Abstract
Obesity is associated with physical inactivity, which exacerbates the negative health consequences of obesity. Despite a wide consensus that people with obesity should exercise more, there are few effective methods for increasing physical activity in people with obesity. This lack is reflected in our limited understanding of the cellular and molecular causes of physical inactivity in obesity. We hypothesize that impairments in dopamine signaling contribute to physical inactivity in people with obesity, as in classic movement disorders such as Parkinson's disease. Here, we review two lines of evidence supporting this hypothesis: (1) chronic exposure to obesogenic diets has been linked to impairments in dopamine synthesis, release, and receptor function, particularly in the striatum, and (2) striatal dopamine is necessary for the proper control of movement. Identifying the biological determinants of physical inactivity may lead to more effective strategies for increasing physical activity in people with obesity, as well as improve our understanding of why it is difficult for people with obesity to alter their levels of physical activity.
Keywords: obesity, dopamine, exercise, physical activity, physical activity promotion, Parkinson's disease, movement disorders
Introduction
Obesity is associated with reductions in motor output, often termed “physical inactivity” (Tudor-Locke et al., 2010; Bouchard et al., 2015), although whether this relationship is causal remains a point of debate (Simon et al., 2008; Haskell et al., 2009; Dwyer-Lindgren et al., 2013; Swift et al., 2014). Despite the importance of physical activity for health, there are few effective methods for increasing physical activity levels in people with obesity, leading some researchers to conclude that, “there are presently no evidence-based interventions that can reliably and sustainably increase the level of physical activity among obese adults” (Ekkekakis et al., 2016). This point is reflected in our limited understanding of the cellular and molecular determinants of physical inactivity in people with obesity. We believe that a cellular understanding of why obesity is associated with physical inactivity is needed to understand, and ultimately alter, the relationship between obesity and physical inactivity. In this review, we propose that impairments in striatal dopamine contribute to physical inactivity in obesity, akin to classic movement disorders such as Parkinson's disease.
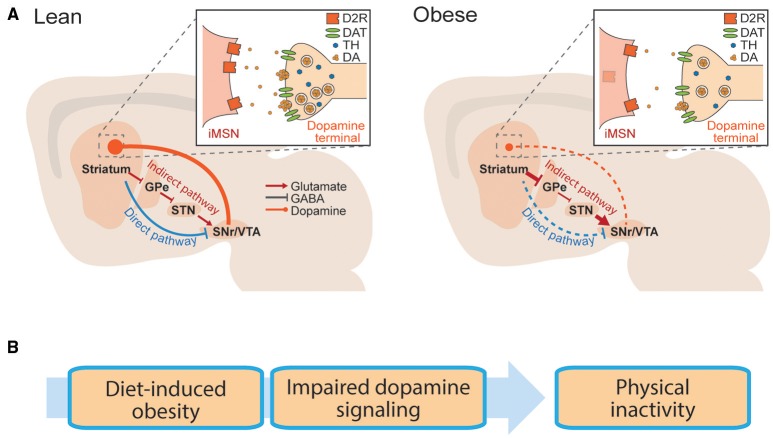
The striatum is a forebrain structure that controls movement, as well as learning and emotional states. There are two main projection cell types in the striatum, the “direct” and the “indirect” pathway medium spiny neurons (dMSNs and iMSNs), as well as several classes of interneurons. dMSNs and iMSNs exhibit distinct protein expression patterns, projection targets, and support distinct behavioral functions (Alexander and Crutcher, 1990; DeLong, 1990; Gerfen et al., 1990; Graybiel et al., 1994; Le Moine and Bloch, 1995; Obeso et al., 2000; Figure 1A). dMSNs express the excitatory Gs-coupled dopamine D1 receptor (D1R), while iMSNs express the inhibitory Gi-coupled dopamine D2 receptor (D2R; Gerfen et al., 1990). Dopamine can facilitate movement by binding to D1Rs and enhancing the output of dMSNs, or binding to D2Rs and inhibiting the output of iMSNs (Sano et al., 2003; Buch et al., 2005; Durieux et al., 2009; Kravitz et al., 2010). In this way, dopaminergic signaling controls the downstream signaling of dMSNs and iMSNs, and resulting motor output. We have simplified this discussion for the purposes of this review, but striatal function is also influenced by several additional layers of complexity (Mink, 1996; Calabresi et al., 2014). For example, the dorsal striatum is commonly linked to motor control, while the ventral striatum is linked to motivation and effortful movement (Mogenson et al., 1980; Voorn et al., 2004; Kreitzer and Malenka, 2008).
Figure 1.
Basal ganglia circuitry in lean and obese conditions. (A) Striatal neurons send projections to the midbrain via the direct pathway or indirect pathway. Schematic is replicated in lean (left) and obese (right) conditions, to show reported dopaminergic alterations in obesity. Inlay: Dopaminergic synapse onto striatal iMSNs. GPe, globus pallidus; STN, subthalamic nucleus; SNr, substantia nigra; VTA, ventral tegmental area. (B) Hypothetical progression by which diet induced obesity is associated with impaired striatal dopamine transmission, leading to physical inactivity.
The importance of dopamine for proper control of movement is evident in neurological disorders. Hypokinetic states such as Parkinson's disease are the result of too little striatal dopamine (Hornykiewicz, 2010), whereas hyperactive states such as bipolar mania are associated with too much (Logan and McClung, 2016). Drugs that increase dopamine release (e.g., amphetamine) increase motor output (Schindler and Carmona, 2002) and dopamine antagonists (used clinically to reduce manic episodes) often result in motor impairments as a side effect (Janno et al., 2004; Parksepp et al., 2016). Genetic manipulations in animals further support the role of striatal dopamine transmission in motor control, as mice lacking dopamine receptors have reduced movement (Drago et al., 1994; Xu et al., 1994; Baik et al., 1995; Kelly et al., 1997; Beeler et al., 2016), whereas those that overexpress dopamine receptors are hyperactive (Ikari et al., 1995; Ingram et al., 1998; Dracheva et al., 1999; Thanos et al., 2001; Trifilieff et al., 2013). In particular, cell-type specific reductions of the D2R in iMSNs reduce open field movement, demonstrating the sufficiency of the D2R to regulate physical activity, by controlling the output of iMSNs (Anzalone et al., 2012; Lemos et al., 2016). In summary, striatal dopamine promotes movement in animals, due to actions on its striatal target neurons.
Obesity is associated with impairments in striatal dopamine function. Reported impairments include deficiencies in dopamine synthesis and release, as well as alterations in striatal dopamine receptors. While alterations in striatal DA transmission are commonly discussed in relation to reward processing (Kenny et al., 2013; Volkow et al., 2013), we hypothesize that these impairments may also contribute to the link between obesity and physical inactivity (Figure 1B).
Obesity and physical inactivity
An inverse relationship between weight gain and physical activity has been observed in humans (Hemmingsson and Ekelund, 2007; Chaput et al., 2012; Hjorth et al., 2014), non-human primates (Wolden-Hanson et al., 1993), domesticated animals (Morrison et al., 2013), and rodents (Jürgens et al., 2006; Bjursell et al., 2008). The cross-species nature of this relationship indicates that it is a conserved phenomenon that may stem from the evolutionary benefit of storing energy in times of caloric excess, a state that is rare in nature. However, in modern environments physical inactivity exacerbates the negative health effects of obesity, increasing the risk of cardiac disease and diabetes (Al Tunaiji et al., 2014; Bao et al., 2014; Bouchard et al., 2015). It is possible that physical inactivity precedes, and thereby contributes to, weight gain (Jürgens et al., 2006; Haskell et al., 2009). Indeed animals with high levels of spontaneous physical activity are partially protected against diet-induced obesity (Teske et al., 2012; Zhang et al., 2012). While pre-existing differences in activity levels may contribute to the relationship between obesity and physical inactivity, at a cellular level it remains unclear why people with obesity are inactive.
Part of the difficulty in understanding this relationship stems from the multifaceted nature of the two variables. For instance, the weight of excess adiposity restricts joint and muscle mobility and increases joint pain, which may make it more difficult for people to move (Belczak et al., 2014; Muramoto et al., 2014). However, weight alone does not appear sufficient to explain physical inactivity in people with obesity. Several researchers have tracked physical activity levels across periods of weight loss, to see whether physical activity levels increase as people lose weight, and experience fewer mobility-restricting effects of excess adiposity. Surprisingly, weight loss is generally associated with decreases, and not increases, in physical activity (de Boer et al., 1986; de Groot et al., 1989; Martin et al., 2007; Redman et al., 2009). These results have been explained in terms of metabolic adaptations, as the body seeks to reduce energy expenditure to compensate for the caloric deficit induced by the diet. However, when subjects were tracked during maintained periods of weight loss lasting a year, physical activity levels still did not increase above pre-diet obese levels (Camps et al., 2013). Similar results have been reported following gastric bypass surgery. Despite large amounts of weight loss (>30 kg), objectively measured physical activity levels did not increase in patients that received gastric bypass surgery, even up to 12 months after the peak of the weight loss (Bond et al., 2010; Ramirez-Marrero et al., 2014; Berglind et al., 2015, 2016). Studies in animals also support these conclusions, as loss of adiposity is again associated with decreases, and not increases, in physical activity (Sullivan and Cameron, 2010; Morrison et al., 2014; Vitger et al., 2016). We conclude that the weight of excess adiposity does not sufficiently explain the association between obesity and physical inactivity. Rather, the evidence suggests that obesity-induced adaptations continue to contribute to physical inactivity, even after weight loss. While these adaptations may include chronic mobility issues in joints or muscles, we hypothesize that motor circuitry in the brain is also a large contributor. Specifically, we hypothesize that deficits in striatal dopaminergic signaling contribute to the persistent reductions in physical activity in obesity.
Further supporting the conclusion that the weight of adiposity does not adequately explain physical inactivity in obesity, not all groups of obese animals, or people with obesity, have low levels of physical activity. Even in studies that report deficits in striatal dopamine, physical activity levels can remain unaltered (Davis et al., 2008). Similar findings have been reported under controlled conditions in humans as well. In an 8-week study in which subjects were over-fed by 1000 calories per day, subjects significantly increased their spontaneous physical activity, despite gaining an average of 4.7 kg. The authors linked this increase to a mechanism for dissipating excess energy to preserve body weight (Levine et al., 1999). A similar increase in physical activity was reported in an 8-week over-eating study, despite an average weight gain of 5.3 kg (Apolzan et al., 2014). While physical inactivity is a correlate of obesity in large populations, there is considerable variability on this point among individuals. This variability may be another avenue for unraveling the cellular underpinnings of the relationship between physical activity and obesity.
Obesity and disruptions in dopamine production and release
A wealth of animal research has described alterations in the dopamine system in obesity. The majority of studies in obese rodents have focused on dopamine transmission in the nucleus accumbens (NAc), which resides in the ventral striatum and is involved in effortful movement (Salamone et al., 2007; Schmidt et al., 2012). Based on this role, the NAc may be particularly important for explaining the lack of vigorous physical activity in obesity (Ekkekakis et al., 2016). Long-term ad libitum high-fat diet decreased tonic dopamine in the NAc of mice (Carlin et al., 2013) as well as dopamine turnover in the NAc of rats (Davis et al., 2008). This specific deficit was distinct from adiposity, as rats that were fed an iso-caloric amount of high-fat diet also had decreased dopamine turnover (Davis et al., 2008). Whereas both chow and high-fat diet increased phasic dopamine in the NAc of lean rats, obese rats had a blunted response to these diets (Geiger et al., 2009). Chronic exposure may be necessary for deficits in phasic dopamine signaling, as they are seen following 6, but not 2, weeks of high-fat diet (Cone et al., 2013). Similar to differences observed in phasic dopamine release in the NAc of obese animals, rats that were bred to be prone to weight gain had reduced dopaminergic responses to both chow (Geiger et al., 2008) and high-fat diet (Rada et al., 2010).
The above deficits in dopamine release may be explained by alterations in genes involved in the synthesis and metabolism of dopamine. Midbrain dopamine regions including the substantia nigra and the ventral tegmental area (VTA) provide the main dopaminergic innervation to the striatum (Figure 1). Expression of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, is reduced in the VTA of mice fed a high-fat diet (Vucetic et al., 2012; Carlin et al., 2013). Again, this did not depend on fat storage, as similar effects were observed in mice that were pair-fed a high fat diet (Li et al., 2009). The effect of high-fat diet on co-acetyl methyl transferase (COMT), a key enzyme responsible for the degradation of dopamine is less clear, with studies reporting either decreased (Carlin et al., 2013) or unchanged (Alsio et al., 2010; Vucetic et al., 2012) expression following diet-induced obesity. Interestingly, in humans, polymorphisms that confer low activity of monoamine-oxidases (the other main enzyme responsible for degrading dopamine) have been linked to obesity (Camarena et al., 2004; Ducci et al., 2006; Need et al., 2006). Overall, the evidence supports two conclusions: (1) exposure to high-fat diets can impair dopamine synthesis and striatal dopamine release and processing, but (2) heterogeneity exists among these reports, indicating that the impact of high-fat diets on the dopamine system is complex and may occur differently among different individuals.
Obesity and dysfunction of dopamine receptors
Multiple researchers have observed alterations in dopamine receptors in people with obesity. Individuals with at least one copy of the drd2 Taq1A allele have reduced brain D2R availability of ~30–40% (Noble et al., 1991; Thompson et al., 1997) and an increased prevalence of obesity (Blum et al., 1996; Stice et al., 2008, 2010; Davis et al., 2009; Carpenter et al., 2013). An inverse relationship between obesity and D2R availability, assayed via positron emission tomography (PET), has also been reported in humans. This was first reported by Wang et al. (2001) and was initially supported by others (Volkow et al., 2008; de Weijer et al., 2011; Kessler et al., 2014; van de Giessen et al., 2014). However, several other groups have failed to replicate this finding (Dunn et al., 2012; Caravaggio et al., 2015; Cosgrove et al., 2015; Karlsson et al., 2015, 2016; Tuominen et al., 2015), or found opposing associations in different regions of the striatum (Guo et al., 2014). Interestingly, Guo and colleagues noted a negative relationship between body mass index (BMI) and D2R binding only in the ventral striatum, which may be linked to effortful movements (Salamone et al., 2007; Schmidt et al., 2012). Several possibilities may account for the discrepancy among studies of D2R binding and BMI. Different D2R radio-ligands were used among these studies, which may bind differentially to D2R or D3Rs (Gaiser et al., 2016). Changes in striatal dopamine tone could impact binding potential (Horstmann et al., 2015). Finally, experimental factors including the amount of time after meal consumption or individual variability among subjects may contribute to observed differences (Small et al., 2003).
Animal studies have more consistently linked impairments in D2Rs to obesity, via analysis of mRNA (Mathes et al., 2010; Zhang et al., 2015), protein (Johnson and Kenny, 2010; Adams et al., 2015), and receptor binding (Huang et al., 2006; Hajnal et al., 2008; Thanos et al., 2008; Michaelides et al., 2012; van de Giessen et al., 2012, 2013; Narayanaswami et al., 2013). Interestingly, rats maintained on an iso-caloric high-fat (but not high-sugar) diet also had lower levels of D2Rs in ventral (but not dorsal) striatum (Adams et al., 2015), supporting the conclusion that exposure to high-fat diet may be a better predictor of dopaminergic dysfunction than weight gain itself (van de Giessen et al., 2013). To date, no published work has examined associations between D1-type dopamine receptors (D1Rs) and obesity in humans, so an evaluation of potential changes here is limited to a small number of animal studies. D1R mRNA was decreased in obese rats relative to lean controls (Vucetic et al., 2012; Zhang et al., 2015), while another study reported a decrease in D1Rs only in female rats (Ong et al., 2013). We conclude that reduced function of D2Rs appears to be a particularly important alteration in obesity, although there is considerable variability in D2R alterations among studies and individuals. Unfortunately, studies of the D1R are too sparse to make strong conclusions about its relationship to obesity.
Do alterations in dopamine function recover with weight loss?
It is unclear whether changes in dopamine signaling in people with obesity persist after weight loss. The few studies that exist on this topic point to dopaminergic alterations being at least partly resistant to change, and at times even exacerbated by weight loss. High-fat diet reduced the levels of several enzymes involved in dopamine production in the VTA and NAc, and switching these obese mice to low-fat chow caused even further decreases in these enzymes (Carlin et al., 2013; Sharma et al., 2013). Two PET imaging studies reported a lack of recovery of D2R binding following Roux-en-Y gastric bypass surgery (RYGB) in humans, with one showing an even further decrease in binding (Dunn et al., 2010; de Weijer et al., 2014). A small study of five women reported a partial recovery of D2R binding 6-weeks after RYGB (Steele et al., 2010). An increase in D2R binding was also reported during food restriction and associated weight alterations in obese rats (Thanos et al., 2008). Although the data on this topic are limited, it appears that diet-induced changes in dopamine function are at least partly persistent following weight loss. Consistent with this conclusion, physical activity levels remain low in people with obesity, even months after the peak of weight loss (Bond et al., 2010; Camps et al., 2013; Ramirez-Marrero et al., 2014; Berglind et al., 2015, 2016). Again, the small number of studies of this topic precludes firm conclusions, and underscores the need for further research on the persistence of dopaminergic alterations in people with obesity.
Obesity and physical inactivity: conclusions
Chronic exposure to obesogenic diets is associated with changes in both physical activity levels and dopaminergic function. Diet-induced changes in the dopamine system may be sufficient to explain the development of physical inactivity in people with obesity. Increased understanding of obesity-related changes in dopamine and related systems may support evidence-based approaches for increasing physical activity in people with obesity. In addition, such an understanding may reveal genetic or environmental contributions to dopaminergic dysfunction, and physical inactivity, in obesity.
Author contributions
AK, TO, and DF conceived of the idea and wrote and edited this manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the NIH Intramural research program. We thank Kavya Devarakonda for comments on this manuscript.
References
- Adams W. K., Sussman J. L., Kaur S., D'Souza A. M., Kieffer T. J., Winstanley C. A. (2015). Long-term, calorie-restricted intake of a high-fat diet in rats reduces impulse control and ventral striatal D2 receptor signalling - two markers of addiction vulnerability. Eur. J. Neurosci. 42, 3095–3104. 10.1111/ejn.13117 [DOI] [PubMed] [Google Scholar]
- Alexander G. E., Crutcher M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. 10.1016/0166-2236(90)90107-L [DOI] [PubMed] [Google Scholar]
- Alsiö J., Olszewski P. K., Norbäck A. H., Gunnarsson Z. E., Levine A. S., Pickering C., et al. (2010). Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 171, 779–787. 10.1016/j.neuroscience.2010.09.046 [DOI] [PubMed] [Google Scholar]
- Al Tunaiji H., Davis J. C., Mackey D. C., Khan K. M. (2014). Population attributable fraction of type 2 diabetes due to physical inactivity in adults: a systematic review. BMC Public Health 14:469. 10.1186/1471-2458-14-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A., Lizardi-Ortiz J. E., Ramos M., De Mei C., Hopf F. W., Iaccarino C., et al. (2012). Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J. Neurosci. 32, 9023–9034. 10.1523/JNEUROSCI.0918-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolzan J. W., Bray G. A., Smith S. R., de Jonge L., Rood J., Han H., et al. (2014). Effects of weight gain induced by controlled overfeeding on physical activity. Am. J. Physiol. Endocrinol. Metab. 307, E1030–E1037. 10.1152/ajpendo.00386.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik J. H., Picetti R., Saiardi A., Thiriet G., Dierich A., Depaulis A., et al. (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428. 10.1038/377424a0 [DOI] [PubMed] [Google Scholar]
- Bao W., Tobias D. K., Bowers K., Chavarro J., Vaag A., Grunnet L. G., et al. (2014). Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern. Med. 174, 1047–1055. 10.1001/jamainternmed.2014.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler J. A., Faust R. P., Turkson S., Ye H., Zhuang X. (2016). Low dopamine D2 receptor increases vulnerability to obesity via reduced physical activity not increased appetitive motivation. Biol. Psychiatry 79, 887–897. 10.1016/j.biopsych.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belczak C. E., de Godoy J. M., Belzack S. Q., Ramos R. N., Caffaro R. A. (2014). Obesity and worsening of chronic venous disease and joint mobility. Phlebology 29, 500–504. 10.1177/0268355513492510 [DOI] [PubMed] [Google Scholar]
- Berglind D., Willmer M., Eriksson U., Thorell A., Sundbom M., Uddén J., et al. (2015). Longitudinal assessment of physical activity in women undergoing Roux-en-Y gastric bypass. Obes. Surg. 25, 119–125. 10.1007/s11695-014-1331-x [DOI] [PubMed] [Google Scholar]
- Berglind D., Willmer M., Tynelius P., Ghaderi A., Näslund E., Rasmussen F. (2016). Accelerometer-measured versus self-reported physical activity levels and sedentary behavior in women before and 9 months after Roux-en-Y gastric bypass. Obes. Surg. 26, 1463–1470. 10.1007/s11695-015-1971-5 [DOI] [PubMed] [Google Scholar]
- Bjursell M., Gerdin A. K., Lelliott C. J., Egecioglu E., Elmgren A., Törnell J., et al. (2008). Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 294, E251–E260. 10.1152/ajpendo.00401.2007 [DOI] [PubMed] [Google Scholar]
- Blum K., Braverman E. R., Wood R. C., Gill J., Li C., Chen T. J., et al. (1996). Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report. Pharmacogenetics 6, 297–305. 10.1097/00008571-199608000-00003 [DOI] [PubMed] [Google Scholar]
- Bond D. S., Jakicic J. M., Unick J. L., Vithiananthan S., Pohl D., Roye G. D., et al. (2010). Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity 18, 2395–2397. 10.1038/oby.2010.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C., Blair S. N., Katzmarzyk P. T. (2015). Less sitting, more physical activity, or higher fitness? Mayo Clin. Proc. 90, 1533–1540. 10.1016/j.mayocp.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Buch T., Heppner F. L., Tertilt C., Heinen T. J., Kremer M., Wunderlich F. T., et al. (2005). A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426. 10.1038/nmeth762 [DOI] [PubMed] [Google Scholar]
- Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. (2014). Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci. 17, 1022–1030. 10.1038/nn.3743 [DOI] [PubMed] [Google Scholar]
- Camarena B., Santiago H., Aguilar A., Ruvinskis E., González-Barranco J., Nicolini H. (2004). Family-based association study between the monoamine oxidase A gene and obesity: implications for psychopharmacogenetic studies. Neuropsychobiology 49, 126–129. 10.1159/000076720 [DOI] [PubMed] [Google Scholar]
- Camps S. G., Verhoef S. P., Westerterp K. R. (2013). Weight loss-induced reduction in physical activity recovers during weight maintenance. Am. J. Clin. Nutr. 98, 917–923. 10.3945/ajcn.113.062935 [DOI] [PubMed] [Google Scholar]
- Caravaggio F., Raitsin S., Gerretsen P., Nakajima S., Wilson A., Graff-Guerrero A. (2015). Ventral striatum binding of a dopamine D2/3 receptor agonist but not antagonist predicts normal body mass index. Biol. Psychiatry 77, 196–202. 10.1016/j.biopsych.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J., Hill-Smith T. E., Lucki I., Reyes T. M. (2013). Reversal of dopamine system dysfunction in response to high-fat diet. Obesity 21, 2513–2521. 10.1002/oby.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. L., Wong A. M., Li Z., Noble E. P., Heber D. (2013). Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity 21, E467–E473. 10.1002/oby.20202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput J. P., Lambert M., Mathieu M. E., Tremblay M. S., O'Loughlin J., Tremblay A. (2012). Physical activity vs. sedentary time: independent associations with adiposity in children. Pediatr. Obes. 7, 251–258. 10.1111/j.2047-6310.2011.00028.x [DOI] [PubMed] [Google Scholar]
- Cone J. J., Chartoff E. H., Potter D. N., Ebner S. R., Roitman M. F. (2013). Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS ONE 8:e58251. 10.1371/journal.pone.0058251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K. P., Veldhuizen M. G., Sandiego C. M., Morris E. D., Small D. M. (2015). Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum. Synapse 69, 195–202. 10.1002/syn.21809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Levitan R. D., Reid C., Carter J. C., Kaplan A. S., Patte K. A., et al. (2009). Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity 17, 1220–1225. 10.1038/oby.2009.52 [DOI] [PubMed] [Google Scholar]
- Davis J. F., Tracy A. L., Schurdak J. D., Tschöp M. H., Lipton J. W., Clegg D. J., et al. (2008). Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav. Neurosci. 122, 1257–1263. 10.1037/a0013111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. O., van Es A. J., Roovers L. C., van Raaij J. M., Hautvast J. G. (1986). Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. Am. J. Clin. Nutr. 44, 585–595. [DOI] [PubMed] [Google Scholar]
- de Groot L. C., van Es A. J., van Raaij J. M., Vogt J. E., Hautvast J. G. (1989). Adaptation of energy metabolism of overweight women to alternating and continuous low energy intake. Am. J. Clin. Nutr. 50, 1314–1323. [DOI] [PubMed] [Google Scholar]
- DeLong M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. 10.1016/0166-2236(90)90110-V [DOI] [PubMed] [Google Scholar]
- de Weijer B. A., van de Giessen E., Janssen I., Berends F. J., van de Laar A., Ackermans M. T., et al. (2014). Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia 57, 1078–1080. 10.1007/s00125-014-3178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weijer B. A., van de Giessen E., van Amelsvoort T. A., Boot E., Braak B., Janssen I. M., et al. (2011). Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 1:37. 10.1186/2191-219x-1-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S., Xu M., Kelley K. A., Haroutunian V., Holstein G. R., Haun S., et al. (1999). Paradoxical locomotor behavior of dopamine D1 receptor transgenic mice. Exp. Neurol. 157, 169–179. 10.1006/exnr.1999.7037 [DOI] [PubMed] [Google Scholar]
- Drago J., Gerfen C. R., Lachowicz J. E., Steiner H., Hollon T. R., Love P. E., et al. (1994). Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc. Natl. Acad. Sci. U.S.A. 91, 12564–12568. 10.1073/pnas.91.26.12564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F., Newman T. K., Funt S., Brown G. L., Virkkunen M., Goldman D. (2006). A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol. Psychiatry 11, 858–866. 10.1038/sj.mp.4001856 [DOI] [PubMed] [Google Scholar]
- Dunn J. P., Cowan R. L., Volkow N. D., Feurer I. D., Li R., Williams D. B., et al. (2010). Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res. 1350, 123–130. 10.1016/j.brainres.2010.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. P., Kessler R. M., Feurer I. D., Volkow N. D., Patterson B. W., Ansari M. S., et al. (2012). Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care 35, 1105–1111. 10.2337/dc11-2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux P. F., Bearzatto B., Guiducci S., Buch T., Waisman A., Zoli M., et al. (2009). D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat. Neurosci. 12, 393–395. 10.1038/nn.2286 [DOI] [PubMed] [Google Scholar]
- Dwyer-Lindgren L., Freedman G., Engell R. E., Fleming T. D., Lim S. S., Murray C. J., et al. (2013). Prevalence of physical activity and obesity in US counties, 2001–2011: a road map for action. Popul. Health Metr. 11:7. 10.1186/1478-7954-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekkekakis P., Vazou S., Bixby W. R., Georgiadis E. (2016). The mysterious case of the public health guideline that is (almost) entirely ignored: call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes. Rev. 17, 313–329. 10.1111/obr.12369 [DOI] [PubMed] [Google Scholar]
- Gaiser E. C., Gallezot J. D., Worhunsky P. D., Jastreboff A. M., Pittman B., Kantrovitz L., et al. (2016). Elevated Dopamine D2/3 Receptor Availability in Obese Individuals: a PET Imaging Study with [11C](+)PHNO. Neuropsychopharmacology. 10.1038/npp.2016.115. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. M., Behr G. G., Frank L. E., Caldera-Siu A. D., Beinfeld M. C., Kokkotou E. G., et al. (2008). Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 22, 2740–2746. 10.1096/fj.08-110759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. M., Haburcak M., Avena N. M., Moyer M. C., Hoebel B. G., Pothos E. N. (2009). Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159, 1193–1199. 10.1016/j.neuroscience.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr., et al. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. 10.1126/science.2147780 [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Aosaki T., Flaherty A. W., Kimura M. (1994). The basal ganglia and adaptive motor control. Science 265, 1826–1831. 10.1126/science.8091209 [DOI] [PubMed] [Google Scholar]
- Guo J., Simmons W. K., Herscovitch P., Martin A., Hall K. D. (2014). Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol. Psychiatry 19, 1078–1084. 10.1038/mp.2014.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A., Margas W. M., Covasa M. (2008). Altered dopamine D2 receptor function and binding in obese OLETF rat. Brain Res. Bull. 75, 70–76. 10.1016/j.brainresbull.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell W. L., Blair S. N., Hill J. O. (2009). Physical activity: health outcomes and importance for public health policy. Prev. Med. 49, 280–282. 10.1016/j.ypmed.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Hemmingsson E., Ekelund U. (2007). Is the association between physical activity and body mass index obesity dependent? Int. J. Obes. 31, 663–668. 10.1038/sj.ijo.0803458 [DOI] [PubMed] [Google Scholar]
- Hjorth M. F., Chaput J. P., Ritz C., Dalskov S. M., Andersen R., Astrup A., et al. (2014). Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int. J. Obes. 38, 959–965. 10.1038/ijo.2013.229 [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. (2010). A Brief history of levodopa. J. Neurol. 257, S249–S252. 10.1007/s00415-010-5741-y [DOI] [PubMed] [Google Scholar]
- Horstmann A., Fenske W. K., Hankir M. K. (2015). Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes. Rev. 16, 821–830. 10.1111/obr.12303 [DOI] [PubMed] [Google Scholar]
- Huang X. F., Zavitsanou K., Huang X., Yu Y., Wang H., Chen F., et al. (2006). Dopamine transporter and D2 receptor binding densities in mice prone or resistant to chronic high fat diet-induced obesity. Behav. Brain Res. 175, 415–419. 10.1016/j.bbr.2006.08.034 [DOI] [PubMed] [Google Scholar]
- Ikari H., Zhang L., Chernak J. M., Mastrangeli A., Kato S., Kuo H., et al. (1995). Adenovirus-mediated gene transfer of dopamine D2 receptor cDNA into rat striatum. Brain Res. Mol. Brain Res. 34, 315–320. 10.1016/0169-328X(95)00185-U [DOI] [PubMed] [Google Scholar]
- Ingram D. K., Ikari H., Umegaki H., Chernak J. M., Roth G. S. (1998). Application of gene therapy to treat age-related loss of dopamine D2 receptor. Exp. Gerontol. 33, 793–804. 10.1016/S0531-5565(98)00043-6 [DOI] [PubMed] [Google Scholar]
- Janno S., Holi M., Tuisku K., Wahlbeck K. (2004). Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am. J. Psychiatry 161, 160–163. 10.1176/appi.ajp.161.1.160 [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Kenny P. J. (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 13, 635–641. 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens H. S., Schürmann A., Kluge R., Ortmann S., Klaus S., Joost H. G., et al. (2006). Hyperphagia, lower body temperature, and reduced running wheel activity precede development of morbid obesity in New Zealand obese mice. Physiol. Genomics 25, 234–241. 10.1152/physiolgenomics.00252.2005 [DOI] [PubMed] [Google Scholar]
- Karlsson H. K., Tuominen L., Tuulari J. J., Hirvonen J., Parkkola R., Helin S., et al. (2015). Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J. Neurosci. 35, 3959–3965. 10.1523/JNEUROSCI.4744-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H. K., Tuulari J. J., Tuominen L., Hirvonen J., Honka H., Parkkola R., et al. (2016). Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol. Psychiatry. 21, 1057–1062. 10.1038/mp.2015.153 [DOI] [PubMed] [Google Scholar]
- Kelly M. A., Rubinstein M., Asa S. L., Zhang G., Saez C., Bunzow J. R., et al. (1997). Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19, 103–113. 10.1016/S0896-6273(00)80351-7 [DOI] [PubMed] [Google Scholar]
- Kenny P. J., Voren G., Johnson P. M. (2013). Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr. Opin. Neurobiol. 23, 535–538. 10.1016/j.conb.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. M., Zald D. H., Ansari M. S., Li R., Cowan R. L. (2014). Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity. Synapse 68, 317–320. 10.1002/syn.21738 [DOI] [PubMed] [Google Scholar]
- Kravitz A. V., Freeze B. S., Parker P. R., Kay K., Thwin M. T., Deisseroth K., et al. (2010). Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626. 10.1038/nature09159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer A. C., Malenka R. C. (2008). Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554. 10.1016/j.neuron.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C., Bloch B. (1995). D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 355, 418–426. 10.1002/cne.903550308 [DOI] [PubMed] [Google Scholar]
- Lemos J. C., Friend D. M., Kaplan A. R., Shin J. H., Rubinstein M., Kravitz A. V., et al. (2016). Enhanced gaba transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 90, 824–838. 10.1016/j.neuron.2016.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. A., Eberhardt N. L., Jensen M. D. (1999). Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283, 212–214. 10.1126/science.283.5399.212 [DOI] [PubMed] [Google Scholar]
- Li Y., South T., Han M., Chen J., Wang R., Huang X. F. (2009). High-fat diet decreases tyrosine hydroxylase mRNA expression irrespective of obesity susceptibility in mice. Brain Res. 1268, 181–189. 10.1016/j.brainres.2009.02.075 [DOI] [PubMed] [Google Scholar]
- Logan R. W., McClung C. A. (2016). Animal models of bipolar mania: the past, present and future. Neuroscience 321, 163–188. 10.1016/j.neuroscience.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. K., Heilbronn L. K., de Jonge L., DeLany J. P., Volaufova J., Anton S. D., et al. (2007). Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 15, 2964–2973. 10.1038/oby.2007.354 [DOI] [PubMed] [Google Scholar]
- Mathes W. F., Nehrenberg D. L., Gordon R., Hua K., Garland T., Jr., Pomp D. (2010). Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav. Brain Res. 210, 155–163. 10.1016/j.bbr.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M., Thanos P. K., Kim R., Cho J., Ananth M., Wang G. J., et al. (2012). PET imaging predicts future body weight and cocaine preference. Neuroimage 59, 1508–1513. 10.1016/j.neuroimage.2011.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink J. W. (1996). The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425. 10.1016/S0301-0082(96)00042-1 [DOI] [PubMed] [Google Scholar]
- Mogenson G. J., Jones D. L., Yim C. Y. (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 14, 69–97. 10.1016/0301-0082(80)90018-0 [DOI] [PubMed] [Google Scholar]
- Morrison R., Penpraze V., Beber A., Reilly J. J., Yam P. S. (2013). Associations between obesity and physical activity in dogs: a preliminary investigation. J. Small Anim. Pract. 54, 570–574. 10.1111/jsap.12142 [DOI] [PubMed] [Google Scholar]
- Morrison R., Reilly J. J., Penpraze V., Pendlebury E., Yam P. S. (2014). A 6-month observational study of changes in objectively measured physical activity during weight loss in dogs. J. Small Anim. Pract. 55, 566–570. 10.1111/jsap.12273 [DOI] [PubMed] [Google Scholar]
- Muramoto A., Imagama S., Ito Z., Hirano K., Tauchi R., Ishiguro N., et al. (2014). Waist circumference is associated with locomotive syndrome in elderly females. J. Orthop. Sci. 19, 612–619. 10.1007/s00776-014-0559-6 [DOI] [PubMed] [Google Scholar]
- Narayanaswami V., Thompson A. C., Cassis L. A., Bardo M. T., Dwoskin L. P. (2013). Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int. J. Obes. 37, 1095–1103. 10.1038/ijo.2012.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need A. C., Ahmadi K. R., Spector T. D., Goldstein D. B. (2006). Obesity is associated with genetic variants that alter dopamine availability. Ann. Hum. Genet. 70, 293–303. 10.1111/j.1529-8817.2005.00228.x [DOI] [PubMed] [Google Scholar]
- Noble E. P., Blum K., Ritchie T., Montgomery A., Sheridan P. J. (1991). Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry 48, 648–654. 10.1001/archpsyc.1991.01810310066012 [DOI] [PubMed] [Google Scholar]
- Obeso J. A., Rodríguez-Oroz M. C., Rodríguez M., Lanciego J. L., Artieda J., Gonzalo N., et al. (2000). Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 23, S8–S19. 10.1016/s1471-1931(00)00028-8 [DOI] [PubMed] [Google Scholar]
- Ong Z. Y., Wanasuria A. F., Lin M. Z., Hiscock J., Muhlhausler B. S. (2013). Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite 65, 189–199. 10.1016/j.appet.2013.01.014 [DOI] [PubMed] [Google Scholar]
- Parksepp M., Ljubajev Ü., Täht K., Janno S. (2016). Prevalence of neuroleptic-induced movement disorders: an 8-year follow-up study in chronic schizophrenia inpatients. Nord. J. Psychiatry 70, 498–502. 10.3109/08039488.2016.1164245 [DOI] [PubMed] [Google Scholar]
- Rada P., Bocarsly M. E., Barson J. R., Hoebel B. G., Leibowitz S. F. (2010). Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol. Behav. 101, 394–400. 10.1016/j.physbeh.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Marrero F. A., Miles J., Joyner M. J., Curry T. B. (2014). Self-reported and objective physical activity in postgastric bypass surgery, obese and lean adults: association with body composition and cardiorespiratory fitness. J. Phys. Act. Health 11, 145–151. 10.1123/jpah.2012-0048 [DOI] [PubMed] [Google Scholar]
- Redman L. M., Heilbronn L. K., Martin C. K., de Jonge L., Williamson D. A., Delany J. P., et al. (2009). Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS ONE 4:e4377. 10.1371/journal.pone.0004377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J. D., Correa M., Farrar A., Mingote S. M. (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191, 461–482. 10.1007/s00213-006-0668-9 [DOI] [PubMed] [Google Scholar]
- Sano H., Yasoshima Y., Matsushita N., Kaneko T., Kohno K., Pastan I., et al. (2003). Conditional ablation of striatal neuronal types containing dopamine D2 receptor disturbs coordination of basal ganglia function. J. Neurosci. 23, 9078–9088. Available online at: http://www.jneurosci.org/content/23/27/9078.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C. W., Carmona G. N. (2002). Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol. Biochem. Behav. 72, 857–863. 10.1016/S0091-3057(02)00770-0 [DOI] [PubMed] [Google Scholar]
- Schmidt L., Lebreton M., Cléry-Melin M. L., Daunizeau J., Pessiglione M. (2012). Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 10:e1001266. 10.1371/journal.pbio.1001266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Fernandes M. F., Fulton S. (2013). Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes. 37, 1183–1191. 10.1038/ijo.2012.197 [DOI] [PubMed] [Google Scholar]
- Simon C., Schweitzer B., Oujaa M., Wagner A., Arveiler D., Triby E., et al. (2008). Successful overweight prevention in adolescents by increasing physical activity: a 4-year randomized controlled intervention. Int. J. Obes. 32, 1489–1498. 10.1038/ijo.2008.99 [DOI] [PubMed] [Google Scholar]
- Small D. M., Jones-Gotman M., Dagher A. (2003). Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19, 1709–1715. 10.1016/S1053-8119(03)00253-2 [DOI] [PubMed] [Google Scholar]
- Steele K. E., Prokopowicz G. P., Schweitzer M. A., Magunsuon T. H., Lidor A. O., Kuwabawa H., et al. (2010). Alterations of central dopamine receptors before and after gastric bypass surgery. Obes. Surg. 20, 369–374. 10.1007/s11695-009-0015-4 [DOI] [PubMed] [Google Scholar]
- Stice E., Spoor S., Bohon C., Small D. M. (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322, 449–452. 10.1126/science.1161550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Bohon C., Marti N., Smolen A. (2010). Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 50, 1618–1625. 10.1016/j.neuroimage.2010.01.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. L., Cameron J. L. (2010). A rapidly occurring compensatory decrease in physical activity counteracts diet-induced weight loss in female monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1068–R1074. 10.1152/ajpregu.00617.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift D. L., Johannsen N. M., Lavie C. J., Earnest C. P., Church T. S. (2014). The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 56, 441–447. 10.1016/j.pcad.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske J. A., Billington C. J., Kuskowski M. A., Kotz C. M. (2012). Spontaneous physical activity protects against fat mass gain. Int. J. Obes. 36, 603–613. 10.1038/ijo.2011.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos P. K., Michaelides M., Piyis Y. K., Wang G. J., Volkow N. D. (2008). Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 62, 50–61. 10.1002/syn.20468 [DOI] [PubMed] [Google Scholar]
- Thanos P. K., Volkow N. D., Freimuth P., Umegaki H., Ikari H., Roth G., et al. (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem. 78, 1094–1103. 10.1046/j.1471-4159.2001.00492.x [DOI] [PubMed] [Google Scholar]
- Thompson J., Thomas N., Singleton A., Piggott M., Lloyd S., Perry E. K., et al. (1997). D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 7, 479–484. 10.1097/00008571-199712000-00006 [DOI] [PubMed] [Google Scholar]
- Trifilieff P., Feng B., Urizar E., Winiger V., Ward R. D., Taylor K. M., et al. (2013). Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol. Psychiatry 18, 1025–1033. 10.1038/mp.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C., Brashear M. M., Johnson W. D., Katzmarzyk P. T. (2010). Accelerometer profiles of physical activity and inactivity in normal weight, overweight, and obese U.S. men and women. Int. J. Behav. Nutr. Phys. Act. 7:60. 10.1186/1479-5868-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen L., Tuulari J., Karlsson H., Hirvonen J., Helin S., Salminen P., et al. (2015). Aberrant mesolimbic dopamine-opiate interaction in obesity. Neuroimage 122, 80–86. 10.1016/j.neuroimage.2015.08.001 [DOI] [PubMed] [Google Scholar]
- van de Giessen E., Celik F., Schweitzer D. H., van den Brink W., Booij J. (2014). Dopamine D2/3 receptor availability and amphetamine-induced dopamine release in obesity. J. Psychopharmacol. 28, 866–873. 10.1177/0269881114531664 [DOI] [PubMed] [Google Scholar]
- van de Giessen E., la Fleur S. E., de Bruin K., van den Brink W., Booij J. (2012). Free-choice and no-choice high-fat diets affect striatal dopamine D2/3 receptor availability, caloric intake, and adiposity. Obesity 20, 1738–1740. 10.1038/oby.2012.17 [DOI] [PubMed] [Google Scholar]
- van de Giessen E., la Fleur S. E., Eggels L., de Bruin K., van den Brink W., Booij J. (2013). High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. Int. J. Obes. 37, 754–757. 10.1038/ijo.2012.128 [DOI] [PubMed] [Google Scholar]
- Vitger A. D., Stallknecht B. M., Nielsen D. H., Bjornvad C. R. (2016). Integration of a physical training program in a weight loss plan for overweight pet dogs. J. Am. Vet. Med. Assoc. 248, 174–182. 10.2460/javma.248.2.174 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Telang F., Fowler J. S., Thanos P. K., Logan J., et al. (2008). Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543. 10.1016/j.neuroimage.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Tomasi D., Baler R. D. (2013). The addictive dimensionality of obesity. Biol. Psychiatry 73, 811–818. 10.1016/j.biopsych.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P., Vanderschuren L. J., Groenewegen H. J., Robbins T. W., Pennartz C. M. (2004). Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 27, 468–474. 10.1016/j.tins.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Vucetic Z., Carlin J. L., Totoki K., Reyes T. M. (2012). Epigenetic dysregulation of the dopamine system in diet-induced obesity. J. Neurochem. 120, 891–898. 10.1111/j.1471-4159.2012.07649.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. J., Volkow N. D., Logan J., Pappas N. R., Wong C. T., Zhu W., et al. (2001). Brain dopamine and obesity. Lancet 357, 354–357. 10.1016/S0140-6736(00)03643-6 [DOI] [PubMed] [Google Scholar]
- Wolden-Hanson T., Davis G. A., Baum S. T., Kemnitz J. W. (1993). Insulin levels, physical activity, and urinary catecholamine excretion of obese and non-obese rhesus monkeys. Obes. Res. 1, 5–17. 10.1002/j.1550-8528.1993.tb00003.x [DOI] [PubMed] [Google Scholar]
- Xu M., Moratalla R., Gold L. H., Hiroi N., Koob G. F., Graybiel A. M., et al. (1994). Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79, 729–742. 10.1016/0092-8674(94)90557-6 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wei N. L., Wang Y., Wang X., Zhang J. G., Zhang K. (2015). Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci. Lett. 589, 1–6. 10.1016/j.neulet.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Zhang L. N., Morgan D. G., Clapham J. C., Speakman J. R. (2012). Factors predicting nongenetic variability in body weight gain induced by a high-fat diet in inbred C57BL/6J mice. Obesity 20, 1179–1188. 10.1038/oby.2011.151 [DOI] [PubMed] [Google Scholar]