi: 10.1073/pnas.84.23.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orco R. T., Anderson L. E. Decline of poly(ADP-ribosyl)ation during in vitro senescence in human diploid fibroblasts. J Cell Physiol. 1991 Feb;146(2):216–221. doi: 10.1002/jcp.1041460205. [DOI] [PubMed] [Google Scholar]
- Desmarais Y., Ménard L., Lagueux J., Poirier G. G. Enzymological properties of poly(ADP-ribose)polymerase: characterization of automodification sites and NADase activity. Biochim Biophys Acta. 1991 Jun 24;1078(2):179–186. doi: 10.1016/0167-4838(91)99007-f. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Panayotou G. N., Bowler L. D., Hardas B. D., Broom T., Walther C., Shall S. ADP-ribosylation is involved in the integration of foreign DNA into the mammalian cell genome. Nucleic Acids Res. 1988 Dec 9;16(23):11319–11326. doi: 10.1093/nar/16.23.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. E., Gray D. A., Berney J. J., Wing M. A., Guimaraes J. E., Hoffbrand A. V. Role of ADP-ribosyl transferase in differentiation of human granulocyte-macrophage progenitors to the macrophage lineage. Blood. 1983 Nov;62(5):1055–1062. [PubMed] [Google Scholar]
- Gradwohl G., Ménissier de Murcia J. M., Molinete M., Simonin F., Koken M., Hoeijmakers J. H., de Murcia G. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2990–2994. doi: 10.1073/pnas.87.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube K., Küpper J. H., Bürkle A. Direct stimulation of poly(ADP ribose) polymerase in permeabilized cells by double-stranded DNA oligomers. Anal Biochem. 1991 Mar 2;193(2):236–239. doi: 10.1016/0003-2697(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Hahn P., Nevaldine B., Morgan W. F. X-ray induction of methotrexate resistance due to dhfr gene amplification. Somat Cell Mol Genet. 1990 Sep;16(5):413–423. doi: 10.1007/BF01233191. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Sacher G. A., Hoskins T. L. DNA repair in a short- and a long-lived rodent species. J Gerontol. 1979 Nov;34(6):808–817. doi: 10.1093/geronj/34.6.808. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Setlow R. B. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi K., Bhatia K., Siwarski D., Klinman D., Cherney B., Smulson M. Sequence and organization of the mouse poly (ADP-ribose) polymerase gene. Nucleic Acids Res. 1989 May 11;17(9):3387–3401. doi: 10.1093/nar/17.9.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima M., Noguchi S., Yamashita R., Ogura T., Sugimura T., Gill D. M., Miwa M. The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J Biol Chem. 1990 Dec 15;265(35):21907–21913. [PubMed] [Google Scholar]
- Jackowski G., Kun E. Age-dependent variation of rates of polyadenosine-diphosphoribose synthesis by cardiocyte nuclei and the lack of correlation of enzymatic activity with macromolecular size distribution of DNA. J Biol Chem. 1981 Apr 25;256(8):3667–3670. [PubMed] [Google Scholar]
- Jacobson E. L., Meadows R., Measel J. Cell cycle perturbations following DNA damage in the presence of ADP-ribosylation inhibitors. Carcinogenesis. 1985 May;6(5):711–714. doi: 10.1093/carcin/6.5.711. [DOI] [PubMed] [Google Scholar]
- Kasid U. N., Stefanik D. F., Lubet R. A., Dritschilo A., Smulson M. E. Relationship between DNA strand breaks and inhibition of poly (ADP-ribosylation): enhancement of carcinogen-induced transformation. Carcinogenesis. 1986 Feb;7(2):327–330. doi: 10.1093/carcin/7.2.327. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Ushiro H., Mitsuuchi Y., Suzuki S., Matsuda M., Matsuda Y., Katunuma N., Kangawa K., Matsuo H., Hirose T. Primary structure of human poly(ADP-ribose) synthetase as deduced from cDNA sequence. J Biol Chem. 1987 Nov 25;262(33):15990–15997. [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Behnke B., Holtlund J., Hilz H. Immunoquantitation and size determination of intrinsic poly(ADP-ribose) polymerase from acid precipitates. An analysis of the in vivo status in mammalian species and in lower eukaryotes. J Biol Chem. 1988 May 25;263(15):6993–6999. [PubMed] [Google Scholar]
- Lönn U., Lönn S. Accumulation of 10-kilobase DNA replication intermediates in cells treated with 3-aminobenzamide. Proc Natl Acad Sci U S A. 1985 Jan;82(1):104–108. doi: 10.1073/pnas.82.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura I., Kurashige T., Taniguchi T. Inhibitory effect of interferon-gamma-dependent induction of major histocompatibility complex class II antigen by expressing exogenous poly(ADP-ribose) synthetase gene. Biochem Biophys Res Commun. 1991 Mar 15;175(2):685–689. doi: 10.1016/0006-291x(91)91620-r. [DOI] [PubMed] [Google Scholar]
- Osiewacz H. D. Molecular analysis of aging processes in fungi. Mutat Res. 1990 Jan;237(1):1–8. doi: 10.1016/0921-8734(90)90026-n. [DOI] [PubMed] [Google Scholar]
- Pero R. W., Holmgren K., Persson L. Gamma-radiation induced ADP-ribosyl transferase activity and mammalian longevity. Mutat Res. 1985 Jan-Feb;142(1-2):69–73. doi: 10.1016/s0165-7992(85)80016-6. [DOI] [PubMed] [Google Scholar]
- Quesada P., Faraone-Mennella M. R., Jones R., Malanga M., Farina B. ADP-ribosylation of nuclear proteins in rat ventral prostate during ageing. Biochem Biophys Res Commun. 1990 Jul 31;170(2):900–907. doi: 10.1016/0006-291x(90)92176-z. [DOI] [PubMed] [Google Scholar]
- Saito I., Hatakeyama K., Kido T., Ohkubo H., Nakanishi S., Ueda K. Cloning of a full-length cDNA encoding bovine thymus poly(ADP-ribose) synthetase: evolutionarily conserved segments and their potential functions. Gene. 1990 Jun 15;90(2):249–254. doi: 10.1016/0378-1119(90)90187-v. [DOI] [PubMed] [Google Scholar]
- Slagboom P. E., Vijg J. Genetic instability and aging: theories, facts, and future perspectives. Genome. 1989;31(1):373–385. doi: 10.1139/g89-057. [DOI] [PubMed] [Google Scholar]
- Su Z. Z., Zhang P. Q., Fisher P. B. Enhancement of viral and DNA mediated transformation of cloned rat embryo fibroblast cells by 3-aminobenzamide. Mol Carcinog. 1990;3(5):309–318. doi: 10.1002/mc.2940030512. [DOI] [PubMed] [Google Scholar]
- Thibodeau J., Gradwohl G., Dumas C., Clairoux-Moreau S., Brunet G., Penning C., Poirier G. G., Moreau P. Cloning of rodent cDNA coding the poly(ADP-ribose) polymerase catalytic domain and analysis of mRNA levels during the cell cycle. Biochem Cell Biol. 1989 Sep;67(9):653–660. doi: 10.1139/o89-097. [DOI] [PubMed] [Google Scholar]
- Tolmasoff J. M., Ono T., Cutler R. G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980 May;77(5):2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Morita T., Sato T., Ogura T., Yamashita R., Noguchi S., Suzuki H., Nyunoya H., Miwa M., Sugimura T. Nucleotide sequence of a full-length cDNA for human fibroblast poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1987 Oct 29;148(2):617–622. doi: 10.1016/0006-291x(87)90921-1. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Waldman B. C. Stimulation of intrachromosomal homologous recombination in mammalian cells by an inhibitor of poly(ADP-ribosylation). Nucleic Acids Res. 1991 Nov 11;19(21):5943–5947. doi: 10.1093/nar/19.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H., Penning C. A., Willis E. H., Wasson D. B., Carson D. A. Characterization of human poly(ADP-ribose) polymerase with autoantibodies. J Biol Chem. 1988 Mar 15;263(8):3879–3883. [PubMed] [Google Scholar]