Abstract
The prion protein (PrPC) has been suggested to operate as a scaffold/receptor protein in neurons, participating in both physiological and pathological associated events. PrPC, laminin, and metabotropic glutamate receptor 5 (mGluR5) form a protein complex on the plasma membrane that can trigger signaling pathways involved in neuronal differentiation. PrPC and mGluR5 are co-receptors also for β-amyloid oligomers (AβOs) and have been shown to modulate toxicity and neuronal death in Alzheimer's disease. In the present work, we addressed the potential crosstalk between these two signaling pathways, laminin-PrPC-mGluR5 or AβO-PrPC-mGluR5, as well as their interplay. Herein, we demonstrated that an existing complex containing PrPC-mGluR5 has an important role in AβO binding and activity in neurons. A peptide mimicking the binding site of laminin onto PrPC (Ln-γ1) binds to PrPC and induces intracellular Ca2+ increase in neurons via the complex PrPC-mGluR5. Ln-γ1 promotes internalization of PrPC and mGluR5 and transiently decreases AβO biding to neurons; however, the peptide does not impact AβO toxicity. Given that mGluR5 is critical for toxic signaling by AβOs and in prion diseases, we tested whether mGlur5 knock-out mice would be susceptible to prion infection. Our results show mild, but significant, effects on disease progression, without affecting survival of mice after infection. These results suggest that PrPC-mGluR5 form a functional response unit by which multiple ligands can trigger signaling. We propose that trafficking of PrPC-mGluR5 may modulate signaling intensity by different PrPC ligands.
Keywords: Alzheimer disease, calcium, laminin, metabotropic glutamate receptor (mGluR), prion, Protein trafficking, metabotropic glutamate receptor 5
Introduction
The prion protein (PrPC)3 was originally discovered as a substrate for prion disease propagation in mammals (1). Several studies on PrPC physiological function revealed dozens of PrPC partners (2–4). In neurons, PrPC interactions with some of these ligands trigger multiple effects, including regulation of protein synthesis, differentiation, neuroprotection, and neuritogenesis (4–17). Given that PrPC is a glycosylphosphatidylinositol (GPI)-anchored protein, signal transduction requires the formation of complexes between PrPC and transmembrane receptors. PrPC interactions have been demonstrated for α7 nicotinic acetylcholine receptor (5), group I metabotropic glutamate receptors (10, 18, 19), ionotropic glutamate receptors (20, 21) and purinergic receptors (22). These results suggest that PrPC functions as an extracellular scaffolding protein, able to organize multiprotein complexes at the cell surface (3, 4). Increasing evidence indicates that such scaffolding can be neurotoxic. For instance, when PrPC binds oligomeric forms of β-amyloid peptides (AβO) (23), mal-adaptive signaling via metabotropic glutamate receptor 5 (mGluR5) is elicited (18), which initiates multiple changes in synaptic homeostasis, leading to excitotoxicity, endoplasmic reticulum stress, and eventually to synaptic degradation and neuronal cell death (18, 23–30). Thus, efforts have been made to disrupt this complex and downstream pathways to prevent neurotoxic consequences. Antibodies specific to certain PrPC regions were efficient in preventing AβO binding to the neuronal surface (31–33), but such antibodies can also trigger toxic neuronal signaling by themselves (34), mediated by PrPC N terminus domain (35). The PrPC N-terminal domain, known as N1 peptide, which is usually secreted into extracellular space by α-secretase action on PrPC, also exhibited a neuroprotective effect, presumably due to the sequestration of AβOs from the extracellular space (36, 37).
Stress-inducible phosphoprotein 1 (STI1), a secreted co-chaperone that can bind and activate PrPC-dependent signaling, has been shown to prevent the toxicity of AβOs in cultured neurons and brain slices (38). mGluR5 ligands (antagonists and modulators) have also been shown to impact on AβO toxicity and cognitive deficits (18, 25, 29, 39, 40). The interaction between laminin γ1 chain and PrPC is of particular interest, given that the PrPC-mediated signaling triggered by laminin γ1 chain-mimicking peptide (1575–1584, Ln-γ1) depends on mGluR5 (10). Of importance, Ln-γ1 signaling via the complex PrPC-mGluR5 leads to neuritogenesis (10), a process known to be disrupted by AβO-induced toxicity (41, 42).
Here we investigated the relationship between Ln-γ1, PrPC, and mGluR5 in AβO toxicity and the role of mGluR5 in prion infection. Our data suggest that a preformed complex containing mGluR5 and PrPC cooperates for Aβ oligomer binding to neurons, and that Ln-γ1 is ineffective to prevent AβO-induced toxicity. Moreover, mGluR5 may also regulate some of the toxicity related to prion infection.
Results
Ln-γ1 Modulates AβO Binding to Neurites
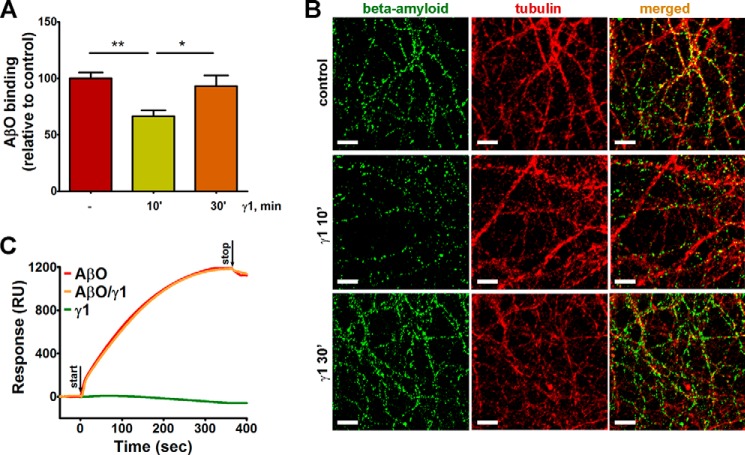
There is accumulating evidence that PrPC binds AβOs on the neuronal surface, initiating toxic signaling pathways (18, 23, 27, 33, 38). Previously, we showed by multiple assays that a PrPC ligand, STI1, decreases AβO binding to the prion protein and prevents neuronal death induced by AβOs (38). Given that similarly to AβOs, Ln-γ1 also engages PrPC and mGluR5, we tested whether the Ln-γ1-dependent activation of PrPC could affect its binding to AβO and toxicity. We observed a 30% decrease in neurite-bound AβOs when neuronal cultures were pretreated with Ln-γ1 laminin peptide for 10 min (Fig. 1, A and B). Surprisingly, there were no changes in AβO binding when neuronal cultures were pretreated with Ln-γ1 peptide for 30 min (Fig. 1, A and B). To further understand the potential mechanisms by which Ln-γ1 interferes with Aβ biding to PrPC, we performed an SPR study (Fig. 1C). Likely due to its small size, the Ln-γ1 peptide did not produce any signals on SPR (Fig. 1C), albeit each preparation was able to cause PrPC-dependent calcium signal in neurons, as described previously (10). Importantly, Ln-γ1 peptide was unable to interfere with AβO binding (Fig. 1C). These results suggest that treatment with Ln-γ1 peptide decreases AβO binding in live cells by a mechanism distinct from competition with AβO for PrPC binding sites.
FIGURE 1.
Ln-γ1 effect on AβO binding to neurites and on-chip PrP. A, levels of the neurite-bound AβOs decrease in cultures treated with Ln-γ1 peptide (RNIAEIIKDI) for 10, but not for 30 min. B, representative images of hippocampal neuronal cultures, immunostained for β-amyloid (green) and tubulin (red), as described under “Experimental Procedures.” Scale bar = 10 μm. C, SPR kinetics of ligands binding to PrP on a Ni-nitrilotriacetic acid chip. Ln-γ1 (green) or AβOs (red) were injected separately or simultaneously (orange). RU, response units.
Ln-γ1 Induces Decrease in Cell Surface PrPC
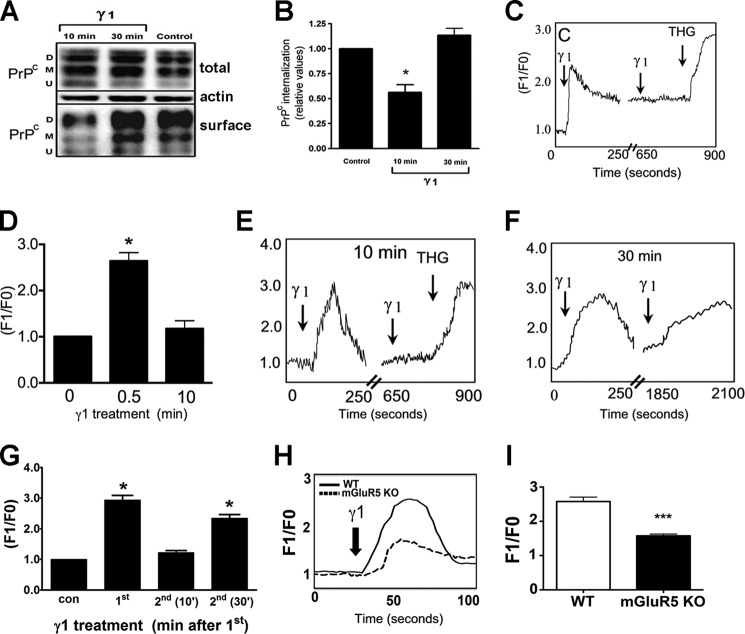
To investigate the potential mechanisms by which Ln-γ1 peptide could modulate PrPC, we incubated neuronal CF10 cells (a PrPC-null cell line) stably transfected with PrPC with Ln-γ1 peptide and measured cell surface PrPC by biotinylation. Treatment of cells with Ln-γ1 for 10 min significantly decreased cell surface PrPC by 30–40%, which then returned to normal levels after 30 min of Ln-γ1 treatment (Fig. 2A and B). This result was confirmed by confocal and total internal reflection fluorescence imaging of HEK293T cells transfected with PrPC fused to GFP, suggesting that Ln-γ1 peptide treatment causes internalization of PrPC (supplemental Experimental Procedures and Fig. S1). Ln-γ1 peptide triggers calcium signaling via PrPC-mGluR5 complex; hence it is possible that the changes in cell surface PrPC levels may reflect a desensitization mechanism. Treatment of PrPC-CF10 cells with Ln-γ1 peptide led to a significant increase in intracellular Ca2+, but a second application of the peptide 10 min after the first treatment failed to elicit a second bout of Ca2+ signaling (Fig. 2, C and D). The same pattern of intracellular Ca2+ release was observed in neurons treated with Ln-γ1 peptide (Fig. 2, E and G). In contrast, when Ln-γ1 peptide was reapplied 30 min after the initial treatment, a robust increase in intracellular Ca2+ was observed (Fig. 2, F and G). Thapsigargin (1 μm), a non-competitive inhibitor of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), which stimulates intracellular calcium mobilization, was used as a positive control (43). These results suggest that about 30–40% of cell surface PrPC is available for signaling by Ln-γ1 peptide, and when this pool of PrPC is internalized, the remaining cell surface protein cannot trigger calcium signaling.
FIGURE 2.
Effect of Ln-γ1 treatment on PrPC localization and intracellular calcium in cells. A and B, Ln-γ1 induces transient PrPC internalization in CF10 cells. A, representative Western blotting image of diglycosylated (D), monoglycosylated (M) and unglycosylated (U) forms of PrPC. B, quantification of surface/total protein levels from at least 3 independent experiments. C and D, Ln-γ1 induces an increase in intracellular Ca2+ levels in CF10 cells, and repeated administration of Ln-γ1 over 10 min has no effect. C, calcium response (relative Fluo-4 fluorescence kinetics) induced by Ln-γ1. Thapsigargin (1 μm) (THG) was used as a positive control for intracellular calcium mobilization. D, quantification of Ca2+ levels, averaged from at least 60 cells measured in at least 3 independent experiments. E–G, Ln-γ1 induces increase in intracellular Ca2+ levels in primary hippocampal neurons. E, relative Fluo-4 fluorescence kinetics of the initial Ln-γ1 treatment and the repeated one in 10 min. F, the same for the repeated γ1 treatment in 30 min after the initial one. G, quantification of Ca2+ levels, averaged from at least 60 cells measured in at least 3 independent experiments. H and I, Ln-γ1-induced increase in intracellular Ca2+ in wild-type and mGluR5−/− neurons. H, relative Fluo-4 fluorescence kinetics. I, quantification of Ca2+ signal amplitude, averaged from at least 60 cells measured in at least 3 independent experiments. Error bars indicate mean ± S.E. *, p < 0.05; ***, p < 0.001.
We have shown previously that mGluR5 antagonists blocked the intracellular calcium signal induced by Ln-γ1/PrPC interaction (10). To further test the role of these two proteins, we used hippocampal cell cultures from mouse embryos lacking either mGlur5 or PrPC or both. Ln-γ1 peptide-induced calcium release is significantly decreased in mGluR5−/− neurons (Fig. 2H). The residual signal probably depends on mGluR1, which also was shown to be involved in Ln-γ1-PrPC pathway (10).
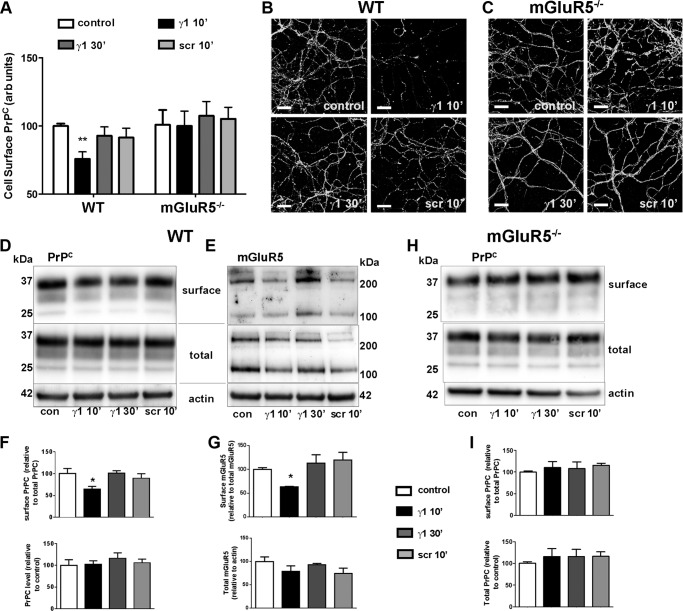
To investigate the relationship between mGluR5 and PrPC in the Ln-γ1-induced PrPC internalization, we treated WT and mGluR5−/− neuronal cultures with Ln-γ1 or scrambled peptide (Ln-SCR) and determined the amount of cell surface PrPC and mGluR5 by immunofluorescence (Fig. 3, A–C) and cell surface biotinylation (Fig. 3, D–I). Similar to CF10 cells, in WT neurons, surface localization of PrPC decreased after treatment with Ln-γ1 peptide for 10 min, but returned to its original level 30 min after the treatment, whereas the scrambled peptide had no effect (Fig. 3, A, B, D, and F). In contrast, cell surface PrPC did not respond to Ln-γ1 peptide treatment in mGluR5−/− neurons (Fig. 3, A, C, H, and I). Similarly to what was observed for PrPC, in WT neurons, surface mGluR5 decreased after 10 min of Ln-γ1 treatment and returned to its original levels after 30 min of treatment (Fig. 3, E and G). The scrambled peptide had no effect on mGluR5 surface localization. These results suggest that PrPC and mGluR5 move together upon treatment with the Ln-γ1 peptide. Moreover, the peptide induced internalization of a fraction of cell surface PrPC (30%), which seems to depend on the complex with mGluR5. It is possible that only the complex PrPC-mGluR5 is able to respond to Ln-γ1 treatment. As a control for the specificity for the mGluR5 antibody, we detected no mGluR5 signal in mGlur5−/− protein extracts (supplemental Fig. S2).
FIGURE 3.
Ln-γ1-induced transient internalization of PrPC and mGluR5 in primary neurons. A–C, WT (A and B) and mGluR5−/− (A and C) hippocampal neuronal cultures were treated with Ln-γ1 for 10 or 30 min, or with Ln-SCR (Ln-γ1 scrambled peptide: IRADIEIKID), and the neuronal cell surface was immunostained with 8H4 antibody. Quantification of surface PrPC was done for eight random fields of view for each condition (A). Primary neuronal cultures were prepared from at least five embryos of each genotype. arb units, arbitrary units. B and C, representative images of WT (B) and mGluR5−/− (C) cultures, corresponding to each treatment. Scale bar = 20 μm. D–I, primary cortical neurons were treated with Ln-γ1 for 10 or 30 min, or with Ln-SCR, after which the surface proteins were biotinylated. Levels of surface PrPC (D and F) and mGluR5 (monomer + dimer, E and G) in WT cultures, and of surface PrPC in mGluR5−/− cultures (H and I), were quantified as the ratio of biotinylated protein to the total protein levels on Western blot (D, E, and H), and then compared using GraphPad 5.0 software (F, G, and I). con, control. Error bars indicate mean ± S.E. *, p < 0.05.
PrPC and mGluR5 Cooperate to Transduce AβO Signals
The finding that 30% of the cell surface pool of PrPC is internalized by Ln-γ1 peptides only in the presence of mGluR5 led us to further investigate how this complex modulates the interaction of AβO with neurons. Interestingly, although Ln-γ1 treatment decreases AβO binding to neuronal surfaces, possibly due to PrPC internalization, this treatment was inefficient against AβO-induced neuronal death (Fig. 4, A and B). This result suggests that indeed Ln-γ1 peptide cannot compete effectively with AβOs to prevent toxicity. We then investigated the impact of PrPC-mGluR5 complex in AβO interaction with neurons. The interaction of AβO with neuronal cell surface was reduced by ∼40% in the absence of PrPC or mGluR5 (Fig. 4, C and D). In contrast, in neuronal cultures from double knock-out mice lacking both PrPC and mGluR5, AβO binding to neurites was reduced by 58% (Fig. 4, C and D). Of note, previous findings in COS-7 cells, overexpressing PrPC and/or mGluR5, suggested that PrPC, but not mGluR5, is needed for AβO binding to the cell surface (18). However, Renner et al. (39) have shown, similar to the present results, that Aβ binding is decreased in mGluR5-null neurons. To confirm the PrPC dependence on the effect of Ln-γ1, we performed the same experiments described above in PrP−/− neurons and in CF10 cells. In both cases, in the absence of PrPC, pretreatment with Ln-γ1 peptide did not alter AβO binding to either PrP−/− neurons or CF10 cells (supplemental Fig. S3). To further explore the role of PrPC-mGluR5 complex in the toxic effects of AβO, we investigated Ca2+ signaling (Fig. 4, E and F). We confirmed previous results from Um et al. (18) that the absence of PrPC almost abolished Ca2+ signaling induced by AβOs. In contrast, some residual Ca2+ signaling was still triggered in the absence of mGluR5 (Fig. 4, E and F). Interestingly, in double-knock-out neurons, lacking both PrPC and mGluR5, Ca2+ signaling was abolished, a similar result as observed for neurons lacking only PrPC (Fig. 4, E and F).
FIGURE 4.
Effect of PrPC and mGluR5 absence and of Ln-γ1 treatment on AβO toxicity hallmarks. A and B, the effect of pretreatment of neuronal cultures with Ln-γ1 or Ln-SCR (scr) on AβO-induced cell death. Cell death was registered for cultures prepared from at least five embryos for each condition (A). Representative images show live (green) and dead (red) cells (B). Scale bar = 100 μm. C and D, AβO binding to neurites lacking PrPC, mGluR5, or both proteins. Representative images showing immunostaining of AβOs (red) and tubulin (green) (C). Levels of bound AβOs were normalized to neurite area (calculated by tubulin immunostaining) from eight random fields of view of neuronal cultures prepared from at least five embryos for each genotype (D). Scale bar = 20 μm. E and F, AβO-induced increase in intracellular Ca2+ in neuron lacking PrPC (red), mGluR5 (blue), or both proteins (purple). E, calcium response (relative to baseline Fluo-4 fluorescence, F1/F0) kinetics in the neurons treated with 500 nm AβOs. F, quantification of the peak signal from E. Data were collected from at least 20 cells in at least 3 independent experiments for each genotype. Statistical comparison was done with one-way analysis of variance followed by Tukey post hoc test. Error bars indicate mean ± S.E. For a and b, p < 0.05; for a and c, p < 0.001.
Prion Disease Onset Is Delayed in mGluR5−/− Mice
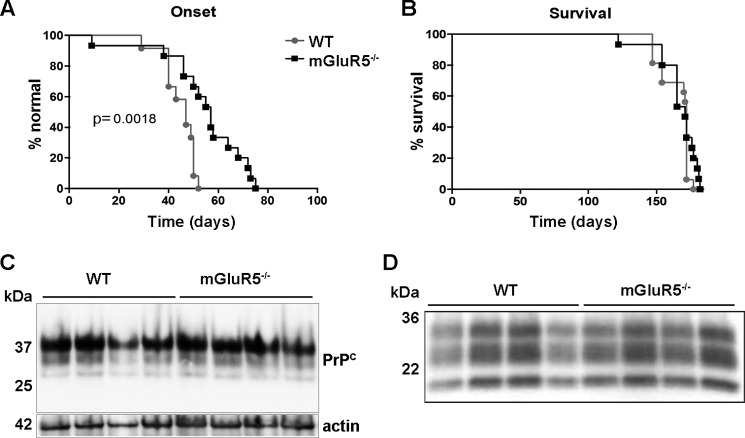
Previous studies suggest that various misfolded proteins, including PrPSc, can interact with and corrupt the signaling mediated by PrPC, similar to AβOs (44). However, it is unknown whether the PrPC-mGluR5 complex has a role in other protein misfolding diseases. This might be particularly important in prion diseases, in which PrPSc toxicity could impact mGluR5 signaling. Therefore, we infected wild-type and mGluR5−/− mice with prions (prion strain RML (named for Rocky Mountain Laboratories)) and followed the mice after infection. Interestingly, there was a significant delay in disease onset, determined by observing clinical symptoms, in mice lacking mGluR5 when compared with control mice (Fig. 5A). This, however, did not affect mouse survival (Fig. 5B). Biochemical evaluation of protease-resistant PrPSc and total levels of PrPC showed no difference between genotypes (Fig. 5, C and D). These results suggest that pharmacological targeting mGluR5 may delay some prion disease symptoms, but it is unlikely to extend the life of prion disease-affected individuals.
FIGURE 5.
Prion infection in WT and mGluR5−/− mice. A, disease onset in infected mice was determined as described under “Experimental Procedures.” B, mouse survival upon prion infection. C, Western blotting of PrPC in non-infected WT and mGluR5−/− mice. D, Western blotting of PrPSc in infected WT and mGluR5−/− mice as determined by proteinase K cleavage assay. Four mouse brains were used for each condition for Western blotting analyses. Data were analyzed and compared by Mantel-Cox log-rank test. A, p = 0.0018, and B, p = 0.3215.
Discussion
In this work, we explored the ensemble PrPC-mGluR5 and its potential ligands Ln-γ1 and AβOs on the pathological mechanisms involved in neurodegeneration. Our results suggest that Ln-γ1 signals via a small portion of cell surface prion protein. This conclusion is based on the fact that after treatment with a peptide mimicking the effects of Ln-γ1 chain, a transient decrease of 30% in cell surface PrPC was observed. This minor decrease of cell surface PrPC prevented subsequent signal by the Ln-γ1 peptide, suggesting that the majority of PrPC at the membrane is not available for Ln-γ1-induced Ca2+ increase. Although the mechanisms involved in this limited signaling by PrPC-mGluR5 are currently unclear, it is possible that Ln-γ1 peptide may not be able to induce the association of PrPC with mGluR5, but rather it only activates the complex if it is already formed.
Evidence that PrPC can be found in a complex with mGluR5 and mGluR1 without any stimulation is abundant. We (10) and others (18, 19, 25) found that PrPC and mGluRs can associate biochemically. We have also reported a functional association between PrPC and mGluR5 in non-neuronal cells for Ln-γ1 peptide signaling (10), whereas others have shown similar functional association for AβOs (18, 25). In the current work, we confirmed and extended these results, in primary neuronal culture from embryos lacking either PrPC or mGluR5 or both, showing that a complex formed between PrPC and mGluR5 is important for AβO interaction with neurons. AβO binding to neurons seems to be partially affected by the lack of PrPC and in the same proportion by the lack of mGluR5. These results are consistent with previous observations that both mGluR5 (10, 18, 25, 39) and PrPC (21, 23, 27, 33, 38) can function as receptors for extracellular ligands, including AβOs and Ln-γ1. Interestingly, in neurons lacking both mGluR5 and PrPC, the decrease in AβO binding was not additive. Previous experiments in neurons showed that in mGluR5− neurons, binding of AβOs is decreased (39), whereas experiments in COS7 cells overexpressing mGluR5 suggests that AβOs cannot bind directly to mGluR5 (18). Overall, our study suggests that although AβO may be able to interact with either PrPC or mGluR5 independently, as proposed previously (23, 39, 45), a proportion of oligomers may also bind to a preformed complex. These results are consistent with the notion that a certain proportion of PrPC may already be in a functional complex with mGluR5 at the cell surface.
Trafficking of the GPI-anchored PrPC has been extensively studied (46–53). Although most GPI-anchored proteins are thought to be internalized via a non-clathrin-mediated mechanism (54), multiple data suggest that PrPC is internalized in dynamin-sensitive vesicles (48) by clathrin-mediated endocytosis (52, 55–58). Constitutive internalization of cell surface PrPC seems to depend on LRP1, a scaffold protein that connects PrPC to clathrin-coated vesicles (59, 60). Here we described a novel mechanism by which Ln-γ1 peptide induces a decrease in only a fraction of cell surface PrPC, and it can also decrease the same proportion of cell surface mGluR5. Interestingly, the absence of mGluR5 impairs Ln-γ1 peptide-induced internalization of PrPC, suggesting that the complex PrPC-mGluR5 may internalize together. mGluR5 can be internalized in a constitutive or an agonist-induced way. Constitutive internalization was found to proceed in a clathrin-independent (61) but rather in a GRK2- (62) and caveolin-1/lipid raft-dependent (63) manner. The mechanisms of agonist-induced endocytosis of mGluR5, which are important for controlling NMDA plasticity in the hippocampus (64), and, possibly, for the roles of mGluR5 in neuropsychiatric disorders (65, 66), are not completely clear. Although PrPC and mGluR5 have not yet been shown to be present in intracellular vesicles together, both proteins can be found in the post-synaptic density. Moreover, PrPC co-immunoprecipitates not only with mGluR5 (10, 18), but also with mGluR5 intracellular mediators, including Homer 1b (19), shown previously to regulate mGluR5 trafficking (67). It is possible, therefore, that at least a portion of the PrPC-mGluR5 complex is internalized together, which could have important implications in mGluR5 functions in healthy organisms and in disease. Of note, AβO treatment of cells can trap both mGluR5 (18) and PrPC (68) at the cell surface, suggesting that part of the AβO-induced toxic signaling may occur due to the longer permanence of complexes formed between mGluR5 and PrPC at the cell surface.
It has been suggested that signaling by PrPC and mGluR5 may be corrupted by AβOs, leading to abnormal activation of Fyn kinase and neurotoxicity (18). Why do different ligands, such as AβO and Ln-γ1, which use similar signaling pathways, have distinct effects? Ln-γ1 interaction with PrPC triggers neuritogenesis, whereas AβOs cause neurotoxic effects. One possibility is that the ability of these two ligands to trigger internalization or increased permanence of the PrPC-mGluR5 complex at the membrane could regulate subsequent signaling in neurons. Interestingly, ligands that can trigger internalization of PrPC, such as Ln-γ1 (present results) or STI1 (46), are usually not toxic and can trigger survival signaling. A second possibility is that Ln-γ1 or its peptide cannot activate the formation of PrPC-mGluR5 complex, whereas AβOs can (25). These results are consistent with the hypothesis that PrPC is an extracellular scaffolding protein able to seed the formation of several multi-protein complexes that underlie neuronal signaling (3). Biasing the interaction of PrPC with other receptors, such as α7 nicotinic acetylcholine receptors (5, 38, 69), may be also an effective way to diminish toxic effects of AβOs.
Despite a critical role for PrPC and mGluR5 in Alzheimer's disease, it seems that for toxicity, due to PrPSc buildup, lack of mGluR5 is not critical. Although drugs that interfere with mGluR5 and genetic elimination of mGluR5 seem to improve the outcomes in mouse models of Alzheimer's disease (19, 70), we observed only marginal improvements in clinical signs during prion infection. Our data suggest a small, but unlikely, life-extending benefit, by inhibiting the activity of mGluR5 in prion diseases. It is possible that other mechanisms triggered by the accumulation of PrPSc, such as endoplasmic reticulum stress (17), which has recently been shown to play a major role in prion and Alzheimer's diseases (71–73), need to be targeted simultaneously with mGluR5 for potential therapeutic benefits in these neurodegenerative diseases.
In summary, we find that Ln-γ1 peptide, which signals via PrPC-mGluR5, does not affect AβO-mediated neurotoxicity. However, our experiments suggest the possible existence of the complex PrPC-mGluR5 at the cell surface even in the absence of any ligands. Further experiments are required to determine by which extent the complex PrPC-mGluR5 can regulate each other's traffic and function in distinct physiological and pathological settings. mGluR5 has been proposed as a target in several psychiatric and developmental conditions, including fragile X syndrome, schizophrenia, and autism spectrum disorders (74–77). It will be important to delineate whether its complex with PrPC also plays a role in these diseases. Our results support the notion that mGluR5 function is coupled to PrPC and that targeting this complex might be beneficial in neurodegenerative diseases.
Experimental Procedures
Animals
Prnp−/− mice in a C57BL/6 background were kindly donated by Dr. Frank Jirik, University of Calgary (78). mGluR5−/− (Grm5tm1Rod/J) mice in a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, stock number 003558) (79). Procedures were conducted in accordance with approved animal use protocols at the University of Western Ontario (2008/127), University of British Columbia (A11-0138), and the A. C. Camargo Cancer Center (037/09) following Canadian Council of Animal Care (CCAC) and National Institutes of Health guidelines.
Peptide Preparations
AβOs were prepared from Aβ(1–42) peptide (rPeptide) as described previously (38). Briefly, the peptide was monomerized in hexafluoroisopropanol, dried in a SpeedVac centrifuge, restored in DMSO to 1 mm solution, and diluted in PBS (for intracellular calcium experiments) or F-12 medium (Invitrogen) (for the neuronal survival experiments) to a final concentration of 100 μm (hereafter monomer concentration used as AβO concentration). After incubation for 24 h at 4 °C, AβOs were used immediately or stored at −80 °C for no more than 4 weeks. Peptide preparation quality was checked by Western blotting with 6E10 (1:2000, Covance) antibody, and similar preparations were fully characterized using size exclusion chromatography as well as by atomic force microscopy (28, 38). Ln-γ1 (RNIAEIIKDI) or Ln-SCR (IRADIEIKID) peptides were synthesized by GenScript and dissolved in PBS to the final concentration of 1 mm and then used immediately or stored for not more than 2 weeks at −20 °C.
Primary Neuronal Cultures
Mouse primary cortical and hippocampal neuronal cultures were prepared from embryonic day 17 embryos as described previously (10, 38). Briefly, cortices and hippocampi were separated from embryonic brains, dissociated in Hanks' balanced salt solution (Invitrogen), and trypsinized (0.25%) for 20 min at 37 °C). Neurons were plated onto dishes coated with 5 μg/ml poly-l-lysine (Sigma) in minimum essential medium Eagle (Invitrogen) containing 10% FBS (Invitrogen) and antibiotics (100 μg/ml streptomycin, 100 units/ml penicillin). Four hours after plating, the medium was replaced with the Neurobasal medium (Invitrogen), supplemented with B-27 (Invitrogen), glutamine (0.5 mm) (Invitrogen), penicillin (100 units/ml), streptomycin (100 μg/ml), and glucose (0.25%).
Calcium Imaging
Mouse primary hippocampal neurons were plated onto poly-l-lysine-coated 35-mm glass-bottom dishes (MatTek, Ashland, MA) at ∼7.5 × 104 cells/dish density. On day 14, cultures were washed with Krebs-Ringer buffer (KRH) (124 mm NaCl, 4 mm KCl, 25 mm HEPES, 1.2 mm MgSO4, and 10 mm glucose), and loaded with 5 μm of the intracellular Ca2+ indicator Fluo-4-AM (Invitrogen), diluted in KRH buffer with 2 mm CaCl2, for 1 h at 37 °C. The cells were washed three times with KRH with (for AβO experiments) or without calcium (Ln-γ1 experiments). Data acquisition was performed in a LSM Meta 510 confocal microscope, with excitation at 488 nm (argon laser), and emission was collected with a 505–530-nm band-pass filter. The fluorescence was normalized as F1/F0 (F1, maximal fluorescence after the treatment; F0, basal fluorescence before the treatment). Imaging analysis was performed using ImageJ software (WCIF ImageJ, National Institutes of Health). Experiments were carried out with ≥3 different dishes, and 20–30 cells were monitored in each experiment.
CF10 Cell Culture and Ca2+ Imaging
CF10, a PrPC-null immortalized cell line, and its counterpart expressing 3F4-tagged mouse PrPC were obtained as described previously (46). CF10 cells were plated onto 35-mm glass-bottom dishes (MatTek) (104 cells), and then serum-starved for 48 h with medium change after 24 h. Cells were loaded with Fluo-4-AM (5 μm/30 min) and then washed three times with KRH buffer. Data acquisition was performed in a confocal Bio-Rad Radiance 2100/Nikon TE2000U microscope, with excitation at 488 nm (argon laser), and emission was collected with a 522–535-nm band-pass filter as described previously (10).
CF10 Cell Surface Protein Biotinylation and Western Blotting
Biotinylation of cell surface proteins was performed as described (46, 80). Briefly, cells were incubated with Ln-γ1 (120 μm) for 10 or 30 min and then transferred to ice, washed, and incubated on ice in PBS/CM (PBS supplemented with 1.0 mm MgCl2, 0.1 mm CaCl2). Cell surface proteins were biotinylated with sulfo-NHS-SS-biotin (Pierce) for 40 min on ice. To quench the biotinylation reaction, cells were washed and incubated for 30 min with cold 100 mm glycine in PBS/CM, followed by three washes with cold PBS/CM, and then proteins were extracted using 100 mm Tris-HCl, 150 mm NaCl, 10 mm EDTA, 0.5% Triton X-100, 0.5% deoxycholic acid, pH 7.4. Biotinylated proteins were separated from non-biotinylated proteins by pulldown of NeutrAvidin beads from equivalent amounts of total cellular protein (800 μg) from each sample. The biotinylated proteins were subjected to SDS-PAGE, followed by electroblotting onto PVDF membrane, and then revealed using a mouse anti-PrPC antibody as described previously (81). For quantification, the major glycosylated band of PrPC in non-saturated blots was analyzed using ImageQuant TL and normalized by the expression of PrPC in the lysates.
Neuronal cell Surface Protein Biotinylation and Western Blotting
Mouse primary cortical neurons were plated on polylysine-coated 60-mm Petri dishes at ∼3 × 106 cells/dish density. On day 8, cultures were treated with Ln-γ1 or Ln-SCR for a specific amount of time after which the cells where washed with ice-cold PBS and the cell surface was biotinylated using the cell surface protein isolation kit (catalogue number 21328, Thermo Scientific) as described in the supplier's manual. In short, cells were incubated with NHS-SS-biotin reagent for 1 h, washed, and lysed. Biotinylated protein was collected from the lysate by incubation with NeutrAvidin agarose, followed by elution with 50 mm dithiothreitol. Untreated lysate was used for total protein quantification. PrPC and mGluR5 in cell surface or total cellular fractions were quantified by Western blotting using 8H4 (1:2500, Abcam ab61409, Lot: GR82819-6) and anti-mGluR5 (1:1000, Millipore AB5675, Lot: 2279534) antibodies, respectively.
Peptide Binding and Protein Internalization Imaging
For AβO binding and PrPC internalization experiments, primary neuronal cultures were plated on glass coverslips at ∼5 × 104 cells/dish density. On day 15, cultures were washed with KRH buffer and treated with 250 nm AβO solution in KRH buffer for 15 min or with 40 μm Ln-γ1 or Ln-SCR solution for 10 or 30 min. After that, cover glasses were washed with ice-cold PBS, permeabilized for 5 min in PBS containing 0.5% Triton X-100, and fixed with 4% paraformaldehyde for 20 min. For immunofluorescence, cover glasses were incubated with 6E10 (for AβO binding, 1:350, Covance, catalogue number SIG-39300, lot number D13EF01399) or 8H4 (for PrPC internalization, 1:350) and anti-tubulin (1:300, Abcam) antibodies overnight at 4 °C, followed by Alexa Fluor 633 anti-mouse IgG and Alexa Fluor 488 anti-rabbit IgG (1:1000, Invitrogen) for 1 h at room temperature. After that, cover glasses were mounted on slides using Immu-mount (Thermo Fisher Scientific) and imaged with an LSM 510 Meta ConfoCor microscope. Integrated fluorescence intensity was normalized by total neurite area calculated from tubulin fluorescence, and then for each treatment normalized toward the wild-type or non-treated controls.
Surface Plasmon Resonance
SPR was measured using a Biacore X system (GE Healthcare Life Sciences) equipped with a nitrilotriacetic acid sensor chip as described previously (38). Briefly, the chip was nickel-charged and uniformly covered with His6-tagged PrP to the final SPR signal of ∼10,000 response units. Ln-γ1, AβO, and their mix were diluted in 25 mm HEPES, 150 mm NaCl, 10 mm imidazole, pH 7.0, and injected at 5 μl/min. On-kinetics were registered for 6 min, followed by off-kinetics registered for 2 min. The chip surface was regenerated between injections by a short injection of 10 mm HCl. SPR curves for binding to PrP were analyzed by maximum signal comparison.
LIVE/DEAD Cell Viability Assay
Mouse primary hippocampal neurons were plated onto poly-l-lysine-coated 4-well dishes and cultured for 10 days. On day 11, AβOs (1 μm) were added to cultures. Some cultures were pretreated with Ln-γ1 or Ln-SCR peptides (40 μm) for 10 or 30 min. Following 48-h treatment, cultures were analyzed with a LIVE/DEAD cell viability assay (Invitrogen) according to the manufacturer's manual. Live and dead cells were counted from fluorescence images taken with Zeiss LSM 510 Meta confocal microscope equipped with 10×/0.3 objective lens, and the cell death rate was calculated as the percentage of dead cells in the total amount of cells.
Prion Infection and Western Blotting
For prion infection the mice were anesthetized with isoflurane. The injection was made through the skull into the brain with a 26-gauge needle attached to a syringe containing the prion material (5% RML strain). The total volume injected per mouse was 20 μl. Following the injection, the mice were allowed to recover from the anesthesia in their home cage. Early signs of mouse prion disease were evaluated including ataxia (lack of coordination) and extension of the hind limbs when mice are hung by their tails. As the disease progressed, affected mice showed hypokinesia, waddling gait, difficulty righting from a supine position, weight loss, and deficient grooming. When held by their tails, some affected mice assume an unusual flexed posture, with all four limbs clasped together. For the onset of the disease, the following parameters were analyzed: 1) foot clasp reflex when picked up by the tail and 2) observation of kyphosis while the mouse was in the cage. These clinical observations were done on a weekly basis prior to onset and daily upon onset of disease. Experimental end point was reached once a mouse no longer could right itself within 30 s. The incubation period when injecting 20 μl of 5% RML strain intracranially is ∼120 days. Once the mice reached the experimental end point (described above), they were euthanized by inhalation of CO2 and their brains were harvested and frozen on dry ice.
For prion scrapie analyses, brain homogenate in PBS (250 μg of total protein) was digested with 50 μg/ml proteinase K for 1 h at 37 °C, and then samples were boiled in SDS loading buffer and separated with 14% SDS-PAGE. After electrotransfer to a PVDF membrane (Immobilon, Millipore) protein bands were probed with SHA31 antibody (1:30000, Spibio Inc., catalogue number A03263, lot number 0107).
Statistics
Western blots and immunocytochemistry data were quantified using the ImageJ software (National Institutes of Health, Bethesda, MD) and statistically analyzed using one-way analysis of variance with Tukey's post hoc or by Student's t test using Prism software (GraphPad, La Jolla, CA). For prion disease onset and progression, data were analyzed and compared by Mantel-Cox log-rank test.
Author Contributions
F. H. B. and V. G. O. conceived and designed the project, acquired data, analyzed and interpreted data, and wrote the manuscript; F. A. C., A. L. S. G., G. D. S. F., N. D., L. B., and K. O. P. C. N. acquired data; J. L. S., D. W., N. R. C., and V. F. P. analyzed and interpreted data; and V. R. M. and M. A. M. P. conceived and designed the project, analyzed and interpreted data, and wrote the manuscript.
Supplementary Material
This work was supported by PrioNet-Canada, Canadian Institutes of Health Research (Grants MOP 93651, MOP 136930, MOP 126000, and MOP 89919), The Alzheimer's Association, the Canadian Foundation for Innovation, and the Ontario Research Fund (to M. A. M. P. and V. F. P.). This work was also supported by the Fundacao de Amparo a Pesquisa do Estado de Sao Paulo – FAPESP (to V. R. M) The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1–S3 and supplemental Experimental Procedures.
- PrPC
- cellular form of PrP
- PrP
- prion protein
- PrPSc
- misfolded form of PrP
- GPI
- glycosylphosphatidylinositol
- Aβ
- β-amyloid
- AβO
- β-amyloid oligomer
- mGluR
- metabotropic glutamate receptor
- Ln
- laminin
- SCR
- scrambled
- STI1
- Stress-inducible phosphoprotein 1
- KRH
- Krebs-Ringer buffer.
References
- 1. Prusiner S. B. (1991) Molecular biology of prion diseases. Science 252, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 2. Lee K. S., Linden R., Prado M. A., Brentani R. R., and Martins V. R. (2003) Towards cellular receptors for prions. Rev. Med. Virol. 13, 399–408 [DOI] [PubMed] [Google Scholar]
- 3. Linden R., Martins V. R., Prado M. A., Cammarota M., Izquierdo I., and Brentani R. R. (2008) Physiology of the prion protein. Physiol. Rev. 88, 673–728 [DOI] [PubMed] [Google Scholar]
- 4. Martins V. R., Beraldo F. H., Hajj G. N., Lopes M. H., Lee K. S., Prado M. A., and Linden R. (2010) Prion protein: orchestrating neurotrophic activities. Curr. Issues Mol. Biol. 12, 63–86 [PubMed] [Google Scholar]
- 5. Beraldo F. H., Arantes C. P., Santos T. G., Queiroz N. G., Young K., Rylett R. J., Markus R. P., Prado M. A., and Martins V. R. (2010) Role of α7 nicotinic acetylcholine receptor in calcium signaling induced by prion protein interaction with stress-inducible protein 1. J. Biol. Chem. 285, 36542–36550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roffé M., Beraldo F. H., Bester R., Nunziante M., Bach C., Mancini G., Gilch S., Vorberg I., Castilho B. A., Martins V. R., and Hajj G. N. (2010) Prion protein interaction with stress-inducible protein 1 enhances neuronal protein synthesis via mTOR. Proc. Natl. Acad. Sci. U.S.A. 107, 13147–13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santos T. G., Beraldo F. H., Hajj G. N., Lopes M. H., Roffe M., Lupinacci F. C., Ostapchenko V. G., Prado V. F., Prado M. A., and Martins V. R. (2013) Laminin-γ1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2+ mobilization in dorsal root ganglia neurons. J. Neurochem. 124, 210–223 [DOI] [PubMed] [Google Scholar]
- 8. Lopes M. H., Hajj G. N., Muras A. G., Mancini G. L., Castro R. M., Ribeiro K. C., Brentani R. R., Linden R., and Martins V. R. (2005) Interaction of cellular prion and stress-inducible protein 1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J. Neurosci. 25, 11330–11339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santuccione A., Sytnyk V., Leshchyns'ka I., and Schachner M. (2005) Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Biol. 169, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beraldo F. H., Arantes C. P., Santos T. G., Machado C. F., Roffe M., Hajj G. N., Lee K. S., Magalhães A. C., Caetano F. A., Mancini G. L., Lopes M. H., Américo T. A., Magdesian M. H., Ferguson S. S., Linden R., et al. (2011) Metabotropic glutamate receptors transduce signals for neurite outgrowth after binding of the prion protein to laminin γ1 chain. FASEB J. 25, 265–279 [DOI] [PubMed] [Google Scholar]
- 11. Mouillet-Richard S., Ermonval M., Chebassier C., Laplanche J. L., Lehmann S., Launay J. M., and Kellermann O. (2000) Signal transduction through prion protein. Science 289, 1925–1928 [DOI] [PubMed] [Google Scholar]
- 12. Mouillet-Richard S., Pietri M., Schneider B., Vidal C., Mutel V., Launay J. M., and Kellermann O. (2005) Modulation of serotonergic receptor signaling and cross-talk by prion protein. J. Biol. Chem. 280, 4592–4601 [DOI] [PubMed] [Google Scholar]
- 13. Mouillet-Richard S., Schneider B., Pradines E., Pietri M., Ermonval M., Grassi J., Richards J. G., Mutel V., Launay J. M., and Kellermann O. (2007) Cellular prion protein signaling in serotonergic neuronal cells. Ann. N.Y. Acad. Sci. 1096, 106–119 [DOI] [PubMed] [Google Scholar]
- 14. Loubet D., Dakowski C., Pietri M., Pradines E., Bernard S., Callebert J., Ardila-Osorio H., Mouillet-Richard S., Launay J. M., Kellermann O., and Schneider B. (2012) Neuritogenesis: the prion protein controls β1 integrin signaling activity. FASEB J. 26, 678–690 [DOI] [PubMed] [Google Scholar]
- 15. Pietri M., Caprini A., Mouillet-Richard S., Pradines E., Ermonval M., Grassi J., Kellermann O., and Schneider B. (2006) Overstimulation of PrPC signaling pathways by prion peptide 106–126 causes oxidative injury of bioaminergic neuronal cells. J. Biol. Chem. 281, 28470–28479 [DOI] [PubMed] [Google Scholar]
- 16. Hirsch T. Z., Hernandez-Rapp J., Martin-Lannerée S., Launay J. M., and Mouillet-Richard S. (2014) PrPC signalling in neurons: from basics to clinical challenges. Biochimie 104, 2–11 [DOI] [PubMed] [Google Scholar]
- 17. Moreno J. A., Radford H., Peretti D., Steinert J. R., Verity N., Martin M. G., Halliday M., Morgan J., Dinsdale D., Ortori C. A., Barrett D. A., Tsaytler P., Bertolotti A., Willis A. E., Bushell M., and Mallucci G. R. (2012) Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature 485, 507–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Um J. W., Kaufman A. C., Kostylev M., Heiss J. K., Stagi M., Takahashi H., Kerrisk M. E., Vortmeyer A., Wisniewski T., Koleske A. J., Gunther E. C., Nygaard H. B., and Strittmatter S. M. (2013) Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron 79, 887–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas L. T., Salazar S. V., Kostylev M. A., Um J. W., Kaufman A. C., and Strittmatter S. M. (2016) Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer's disease. Brain 139, 526–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watt N. T., Taylor D. R., Kerrigan T. L., Griffiths H. H., Rushworth J. V., Whitehouse I. J., and Hooper N. M. (2012) Prion protein facilitates uptake of zinc into neuronal cells. Nat. Commun. 3, 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. You H., Tsutsui S., Hameed S., Kannanayakal T. J., Chen L., Xia P., Engbers J. D., Lipton S. A., Stys P. K., and Zamponi G. W. (2012) Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 109, 1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carneiro M. V., Americo T. A., Guimarães M. Z., and Linden R. (2016) The prion protein selectively binds to and modulates the content of purinergic receptor P2X4R. Biochem. Biophys. Res. Commun. 472, 293–298 [DOI] [PubMed] [Google Scholar]
- 23. Laurén J., Gimbel D. A., Nygaard H. B., Gilbert J. W., and Strittmatter S. M. (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gimbel D. A., Nygaard H. B., Coffey E. E., Gunther E. C., Laurén J., Gimbel Z. A., and Strittmatter S. M. (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 30, 6367–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haas L. T., Kostylev M. A., and Strittmatter S. M. (2014) Therapeutic molecules and endogenous ligands regulate the interaction between brain cellular prion protein (PrPC) and metabotropic glutamate receptor 5 (mGluR5). J. Biol. Chem. 289, 28460–28477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bate C., and Williams A. (2011) Amyloid-β-induced synapse damage is mediated via cross-linkage of cellular prion proteins. J. Biol. Chem. 286, 37955–37963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kudo W., Lee H. P., Zou W. Q., Wang X., Perry G., Zhu X., Smith M. A., Petersen R. B., and Lee H. G. (2012) Cellular prion protein is essential for oligomeric amyloid-β-induced neuronal cell death. Hum. Mol. Genet. 21, 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ostapchenko V. G., Beraldo F. H., Guimarães A. L., Mishra S., Guzman M., Fan J., Martins V. R., Prado V. F., and Prado M. A. (2013) Increased prion protein processing and expression of metabotropic glutamate receptor 1 in a mouse model of Alzheimer's disease. J. Neurochem. 127, 415–425 [DOI] [PubMed] [Google Scholar]
- 29. Hu N. W., Nicoll A. J., Zhang D., Mably A. J., O'Malley T., Purro S. A., Terry C., Collinge J., Walsh D. M., and Rowan M. J. (2014) mGlu5 receptors and cellular prion protein mediate amyloid-β-facilitated synaptic long-term depression in vivo. Nat. Commun. 5, 3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larson M., Sherman M. A., Amar F., Nuvolone M., Schneider J. A., Bennett D. A., Aguzzi A., and Lesné S. E. (2012) The complex PrPC-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer's disease. J. Neurosci. 32, 16857–16871a [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Chung E., Ji Y., Sun Y., Kascsak R. J., Kascsak R. B., Mehta P. D., Strittmatter S. M., and Wisniewski T. (2010) Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer's disease model mouse. BMC Neurosci. 11, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barry A. E., Klyubin I., Mc Donald J. M., Mably A. J., Farrell M. A., Scott M., Walsh D. M., and Rowan M. J. (2011) Alzheimer's disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J. Neurosci. 31, 7259–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freir D. B., Nicoll A. J., Klyubin I., Panico S., Mc Donald J. M., Risse E., Asante E. A., Farrow M. A., Sessions R. B., Saibil H. R., Clarke A. R., Rowan M. J., Walsh D. M., and Collinge J. (2011) Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nat. Commun. 2, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solforosi L., Criado J. R., McGavern D. B., Wirz S., Sánchez-Alavez M., Sugama S., DeGiorgio L. A., Volpe B. T., Wiseman E., Abalos G., Masliah E., Gilden D., Oldstone M. B., Conti B., and Williamson R. A. (2004) Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303, 1514–1516 [DOI] [PubMed] [Google Scholar]
- 35. Sonati T., Reimann R. R., Falsig J., Baral P. K., O'Connor T., Hornemann S., Yaganoglu S., Li B., Herrmann U. S., Wieland B., Swayampakula M., Rahman M. H., Das D., Kav N., Riek R., et al. (2013) The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature 501, 102–106 [DOI] [PubMed] [Google Scholar]
- 36. Guillot-Sestier M. V., Sunyach C., Ferreira S. T., Marzolo M. P., Bauer C., Thevenet A., and Checler F. (2012) α-Secretase-derived fragment of cellular prion, N1, protects against monomeric and oligomeric amyloid β (Aβ)-associated cell death. J. Biol. Chem. 287, 5021–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Béland M., Bédard M., Tremblay G., Lavigne P., and Roucou X. (2014) Aβ induces its own prion protein N-terminal fragment (PrPN1)-mediated neutralization in amorphous aggregates. Neurobiol. Aging 35, 1537–1548 [DOI] [PubMed] [Google Scholar]
- 38. Ostapchenko V. G., Beraldo F. H., Mohammad A. H., Xie Y. F., Hirata P. H., Magalhaes A. C., Lamour G., Li H., Maciejewski A., Belrose J. C., Teixeira B. L., Fahnestock M., Ferreira S. T., Cashman N. R., Hajj G. N., et al. (2013) The prion protein ligand, stress-inducible phosphoprotein 1, regulates amyloid-β oligomer toxicity. J. Neurosci. 33, 16552–16564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Renner M., Lacor P. N., Velasco P. T., Xu J., Contractor A., Klein W. L., and Triller A. (2010) Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 66, 739–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rammes G., Hasenjäger A., Sroka-Saidi K., Deussing J. M., and Parsons C. G. (2011) Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology 60, 982–990 [DOI] [PubMed] [Google Scholar]
- 41. Petratos S., Li Q. X., George A. J., Hou X., Kerr M. L., Unabia S. E., Hatzinisiriou I., Maksel D., Aguilar M. I., and Small D. H. (2008) The β-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain 131, 90–108 [DOI] [PubMed] [Google Scholar]
- 42. Calkins M. J., and Reddy P. H. (2011) Amyloid β impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim. Biophys, Acta 1812, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., and Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Resenberger U. K., Harmeier A., Woerner A. C., Goodman J. L., Müller V., Krishnan R., Vabulas R. M., Kretzschmar H. A., Lindquist S., Hartl F. U., Multhaup G., Winklhofer K. F., and Tatzelt J. (2011) The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 30, 2057–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Um J. W., Nygaard H. B., Heiss J. K., Kostylev M. A., Stagi M., Vortmeyer A., Wisniewski T., Gunther E. C., and Strittmatter S. M. (2012) Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 15, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caetano F. A., Lopes M. H., Hajj G. N., Machado C. F., Pinto Arantes C., Magalhães A. C., Vieira Mde P., Américo T. A., Massensini A. R., Priola S. A., Vorberg I., Gomez M. V., Linden R., Prado V. F., Martins V. R., and Prado M. A. (2008) Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1. J. Neurosci. 28, 6691–6702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang Y. S., Zhao X., Lovaas J., Eisenberg E., and Greene L. E. (2009) Clathrin-independent internalization of normal cellular prion protein in neuroblastoma cells is associated with the Arf6 pathway. J. Cell Sci. 122, 4062–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Magalhães A. C., Silva J. A., Lee K. S., Martins V. R., Prado V. F., Ferguson S. S., Gomez M. V., Brentani R. R., and Prado M. A. (2002) Endocytic intermediates involved with the intracellular trafficking of a fluorescent cellular prion protein. J. Biol. Chem. 277, 33311–33318 [DOI] [PubMed] [Google Scholar]
- 49. Lee K. S., Magalhães A. C., Zanata S. M., Brentani R. R., Martins V. R., and Prado M. A. (2001) Internalization of mammalian fluorescent cellular prion protein and N-terminal deletion mutants in living cells. J. Neurochem. 79, 79–87 [DOI] [PubMed] [Google Scholar]
- 50. Yim Y. I., Park B. C., Yadavalli R., Zhao X., Eisenberg E., and Greene L. E. (2015) The multivesicular body is the major internal site of prion conversion. J. Cell Sci. 128, 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martins V. R., and Prado M. A. (2016) Prion protein in exosomes: partnering Aβ peptides and driving fibrilization. J. Neurochem. 137, 9–11 [DOI] [PubMed] [Google Scholar]
- 52. Sunyach C., Jen A., Deng J., Fitzgerald K. T., Frobert Y., Grassi J., McCaffrey M. W., and Morris R. (2003) The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 22, 3591–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaneko K., Vey M., Scott M., Pilkuhn S., Cohen F. E., and Prusiner S. B. (1997) COOH-terminal sequence of the cellular prion protein directs subcellular trafficking and controls conversion into the scrapie isoform. Proc. Natl. Acad. Sci. U.S.A. 94, 2333–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simons K., and Ikonen E. (1997) Functional rafts in cell membranes. Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 55. Shyng S. L., Heuser J. E., and Harris D. A. (1994) A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J. Cell Biol. 125, 1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shyng S. L., Moulder K. L., Lesko A., and Harris D. A. (1995) The N-terminal domain of a glycolipid-anchored prion protein is essential for its endocytosis via clathrin-coated pits. J. Biol. Chem. 270, 14793–14800 [DOI] [PubMed] [Google Scholar]
- 57. Taylor D. R., Watt N. T., Perera W. S., and Hooper N. M. (2005) Assigning functions to distinct regions of the N-terminus of the prion protein that are involved in its copper-stimulated, clathrin-dependent endocytosis. J. Cell Sci. 118, 5141–5153 [DOI] [PubMed] [Google Scholar]
- 58. Sarnataro D., Caputo A., Casanova P., Puri C., Paladino S., Tivodar S. S., Campana V., Tacchetti C., and Zurzolo C. (2009) Lipid rafts and clathrin cooperate in the internalization of PrP in epithelial FRT cells. PLoS ONE 4, e5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morris R. J., Parkyn C. J., and Jen A. (2006) Traffic of prion protein between different compartments on the neuronal surface, and the propagation of prion disease. FEBS Lett. 580, 5565–5571 [DOI] [PubMed] [Google Scholar]
- 60. Taylor D. R., and Hooper N. M. (2007) The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem. J. 402, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fourgeaud L., Bessis A. S., Rossignol F., Pin J. P., Olivo-Marin J. C., and Hémar A. (2003) The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J. Biol. Chem. 278, 12222–12230 [DOI] [PubMed] [Google Scholar]
- 62. Ribeiro F. M., Ferreira L. T., Paquet M., Cregan T., Ding Q., Gros R., and Ferguson S. S. (2009) Phosphorylation-independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein-coupled receptor kinase 2 in neurons. J. Biol. Chem. 284, 23444–23453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Francesconi A., Kumari R., and Zukin R. S. (2009) Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J. Neurosci. 29, 3590–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hunt D. L., Puente N., Grandes P., and Castillo P. E. (2013) Bidirectional NMDA receptor plasticity controls CA3 output and heterosynaptic metaplasticity. Nat. Neurosci. 16, 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. D'Antoni S., Spatuzza M., Bonaccorso C. M., Musumeci S. A., Ciranna L., Nicoletti F., Huber K. M., and Catania M. V. (2014) Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism. Neurosci. Biobehav. Rev. 46, 228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matosin N., Fernandez-Enright F., Lum J. S., Andrews J. L., Engel M., Huang X. F., and Newell K. A. (2015) Metabotropic glutamate receptor 5, and its trafficking molecules Norbin and Tamalin, are increased in the CA1 hippocampal region of subjects with schizophrenia. Schizophr. Res. 166, 212–218 [DOI] [PubMed] [Google Scholar]
- 67. Roche K. W., Tu J. C., Petralia R. S., Xiao B., Wenthold R. J., and Worley P. F. (1999) Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J. Biol. Chem. 274, 25953–25957 [DOI] [PubMed] [Google Scholar]
- 68. Caetano F. A., Beraldo F. H., Hajj G. N., Guimaraes A. L., Jürgensen S., Wasilewska-Sampaio A. P., Hirata P. H., Souza I., Machado C. F., Wong D. Y., De Felice F. G., Ferreira S. T., Prado V. F., Rylett R. J., Martins V. R., and Prado M. A. (2011) Amyloid-β oligomers increase the localization of prion protein at the cell surface. J. Neurochem. 117, 538–553 [DOI] [PubMed] [Google Scholar]
- 69. Jeong J. K., and Park S. Y. (2015) Neuroprotective effect of cellular prion protein (PrPC) is related with activation of α7 nicotinic acetylcholine receptor (α7nAchR)-mediated autophagy flux. Oncotarget. 6, 24660–24674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hamilton A., Esseltine J. L., DeVries R. A., Cregan S. P., and Ferguson S. S. (2014) Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer's disease. Mol. Brain 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ma T., Trinh M. A., Wexler A. J., Bourbon C., Gatti E., Pierre P., Cavener D. R., and Klann E. (2013) Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat. Neurosci. 16, 1299–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moreno J. A., Halliday M., Molloy C., Radford H., Verity N., Axten J. M., Ortori C. A., Willis A. E., Fischer P. M., Barrett D. A., and Mallucci G. R. (2013) Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5, 206ra138. [DOI] [PubMed] [Google Scholar]
- 73. Lourenco M. V., Ferreira S. T., and De Felice F. G. (2015) Neuronal stress signaling and eIF2α phosphorylation as molecular links between Alzheimer's disease and diabetes. Prog. Neurobiol. 129, 37–57 [DOI] [PubMed] [Google Scholar]
- 74. Guo W., Molinaro G., Collins K. A., Hays S. A., Paylor R., Worley P. F., Szumlinski K. K., and Huber K. M. (2016) Selective disruption of metabotropic glutamate receptor 5-homer interactions mimics phenotypes of fragile X syndrome in mice. J. Neurosci. 36, 2131–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fatemi S. H., and Folsom T. D. (2015) GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr. Res. 167, 42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aguilar-Valles A., Matta-Camacho E., Khoutorsky A., Gkogkas C., Nader K., Lacaille J. C., and Sonenberg N. (2015) Inhibition of group I metabotropic glutamate receptors reverses autistic-like phenotypes caused by deficiency of the translation repressor eIF4E binding protein 2. J. Neurosci. 35, 11125–11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. de Esch C. E., van den Berg W. E., Buijsen R. A., Jaafar I. A., Nieuwenhuizen-Bakker I. M., Gasparini F., Kushner S. A., and Willemsen R. (2015) Fragile X mice have robust mGluR5-dependent alterations of social behaviour in the Automated Tube Test. Neurobiol. Dis. 75, 31–39 [DOI] [PubMed] [Google Scholar]
- 78. Tsutsui S., Hahn J. N., Johnson T. A., Ali Z., and Jirik F. R. (2008) Absence of the cellular prion protein exacerbates and prolongs neuroinflammation in experimental autoimmune encephalomyelitis. The Am. J. Pathol. 173, 1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu Y. M., Jia Z., Janus C., Henderson J. T., Gerlai R., Wojtowicz J. M., and Roder J. C. (1997) Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 17, 5196–5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ribeiro F. M., Black S. A., Cregan S. P., Prado V. F., Prado M. A., Rylett R. J., and Ferguson S. S. (2005) Constitutive high-affinity choline transporter endocytosis is determined by a carboxyl-terminal tail dileucine motif. J. Neurochem. 94, 86–96 [DOI] [PubMed] [Google Scholar]
- 81. Zanata S. M., Lopes M. H., Mercadante A. F., Hajj G. N., Chiarini L. B., Nomizo R., Freitas A. R., Cabral A. L., Lee K. S., Juliano M. A., de Oliveira E., Jachieri S. G., Burlingame A., Huang L., Linden R., et al. (2002) Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J. 21, 3307–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.