Abstract
The objective of this study is to investigate whether stem cell delivery of secreted Klotho (SKL), an aging-suppressor protein, attenuates monocrotaline (MCT)-induced pulmonary vascular dysfunction and remodelling. Overexpression of SKL in mesenchymal stem cells (MSCs) was achieved by transfecting MSCs with lentiviral vectors expressing SKL-GFP. Four groups of rats were treated with MCT, while an additional group was given saline (control). Three days later, four MCT-treated groups received IV delivery of non-transfected MSCs, MSC-GFP, MSC-SKL-GFP, and PBS, respectively. Ex vivo vascular relaxing responses to acetylcholine were diminished in small pulmonary arteries (PA) in MCT-treated rats, indicating pulmonary vascular endothelial dysfunction. Interestingly, delivery of MSCs overexpressing SKL (MSC-SKL-GFP) abolished MCT-induced pulmonary vascular endothelial dysfunction and PA remodelling. MCT significantly increased right ventricular (RV) systolic blood pressure, which was attenuated significantly by MSC-SKL-GFP, indicating improved pulmonary arterial hypertension (PAH). MSC-SKL-GFP also attenuated RV hypertrophy. Non-transfected MSCs slightly, but not significantly, improved PAH and pulmonary vascular endothelial dysfunction. MSC-SKL-GFP attenuated MCT-induced inflammation, as evidenced by decreased macrophage infiltration around PAs. MSC-SKL-GFP increased SKL levels which rescued the downregulation of SIRT1 expression and eNOS phosphorylation in the lungs of MCT-treated rats. In cultured endothelial cells, SKL abolished MCT-induced downregulation of eNOS activity and NO levels and enhanced cell viability. Therefore, stem cell delivery of SKL is an effective therapeutic strategy for pulmonary vascular endothelial dysfunction and PA remodelling. SKL attenuates MCT-induced PA remodelling and PASMC proliferation, likely by reducing inflammation and restoring SIRT1 levels and eNOS activity.
Keywords: pulmonary arterial hypertension, Klotho, mesenchymal stem cell, SIRT1, stem cell therapy, gene therapy
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease involving an increase in pulmonary vascular resistance and pulmonary arterial pressure (PAP), which leads to right ventricular (RV) dysfunction and failure and ultimately death. Although fairly uncommon (15 cases per million),1 PAH is associated with high mortality (5-year survival rates ranging from 34–58%).2 Treatment is expensive and is often based on vasodilators, which relieve the symptoms but do not cure the disease. PAH pathogenesis is complex and involves multiple factors (metabolism, the immune system/inflammation, right ventricle function) that contribute to disease progression.3 Monocrotaline (MCT)-induced PAH in rats involves initial endothelial dysfunction followed by rapid medial remodeling in small pulmonary arteries, which leads to increased PAP, RV dysfunction, and death in animals at 4–6 weeks after insult. The MCT model is associated with endothelial toxicity, endothelial dysfunction, increased endothelial permeability in small pulmonary arteries (PA), significant inflammation in lungs, over-proliferation of PA smooth muscle cells, and PA medial remodeling.
Several types of progenitor cells, such as endothelial progenitor cells (EPCs), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs) have been investigated for the treatment of PAH. Some cell-based therapies are at various stages of preclinical or clinical trials.4 MSCs were reported to have anti-inflammatory and immune-modulating properties5–6 and were shown to prevent or repair endothelial injury.7–8 Thus, MSC therapy is an attractive strategy for PAH treatment, as inflammation and endothelial injury play an important role in the pathogenesis of PAH. It was previously reported that MSCs improve PAH, lung pathology, and RV dysfunction,9–12 PA remodeling,11, 13 and impaired PA responses to vasodilators9 in MCT models of PAH in rats. We chose to use MSCs in this study, because they also have other characteristics that facilitate their application, such as their expansion potential, ease of collection, and decreased susceptibility to genetic mutations during in vitro passaging.14 MSCs engineered with the desired therapeutic genes may expand and have enhanced therapeutic potential.14 Genetically modified MSCs overexpressing genes of interest, for example, eNOS15 or prostacyclin synthase,16 have also been used in the treatment of MCT-induced PAH in rats. Nevertheless, MSC-based therapy has been less effective than expected.
Klotho is an anti-aging gene that causes extensive premature aging phenotypes and shortens lifespan when disrupted17 and slows the aging process and extends lifespan when overexpressed.18–19 Klotho gene mutation causes lung inflammation and emphysema,19 while unmutated Klotho has been shown to reduce inflammation and oxidative stress in kidneys.19–21 Secreted Klotho (SKL) was reported to protect against endothelial dysfunction22–23 and endothelial cell (EC) apoptosis24 and attenuate vascular remodeling associated with systemic hypertension.23 Mesenchymal stem cells entail have secretory function.14 In this study, we engineered mouse MSCs to overexpress SKL and intravenously injected the modified MSCs into MCT-treated rats. To our knowledge, this is the first study to investigate whether MSC delivery of SKL attenuates MCT-induced PAH.
Materials and Methods
A detailed method section is available in the Online Supplemental Methods and Data.
Generation of MSCS overexpressing SKL
MSCs overexpressing SKL were generated by transducing MSCs with lentiviral-based transfer of SKL gene (MSC-SKL-GFP) or GFP gene (MSC-GFP) (see Online Supplemental Methods).
Animal studies
Briefly, five groups of rats (six rats per group) were used for the study: Saline, MCT, MCT + MSC, MCT + MSC-GFP and MCT + MSC-SKL-GFP. Four groups were given monocrotaline (MCT) daily (60 mg/kg) via subcutaneous injections, while the last group was given saline and served as a control. After 3 days of MCT injections, three MCT-treated groups were administered MSCs, MSCs carrying eGFP (MSC-GFP), and MSCs carrying eGFP-SKL (MSC-SKL-GFP), respectively, via injection into the right jugular vein (3.5 × 106 cells/rat), while one MCT-treated group received no treatment and served as a control. Twenty-one days post MCT injection, RV pressures were measured under anesthesia. The scheme of the experimental protocol is shown in Supplemental Figure S1 (Fig. S1).
Immunohistochemical (IHC) analysis of the lungs
The IHC was performed as described recently.25–28 A detailed protocol is provided in the Online Supplemental Methods.
Western blot for SKL, SIRT1, eNOS, and p-eNOS expression in serum and lung lysates
Western blotting was performed as we described previously.29,30–33 A detailed protocol is provided in the Online Supplemental Methods.
Cell culture experiments
A detailed protocol is provided in the Online Supplemental Methods.
Statistical analysis
All data are presented as mean ± SEM unless otherwise specified. Data were analyzed using one-way ANOVA and Student’s t-test. The Newman–Keuls procedure was used to assess the significance of differences between groups, and p<0.05 was considered statistically significant.
Results
Transfection of mesenchymal stem cells (MSCs) with the mouse secreted Klotho (SKL) gene
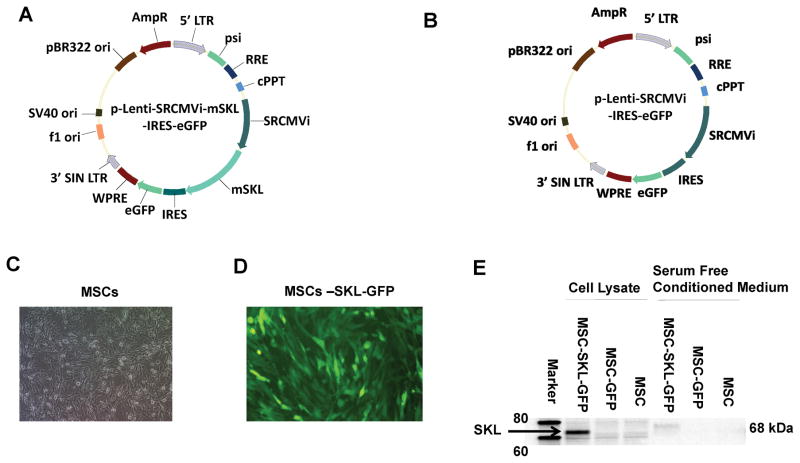
A lentiviral vector construct, pLenti-SRCMVi-mSKL-GFP (Fig. 1A), was constructed in which the expression of mSKL was driven by a CMV promoter, with extra enhancers from SV40 and RSV promoters and an intron from the backbone of the pAAV-MCS plasmid for enhancing expression of the mSKL gene. Besides mSKL, it also expresses an internal ribosome-entering site (IRES)-mediated eGFP for easy detection of the transduction of target cells. A lentiviral vector construct, pLenti-SRCMVi-GFP, was also constructed as a control construct (Fig. 1B). MSCs were in a healthy condition (Fig. 1C). Following transfection of 293T cells, robust expression of eGFP was observed as an indication of efficient production of infectious lentiviral vectors, and the target MSCs were transduced with nearly 100% efficiency following infection of suspended cells at a multiplicity of infection (MOI) of 10–20 (Fig. 1D). Western blot analysis of SKL protein expression in MSC lysates and serum-free conditioned medium (SFCM) from the transduced MSCs and untransfected MSCs showed that mSKL was expressed in these cells and was also secreted into the medium (Fig. 1E). The full-length Klotho protein (130 kDa) was not detectable in cell lysates and medium. Overexpression of mSKL increased SKL protein levels in cell lysates and medium (Fig. 1E). Due to attachment with Flag-tag and His-tag in the c-terminal, the size of the transgenic SKL protein is ~3 kDa larger than the endogenous SKL protein. The slightly increased size of the secreted SKL protein in the medium is likely attributed to the post-translational modifications (e.g., glycosylation).
Figure 1. Transfection of mesenchymal stem cells (MSCs) with the mouse secreted Klotho (mSKL) gene.
A) Map of lentiviral vector expressing the mSKL and eGFP genes. LTR, long terminal repeat; psi, ψ domain; RRE, Rev responsive element; cPPT, central polypurine tract; SRCMVi, a CMV promoter plus enhancers from RSV and SV40 promoters and a human β-globin intron; mSKL, mouse secreted Klotho gene; IRES, internal ribosome entry site; eGFP, enhanced green fluorescent protein gene; WPRE, woodchuck hepatitis virus post-transcriptional regulatory element; SIN, self-inactivating LTR as a result of the deletion of the U3 promoter. B) Map of the control lentiviral vector expressing eGFP only. C) Phase-contrast image of MSCs in culture. D) eGFP expression in Lenti-SKL-GFP-transduced MSCs. E) Western blot analysis of SKL protein expression in cell lysates and conditioned medium from transfected and non-transfected MSCs.
MSCs overexpressing SKL abolished MCT-induced pulmonary vascular dysfunction
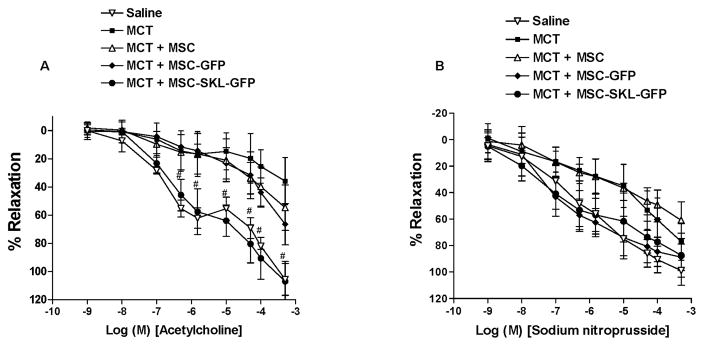
To measure PA responses to vasodilators in our experimental model of PAH, we isolated small intralobar PA rings (3rd order) from the lungs at 3 weeks after MCT injection and mounted them on a wire myograph (DMT) for measuring PA relaxation responses to the endothelium-dependent vasodilator ACh or the endothelium-independent vasodilator SNP. The 3rd-order branches are regarded as resistance PAs. Compared with PAs from the Saline group, PAs from the MCT group had markedly decreased relaxation in response to ACh (Fig. 2A), indicating that MCT impairs pulmonary vascular endothelial function. PAs from the MCT + MSC and MCT + MSC-GFP groups had slightly, but not significantly, better relaxation responses to ACh than PAs from the MCT group. It is noteworthy that the PAs from the MCT + MSC-SKL-GFP group showed a significantly greater relaxation in response to ACh, which was comparable to that of the Saline group (Fig. 2A). This result suggests that implantation of MSCs overexpressing SKL rescues MCT-induced pulmonary vascular endothelial dysfunction.
Figure 2. MSCs overexpressing SKL abolished MCT-induced pulmonary vascular dysfunction.
A) Responses of small pulmonary arteries (PAs) to ACh. B) Responses of small PAs to sodium nitroprusside (SNP). #p<0.05 vs. the MCT group. n=6. Data = means±SEM.
There was no significant difference in PA relaxation responses to the vasodilator SNP among all groups (Fig. 2B), suggesting that endothelium-independent PA relaxation function is not affected significantly by administration of MCT or implantation of MSCs overexpressing SKL.
MSCs overexpressing SKL attenuated MCT-induced pulmonary arterial remodeling
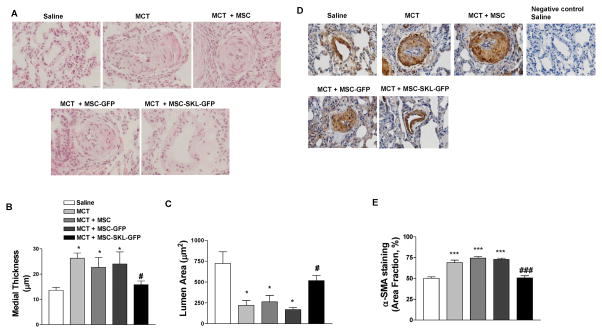
Human PAH is characterized by significant medial hyperplasia/hypertrophy in small pulmonary arteries.34 (REF, cite Patrick papers). We analyzed PA sections using H&E staining, and medial hyperplasia was quantified by measuring the medial thickness and lumen area in the small 3rd-order PAs (diameter 50–80 μm). MCT significantly increased medial thickness and decreased lumen area (Fig. 3A–C), indicating medial hypertrophy and occlusion of small PAs. MCT-induced PA remodeling was abolished by MSC-SKL-GFPs. The MCT-induced medial thickening or hypertrophy was likely due to increased proliferation of PA-SMCs, as MCT significantly increased expression of medial α-smooth muscle actin (α-SMA) (Fig. 3D, E). α-SMA is a marker of SMCs, and MCT-induced SMC over-proliferation was eliminated by MSC-SKL-GFPs. Thus, implantation of MSCs overexpressing SKL effectively rescued MCT-induced PA-SMC proliferation and PA remodeling.
Figure 3. MSCs overexpressing SKL attenuated MCT-induced pulmonary arterial remodeling.
A) Representative H&E staining images of small pulmonary artery (PA) cross-sections (60–80 μM) in rat lungs. B) Quantitative analysis of medial thickness. C) Lumen area in rat small PA cross-sections. D) α-SMA staining using IHC in small PA cross-sections. E) Quantification of α-SMA staining. Scale bar, 20 μm. *p<0.05 vs. Saline; #p<0.05 vs. MCT. n=6. Data = means±SEM.
MSCs overexpressing SKL attenuated MCT-induced pulmonary arterial hypertension (PAH) and RV hypertrophy
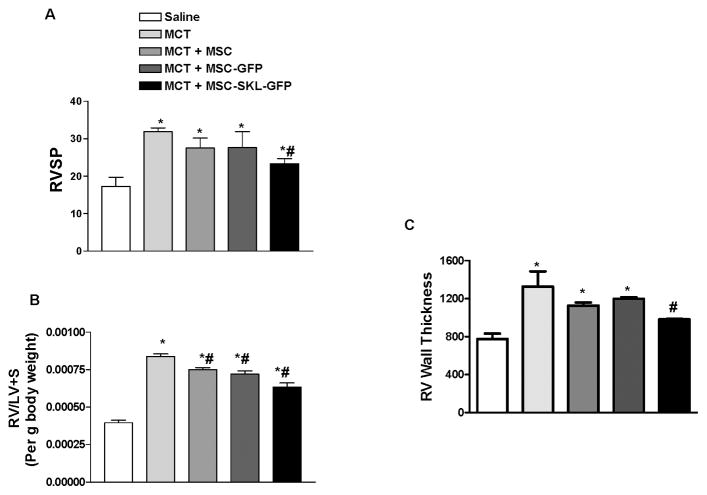
Right ventricular systolic pressure (RVSP) was significantly higher in rats from the MCT group than from the Saline group (31.88 ± 1.39 vs. 17.27 ± 4.86 mmHg) (Fig. 4A), indicating PAH. Treatment with control MSCs slightly, but not significantly, attenuated the RVSP increase. By contrast, treatment with MSC-SKL-GFP (23.35 ± 3.26 mmHg) significantly attenuated the MCT-induced increase in RVSP but not to the control level (Fig. 4A). The ratio of RV to LV+S weights was increased significantly in the MCT group compared with the Saline group, indicating RV hypertrophy. Treatment with MSCs, especially MSC-SKL-GFP, decreased RV hypertrophy in MCT rats (Fig. 4B). RV wall thickness, measured in H&E-stained heart sections, was also used as an indicator of RV hypertrophy. MCT significantly increased RV wall hypertrophy, and treatment with MSCs slightly, but significantly, attenuated RV hypertrophy (Fig. 4C). By contrast, treatment with MSC-SKL-GFP more effectively attenuated RV hypertrophy than did untransduced MSCs (Fig. 4C). Thus, MSCs overexpressing SKL improved MCT-induced PAH and RV hypertrophy.
Figure 4. MSCs overexpressing SKL attenuated MCT-induced pulmonary arterial hypertension (PAH) and RV hypertrophy.
A) Right ventricular systolic blood pressure (RVSP). B) The RV/LV+S weight ratio. C) Quantification of RV wall thickness. *p<0.05 vs. Saline; #p<0.05, ##p<0.01 vs. MCT. n=6. Data = means±SEM.
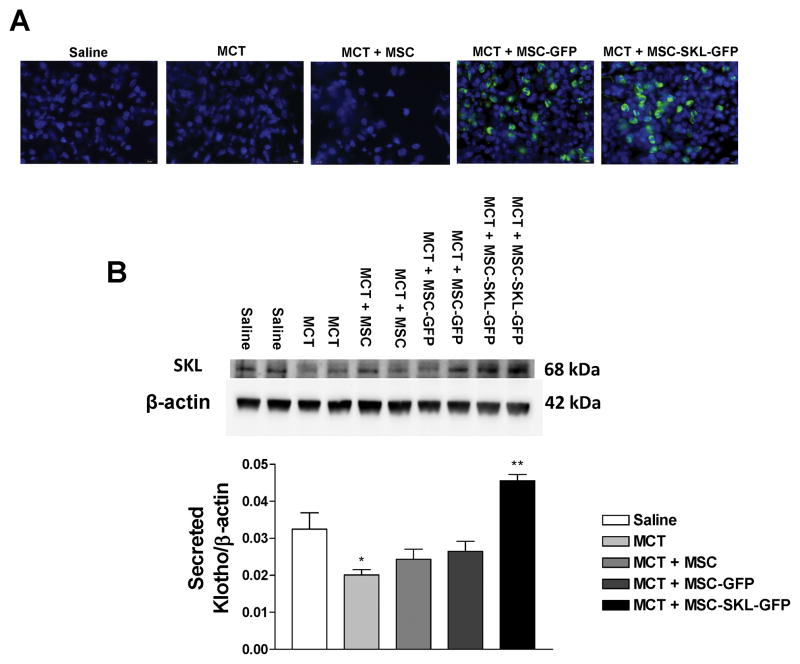
MSCs engrafted into the lung parenchyma and expressed SKL
Eighteen days after IV delivery of MSCs (via the right jugular vein), eGFP-positive MSCs were found in clusters or as single cells in the lung parenchyma in OCT-embedded lung sections from the MCT + MSC-SKL-GFP and MCT + MSC-GFP groups (Fig. 5A). This result suggests that MSCs homed to the lung. eGFP-positive cells were not seen in the Saline, MCT, or MCT + MSC groups. The transgenic cells did not engraft into the pulmonary arteries (PAs) as eGFP-positive cells and were not seen in the vasculature (Fig. S2). Unexpectedly, the transgenic MSCs did not differentiate into epithelial cells, endothelial cells, or smooth muscle cells.
Figure 5. MSCs engrafted into the lung parenchyma and expressed SKL.
A) GFP-positive cells in the lungs of MCT + MSC-SKL-GFP and MCT + MSC-GFP groups. B) Western blot analysis of SKL protein levels in the lung lysates. *p<0.05 vs. Saline. n=6. Data = means±SEM.
Western blot analysis showed that SKL expression was significantly decreased by MCT (Fig. 5B). SKL levels in the lung were significantly higher in the MCT + MSC-SKL-GFP group than in the other groups (Fig. 5B), indicating increased SKL expression. The full-length Klotho protein (130 kDa) was not detectable in the lung. SKL concentrations in the serum were slightly but not significantly increased in the MCT + MSC-SKL-GFP group (Fig. S3). These results suggest that the MSCs secreted SKL to the lungs in a paracrine fashion and that only a minor amount of the secreted SKL entered the circulation. Thus, MSC-SKL-GFP effectively rescued the downregulation of SKL levels in the lungs in MCT-treated rats.
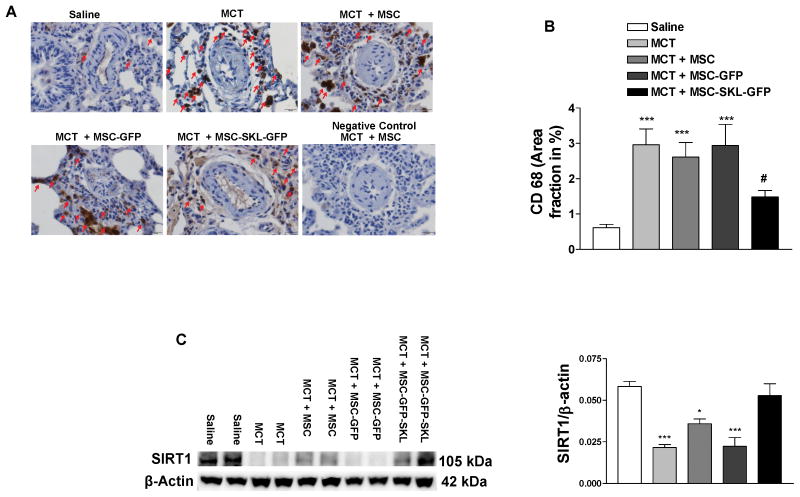
MSCs overexpressing SKL attenuated MCT-induced inflammation in PAs and abolished downregulation of SIRT1 expression and eNOS activity in the lungs
IHC analysis revealed extensive infiltration of macrophages (CD68+) around small PAs and in the lungs of MCT-treated rats (Fig. 6A, B), indicating inflammation. Treatment with MSC-SKL-GFP significantly attenuated MCT-induced macrophage infiltration. By contrast, MSCs or MSC-GFP did not affect macrophage infiltration significantly (Fig. 6A, B). Thus, implantation of MSCs overexpressing SKL effectively attenuated inflammation in PAs due to MCT insult. These results suggest that SKL enhances the anti-inflammatory capacity of MSCs, which contributes to its beneficial effect on pulmonary vascular dysfunction and PA remodeling.
Figure 6. MSCs overexpressing SKL attenuated MCT-induced inflammation in pulmonary arteries (PAs) and abolished downregulation of SIRT1 expression in the lungs.
A) IHC analysis of macrophages around small PAs (dark brown staining, CD68 marker). B) Quantification of CD68 staining. C) Western blot analysis of SIRT1 expression in lung lysates. *p<0.05, **p<0.01, **p<0.001 vs. Saline; #p<0.05 vs. MCT. n=6. Data = means±SEM.
Western blot analysis indicated that the expression level of SIRT1, an important deacetylase, was diminished in the lungs of all MCT-treated groups (Fig. 6C). Interestingly, MSC-SKL–GFP effectively restored SIRT1 expression to the control level (Fig. 6C). The results suggest for the first time that MSCs overexpressing SKL regulate SIRT1 expression in the lungs of MCT-treated rats. Western blot analysis showed that eNOS phosphorylation (p-eNOS) was significantly decreased in the lungs of MCT-treated rats, suggesting that MCT suppresses eNOS activity (Fig. S4A). Interestingly, MSC-SKL-GFP also rescued MCT-induced downregulation of eNOS activity in lungs (Fig. S4A).
SKL prevented MCT-induced impairment in endothelial cell viability and downregulation of nitric oxide bioavailability in HUVECs
To further explore whether SKL affects endothelial cell function, we treated HUVECs with different concentrations of MCT for a period of 48 hours. The MTT assay showed a dose-dependent decrease in HUVEC viability in response to MCT treatment (Fig. S4B, C). Interestingly, the addition of SKL (0.5 μg/ml) to culture medium rescued MCT-induced impairment in cell viability (Fig. S4D). MCTP, a metabolic product of MCT, diminished NO production, as measured by the DAF-2DA assay, at four days after treatment (Fig. S4E). Interestingly, the addition of SKL abolished MCTP-induced downregulation of NO production (Fig. S4E). L-NAME decreased NO levels (Fig. S4E) and prevented an SKL-induced increase in NO production in MCTP-treated HUVECs (not shown), suggesting that SKL stimulates NO production via activation of eNOS.
Discussion
This study demonstrates for the first time that intravenous delivery of MSCs overexpressing SKL effectively prevented MCT- induced pulmonary vascular endothelial dysfunction and PA remodeling. MSC-SKLs also attenuated the elevation of RV pressure and RV hypertrophy in MCT-treated rats. This finding is significant, because it provides a new and effective therapeutic approach for PAH, a devastating disease with no cure. By contrast, untransduced MSCs did not significantly improve pulmonary vascular endothelial dysfunction or PA remodeling. Thus, overexpression of secreted Klotho (SKL) enhances the therapeutic potential of MSCs. The beneficial effects of MSCs overexpressing SKL may be achieved through increasing SKL levels in the lungs.
Klotho was originally identified as an aging-suppressor gene that is primarily expressed in kidney distal tubule epithelial cells and brain choroid plexuses. In this study, we found that SKL is also expressed in the lungs, which was diminished by MCT (Fig. 5). Interestingly, MSC-SKLs rescued MCT-induced downregulation of SKL, suggesting that implantation of MSCs overexpressing SKL effectively increased SKL levels in the lung. MSCs have the ability to secrete paracrine factors, leading to the improvement of injured tissue.12,14, 35 Although MSCs are known to secrete a variety of regulatory and trophic factors, the complete MSC secretome remains to be determined.36 This study demonstrates that the engineered MSCs exhibit paracrine activity, as the released SKL was limited to the lung (Fig. 5), with a minor amount of SKL entering the circulation (Supplemental Figure S3). Although Klotho deficiency has been implicated in systemic hypertension,21, 37–38 this study provides the first evidence that MSCs overexpressing SKL significantly improve pulmonary vascular endothelial dysfunction and PA remodeling. These results also suggest that downregulation of SKL in the lungs contributes to MCT-induced pulmonary vascular endothelial dysfunction, PA remodeling, and PAH.
MSCs are unique in possessing the potential to differentiate into other cell types and home to the injured tissue.12 They can enter the circulation and follow chemotactic gradients to home to sites of injury or inflammation, participating in wound healing and tissue repair via their regenerative and paracrine functions.39–42 We reported recently that SKL regulates adipogenic stem cell (ADSC) proliferation and differentiation.43 Silencing of SKL impairs ADSC differential potential, while overexpression of SKL enhances ADSC viability.43 Nevertheless, whether overexpression of SKL increases MSC therapeutic potential for PAH has never been assessed. In this study, we demonstrated that the engineered MSCs overexpressing SKL were engrafted in the lung parenchyma in MCT-treated rats (Fig. 5), suggesting effective homing to the injured lungs. Unexpectedly, these MSCs did not differentiate into any types of lung cells (alveolar epithelial cells type I or II), pulmonary vascular endothelial cells, or smooth muscle cells (Fig S2). These results suggest that the beneficial effect of MSCs overexpressing SKL cannot be attributed to their regenerative capacity. Instead, this is likely mediated by their paracrine function, for example, by release of SKL.
Inflammation is a key mediator in MCT-induced PASMC proliferation, PA remodeling, and PAH.44 Interestingly, MSCs overexpressing SKL effectively attenuated MCT-induced inflammation, as evidenced by decreased infiltration of macrophages around small PAs (Fig. 6). Therefore, the therapeutic effect of MSCs overexpressing SKL may be mediated, at least in part, by their anti-inflammatory effect. We reported recently that haplodeficiency of Klotho increases the release of chemokines (e.g., MCP-1) and cytokines (e.g., TNFα), leading to macrophage infiltration and inflammation in kidneys.37 The current study showed that overexpression of SKL enhances the anti-inflammatory effect of MSCs in PAs and lungs (Fig. 6). This finding is supported by a recent report that Klotho may suppress inflammation.45 On the other hand, we cannot exclude the possibility that SKL also induces MSCs to release other paracrine factors, such as anti-inflammatory cytokines (e.g., IL-10). This hypothesis, however, needs to be validated. Recently, SIRT1 has come to the attention of researchers in the field, as it may protect against lung inflammation and PASMC proliferation.46–48 Resveratrol, a SIRT1 activator, has been shown to prevent or rescue MCT-induced PAH in rats by reducing inflammation and oxidative stress and inhibiting PASMC proliferation.46–48 Inhibition of SIRT1 increased SMC proliferation48, while resveratrol prevented SMC proliferation.47–48 Activation of SIRT1 protects endothelial cells and improves endothelial function.49–50 Interestingly, we found that MCT depleted SIRT1 levels in the lungs, which were rescued by MSCs overexpressing SKL (Fig. 6). To our knowledge, this is the first study demonstrating that SKL regulates SIRT1 expression in vivo. Thus, it is expected that the enhanced SIRT1 levels contribute to the beneficial effect of MSCs overexpressing SKL on pulmonary vascular endothelial dysfunction and PA remodeling.
Another interesting finding is that MSCs overexpressing SKL almost rescued MCT-induced downregulation of eNOS activity (Fig. S4). Consistently, the pulmonary vascular relaxation response to stimulation of eNOS by acetylcholine was increased by MSCs overexpressing SKL in MCT-treated rats (Fig. 2), suggesting an enhanced ability of endothelial cells to generate nitric oxide (NO). NO not only leads to PA vasodilation but also inhibits PASMC proliferation.34, 44 Indeed, MSCs overexpressing SKL significantly improved pulmonary vascular endothelial dysfunction, PASMC proliferation, and PA remodeling. It was reported that inhalation of NO improves PAH in patients44. Implantation of MSCs overexpressing eNOS also attenuates PAH and RV hypertrophy15. Thus, impaired eNOS activity and NO production may be involved in the pathogenesis of PAH34, 44. We further assessed whether SKL has a direct protective effect in endothelial cells challenged by MCT. Interestingly, SKL significantly improved MCT-induced impairment in endothelial cell viability (Fig. S4). It is noteworthy that SKL abolished the MCT-induced decrease in NO levels in endothelial cells, suggesting for the first time that SKL rescues the downregulation of eNOS activity.
Engineering MSCs for overexpressing therapeutic genes seems to be a promising strategy for the treatment of PAH. In this study, MSCs were genetically engineered to provide a source for the therapeutic protein SKL. Takemiya et al. genetically modified MSCs to overexpress prostacyclin synthase16 and found that the transgenic MSCs significantly attenuated PAH-related morbidity in MCT rats, while the untransduced MSCs failed to alleviate PAH-associated pathogenic changes. Implantation of MSCs overexpressing eNOS attenuated MCT-induced PAH.15 The engineered MSCs overexpressing eNOS provided moderately better treatment outcomes (RV hypertrophy, survival) compared with untransduced MSCs.
Some reports indicated that delivery of MSCs partially decreases PAH and attenuates PA remodeling.9, 11–12 In this study, untransduced MSCs slightly, but not significantly, attenuated pulmonary vascular dysfunction, PAH, or PA remodeling (Figs. 2–4). Similarly, Takemiya reported that untransduced MSCs failed to attenuate MCT-induced PAH and PA remodeling.16 The variation in the treatment effect of MSCs is likely due to the disparity in the function of the allogeneic MSCs. On the other hand, the untransduced MSCs significantly improved MCT-induced hypertrophy (Fig. 3), suggesting that MSCs improve the RV impairment, which is independent of PAH. The untransduced MSCs may repair heart damage via their paracrine function.14
Perspective
PAH is a life-threatening disease with high mortality. The 5-year survival rate ranges from 34–58%, and the current therapy relieves only the symptoms and does not cure the disease. In this study, we demonstrated that MSCs overexpressing SKL effectively attenuated MCT-induced PA endothelial dysfunction, PAH, PA remodelling, and RV hypertrophy. SKL augments the therapeutic effects of MSCs in PAH by: 1) decreasing inflammation and rescuing downregulation of SIRT1 expression and eNOS activity and 2) improving endothelial cell survival and function. This is, to our knowledge, the first report demonstrating the great efficacy of Klotho gene-engineered MSCs for the treatment of PAH.
Supplementary Material
Novelty and Significance.
1. What is new?
It is new and interesting that mesenchymal stem cell delivery of aging-suppressor protein SKL abolished MCT-induced pulmonary vascular endothelial dysfunction, PAH, and RV hypertrophy.
This study demonstrates, for the first time, that SKL attenuates MCT-induced PA remodelling and PASMC proliferation, likely by reducing inflammation and restoring SIRT1 levels and eNOS activity.
2. What is relevant?
It is significant that a decrease in lung SKL is associated with pulmonary vascular endothelial dysfunction, PA remodeling, and PAH.
This study reveals that stem cell delivery of SKL is an effective therapeutic strategy for pulmonary vascular endothelial dysfunction and PA remodelling.
3. Summary
This study provides the first evidence that Klotho deficiency is linked to pulmonary vascular endothelial dysfunction and pulmonary hypertension. SKL attenuates MCT-induced PA remodelling and PASMC proliferation by reducing inflammation and restoring SIRT1 levels and eNOS activity.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health Grants R01 HL116863, HL122166, DK093403, HL118558, AG049780, HL105302, and HL102074.
This publication was made possible by NIH Grant Number 9P20GM104934-06 from the COBRE Program of the National Institute of General Medical Sciences.
Footnotes
Disclosures
None.
Author Contribution
Conception design: ZS; Performing experiments: RV, QA, CW; Drafting manuscript: ZS, RV
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Pauwaa S, Machado RF, Desai AA. Survival in pulmonary arterial hypertension: A brief review of registry data. Pulm Circ. 2011;1:430–431. doi: 10.4103/2045-8932.87314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurtu V, Michelakis ED. Emerging Therapies and Future Directions in Pulmonary Arterial Hypertension. Can J Cardiol. 2015;31:489–501. doi: 10.1016/j.cjca.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Foster WS, Suen CM, Stewart DJ. Regenerative cell and tissue-based therapies for pulmonary arterial hypertension. Can J Cardiol. 2014;30:1350–1360. doi: 10.1016/j.cjca.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526–539. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin MD, Elliman SJ, Cahill E, English K, Ceredig R, Ritter T. Concise review: Adult mesenchymal stromal cell therapy for inflammatory diseases: How well are we joining the dots? STEM CELLS. 2013;31:2033–2041. doi: 10.1002/stem.1452. [DOI] [PubMed] [Google Scholar]
- 7.Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol & Therapeutics. 2014;143:181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Nassiri SM, Rahbarghazi R. Interactions of Mesenchymal Stem Cells with Endothelial Cells. Stem Cells and Develop. 2013;23:319–332. doi: 10.1089/scd.2013.0419. [DOI] [PubMed] [Google Scholar]
- 9.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Song XH, Liu P, Zeng CL, Huang ZS, Zhu LJ, Jiang YZ, Ouyang HW, Hu H. Platelet-mediated mesenchymal stem cells homing to the lung reduces monocrotaline-induced rat pulmonary hypertension. Cell Transplant. 2012;21:1463–1475. doi: 10.3727/096368912X640529. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZH, Lu Y, Luan Y, Zhao JJ. Effect of bone marrow mesenchymal stem cells on experimental pulmonary arterial hypertension. Exp Ther Med. 2012;4:839–843. doi: 10.3892/etm.2012.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, Schalij MJ, van der Wall EE, van der Laarse A. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Hu D, Niu L, Qu S, Wang S, Liu S. Mesenchymal stem cells attenuate vascular remodeling in monocrotaline-induced pulmonary hypertension rats. J Huazhong Univ Sci Technolog Med Sci. 2012;32:810–817. doi: 10.1007/s11596-012-1039-x. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1:113–119. doi: 10.1016/j.gendis.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114:I181–185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 16.Takemiya K, Kai H, Yasukawa H, Tahara N, Kato S, Imaizumi T. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol. 2010;105:409–417. doi: 10.1007/s00395-009-0065-8. [DOI] [PubMed] [Google Scholar]
- 17.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 18.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Sun Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y-i, Nagai R. Klotho Protein Protects against Endothelial Dysfunction. Biochem Biophys Res Commun. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Mao H, Yu X, Sun B, Zeng M, Zhao X, Qian J, Liu J, Xing C. Effect of secondary hyperparathyroidism serum on endothelial cells and intervention with Klotho. Mol Med Rep. 2015;12:1983–1990. doi: 10.3892/mmr.2015.3606. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, Chen J, Sun Z. Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension. 2016;67:564–573. doi: 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crosswhite P, Chen K, Sun Z. AAV delivery of tumor necrosis factor-alpha short hairpin RNA attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling. Hypertension. 2014;64:1141–1150. doi: 10.1161/HYPERTENSIONAHA.114.03791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Sun Z. In Vivo Pancreatic beta-Cell-Specific Expression of Antiaging Gene Klotho: A Novel Approach for Preserving beta-Cells in Type 2 Diabetes. Diabetes. 2015;64:1444–1458. doi: 10.2337/db14-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Sun Z. Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens. 2014;32:1629–1636. doi: 10.1097/HJH.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Kuro-o M, Sun Z. Genetic Deficiency of Anti-Aging Gene Klotho Exacerbates Early Nephropathy in STZ-Induced Diabetes in Male Mice. Endocrinology. 2013;154:3855–3863. doi: 10.1210/en.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Chen K, Wang Y, Schuman M, Lei H, Sun Z. Antiaging Gene Klotho Regulates Adrenal CYP11B2 Expression and Aldosterone Synthesis. J Am Soc Nephrol. 2016;27:1765–1776. doi: 10.1681/ASN.2015010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wang Q, Sun Z. Normal IgG downregulates the intracellular superoxide level and attenuates migration and permeability in human aortic endothelial cells isolated from a hypertensive patient. Hypertension. 2012;60:818–826. doi: 10.1161/HYPERTENSIONAHA.112.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Skelley L, Wang B, Mejia A, Sapozhnikov V, Sun Z. AAV-Based RNAi Silencing of NADPH Oxidase gp91(phox) Attenuates Cold-Induced Cardiovascular Dysfunction. Hum Gene Ther. 2012;23:1016–1026. doi: 10.1089/hum.2012.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Lin Y, Sun Z. Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKalpha-mediated activation of RUNX2. Aging Cell. 2016 May 31; doi: 10.1111/acel.12494. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crosswhite P, Sun Z. Molecular mechanisms of pulmonary arterial remodeling. Mol Med. 2014;20:191–201. doi: 10.2119/molmed.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krause K, Schneider C, Kuck KH, Jaquet K. Stem cell therapy in cardiovascular disorders. Cardiovasc Ther. 2010;28:e101–110. doi: 10.1111/j.1755-5922.2010.00208.x. [DOI] [PubMed] [Google Scholar]
- 36.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Chen K, Lei H, Sun Z. Klotho Gene Deficiency Causes Salt-Sensitive Hypertension via Monocyte Chemotactic Protein-1/CC Chemokine Receptor 2-Mediated Inflammation. J Am Soc Nephrol. 2015;26:121–132. doi: 10.1681/ASN.2013101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K, Zhou X, Sun Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension. 2015;66:1006–1013. doi: 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weil BR, Manukyan MC, Herrmann JL, Abarbanell AM, Poynter JA, Wang Y, Meldrum DR. The immunomodulatory properties of mesenchymal stem cells: implications for surgical disease. J Surg Res. 2011;167:78–86. doi: 10.1016/j.jss.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapel A, Bertho JM, Bensidhoum M, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 41.Chavakis E, Dimmeler S. Homing of progenitor cells to ischemic tissues. Antioxid Redox Signal. 2011;15:967–980. doi: 10.1089/ars.2010.3582. [DOI] [PubMed] [Google Scholar]
- 42.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Fan J, Sun Z. The Antiaging Gene Klotho Regulates Proliferation and Differentiation of Adipose-Derived Stem Cells. Stem Cells. 2016;34:1615–1625. doi: 10.1002/stem.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crosswhite P, Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens. 2010;28:201–212. doi: 10.1097/HJH.0b013e328332bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Y, Sun Z. Antiaging Gene Klotho Attenuates Pancreatic beta-Cell Apoptosis in Type 1 Diabetes. Diabetes. 2015;64:4298–4311. doi: 10.2337/db15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang DL, Zhang HG, Xu YL, Gao YH, Yang XJ, Hao XQ, Li XH. Resveratrol inhibits right ventricular hypertrophy induced by monocrotaline in rats. Clin Exp Pharmacol Physiol. 2010;37:150–155. doi: 10.1111/j.1440-1681.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- 47.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol Prevents Monocrotaline-induced Pulmonary Hypertension in Rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paffett ML, Lucas SN, Campen MJ. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: A potential role for atrogin-1 in smooth muscle. Vasc Pharmacol. 2012;56:64–73. doi: 10.1016/j.vph.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640–647. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.