Abstract
Background
The etiology of chronic urticaria (CU) remains unknown in most patients. Possible causes in some cases include food, but the role of allergy to food antigens in patients with CU remains controversial.
Objective
The aim of this study was to evaluate the association between food allergy and CU.
Methods
Korean patients with CU were assessed for a previous history of food allergy that caused symptoms of CU. Blood samples were taken from 350 patients to measure food allergen-specific IgE. Based on history and laboratory results, open oral food challenge (OFC) tests were performed.
Results
Of 350 participants, 46 (13.1%) claimed to have experienced previous food hypersensitivity. Pork (n=16) was the main food mentioned, followed by beef (n=7), shrimp (n=6), and mackerel (n=6). We found that 73 participants (20.9%) had elevated levels of food-specific IgE, with pork (n=30), wheat (n=25), and beef (n=23) being the most common. However, when the open OFC tests were conducted in 102 participants with self-reported food hypersensitivity or raised levels of food-specific IgE, only four participants showed a positive reaction to pork (n=3) or crab (n=1).
Conclusion
Although some participants claimed to have a history of CU related to food intake, when an open OFC test was conducted, few of them had positive results. We therefore conclude that food allergy is an uncommon cause of chronic CU.
Keywords: Food allergy, Prevalence, Urticaria
INTRODUCTION
Chronic urticaria (CU) is defined as spontaneous urticaria that lasts over six weeks without improvement. It is a heterogeneous disease in which allergy may be involved1. A food allergy is defined as an adverse effect arising from a specific immune response, which occurs reproducibly on exposure to a given food2,3,4. The skin diseases that are related to food allergy include urticaria, oral allergy syndrome, and atopic dermatitis4.
In CU, food allergy is known to be a rare cause. It is known that in less than 2% of cases is food itself, or food additives, the reason for CU5. However, many people think that foods are the reason, or an aggregating factor, in urticaria and undergo inappropriate food restriction. Some who do this, suffer nutritional imbalance and decline in quality of life. Although physicians in clinical practice educate their patients with CU that there is a low probability that the suspected food is the cause of urticaria, many patients constantly try to associate the presence of their symptoms with specific foods.
There have been several studies on the correlation between CU and the prevalence of food allergy, but few large-scale studies have been reported6,7,8. Moreover, many of these studies have been based only on patients' self-perception of food reactions. Specifically, there have been a few studies about the prevalence of food allergy in CU patients in Korea, involving the use of medical history, questionnaires, food specific IgE test, and skin prick test9,10. However, there was only one study about food allergy using oral food challenge (OFC) test in small number of patients with CU11. For these reasons, the relation between food and CU needs to be determined. Therefore, we carried out an investigation about the prevalence of food allergy and correlation of food with CU in Korean participants using the open OFC test.
MATERIALS AND METHODS
Patients
This study included 350 Korean patients with CU who attended the Department of Dermatology, Hallym University Kangnam Sacred Heart Hospital between October 2011 and August 2014. There were 143 men (40.9%) and 207 women (59.1%). The average age was 39.66±17.20 years (range, 2~79 years). Prevalence period of disease was 23.89±46.59 months (range, 2~240 months). All patients had been medicated with one or several antihistamines (fexofenadine, levocetirizine, or other) before the food challenge. Of the 350 patients, 49 (14.0%) were on immunosuppressant therapy (cyclosporine and/or methylprednisolone). There were no significant differences between the patient group with and without past history of food allergy (n=9, 19.6% vs. n=40, 13.2%, p=0.243). The patient demographics are described in Table 1. CU was defined as urticaria that has been continuously or intermittently present for at least six weeks. Each patient studied, had active urticaria at the time of challenge. For those with persistent urticaria, despite the use of antihistamines and/or immunosuppressants at the time of enrollment, no changes were made in medications. For patients whose hives were completely suppressed by their current medication, their antihistamines and/or immunosuppressants doses were reduced until hives returned at a tolerable level. This was defined as the minimum effective dose, which was individualized for each patient12. Exclusion criteria included pure physical urticaria, infectious disease, vasculitis, thyroid disease, systemic lupus erythematosus and malignancy. The protocol was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital (IRB no. 2015-05-61). Informed consent was obtained from each participant and from their parents, if they were 18 years old or less.
Table 1. Patient demographics.
| Variable | Chronic urticaria | p-value | |
|---|---|---|---|
| The patients with food allergy history (n=46) | The patients without food allergy history (n=304) | ||
| Sex | 0.302 | ||
| Male | 22 (47.8) | 121 (39.8) | |
| Female | 24 (52.2) | 183 (60.2) | |
| Age (yr) | 36.37±16.69 | 40.16±17.26 | 0.164 |
| Disease duration (mo) | 28.96±58.79 | 23.15±44.61 | 0.492 |
| Immunosuppressive medication history | 0.243* | ||
| Nonusers | 37 (80.4) | 264 (86.8) | |
| Users (mg/d) | 9 (19.6) | 40 (13.2) | |
| CsA (100)+MPD (4) | 1 (2.2) | 1 (0.3) | |
| CsA (25)+MPD (4) | 1 (2.2) | 2 (0.7) | |
| CsA (100) | 2 (4.3) | 8 (2.6) | |
| CsA (25) | 3 (6.5) | 19 (6.3) | |
| MPD (4) | 2 (4.3) | 10 (3.3) | |
Values are presented as number (%) or mean±standard deviation. CsA: cyclosporine, MPD: methylprednisolone. *There were no significant differences between the patient group on immunosuppressant therapy with and without past history of food allergy.
Patient history of food allergy
We gathered a detailed medical history from each patient that contained the duration of urticaria, past history of food allergy, suspected food antigens, duration of symptoms and latency period prior to urticarial reaction (such as pruritus, wheals, flares, or swelling). In terms of food allergy, some people were regarded as negative: those who showed inconsistent reaction to certain foods, whose foods contained many artificial additives, those for whom food reactions were complicated by other factors (e.g., exacerbation by physical exercise after meals), and those who reported no food allergy history. An experienced dermatologist was responsible for interpreting the history of food allergy.
Measurement of serum food-specific immunoglobulin E levels
Blood samples were taken from the patients to measure their allergen-specific IgE using a Korean-food-panel multiple allergosorbent test-chemiluminescent assay (MAST-CLA) allergy system (MAST Immunosystems, Mountain View, CA, USA). This assay can simultaneously measure 35 different specific IgE antibodies. Associated allergens consisted of those from food as well as inhalant allergens, including mold and pollen, all of which frequently provoke a positive reaction from Korean people. Based on hospital experience, results from standards, and other data that showed that Class 1 results might be observed in healthy subjects who lacked clinical evidence of allergic disease13,14,15, a MAST class of '2' or above (Allergen-specific IgE content ≥0.70 IU/ml) was considered positive.
Open oral food challenge test
We selected foods for open OFC testing based on food allergy history and serum food-specific IgE levels. Subjects who were negative for both history and serum food-specific IgE levels did not undergo open OFC testing. Foods that were suspected of food allergy were forbidden for 14 days before the open OFC test16. On the test day, patients ate their regular amount of the suspected foods, which means the amount they usually ate at one meal, in the morning, from a fasting state. If the patient did not show any reaction, he or she was tested again on the following morning. A test was considered positive if urticaria (wheal-and-flare) were noticed with aggravation of pruritus, by an experienced dermatologist during the 6-hour period after the test17, and systemic symptoms such as in the digestive system or respiratory system were studied together. The patients were instructed to continue to check skin at 9 and 18 hours after ingestion at home, and to call the physician the following day if additional skin symptoms arose. If open OFC tests were conducted for other foods on the same patient, this was done within an interval of 1~2 days.
Statistical analysis
The results were expressed as means±standard deviations. The chi-square test for nominal variables and the Student's t-test for continuous variables were used to determine the significance of differences. Significance levels for all analyses were set at p<0.05. All statistical analyses were conducted using PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA).
RESULTS
Patient history of food allergy
Of the 350 patients, 46 (13.2%) had a self-reported history of food allergy. There was a tendency that the patient group with food allergy past history had longer disease periods, but this was not statistically significant (28.96±58.79 months vs. 23.15±44.61 months). Neither sex nor age appeared statistically significant. The most common suspicious foods reported were pork (n=16, 4.6%), beef (n=7, 2.0%), shrimp and mackerel (n=6, 1.7% for each), crab (n=4, 1.1%), dog meat soup (n=3, 0.9%), egg, chicken, wheat, and peanuts (n=2, 0.6% for each), salmon, buckwheat, beer, coffee, ramen, and fermented soybean paste (Cheonggukjang) (n=1, 0.3% for each) (Table 2).
Table 2. Frequency of positive reactions to food allergens in self-reported history.
| Variable | No. of patients (%) |
|---|---|
| Number of suspicious foods | |
| 2 | 10 (2.9) |
| 1 | 36 (10.3) |
| 0 | 304 (86.9) |
| Total | 350 (100.0) |
| Types of suspicious foods | |
| Pork | 16 (4.6) |
| Beef | 7 (2.0) |
| Shrimp | 6 (1.7) |
| Mackerel | 6 (1.7) |
| Crab | 4 (1.1) |
| Dog meat soup | 3 (0.9) |
| Egg | 2 (0.6) |
| Chicken | 2 (0.6) |
| Wheat | 2 (0.6) |
| Peanut | 2 (0.6) |
| Salmon | 1 (0.3) |
| Buckwheat | 1 (0.3) |
| Beer | 1 (0.3) |
| Coffee | 1 (0.3) |
| Ramen | 1 (0.3) |
| Fermented soybean paste (Cheonggukjang) | 1 (0.3) |
Serum food-specific immunoglobulin E levels
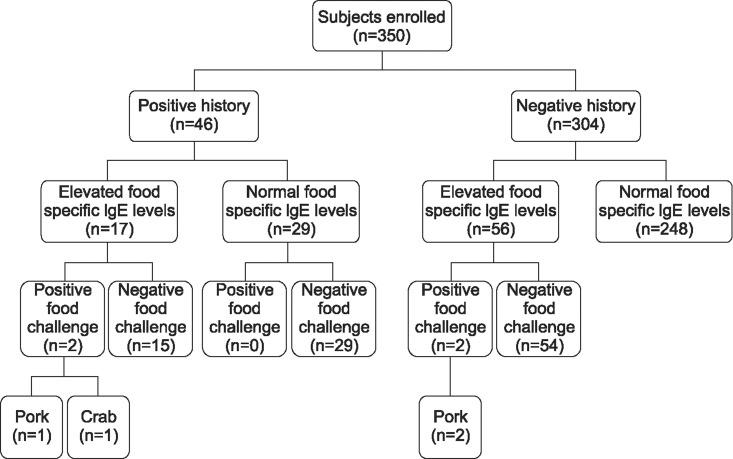
Of the 350 patients, 73 (20.9%) showed elevated serum food-specific IgE levels. Of the 73 patients, 41 were male and 32 were female. The mean age was 33.52±18.16 years (range, 2~70 years), and the mean duration of disease was 26.89±62.92 months (range, 3~240 months). Pork-specific IgE was highest (n=30, 8.6%), followed by wheat (n=25, 7.1%), beef (n=23, 6.6%), garlic (n=20, 5.7%), crab and milk (n=11, 3.1% for each), peach (n=5, 1.4%), egg, cheese, crab, and barley (n=3, 0.9%), shrimp (n=2, 0.6%), salmon, lemon/lime/orange, and peanuts (n=1, 0.3% for each) (Table 3). Seventeen (37.0%) of the 46 patients with a history of food allergy showed elevated serum food-specific IgE levels, while 56 (18.4%) of the 304 patients without a history showed elevated serum food-specific IgE levels (Fig. 1).
Table 3. Frequency of positive reactions to food allergens on multiple allergosorbent test-chemiluminescent assay.
| Variable | No. of patients (%) |
|---|---|
| Number of positive food allergens | |
| 5 | 4 (1.1) |
| 4 | 4 (1.1) |
| 3 | 12 (3.4) |
| 2 | 17 (4.9) |
| 1 | 36 (10.3) |
| 0 | 277 (79.1) |
| Total | 350 (100.0) |
| Types of positive food allergens | |
| Pork | 30 (8.6) |
| Wheat | 25 (7.1) |
| Beef | 23 (6.6) |
| Garlic | 20 (5.7) |
| Crab | 11 (3.1) |
| Milk | 11 (3.1) |
| Peach | 5 (1.4) |
| Egg | 3 (0.9) |
| Cheese | 3 (0.9) |
| Crab | 3 (0.9) |
| Barley | 3 (0.9) |
| Shrimp | 2 (0.6) |
| Salmon | 1 (0.3) |
| Lemon/lime/orange | 1 (0.3) |
| Peanut | 1 (0.3) |
Fig. 1. Results of the various tests run on the patient group.
Open oral food challenge test
The open OFC test was performed on 102 patients based on their self-reported history of food hypersensitivity (n=46), and/or on their increased food-specific IgE (n=56). Among 102 patients, four showed positive response. The offending food was pork (n=3), followed by crab (n=1). Two (4.3%) of the 46 patients with a history of food allergy showed positive response, while two (0.7%) of the 304 patients without a history showed positive response. Two of four patients with positive open OFC test results showed a history of food hypersensitivity and elevated serum food-specific IgE levels; two patients showed only elevated serum food-specific IgE levels without positive history (Fig. 1). After four patients who showed a positive reaction on open OFC did not consume the offending food, their skin symptoms, such as wheals and pruritus, were improved and the stable conditions persisted for four weeks or more without antihistamines.
Thus, the overall prevalence of true food allergy in the study patients was 1.1% (4/350). Detailed information for each patient is described in Table 4.
Table 4. Patients with chronic urticaria and positive reactions to oral food challenge tests.
| No. | Age (yr) | Sex | Duration (mo) | History of hypersensitivity | Food-specific IgE (Class) | Oral food challenge | Challenge results | Other symptoms |
|---|---|---|---|---|---|---|---|---|
| 1 | 15 | Male | 12 | None | Pork (2) | Pork | Wheals, pruritus (20 minutes later) | None |
| 2 | 44 | Male | 2 | Pork | Pork (2) | Pork | Wheals, pruritus (immediate) | None |
| 3 | 26 | Female | 12 | None | Pork (2) | Pork | Wheals, pruritus (2 hours later) | None |
| 4 | 28 | Male | 36 | Crab | Crab (3), rice (3), garlic (6), onion (2) | Crab, rice, garlic, onion | Crab-wheals, pruritus (immediate) | None |
DISCUSSION
The prevalence of patients' self-reported history of food allergy differs by study. Bock18 found 28% of the children reported adverse reactions to foods. In the study by Young et al.19, 20% of 15,000 households perceived adverse reactions to food. The new US guidelines summarized a self-report rate of 12% to 13%2. Targeting only patients with urticaria, 30%~40% of patients with CU thought their symptoms were related to food20. In research in Korea, patients with CU responded that 50.6% of them experienced that CU got worse in relation to food10. In our previous research, 35% of patients with urticaria had a medical history of food allergy11. Putting the results of previous research together, patients with urticaria showed a higher self-reported history of food allergy than did the normal population. However, in our study, 13.1% of patients with CU reported a history of food allergy, a rate similar to that of the normal population in other research2,21.
Since people tend to overestimate food as a reason for allergy, to diagnose food allergy, skin prick tests and allergen-specific IgE tests are necessary. The skin prick test is being widely used for determining specific antigens and is considered the gold standard. However, this test has several limitations, including a high percentage of false positive reactions, interference by medications such as antihistamines, and invasiveness22,23. Hence, various in vitro tests measuring serum allergen-specific IgE antibody levels have been introduced. The radioallergosorbent test (RAST), a typical in vitro test using patient sera, has been extensively used for examination of allergens. However, this test has an important handicap in that the examiner is at risk for radiation exposure. Since the evolution and development of fluorescent enzyme immunoassay, the ImmunoCAP system has been widely accepted as a reference method of allergen-specific IgE measurement because of its reliability, reproducibility, and good accordance with skin test results. However, each individual ImmunoCAP test can only detect IgE against a single allergen, making it quite expensive to use in a clinical setting24. Accordingly, several multiple allergosorbent test-chemiluminescent assay (MAST) were developed. The MAST-CLA has the advantage that it can test for 35 common allergens simultaneously at a relatively low cost, and thus it is suitable as screening test. Employing a very sensitive chemoluminescence technique, MAST-CLA is safer than other methods that use a radioactive isotope. In addition, MAST-CLA is highly sensitive and specific, and has evidenced good agreement rates of results with the skin prick test or immunoCAP 13,25,26,27. Thus, we used MAST-CLA as a screening tool for detection of food allergens.
For definite diagnosis, food elimination and food challenge tests are also needed. Food challenge ultimately confirms specific foods as the cause of clinical disease3. The double blind placebo-controlled food challenge (DBPCFC) is the gold standard method by which food allergy is determined28. However, the DBPCFC is time-consuming and complicated, requiring 6~8 hours of physician-observed time for a full challenge. For these reasons, the DBPCFC is unsuitable for use with outpatients. Open OFC tests are much easier to perform than DBPCFC, and are widely used for office diagnosis. Previous studies demonstrated that open OFC is a useful method for identifying or confirming food allergy, and that it is nearly as efficient as DBPCFC29,30. Therefore, OFC was chosen for this study. It is difficult to perform food challenges and interpret the results of food challenge tests for patients with CU because most patients with CU take antihistamines or immunosuppressants consistently to control erythema and wheal with pruritus. Moreover, CU often tends to wax and wane naturally. Many prior studies have been performed after withholding antihistamines31. If antihistamines are withheld before a challenge, then coincidental increase in skin symptoms may occur, although unrelated to the challenge. Therefore, it may be advantageous to continue use of antihistamines during challenge32. Another concern is the continuation of antihistamines through other studies, which may have led to false-negative results. To circumvent both of these issues in this study, antihistamines and/or immunosuppresants were reduced to their minimum effective dose12.
Although the prevalence rate of food allergy as demonstrated by OFC is not accurately known, in young children (<4 years) it is reported to be 6%~8%, with the rate going down with increasing age. The food allergy frequency of adults is estimated to be about 1.4%~2.0%18,19,33,34. The proportion of food allergy as demonstrated by OFC among urticaria patients differs from researcher to researcher, estimates range from 2.2% to 4.9%. Because the ratio in patients with CU is lower than with acute urticaria patients, it is known that only 1.4% of them are involved 35,36. In our study, the prevalence of food allergy in patients with CU was 1.1%. This result was not much different from other research on patients with CU. Putting this result, a significantly higher food allergy prevalence rate was not observed in Korean patients with CU. As a result, in our study, a relation between CU and food allergy was not found.
In our study, for patients who had a history of food allergy, the probability of elevated serum food-specific IgE levels was high and statistically significant. Such patients also had a higher probability of being positive for OFC tests, compared to those without such histories, though this was not statistically significant. Although 102 patients out of 350, had a history of food allergy or elevated food-specific IgE levels, only 4 (1.1%) received a definite diagnosis of food allergy by OFC. Two of these four patients showed corresponding results from their food allergy history, MAST-CLA, and OFC; the other two patients showed positive reaction to MAST-CLA and OFC, without histories of food allergy. Although many patients with CU do not know exactly which food was the cause, they think their symptoms are related to what they ate. As pointed out in recent large reviews, guidelines, and meta-analyses, self-reported adverse reaction rates exceed rates based on OFC tests5. Because of the difficulty of formal examination, suspected foods are usually restricted without food challenge tests. Avoiding suspected foods is not always the best option, because inappropriate dietary restrictions may cause imbalanced nutrition. Therefore, open OFC testing is necessary to diagnose food allergy in patients with CU, who have suspicious food allergy histories.
In our study, the most common food considered allergenic by Korean patients with CU was pork (4.6%). The food most commonly showing a positive result with the serum food-specific IgE test was pork (8.6%). In our OFC test, pork was also the most common food allergen in Korean patients with CU and this result is worthy of notice. However, pork allergy has rarely been reported in the medical literature. It may manifest in a variety of ways: urticaria, oral allergy syndrome, and exacerbation of an atopic dermatitis37,38. Over the last 30 years, per capita meat consumption in Korea has increased dramatically due to economic growth and westernized eating habits. In late 2003, with the occurrence of mad cow disease in the US, the demand for pork sharply increased. Currently, pork accounts for half of all meat consumed in Korea39. This increased consumption of pork might be accompanied by more frequent reports of adverse reactions to pork.
Adverse reactions to food can occur through the many heterogeneous mechanisms. These include not only immunological IgE-mediated food allergy and non-immunological reactions like pseudoallergy40. Pseudoallergic reactions are defined as clinical reactions whose symptoms resemble allergic reactions without identifiable immunologic sensitization. CU may have also been associated with pseudoallergic reactions to food ingredients41. In our study, 29 patients showed negative serum food-specific IgE and OFC tests. However they complained to have adverse reaction to certain foods. Further study will be needed to rule out pseudoallergy for these patients by a pseudoallergen-free diet.
This study was conducted to determine the correlation of food allergy and CU in 350 Korean patients. Because only 4/350 (1.1%) patients had a positive response to OFC tests, our study suggests that CU in Koreans is not significantly related to food. Based on our study results, we advised our patients worrying about food allergy not to limit their diets indiscriminately, without diagnosis confirmed by OFC tests.
ACKNOWLEDGMENT
This study was supported by National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3A04049491) and Hallym University Research Fund 2014 (HURF-2014-53, HURF-2014-58).
References
- 1.Grattan CE, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645–657. doi: 10.1067/mjd.2002.122759. quiz 657-660. [DOI] [PubMed] [Google Scholar]
- 2.NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. quiz 820. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–728. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 5.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Hsu ML, Li LF. Prevalence of food avoidance and food allergy in Chinese patients with chronic urticaria. Br J Dermatol. 2012;166:747–752. doi: 10.1111/j.1365-2133.2011.10733.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaeser P, Revelly ML, Frei PC. Prevalence of IgE antibodies specific for food allergens in patients with chronic urticaria of unexplained etiology. Allergy. 1994;49:626–629. doi: 10.1111/j.1398-9995.1994.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 8.Moneret-Vautrin DA. Allergic and pseudo-allergic reactions to foods in chronic urticaria. Ann Dermatol Venereol. 2003;130 Spec No 1:1S35–1S42. [PubMed] [Google Scholar]
- 9.Lee YJ, Park CW, Lee CH. A study of patients with chronic urticaria using the chemiluminescent assay and prick test. Korean J Dermatol. 1995;33:260–267. [Google Scholar]
- 10.Kim MJ, Choi GS, Ye YM, Lee HY, Sung JM, Nahm DH, et al. Association of chronic urticaria and foods by using a food questionnaire. Korean J Asthma Allergy Clin Immunol. 2009;29:186–193. [Google Scholar]
- 11.Kang KS, Han HJ, Lee JO, Park CW, Lee CH. A study of food allergy in patients with urticaria. Korean J Dermatol. 2004;42:1106–1113. [Google Scholar]
- 12.Rajan JP, Simon RA, Bosso JV. Prevalence of sensitivity to food and drug additives in patients with chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2014;2:168–171. doi: 10.1016/j.jaip.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc. 2003;24:253–258. [PubMed] [Google Scholar]
- 14.Lee S, Lim HS, Park J, Kim HS. A new automated multiple allergen simultaneous test-chemiluminescent assay (MAST-CLA) using an AP720S analyzer. Clin Chim Acta. 2009;402:182–188. doi: 10.1016/j.cca.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Park HS, Kim JW, Chung DH, Kim YJ. Relationship between skin prick test, radioallergosorbent test, and chemiluminescent assay in allergic patients. Allergy. 1991;11:347–354. [Google Scholar]
- 16.Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, et al. Work group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123:S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Genton C, Frei PC, Pécoud A. Value of oral provocation tests to aspirin and food additives in the routine investigation of asthma and chronic urticaria. J Allergy Clin Immunol. 1985;76:40–45. doi: 10.1016/0091-6749(85)90802-4. [DOI] [PubMed] [Google Scholar]
- 18.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987;79:683–688. [PubMed] [Google Scholar]
- 19.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994;343:1127–1130. doi: 10.1016/s0140-6736(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 20.Guida B, De Martino CD, De Martino SD, Tritto G, Patella V, Trio R, et al. Histamine plasma levels and elimination diet in chronic idiopathic urticaria. Eur J Clin Nutr. 2000;54:155–158. doi: 10.1038/sj.ejcn.1600911. [DOI] [PubMed] [Google Scholar]
- 21.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 22.Kniker WT. Is the choice of allergy skin testing versus in vitro determination of specific IgE no longer a scientific issue? Ann Allergy. 1989;62:373–374. [PubMed] [Google Scholar]
- 23.Pipkorn U. Pharmacological influence of antiallergic medication on in vivo allergen testing. Allergy. 1988;43:81–86. doi: 10.1111/j.1398-9995.1988.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 24.Ollert M, Weissenbacher S, Rakoski J, Ring J. Allergen-specific IgE measured by a continuous random-access immunoanalyzer: interassay comparison and agreement with skin testing. Clin Chem. 2005;51:1241–1249. doi: 10.1373/clinchem.2004.046565. [DOI] [PubMed] [Google Scholar]
- 25.Finnerty JP, Summerell S, Holgate ST. Relationship between skin-prick tests, the multiple allergosorbent test and symptoms of allergic disease. Clin Exp Allergy. 1989;19:51–56. doi: 10.1111/j.1365-2222.1989.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 26.Agata H, Yomo A, Hanashiro Y, Muraki T, Kondo N, Orii T. Comparison of the MAST chemiluminescent assay system with RAST and skin tests in allergic children. Ann Allergy. 1993;70:153–157. [PubMed] [Google Scholar]
- 27.Shin JW, Jin SP, Lee JH, Cho S. Analysis of MAST-CLA results as a diagnostic tool in allergic skin diseases. Ann Dermatol. 2010;22:35–40. doi: 10.5021/ad.2010.22.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson HA. Immunologically mediated food allergy: the importance of food challenge procedures. Ann Allergy. 1988;60:262–269. [PubMed] [Google Scholar]
- 29.Wang de Y, Gordon BR, Chan YH, Yeoh KH. Potential non-immunoglobulin E-mediated food allergies: comparison of open challenge and double-blind placebo-controlled food challenge. Otolaryngol Head Neck Surg. 2007;137:803–809. doi: 10.1016/j.otohns.2007.06.714. [DOI] [PubMed] [Google Scholar]
- 30.Pelikan Z, Pelikan-Filipek M. Bronchial response to the food ingestion challenge. Ann Allergy. 1987;58:164–172. [PubMed] [Google Scholar]
- 31.Juhlin L. Recurrent urticaria: clinical investigation of 330 patients. Br J Dermatol. 1981;104:369–381. doi: 10.1111/j.1365-2133.1981.tb15306.x. [DOI] [PubMed] [Google Scholar]
- 32.Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FER, Lemanske RF. Middleton's allergy: principles and Practice. 7th ed. Philadelphia: Mosby; 2009. pp. 1169–1187. [Google Scholar]
- 33.Osterballe M, Mortz CG, Hansen TK, Andersen KE, Bindslev-Jensen C. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20:686–692. doi: 10.1111/j.1399-3038.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 34.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354–359. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- 35.Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. Br J Dermatol. 1998;138:635–638. doi: 10.1046/j.1365-2133.1998.02175.x. [DOI] [PubMed] [Google Scholar]
- 36.Champion RH. Urticaria: then and now. Br J Dermatol. 1988;119:427–436. doi: 10.1111/j.1365-2133.1988.tb03246.x. [DOI] [PubMed] [Google Scholar]
- 37.Atanasković-Marković M, Gavrović-Jankulović M, Jankov RM, Vuèković O, Nestorović B. Food allergy to pork meat. Allergy. 2002;57:960–961. doi: 10.1034/j.1398-9995.2002.23832_5.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim HO, Cho SI, Kim JH, Chung BY, Cho HJ, Park CW, et al. Food hypersensitivity in patients with childhood atopic dermatitis in Korea. Ann Dermatol. 2013;25:196–202. doi: 10.5021/ad.2013.25.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KJ, Cho MS. Transition of Korean meat consumption and consumption trends after modern times-focused on beef and pork. J Korean Soc Food Cult. 2012;27:422–433. [Google Scholar]
- 40.Zuberbier T, Edenharter G, Worm M, Ehlers I, Reimann S, Hantke T, et al. Prevalence of adverse reactions to food in Germany - a population study. Allergy. 2004;59:338–345. doi: 10.1046/j.1398-9995.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 41.Reese I, Zuberbier T, Bunselmeyer B, Erdmann S, Henzgen M, Fuchs T, et al. Diagnostic approach for suspected pseudoallergic reaction to food ingredients. J Dtsch Dermatol Ges. 2009;7:70–77. doi: 10.1111/j.1610-0387.2008.06894.x. [DOI] [PubMed] [Google Scholar]